Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, has infected several animal species, including dogs, presumably via human-to-animal transmission. Most infected dogs reported were asymptomatic, with low viral loads. However, in this case we detected SARS-CoV-2 in a dog from the North African coastal Spanish city of Ceuta presenting hemorrhagic diarrhea, a disease also reported earlier on in an infected dog from the USA.
Case presentation
In early January 2021, a West Highland Terrier pet dog from Ceuta (Spain) presented hemorrhagic diarrhea with negative tests for candidate microbial pathogens. Since the animal was in a household whose members suffered SARS-CoV-2 in December 2020, dog feces were analyzed for SARS-CoV-2, proving positive in a two-tube RT-PCR test, with confirmation by sequencing a 399-nucleotide region of the spike (S) gene. Furthermore, next-generation sequencing (NGS) covered > 90% SARS-CoV-2 genome sequence, allowing to classify it as variant B.1.177. Remarkably, the sequence revealed the Ile402Val substitution in the spike protein (S), of potential concern because it mapped in the receptor binding domain (RBD) that mediates virus interaction with the cell. NGS reads mapping to bacterial genomes showed that the dog fecal microbiome fitted best the characteristic microbiome of dog’s acute hemorrhagic diarrhea.
Conclusion
Our findings exemplify dog infection stemming from the human SARS-CoV-2 pandemic, providing nearly complete-genome sequencing of the virus, which is recognized as belonging to the B.1.177 variant, adding knowledge on variant circulation in a geographic region and period for which there was little viral variant characterization. A single amino acid substitution found in the S protein that could have been of concern is excluded to belong to this category given its rarity and intrinsic nature. The dog’s pathology suggests that SARS-CoV-2 could affect the gastrointestinal tract of the dog.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-022-03453-8.
Keywords: SARS-CoV-2, B.1.177, Ile402Val S protein substitution, Dog COVID-19, Zoonosis, One Health
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged on a wholesale market in Wuhan (China) on December, 2019 [1]. It is assumed to be a zoonotic pathogen that could have originated in non-clarified wild animals and transmitted to humans via a yet undetermined intermediate host [2], which led to a global pandemic through human-to-human transmission [3]. In addition to humans, human-related infections have been reported in several carnivore species of domestic pets and captive animals, including several species of felines (cats, tigers, lions, snow leopards, a cougar, an Amur leopard and a fishing cat) and mustelids (ferrets, American mink and Asian small-clawed otters), dogs, spotted hyenas and a binturong [4]. SARS-CoV-2 was also detected in omnivorous and herbivorous zoo animals, such as gorillas, a South American coati, hippos [4] and Antillean manatees [5]. It is interesting to note that it has also reached non-captive animals, having been detected in free-ranging white-tailed deer [6], feral American mink [7] and European wild otter [8].
Among pets, ferrets and cats appear to be those most vulnerable to contagion [9]. Natural infections of these animals in most cases were asymptomatic or caused mild disease [10–13]. However, a cat was reported [14] to develop more serious disease, with severe respiratory distress and thrombocytopenia, and had to be humanely euthanized. Regarding dogs, their susceptibility to SARS-CoV-2 infection is considered lower than that of felines due to poor viral replication in this species [9]. The first two dogs reported [15] as SARS-CoV-2-positive were detected early in the COVID-19 pandemic, in Hong Kong, living with infected people. They were asymptomatic, had relatively low viral loads, and their viral sequences were identical to those found in their respective owners. Later on, other dogs infected with prevalent SARS-CoV-2 variants have been detected throughout the world [4]. As already indicated, the dogs generally were asymptomatic or presented mild disease, although respiratory, gastrointestinal and cardiac complication, sometimes severe, have also been reported in these carnivores [16–21]. Of interest because of the symptomatic similarity with the present case, was the finding of hemorrhagic diarrhea in an infected dog in the USA with low viral load (13 copies RNA/μl) [16].
We now report a dog with hemorrhagic diarrhea presenting SARS-CoV-2 infection revealed by RT-PCR, Sanger DNA sequencing of a partial 399-nt region of the S gene for the SARS-CoV-2 spike protein, and next generation sequencing (NGS) of >90% of the viral genome, which allowed its identification as belonging to the B.1.177 variant. Interestingly, both sequencing methodologies revealed the Ile402Val substitution in the S protein sequence, of potential concern since it affected the Receptor Binding Domain (RBD) of S. In addition, NGS reads for genomes of fecal bacteria have also provided insight on the fecal microbiome of this dog, which agrees with that for canine acute hemorrhagic diarrhea [22].
Case presentation
In early January 2021, a well-cared properly vaccinated and regularly wormed 11-year-old West Highland Terrier pet dog in a family in the North African Autonomous Spanish city of Ceuta started producing pasty stools suggestive of mucous enteritis which, after a few days, transformed into persistent mucohemorrhagic enteritis with pasty bloody stools. Physical examination was unremarkable and did not substantiate altered body temperature, the presence of swollen lymph nodes or pain upon abdominal palpation, while cardiac and pulmonary auscultation were normal. Complete blood cell count (CBC) and routine blood biochemistry were uninformative, and stool examination and analysis did not reveal gross steatorrhea, amylorrhea, or the presence of common parasites (Giardia, nematodes, cestodes or coccidia). Colonoscopy showed a moist pink mucosa, with a capillary refill time (CRT) < 2 seconds and no ulcers, tumors or other gross macroscopic pathological alterations. Histopathological analysis of a mucosal biopsy sample revealed areas of superficial erosion of the epithelium lining the lumen and a moderate inflammatory infiltrate throughout the lamina propria and between the intestinal crypts of the colic mucosa. The infiltrating cells were essentially mononuclear, largely lymphocytes with some plasma cells, leading to the histopathological diagnosis of lymphoplasmacytic colitis of grade II with superficial focal erosions.
Since the dog was in close contact with human household members who suffered symptomatic and RT-PCR-proven COVID-19 at the end of December 2020, a stool sample from the dog was used for SARS-CoV-2 assay. Total fecal RNA was isolated (see Supplementary Methods) and was used first in a one-step commercial SARS-CoV-2 RT-PCR test (Viasure, CerTest Biotec, Zaragoza, Spain) with negative results. It was also used in a more sensitive two-tube RT-PCR assay ([7] and Supplementary Methods) whose results were consistently and repeatedly positive. RNA from the stools from other four dogs studied in parallel were negative in both assays. These results replicated our own previous findings [7] with two feral mink in which the commercial test was negative but the two-tube test was positive. This two-tube assay focuses on a partial 399 base pairs (bp) sequence of the SARS-CoV-2 viral spike glycoprotein gene (S) (Figs. 1 and 2, top). The low load of viral RNA reflected in the high Ct value (average of 32.95 in six different repeats of the test, three of them on each one of two separate RNA extractions and retrotranscription assays from the same stool) was consistent with the negativity of the commercial test, as previously experience in mink [7]. The positivity of the two-tube test was corroborated physically by subsequent visualization by agarose gel electrophoresis of the amplified product, which was of the expected size, and by Sanger sequencing, which yielded the expected sequence for the intended amplicon (Figs. 1 and 2, top).
Fig. 1.
Alignment of the partial S gene sequences. The sequences shown are, from top to bottom, the following ones: consensus early Wuhan (GenBank ID: NC_045512.2); present dog fecal sequence (in bold; GenBank and GISAID IDs: MW872017 and EPI_ISL_1490671, respectively); the positive control used (GISAID ID: EPI_ISL_433320); the variants indicated with their trivial and canonic designations (Alpha, GISAID ID: EPI_ISL_581117; Beta, GISAID ID: EPI_ISL_660605; Gamma, GISAID ID: EPI_ISL_792680). Bases exhibiting complete conservation are marked at the bottom by asterisks. The base deviations from the Wuhan sequence are green-shadowed and squared. The alignment was performed using Bio-Edit [23]
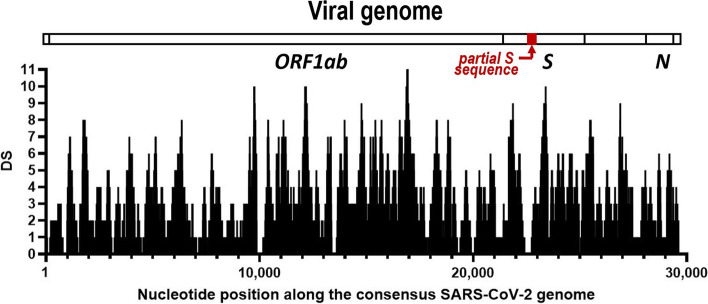
Fig. 2.
SARS-CoV-2 genomic coverage obtained by Next-generation sequencing (NGS). The horizontal axis corresponds to the position of the SARS-CoV-2 consensus genome and the vertical one to the depth of the sequence (DS) at a given position. On the top, aligned over the nucleotide positions, the bar shows a linear diagram of the entire genome, showing the span of the ORF1ab, spike (S) and nucleocapsid (N) genes as well as the region amplified of the S gene that was subjected to Sanger sequencing (in black, labeled “partial S sequence”)
Alignment with the SARS-CoV-2 consensus sequence (GenBank ID NC_045512.2) of the partial S gene sequence obtained from our dog (GenBank accession number MW872017) revealed two nucleotide changes relative to the reference sequence (Wuhan original sequence) (Fig. 1). These changes, at positions 40 and 342 of the amplicon, were the A>G and T>C transitions (genome nucleotides 22766 and 23068, Figs. 1 and 2, top; S gene coding sequence positions 1204 and 1506, at codons 402 and 502, respectively). Only the first of these two substitutions was non-synonymous, causing the amino acid change Ile402Val in the S protein, a change that maps in the receptor binding domain (RBD) of this protein. The RBD is the part of the spike protein that interacts with the angiotensin-converting enzyme 2 (ACE2) cell receptor for SARS-CoV-2 [24], stressing the possibility that amino acid changes in this domain could affect the ability of the virus to infect cells.
Despite de low Ct value most likely reflecting a low viral load in the analyzed fecal sample, we attempted NGS on the isolated RNA (see Supplementary Methods). This approach yielded 623,796 reads, but, after application of the quality and sequence length filters indicated in the Supplementary Methods, and following removal of 54,085 reads that mapped with the dog genome, 116,637 high-quality reads remained (given the high number of small reads). These high-quality reads reflected the entire population of microorganisms present in the stool sample, with only 1,290 of them mapping to the SARS-CoV-2 consensus genome (Fig. 2). Independently of the sequencing depth (number of times each position was found in the SARS-CoV-2 mapped reads) these reads covered 90.6% of the entire viral genome, with just 2,813 positions of the 29,903 nucleotides of the whole genome not covered. Bioinformatic analysis revealed a number of nucleotide substitutions relative to the consensus SARS-CoV-2 genome sequence (Table 1). Submission of the sequence to GISAID (https://www.gisaid.org/) resulted in the assignment of the virus to the B.1.177 variant. Indeed, the sequence hosted the three characteristic mutations of this variant [25], Ala222Val, Ala220Val and Val30Leu in the S, N and 10 proteins, respectively (Table 1). It is to be stressed that the two nucleotide substitutions detected by Sanger sequencing (see above) were also detected by NGS.
Table 1.
Base substitutions and corresponding amino acid substitutions in the genome of the SARS-CoV-2 virus found in the infected dog of this study
| Genome Positiona | Gene | Base changeb | Protein | Non-Synonymous substitution |
|---|---|---|---|---|
| 241 | 5' UTR | C > T | - | - |
| 445 | ORF1ab | T > C | NSP1 | - |
| 3037 | C > T | NSP3 | - | |
| 6286 | C > T | - | ||
| 14408 | C > T | NSP12 | Pro323Leu | |
| 15313 | G > A | Ala625Thr | ||
| 15418 | G > T | Ala660Ser | ||
| 21255 | G > C | NSP16 | - | |
| 21776 | S | G > C | S | Gly72Arg |
| 22227 | C > T | Ala222Val | ||
| 22766 | A > G | Ile402Val | ||
| 23068 | T > C | - | ||
| 23403 | A > G | Asp614Gly | ||
| 24034 | C > T | - | ||
| 26530 | M | A > G | M | Asp3Gly |
| 27944 | ORF8 | C > T | 8 | - |
| 28083 | G > T | Glu64STOP | ||
| 28378 | N | G > T | N | - |
| 28703 | G > C | Asp144His | ||
| 28932 | C > T | Ala220Val | ||
| 29645 | ORF10 | G > T | 10 | Val30Leu |
aPosition in the consensus genome (GenBank NC_045512.2)
bSubstitution relative to the base in the consensus genome. As the sequencing was based on the genome retrotranscribed to DNA, thymine should correspond to uracil in the viral RNA genome
For completeness of the analysis, we assessed the phylogenetic relation of the near-complete viral genome sequence with those of other SARS-CoV-2 variants that have infected humans, dogs or other animals (Fig. 3). The phylogenetic tree shows that all SARS-CoV-2 variants were included in the same cluster, different from the clusters in which non-SARS-CoV-2 coronavirus sequences were found. Furthermore, our sequence was more closely related to the B.1.177 variant than to other variants, again supporting its classification as belonging to the B.1.177 variant.
Fig. 3.
Phylogenetic analysis. Maximum Likelihood Tree based on the complete SARS-CoV-2 genome. The analysis was carried out as reported [7] and is detailed in Supplementary Methods. The evolutionary history was inferred by the Neighbor-Joining method. The optimal tree is shown. The percentage of trees in which the associated taxa clustered together is shown next to branches. The tree is drawn to scale, with branch lengths proportional to the number of substitutions per site. This analysis involved 34 nucleotide sequences. There were a total of 32,122 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 [26]. The name of each sequence is composed of four elements separated by vertical lines: the host where the virus was isolated; the virus strain name (for the SARS-CoV-2 sequences isolated from animals other than humans, countries where they were found are shown in brackets); the specific SARS-CoV-2 variant that they belonged to; and the GenBank or GISAID Accession ID. Bold names indicate sequences belonging to the B.1.177 variant. The black dot (●) marks the present dog SARS-CoV-2 sequence
On the other hand, the Ile402Val substitution of the S protein, which is not specific of the B.1.177 variant, has been reported in only 316 SARS-CoV-2 sequences of the GISAID database (accessed on May 17, 2022). Fifteen of these occurrences correspond to genomes of the B.1.177.4 variant, which is closely related to the B.1.177 variant, sharing the three specific mutations of this last variant (see above). These B.1.177.4 sequences were collected in England between October and November 2020, before the detection of the virus in our dog (January 2021), but none of them arose from Spain or Morocco (the country of our dog and the land region surrounding Ceuta, respectively).
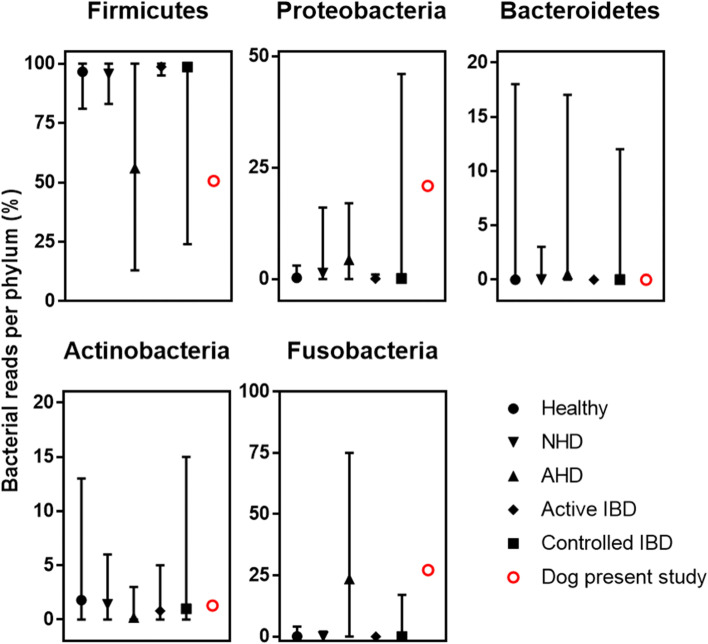
We also exploited the NGS data to estimate the abundance in the stool of bacteria belonging to the five major phyla of Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria and Fusobacteria. This information could be gathered because the vast majority of the >115,347 high-quality reads remaining after subtraction of the reads mapping to the dog and SARS-CoV-2 genomes were sequences mapping to microbial genomes, mostly to bacterial genomes (see Supplementary Methods). We compared our results (Fig. 4) with those reported by Suchodolski et al. [22] on 32 healthy dogs or on dogs with signs of either acute non-hemorrhagic diarrhea (NHD; n = 12), acute hemorrhagic diarrhea (AHD, n = 13), active inflammatory bowel disease (Active IBD; n = 9), or therapeutically controlled clinically insignificant inflammatory bowel disease (Controlled IBD; n = 10). In our dog, the distribution of reads among bacteria pertaining to these phyla, as percentage of recognized reads for bacteria from these phyla, was 50.7, 20.9, 0, 1.3 and 27.1 for Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria and Fusobacteria, respectively. Except for Proteobacteria, the proportion of reads in our dog closely resembled the median frequency of reads for these phyla in the AHD dog group, clearly distinguishing the intestinal microbiota of our dog from those of healthy dogs, of dogs with non-hemorrhagic diarrhea or of those with active inflammatory bowel disease.
Fig. 4.
Comparison of fecal abundance of main bacterial phyla in the present dog with the abundances reported [22] in dogs with acute diarrhea and inflammatory bowel disease. For details on microbiome monitoring, see the text and the Supplementary Methods. Data from our dog stool are shown in red. All other results are from [22]. NHD, acute non-hemorrhagic diarrhea (n = 12); AHD, acute hemorrhagic diarrhea (n = 13); IBD, inflammatory bowel disease (active disease, n = 9; therapeutically controlled and thus clinically insignificant, n = 10). The symbols give the median for the percent abundance in each dog, and the whiskers give the range
Discussion and Conclusions
This report supports the notion that dogs can be SARS-CoV-2-infected when intimately related with COVID-19 affected humans [15, 16]. The presentation of clinically relevant hemorrhagic diarrhea, and the previous description of the same pathology in an unrelated dog that was reported to be infected with SARS-CoV-2 [16] raises the possibility that this intestinal alteration, considered related to digestive tract microbial dysbiosis [27], was favored or even caused by SARS-CoV-2. The fact that the proportions of five major bacterial phyla in the fecal microbiome of our dog do not substantially differ from those in dogs with acute hemorrhagic diarrhea [22] does not support the occurrence of a specific fecal microbiome disturbance for SARS-CoV-2 infection. In any case, our results, together with those from another dog with SARS-CoV-2 infection and hemorrhagic diarrhea [16], call for a study of the potential connection between SARS-CoV-2, dog hemorrhagic diarrhea, and promotion of the bacterial dysbiosis associated with this diarrhea. After all, there is already the precedent that the canine respiratory coronavirus (CRCoV) affects the dog gastrointestinal tract [28].
The virus found in the feces of this dog by RT-PCR amplification, partial Sanger sequencing of the S gene and almost complete NGS genome sequencing, is clearly proven to be SARS-CoV-2 by clustering of this sequence, in a molecular phylogenetic tree, with SARS-CoV-2 virus variants, whereas it was more distant from other human or canine coronaviruses. Furthermore, it was classified as B.1.177 variant. This variant has circulated widely in many countries of Europe including Spain [25], being the most prevalent SARS-CoV-2 variant in the Balearic Islands between August 2020 and February 2021 [29], the period in which the dog of this study was infected (January 2021).
Thus, from the antecedent of human infection in the family hosting this pet dog and the intense contact between Ceuta and peninsular Spain, the dog likely was infected from his human hosts, who may have been infected by the then widely circulating in Spain B.1.177 variant. Unhappily, the SARS-CoV-2 virus that infected the humans cohabiting with this pet was not sequenced, in line with the low viral genomic sequencing in Ceuta in 2020 and early 2021 [30, 31] as well as in Morocco in the same period [32].
On the other hand, the Ile402Val substitution was apparently unsuccessful in spreading, given its lack of widespread reporting until now, more than one and a half years after its occurrence in the fecal specimen analyzed here. This is attested by the fact that, among the almost 11 million sequences collected in the GISAID database, this substitution has only been detected in 317 sequences (including the one from our dog). It is true that, in principle, the Ile402Val substitution, because it maps in the RBD, could affect virus/receptor interactions. However, the mildness of the amino acid substitution involved may dispel the possibility of a drastic effect. Both Ile and Val are branched hydrophobic aliphatic amino acids differing only in one carbon length in their side chains, leading to the consideration of the Ile>Val substitutions as a conservative replacement. This would agree with the lack of success in spreading of viral variants hosting this substitution, finally making unlikely the conceivable possibility that this substitution is a change of concern.
In conclusion, our results not only add another example for the view that dogs can be SARS-CoV-2-infected when intimately related with COVID-19 affected humans, but they also provide evidence of B.1.177 variant circulation in a North African city in a period for which data were very limited in the city’s region. We also virtually exclude that the Ile402Val substitution in the S protein present in the virus infecting this dog, which conceivably could be a variant of concern, actually posed a particular threat. The presentation of clinically relevant hemorrhagic diarrhea calls for further studies to evaluate the implication of SARS-CoV-2 in this canine pathology.
Supplementary Information
Additional file 1. Supplementary methods.
Acknowledgements
We thank to Helen Warburton for proofreading and editing the English language.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- AHD
Acute hemorrhagic diarrhea
- CBC
Complete blood cell count
- COVID-19
Coronavirus disease 2019
- CRT
Capillary refill time
- HCoV-HKU1
Human coronavirus HKU1
- HCoV-OC43
Human coronavirus OC43
- IBD
Inflammatory bowel disease
- INCLIVA
Health Research Institute of the Hospital Clínico de Valencia
- MERS-CoV
Middle East respiratory syndrome coronavirus
- NGS
Next-generation sequencing
- NHD
Acute non-hemorrhagic diarrhea
- RBD
Receptor binding domain
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SARSr-CoV
Severe acute respiratory syndrome related coronavirus
Authors’ contributions
SV, CM, EM and CR-G conceived the study, AM carried out the veterinary examination and collected the stool sample, MP-B and TL did the molecular analyses, MP-B, CI and VR performed bioinformatic analyses, LE advised in all the steps and contributed to data analyses and to extraction of conclusions, and MP-B, EM, VR and CR-G were responsible for writing the paper, although all authors contributed to this task, making substantial intellectual contributions, and having read, corrected and approved the manuscript.
Funding
This research received external funding to CR-G and EM from “AgenciaValenciana de Innovación: COVID-19. Ayudas de concesión directa a soluciones científico-innovadoras directamente relacionadas con la lucha contra la COVID-19,” (Ref COVID-19-203); from the “Conselleria de Innovación Universidades, Ciencia y Sociedad Digital: Subvenciones a grupos de investigación emergentes” (Ref, GV/2021/163); and from a grant to VR (PID2020-120322RB-C21) from the Spanish Agencia Estatal de Investigación (Plan Estatal de I+D+i). These funding bodies had no role in the design of the study, in the collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The partial 399-bp sequence of SARS-CoV-2 S gene detected in the dog’s stool sample has been deposited in the NCBI GenBank under the Accession Number MW872017 and in the GISAID database with the Accession ID EPI_ISL_1490671. The complete genome sequence was also submitted to GISAID (Accession ID EPI_ISL_12695538). Finally, all raw sequencing data from the dog’s stool sample obtained by NGS have been submitted to the NCBI Sequence Read Archive (SRA) repository, under the BioProject accession number PRJNA810957.
Declarations
Ethics approval and consent to participate
The animal study was reviewed and approved by the Animal Ethics Committees of UCH-CEU University (research permit no. CEEA 21/007). Written informed consent was obtained from the owners for the participation of their animals in this study.
Consent for publication
Written consent to publish this case report was obtained from the owner of the dog.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Santiago Vega, Email: svega@uchceu.es.
Vicente Rubio, Email: rubio@ibv.csic.es.
Consuelo Rubio-Guerri, Email: consuelo.rubio@uchceu.es.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez-Morales AJ, MacGregor K, Kanagarajah S, Patel D, Schlagenhauf P. Going global – travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:101578. doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OIE, 2022. https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/#ui-id-3. Accessed on 17 May 2022.
- 5.Lopes Melo F, Bezerra B, Oliveira Luna F, Navarrete Barragan NA, Loyo Arcoverde RM, Umeed R, et al. Coronavirus (SARS-CoV-2) in Antillean manatees (Trichechus manatus manatus). Res Square. 2022. 10.21203/rs.3.rs-1065379/v1.
- 6.Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602:481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguiló-Gisbert J, Padilla-Blanco M, Lizana V, Maiques E, Muñoz-Baquero M, Chillida-Martínez E, et al. First description of SARS-CoV-2 infection in two feral American mink (Neovison vison) caught in the wild. Animals (Basel) 2021;11:1422. doi: 10.3390/ani11051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padilla-Blanco M, Aguiló-Gisbert J, Rubio V, Lizana V, Chillida-Martínez E, Cardells J, et al. The finding of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) in a wild Eurasian river otter (Lutra lutra) highlights the need for viral surveillance in wild mustelids. Front Vet Sci. 2022;9:826991. doi: 10.3389/fvets.2022.826991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garigliany M, Laere A-SV, Clercx C, Giet D, Escriou N, Huon C, et al. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg Infect Dis. 2020;26:3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giner J, Villanueva-Saz S, Tobajas AP, Pérez MD, González A, Verde M, et al. SARS-CoV-2 seroprevalence in household domestic ferrets (Musterla putorius furo) Animals (Basel) 2021;11:667. doi: 10.3390/ani11030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gortázar C, Barroso-Arévalo S, Ferreras-Colino E, Isla J, de la Fuente G, Rivera B, et al. Natural SARS-CoV-2 infection in kept ferrets, Spain. Emerg Infect Dis. 2021;27:1994–1996. doi: 10.3201/eid2707.210096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sailleau C, Dumarest M, Vanhomwegen J, Delaplace M, Caro V, Kwasiborski A, et al. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound Emerg Dis. 2020;67:2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segalés J, Puig M, Rodon J, Avila-Nieto C, Carrillo J, Cantero G, et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc Natl Acad Sci U S A. 2020;117:24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sit THC, Brackman CJ, Ip SM, Tam KWS, Law PYT, To EMW, et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferasin L, Fritz M, Ferasin H, Becquart P, Corbet S, Gouilh MA, et al. Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet Rec. 2021;189(e944). [DOI] [PMC free article] [PubMed]
- 17.van Aart AE, Velkers FC, Fischer EAJ, Broens EM, Egberink H, Zhao S, et al. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound Emerg Dis. 2021. 10.1111/tbed.14173. [DOI] [PMC free article] [PubMed]
- 18.Calvet GA, Pereira SA, Ogrzewalska M, Pauvolid-Corrêa A, Resende PC, Tassinari WDS, et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS One. 2021;16:e0250853. doi: 10.1371/journal.pone.0250853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamer SA, Ghai RR, Zecca IB, Auckland LD, Roundy CM, Davila E, et al. SARS-CoV-2 B.1.1.7 variant of concern detected in a pet dog and cat after exposure to a person with COVID-19, USA. Transbound Emerg Dis. 2022;69:1656–1658. doi: 10.1111/tbed.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barroso-Arévalo S, Rivera B, Domínguez L, Sánchez-Vizcaíno JM. First detection of SARS-CoV-2 B.1.1.7 variant of concern in an asymptomatic dog in Spain. Viruses. 2021;13:1379. doi: 10.3390/v13071379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miró G, Regidor-Cerrillo J, Checa R, Diezma-Díaz C, Montoya A, García-Cantalejo J, et al. SARS-CoV-2 infection in one cat and three dogs living in COVID-19-positive households in Madrid, Spain. Front Vet Sci. 2021;8:779341. doi: 10.3389/fvets.2021.779341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchodolski JS, Markel ME, Garcia-Mazcorro JF, Unterer S, Heilmann RM, Dowd SE, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7:e51907. doi: 10.1371/journal.pone.0051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 24.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 25.Hodcroft EB, Zuber M, Nadeau S, Vaughan TG, Crawford KHD, et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2020;595:707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeineldin M, Aldridge B, Lowe J. Dysbiosis of the fecal microbiota in feedlot cattle with hemorrhagic diarrhea. Microb Pathog. 2018;115:123–130. doi: 10.1016/j.micpath.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 28.Haake C, Cook S, Pusterla N, Murphy B. Coronavirus infections in companion animals: virology, epidemiology, clinical and pathologic features. Viruses. 2020;12:1023. doi: 10.3390/v12091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Cusapé C, Fraile-Ribot PA, Jiménez-Serrano S, Cabot G, Del Barrio-Tofiño E, Prado MC, et al. A genomic snapshot of the SARS-CoV-2 pandemic in the Balearic Islands. Front Microbiol. 2022;12:803827. doi: 10.3389/fmicb.2021.803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comas I, González F (2020) SeqCOVID, genomic epidemiology of SARS-CoV-2 in Spain. https://seqcovid.csic.es/. Accessed 17 May 2022.
- 31.NextSpain: Spanish site for Nextstrain: consortium for genomic epidemiology of pathogens. (2020) FISABIO Sequencing and Bioinformatics Service. http://seqcovid.csic.es/nextspain/. Accessed 17 May 2022.
- 32.Badaoui B, Sadki K, Talbi C, Salah D, Tazi L. Genetic diversity and genomic epidemiology of SARS-CoV-2 in Morocco. Biosaf Health. 2021;3:124. doi: 10.1016/j.bsheal.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary methods.
Data Availability Statement
The partial 399-bp sequence of SARS-CoV-2 S gene detected in the dog’s stool sample has been deposited in the NCBI GenBank under the Accession Number MW872017 and in the GISAID database with the Accession ID EPI_ISL_1490671. The complete genome sequence was also submitted to GISAID (Accession ID EPI_ISL_12695538). Finally, all raw sequencing data from the dog’s stool sample obtained by NGS have been submitted to the NCBI Sequence Read Archive (SRA) repository, under the BioProject accession number PRJNA810957.