Take Home Message
In this retrospective cohort study of 1895 patients, 36% of COVID-positive patients, defined as the presence of COVID antibodies on serology or positivity on a polymerase chain reaction test of a nasopharyngeal swab, had clinically and statistically significant de novo or worsening overactive bladder (OAB) symptoms after infection. Of these cases, 22% were de novo OAB. This indicates that patients with prior COVID-19 infection are at greater risk of developing OAB symptoms.
Keywords: COVID-19, Cystitis, SARS-CoV-2, Urinary incontinence, Urinary bladder, Overactive bladder
Abstract
Background
Literature is sparse on COVID-19-associated cystitis (CAC), a novel condition comprising frequency, urgency, and nocturia after COVID-19 infection.
Objective
To determine the incidence of CAC and correlation with SARS-CoV-2 antibody levels.
Design, setting, and participants
This was a retrospective study in which urinary symptoms were scored using the International Consultation on Incontinence Questionnaire-overactive bladder (ICIQ-OAB) at three time points: before the pandemic (January 2020), 2 mo after COVID-19 infection (if applicable), and at the time of the study (May 2021). The setting was a regional health care system. The 18 785 healthcare employees who took part in the BLAST COVID study group were invited to participate, of whom 1895 responded.
Outcome measurements and statistical analysis
The outcome measured was the percentage of COVID-positive patients with a significant change on ICIQ-OAB over time. Pearson’s χ2 test was used for comparison of categorical data, and one-way analysis of variance for continuous data and multivariate analysis. A sample size of 618 was calculated for power of 80% and α = 0.05.
Results and limitations
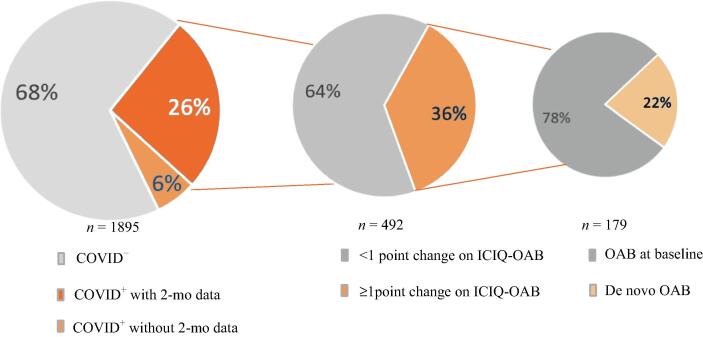
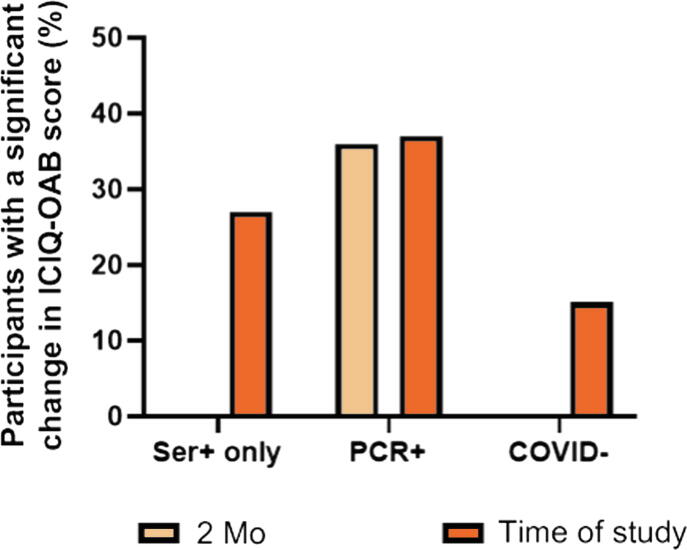
Of the 1895 participants, 31.9% (n = 605) were positive for COVID-19 according to positive serology or a polymerase chain reaction (PCR) test. Of these, 492 were PCR-positive and had 2-mo postinfection data, with 36.4% (179/492) reporting an increase of ≥1 point on the ICIQ-OAB compared to baseline (before the pandemic), with de novo OAB in 22% of these cases (40/179). Comparison of symptoms between baseline and the study time revealed that 27.4% (31/113) of those with positive serology only (asymptomatic COVID) and 37.8% (186/492) of those with PCR positivity (symptomatic COVID) had an increase of ≥1 point on the ICIQ-OAB, compared to 15.8% (n = 204) of uninfected patients, with odds ratios of 2.013 (95% confidence interval [CI] 1.294–3.138; p = 0.0015) and 3.236 (95% CI 2.548–4.080; p < 0.0001), respectively. The retrospective nature of the study and the volunteer sample are limitations.
Conclusions
COVID-19 infection increases the risk of developing new or worsening OAB symptoms.
Patient summary
We compared overactive bladder symptoms in a large group of participants between individuals with and without a previous COVID-19 infection. We found that symptomatic infection was associated with a three times greater risk of developing new or worsening overactive bladder symptoms among COVID-19 patients.
1. Introduction
In late 2019, the first cases of SARS coronavirus-2 (SARS-CoV-2) infection were reported from Wuhan, China [1]. Despite efforts to keep the infection contained, SARS-CoV-2 rapidly spread around the globe, resulting in a pandemic that has impacted the majority of the world. To date, an estimated 611 million people worldwide have been infected with SARS-CoV-2 and approximately 6.52 million people have died of COVID-19 [2]. The most common presenting symptoms of COVID-19 are fever, cough, and shortness of breath [3], although disease presentation can range from asymptomatic to mild to an overwhelming inflammatory response leading to acute respiratory distress syndrome, cytokine release syndrome, and ultimately death. As new variants emerge, such as the delta and omicron variants, symptomatology can vary to include conjunctivitis [4], headache, rhinorrhea, and fatigue [5].
The COVID-19 recovery time ranges from up to 7 d for mild illness to >6 wk for severe or critical cases [6]. However, an estimated 80% of recovered patients have one or more long-lasting symptoms [6]. More than 50 long-term side effects have been reported thus far and include neurocognitive difficulties, metabolic imbalances, gastrointestinal upset, and thromboembolic events [7], [8]. Apart from renal endothelial injury, urological symptoms were not reported in these early studies, suggesting that this is an overlooked area. Several other viruses are known to cause cystitis, such as Epstein-Barr virus, which is implicated in interstitial cystitis, a bladder condition defined by pelvic pain, urinary frequency, and urgency [9], [10]. Infection with human T-cell lymphotropic virus-1 or human immunodeficiency virus can also result in an increase in lower urinary tract symptoms (LUTS) such as frequency, urgency, and nocturia [11], [12]. It has also been shown that adenovirus and BK virus can induce hemorrhagic cystitis, in which patients present with hematuria, urinary urgency, frequency, and dysuria [13], [14]. It is postulated that the pathogenesis with these viral actors involves persistent procytokine release leading to chronic inflammation [9] or reactivation of latent viruses [13]. Early observational studies reported increases in urinary frequency, urgency, and nocturia after COVID-19 infection and termed this condition COVID-associated cystitis (CAC) on the basis of a postulated inflammatory etiology [15]. However, given the clinical symptomatology, COVID-related LUTS could be another suitable descriptive term.
The aim of this study was to establish the incidence of worsening or de novo CAC/COVID-related LUTS in symptomatic versus asymptomatic COVID-infected patients, and to determine the change in International Consultation on Incontinence Questionnaire-overactive bladder (ICIQ-OAB) score in the presence of comorbidities.
2. Patients and methods
2.1. Study population
This was a retrospective observational cohort study conducted with full approval from the Beaumont internal review committee. Individuals from the BLAST COVID study (approval reference 2020-134, ClinicalTrials.gov NCT04349202) were invited to participate in a follow-up study, to which 1895 of the 20 614 invitees responded. At the peak of the COVID-19 outbreak in April 2020, Beaumont Health conducted a large serology study involving 20 614 (BLAST COVID study group) of its 43 000 employees to determine COVID-19 seropositivity and asymptomatic rates among health care workers [16]. Almost half of the workforce participated and had two or more blood draws 2–4 wk apart to test for the presence of anti-SARS-CoV-2 IgG between April 13 and May 28, 2020. We obtained data on demographics, medical history, symptom severity (ranging from asymptomatic to hospitalization), serology status, and antibody levels from this database.
Inclusion criteria for the present study were participation in the BLAST COVID study, age ≥18 yr, and agreement to participate in future studies. Exclusion criteria included decisional impairment and those unable to complete the electronic survey.
2.2. COVID-19 serology testing
Serology results were obtained using a SARS-CoV-2 IgG and IgA serology assay (EUROIMMUN, Lübeck, Germany) running on an automated EURO Lab Workstations. Each EUROIMMUN assay consists of two separate tests: one measures IgG (the memory antibody that should give long-acting resistance in individuals who mount an immune response) and the other measures IgA (the secretory antibody that should protect against infection through mucous membranes). Each test returns a value for the level of antibody in comparison to a control with no antibodies. A significant result is an antibody/control ratio of 1.1 or greater. Internal validation demonstrated specificity and sensitivity (16 d after PCR diagnosis) of 99.35% (95% confidence interval [CI] 97.93–99.86%) and 98.14% (95% CI 97.75–99.22%), respectively.
2.3. Study questionnaires
After study enrollment, participants were retrospectively asked to score their OAB symptoms at three different time points: before the pandemic, 2 mo after their COVID-19 infection (for participants who had a positive PCR history), and at the time of the study (May 2021). Genitourinary symptoms for these time points were assessed using the ICIQ-OAB. The ICIQ-OAB was chosen as a concise grade A validated questionnaire for evaluating both symptom severity and bother. Questions evaluated frequency (“How often do you pass urine during the day?” 0 = 1–6 times; 1 = 7–8 times; 2 = 9–10 times; 3 = 11–12 times; 4 = 13 or more times), nocturia (“During the night, how many times do you have to get up to urinate, on average? 0 = none; 1 = 1 time; 2 = 2 times; 3 = 3 times; 4 = 4 or more times), urgency (“Do you have to rush to the toilet to urinate?” 0 = never; 1 = occasionally; 2 = sometimes; 3 = most of the time; 4 = all of the time), and urge incontinence (“Does urine leak before you can get to the toilet?” 0 = never; 1 = occasionally; 2 = sometimes; 3 = most of the time; 4 = all of the time). Bother scored were also evaluated for each symptom, with responses ranging from 0 (not at all) to 10 (a great deal). With a minimal important difference (MID) of 1 point, the ICIQ-OAB is an ideal tool for evaluating clinically significant changes. MID is defined as the smallest change that is clinically significant [17]. To capture those who were infected and symptomatic, participants were asked “Have you been diagnosed with COVID?” Those who responded in the affirmative were asked to evaluate the status of their symptoms at 2 mo after their COVID infection.
2.4. Statistical analysis
Statistical analysis was conducted using IBM SPSS v28.0 and R. Categorical data (eg, demographics) were analyzed using Pearson’s χ2 test. Continuous data (eg, ICIQ-OAB individual and total symptoms scores) were averaged and the standard deviation (SD) was calculated; these results are reported as the mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA). A p value <0.05 is considered significant. The effect of comorbidities on the change in ICIQ-OAB score stratified by COVID diagnosis was assessed using one-way ANOVA followed by a Tukey post hoc test.
A sample size of 618 COVID-positive individuals was calculated for power of 80% and α = 0.05 with regard to the primary objective, which was the incidence of de novo or worsening OAB symptoms in participants with symptomatic and asymptomatic previous COVID infection. A secondary objective was to assess the change in ICIQ-OAB score for patients with comorbidities.
3. Results
3.1. Study population and demographics
Invitations were sent to 18 785 participants from the BLAST COVID study who indicated they would be willing to partake in future research. Of these, 1895 consented and completed the survey between May 21, 2021 and July 14, 2021 (115 d). The majority of the study participants were female (n = 1548; 81.7%), with 16.5% (n = 312) identifying as male. Most were Caucasian (85.8%), followed by African American (4.1%), Asian (3.8%), Hispanic (1.4%), and other/unknown (2.1%) ethnicities. Overall, only a small proportion of participants had significant comorbidities, of which hypertension was the most common (18.4%). There were no significant demographic differences between the COVID-positive and COVID-negative groups (Table 1).
Table 1.
Demographics of the study cohort stratified by COVID-19 diagnosisa
| Parameter | Patients, n (%) |
p value | ||
|---|---|---|---|---|
| Overall (n = 1895) |
COVID+ (n = 605) |
COVID− (n = 1290) |
||
| Diagnosis | ||||
| COVID− (Ser− and PCR−) | – | 1290 (68)b | ||
| COVID+ (Ser+ and/or PCR+) | 605 (32)b | – | ||
| Ser+ alone (PCR−; asymptomatic) | 113 (18.7)b | – | ||
| PCR+ with/without Ser+ (symptomatic) | 492 (81.3)b | – | ||
| Race | 0.677 | |||
| Asian | 72 (3.8) | 26 (4.3) | 46 (3.6) | |
| Black | 78 (4.1) | 30 (5.0) | 48 (3.7) | |
| Hispanic | 26 (1.4) | 8 (1.3) | 18 (1.4) | |
| White | 1325 (85.8) | 511 (84.5) | 1114 (86.4) | |
| Other/unknown | 94 (5.0) | 30 (4.9) | 64 (5.0) | |
| Sex | 0.733 | |||
| Female | 1548 (81.7) | 499 (82.5) | 1049 (81.3) | |
| Male | 312 (16.5) | 94 (15.5) | 218 (16.9) | |
| Other/unknown | 35 (1.9) | 12 (2.0) | 23 (1.8) | |
| Comorbidities | ||||
| Diabetes | 99 (5.2) | 39 (6.5) | 60 (4.7) | 0.121 |
| Cardiovascular disease | 59 (3.1) | 13 (2.2) | 46 (3.6) | 0.118 |
| Lung disease | 104 (5.5) | 34 (5.6) | 70 (5.4) | 0.914 |
| Kidney disease | – | – | – | – |
| Hypertension | 348 (18.4) | 118 (19.5) | 230 (17.8) | 0.408 |
| Immunodeficiency | 27 (1.4) | 7 (1.2) | 20 (1.6) | 0.678 |
PCR = polymerase chain reaction; Ser = serology; + and − superscripts denote positive and negative status, respectively.
Almost one-third of the cohort was COVID+, defined as positivity on a serology antibody test or a PCR test on a nasopharyngeal swab, or both. Asymptomatic participants were defined as patients with only a positive serology test (Ser+ only), while symptomatic participants were defined as individuals with a positive PCR test, with or without a positive serology test. The p value is based on Fisher’s exact test for squared contingency tables and on a χ2 test for all other nonsquared tables.
Percentage of the overall cohort.
3.2. COVID-19 diagnosis
COVID positivity was defined as either positive serology (serology+) or an affirmative response to the question “Have you been diagnosed with COVID-19?”, denoted as PCR-positive (PCR+), to capture those who may have been infected after the serology tests and were symptomatic. One-third of the study participants (n = 605, 31.9%) were COVID-positive, defined as positive on serology or a PCR test (Table 1). More than 80% (492/605) of the COVID+ cohort were diagnosed via PCR, while 18.7% (113/605) were diagnosed via a positive serology test only.
3.3. Change in OAB symptoms over time
Data on OAB scores at 2 mo after COVID infection were only available for those who reported a previous COVID diagnosis (n = 492; PCR+ only; Fig. 1) as the time of infection was known for this cohort. Thus, this excludes participants whose positive COVID status was on the basis of positive serology alone, who may have had an asymptomatic COVID infection. Of the 492 participants with 2-mo data available, 36.6% (n = 180) reported an increase of ≥1 point in total ICIQ-OAB score compared to baseline (before the pandemic), with a mean score of 6.2 ± 2.5 (range 2–15; Fig. 2, Table 2). For the individual OAB symptoms, the greatest mean increase in score was observed for both frequency (0.8 ± 0.9) and urgency (0.8 ± 0.8), with smaller mean increases for urge urinary incontinence (0.7 ± 0.8) and nocturia (0.6 ± 0.8). The increase in average bother score was similar for all symptoms (Table 2). Of the PCR+ participants, 22% (40/179) had de novo OAB symptoms (Fig. 1). At the time of the study, 37.8% (186/492) of PCR+ cases reported an increase of ≥1 point in total ICIQ-OAB score compared to baseline, with an odds ratio (OR) for de novo or worsening OAB symptoms of 3.236 (95% CI 2.548–4.080; p < 0.0001) versus COVID− participants. In comparison, 27.4% (31/82) of participants who only had serology positivity reported a significant change in OAB symptoms at the time of the study, with an OR of 2.013 (95% CI 1.294–3.138; p = 0.0015; Fig. 2).
Fig. 1.
More than one-third of patients experienced worsening overactive bladder (OAB) symptoms after COVID-19 infection. Out of 1895 respondents, 32% were COVID-positive. Of these, 492 had 2-mo postinfection data, of whom 36.4% (179/492) reported an increase of ≥1 point in OAB symptom score compared to baseline (before the pandemic). In this subgroup, the OAB symptoms were de novo in 22% of cases (40/179). ICIQ = International Consultation on Incontinence Questionnaire.
Fig. 2.
Individuals with COVID positivity are at higher risk of having worsening overactive bladder (OAB) symptoms. The percentage of patients with a significant change in OAB symptom score from baseline (January 2020) to the study time (May 2021) on the ICIQ-OAB are compared for the cohorts. The percentage of patients with a significant changes from baseline to 2 mo after infection is also included for the cohort testing positive on PCR, as the time of infection is known for this group. In comparison to the COVID-negative cohort, the odds ratio for COVID-associated cystitis is 2.013 (95% confidence interval [CI] 1.294–3.138; p = 0.0015) in the cohort with only serology positivity (asymptomatic COVID) and 3.236 (95% CI 2.548–4.080; p < 0.0001) for the PCR-positive cohort (symptomatic COVID). Pearson’s χ2 test was used for statistical analysis. ICIQ-OAB = International Consultation on Incontinence Questionnaire; PCR = polymerase chain reaction; Ser = serology.
Table 2.
Changes in ICIQ-OAB total and subdomain scores from before the COVID-19 pandemic to 2 mo after COVID infection or at the time of the study
| Time point for severity score | Mean score ± standard deviation (range) |
||||
|---|---|---|---|---|---|
| Frequency | Nocturia | Urgency | UUI | Totala | |
| Severity at BL (before the pandemic) | 0.4 ± 0.7 | 1.2 ± 0.5 | 0.9 ± 0.9 | 0.7 ± 0.8 | 3.1 ± 2.1 (1–12)* |
| Severity at 2 mo after COVID infection (n = 179)b | 1.2 ± 1.0 | 1.8 ± 0.9 | 1.7 ± 1.0 | 1.4 ± 1.1 | 6.2 ± 2.5 (2–15)* |
| Change in severity score from BL | 0.8 ± 0.9 (−1 to 3) * |
0.6 ± 0.8 (−1 to 3)* |
0.8 ± 0.8 (−1 to 4)* |
0.7 ± 0.8 (−1 to 3)* |
2.9 ± 2.0 (−1 to 10)* |
| Change in bother score from BL | 2.1 ± 2.7 (−1 to 10)* |
2.3 ± 3.3 (−5 to 10)* |
2.1 ± 3.2 (−5 to 10)* |
2.2 ± 3.5 (−5 to 10)* |
N/A |
| Severity at the time of the study (n = 216)c | 1.3 ± 1.1 | 1.6 ± 0.9 | 1.6 ± 1.0 | 1.3 ± 1.0 | 5.8 ± 2.5 (2 to 15)* |
| Change in severity score from BL | 0.9 ± 0.9 (−1 to 3)* |
0.5 ± 0.7 (0 to 3)* |
0.7 ± 0.8 (−1 to 4)* |
0.6 ± 0.8 (−1 to 3)* |
2.7 ± 2.0 (−1 to 11)* |
| Change in bother score from BL | 1.9 ± 2.7 (−5 to 10)* |
2.4 ± 3.2 (−2 to 10)* |
2 ± 2.9 (−5 to 10)* |
2.2 ± 3.5 (−2 to 10)* |
N/A |
BL = baseline; ICIQ-OAB = International Consultation on Incontinence Questionnaire-overactive bladder; N/A = not applicable; UUI = urge urinary incontinence.
The total score was calculated via a validated methodology as the sum of the symptom severity scores (range 1–16) for all domains without including the bother score.
Participants with a positive polymerase chain reaction test who had 2-mo postinfection data and an increase of ≥1 points for the total ICIQ-OAB score.
Participants with positive COVID status who had an increase of ≥1 points for the total ICIQ-OAB score.
Significant at p < 0.0001 on one-way analysis of variance.
Of the COVID+ cohort, 35.7% (n = 216) had a change of ≥1 point in ICIQ-OAB score from baseline to the time of the study; the mean change was 5.8 ± 2.5 (range 2–15; Fig. 1, Table 2). The greatest increases were for frequency (0.9 ± 0.9; p < 0.0001) and urgency (0.7 ± 0.8; p < 0.0001; Table 2). By contrast, only 15.7% (202/1290) of the COVID− group reported a change of ≥1 point in ICIQ-OAB score (OR 2.99, 95% CI 2.21–4.05; p < 0.001).
3.4. Multivariate analysis of comorbidities
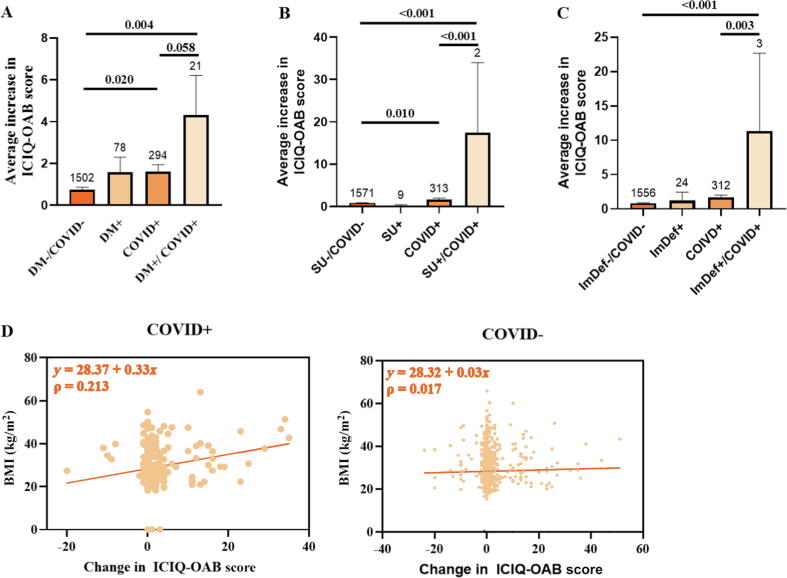
COVID+ patients with baseline diabetes mellitus (p = 0.004) or chronic steroid use (p < 0.001) or on immunosuppression (p < 0.001) were more likely to experience an increase in ICIQ-OAB score than those who were COVID− and without co-morbidities. Body mass index (BMI) was positively correlated with symptom severity in the COVID+ cohort: higher BMI led to worse OAB symptoms (ρ = 0.213; Fig. 3D).
Fig. 3.
COVID-positive patients with (A) baseline diabetes mellitus (DM; p = 0.004), (B) chronic steroid use (SU; p < 0.001), or (C) on immunosuppressive therapy (p < 0.001) were more likely to have an increase in ICIQ-OAB score than those who were COVID+ without comorbidities. (D) BMI was positively correlated with symptom severity in the COVID+ cohort, and patients with a higher BMI were more likely to develop worse overactive bladder symptoms (r = 0.213). Group sizes are indicated above the bars. BMI = body mass index; ICIQ-OAB = International Consultation on Incontinence Questionnaire-overactive bladder; ImDef = immunodeficient.
3.5. Correlation of OAB symptoms with serum antibody levels
The correlation between COVID serology status and OAB symptoms was analyzed. The mean antibody ratio was 1.17 (range 0.05–18.48) for the entire cohort, 3.02 (range 0.07–18.48) for the COVID+ cohort, and 0.31 (range 0.05–1.08) for the COVID− cohort. No correlation was seen between the change in OAB symptoms at 2 mo after infection and antibody levels in the COVID+ cohort (r = 0.01).
4. Discussion
Our study demonstrates CAC incidence of 36.6% among individuals with previous symptomatic COVID-19 infection, which involved de novo OAB in 22% of these cases. Symptomatic COVID infection was associated with a threefold greater risk of having worsening OAB symptoms in comparison to the COVID-negative cohort, while the risk was only doubled for those with asymptomatic COVID. The average change in ICIQ-OAB score for our cohort was an increase of 6.5 points (121% increase) from baseline at the time of the study, with the greatest changes seen for frequency and urgency scores. Those who were COVID+ reported an average urinary frequency of 7–8 episodes/d, with urgency “occasionally” or “sometimes”. This is less severe than reported by Lamb and colleagues [15]: most patients in their study, assessed using the OAB tool [18], had a frequency of ≥13 episodes/24 h (84.6%) and nocturia of ≥4 episodes/night (87.2%). Mumm et al. [19] observed a notable increase in urinary frequency among patients with COVID-19. After treating a male patient for suspected urosepsis who was later confirmed to have a negative urine culture and positive COVID-19 status, the authors retrospectively evaluated urinary symptoms in their COVID-19 cohort and found that 7/57 of their COVID patients had urinary frequency as a presenting symptom. Upper and lower urinary tract bacterial infections as causes were excluded via urine analysis and urinary viral RNA, serum creatinine, and prostate-specific antigen assays [19].
The exact pathophysiology of CAC is yet to be discovered, although others have hypothesized that the increase in systemic inflammation on COVID-19 infection can lead to bladder inflammation and thus bothersome urinary symptoms [15]. It is well established that the primary cause of cystitis can be multifactorial (bacterial, viral, medication, chemicals, radiation, or idiopathic), but once the bladder mucosa is damaged, this can trigger a cascade of events including the release of proinflammatory cytokines. Once released, these cytokines can activate afferent nerves and detrusor muscle contractions that produce bothersome LUTS. This has been shown by Tyagi et al. [20], who reported elevation of urinary cytokines such as MCP-1, sCD40L, IL-12 p70/p40, EGF, and IL-10 in patients with OAB. In those with interstitial cystitis, higher levels of IL-6, IL-8, and growth-related oncogenes were also detected in urine [21]. Analogously, other viruses, such as Epstein-Barr virus, cause solid organ inflammation (hepatitis, gastritis, interstitial cystitis) by inducing T-cell release of cytokines such as IL-8 and chemokine ligands CCL2, CCL3, CCL4, and CCL5 [9], [22]. Specifically for SARS-CoV-2, infection has been linked to multisystem inflammatory responses: elevated IL-6 is the strongest prognostic factor for severity and a novel pediatric condition termed multisystem inflammatory syndrome has been identified [23], [24]. It is also possible that COVID-19 may directly affect urogenital organs given that SARS-CoV-2 binds to ACE2, which is found not only in the lungs, heart, and ileum but also in bladder urothelial cells at high expression levels [25]. The potential impact of COVID-19 on urogenital pathology cannot be understated [26], and although the focus here is not on the mechanism of action, clinical outcomes are reported from the largest cohort to date. It is also likely that our cohort of health care employees has the professional knowledge to accurately answer the questionnaires.
Using data from the BLAST COVID database, the largest COVID serology study to date, we found no correlation between SARS-CoV-2 antibody levels and OAB symptoms among patients with a prior COVID-19 infection. Because the serology data were obtained before vaccinations were approved and available for mass inoculation (December 2020), antibody ratios >1.1 were indicative of infection regardless of symptoms.
Reiterating the current understanding of risks factors for COVID-19 severity, our study shows that high BMI and diabetes are significant prognostic factors for CAC. In a systematic review and meta-analysis, Zheng et al. [27] reported that baseline hypertension, diabetes, and cardiovascular and respiratory diseases portend the highest risk of severe COVID-19 infection in comparison to those with noncritical symptomatology. Wolff et al. [28] reported that smoking and obesity were the most common lifestyle factors found among infected patients. Higher BMI and diabetes are already well established as prognostic factors for OAB, so patients with these conditions are at higher risk of developing CAC.
Our study has several limitations. Our rate of COVID positivity (31.9%) is higher than rates reported in the literature, such as the seropositivity rate of 8.8% (1818/20 614) among health care workers in the BLAST COVID study [16] and the 9.2% seropositive rate among prevaccinated patients in Michigan Medicine, a primary regional health system, in a study by Zhao et al. [29], suggesting that our population may be self-selective. In other words, those who were diagnosed with COVID, had COVID-like symptoms, or were seropositive were more likely to participate. Other limitations include the risk of recall bias given the retrospective nature of the study, which may have limited the extent of symptom reporting. Moreover, a positive correlation between psychological stressors and OAB symptoms has been established [30]. Given the increase in stress related to the pandemic, especially for health care workers [31], it is possible that these are additional confounding factors in the increase in OAB symptoms. Moreover, in our multivariate analysis of comorbidities the numbers of study participants with a positive COVID diagnosis who had chronic steroid use or a suppressed immune system were very low, so the findings for these groups in particular need validation. Finally, our study did not perform urine studies to rule out infectious or inflammatory causes; hence, the term CAC may be a misnomer and COVID-related LUTS may be more accurate. Further research is necessary to better understand this mechanism of action for guiding proper diagnosis and effective treatment of the condition.
5. Conclusions
Our study shows that patients with a prior COVID-19 infection are at greater risk of developing CAC/COVID-related LUTS, with an incidence of 36.6% for patients who had a symptomatic COVID infection. Further work on the natural progression of CAC is ongoing.
Author contributions: Ly Hoang Roberts had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Padmanabhan, Hoang Roberts.
Acquisition of data: Hoang Roberts.
Analysis and interpretation of data: Hoang Roberts, Padmanabhan, Zwaans.
Drafting of the manuscript: Hoang Roberts.
Critical revision of the manuscript for important intellectual content: Hoang Roberts, Padmanabhan, Zwaans, Chancellor, Peters.
Statistical analysis: Zwaans.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Padmanabhan, Chancellor, Peters.
Other: None.
Financial disclosures: Ly Hoang Roberts certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Kenneth M. Peters holds equity in Micron Medical and is a paid consultant for Urogen and Urovant. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: None.
Associate Editor: Silvia Proietti
References
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worldometer. COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus.
- 3.Centers for Disease Control and Prevention. Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- 4.Cheema M., Aghazadeh H., Nazarali S., et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–e129. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacobucci G. Covid-19: Runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ. 2021;375:n3103. doi: 10.1136/bmj.n3103. [DOI] [PubMed] [Google Scholar]
- 6.Raveendran A., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenhalgh T., Knight M., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 9.Jhang J.F., Hsu Y.H., Peng C.W., Jiang Y.H., Ho H.C., Kuo H.C. Epstein-Barr virus as a potential etiology of persistent bladder inflammation in human interstitial cystitis/bladder pain syndrome. J Urol. 2018;200:590–596. doi: 10.1016/j.juro.2018.03.133. [DOI] [PubMed] [Google Scholar]
- 10.Winter B.J., O’Connell H.E., Bowden S., Carey M., Eisen D.P. A case control study reveals that polyomaviruria is significantly associated with interstitial cystitis and vesical ulceration. PLoS One. 2015;10:e0137310. doi: 10.1371/journal.pone.0137310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breyer B.N., Van Den Eeden S.K., Horberg M.A., et al. HIV status is an independent risk factor for reporting lower urinary tract symptoms. J Urol. 2011;185:1710–1715. doi: 10.1016/j.juro.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro N.M., Rodrigues W., Jr, Freitas D.M., Muniz A., Oliveira P., Carvalho E.M. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: evidence of urinary manifestations in large group of HTLV-I carriers. Urology. 2007;69:813–818. doi: 10.1016/j.urology.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Pinto M., Dobson S. BK and JC virus: a review. J Infect. 2014;68:S2–S8. doi: 10.1016/j.jinf.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Mufson M.A., Belshe R.B. A review of adenoviruses in the etiology of acute hemorrhagic cystitis. J Urol. 1976;115:191–194. doi: 10.1016/s0022-5347(17)59130-2. [DOI] [PubMed] [Google Scholar]
- 15.Lamb L.E., Dhar N., Timar R., Wills M., Dhar S., Chancellor M.B. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC) Med Hypotheses. 2020;145:110375. doi: 10.1016/j.mehy.2020.110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims M.D., Maine G.N., Childers K.L., et al. COVID-19 seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. 2021;73(Suppl 2):S154–S162. doi: 10.1093/cid/ciaa1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeschke R., Singer J., Guyatt G.H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 18.Urology Care Foundation. Overactive bladder assessment tool. https://www.urologyhealth.org/educational-resources/overactive-bladder-assessment-tool.
- 19.Mumm J.N., Osterman A., Ruzicka M., et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyagi P., Barclay D., Zamora R., et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42:629–635. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 21.Lamb L.E., Janicki J.J., Bartolone S.N., Peters K.M., Chancellor M.B. Development of an interstitial cystitis risk score for bladder permeability. PLoS One. 2017;12:e0185686. doi: 10.1371/journal.pone.0185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman C.B., Wohlford E.M., Smith N.A., et al. Epstein-Barr virus type 2 latently infects T cells, inducing an atypical activation characterized by expression of lymphotactic cytokines. J Virol. 2015;89:2301–2312. doi: 10.1128/JVI.03001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T., Zhang J., Yang Y., et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoste L., Van Paemel R., Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 2021;180:2019–2034. doi: 10.1007/s00431-021-03993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z.-S., Zhang Z.-Q., Wu S. Focus on the crosstalk between COVID-19 and urogenital systems. J Urol. 2020;204:7–8. doi: 10.1097/JU.0000000000001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff D., Nee S., Hickey N.S., Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., Salerno S., Shi X., Lee S., Mukherjee B., Fritsche L.G. Understanding the patterns of serological testing for COVID-19 pre-and post-vaccination rollout in Michigan. J Clin Med. 2021;10:4341. doi: 10.3390/jcm10194341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai H., Gardner V., Vetter J., Andriole G.L. Correlation between psychological stress levels and the severity of overactive bladder symptoms. BMC Urol. 2015;15:14. doi: 10.1186/s12894-015-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu W., Zhang Y., Wang P., et al. Psychological stress of medical staffs during outbreak of COVID19 and adjustment strategy. J Med Virol. 2020;92:1962–1970. doi: 10.1002/jmv.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]