Abstract
The proper extracytoplasmic localization of proteins is an important aspect of mycobacterial physiology and the pathogenesis of Mycobacterium tuberculosis. The protein export systems of mycobacteria have remained unexplored. The Sec-dependent protein export pathway has been well characterized in Escherichia coli and is responsible for transport across the cytoplasmic membrane of proteins containing signal sequences at their amino termini. SecA is a central component of this pathway, and it is highly conserved throughout bacteria. Here we report on an unusual property of mycobacterial protein export—the presence of two homologues of SecA (SecA1 and SecA2). Using an allelic-exchange strategy in Mycobacterium smegmatis, we demonstrate that secA1 is an essential gene. In contrast, secA2 can be deleted and is the first example of a nonessential secA homologue. The essential nature of secA1, which is consistent with the conserved Sec pathway, leads us to believe that secA1 represents the equivalent of E. coli secA. The results of a phenotypic analysis of a ΔsecA2 mutant of M. smegmatis are presented here and also indicate a role for SecA2 in protein export. Based on our study, it appears that SecA2 can assist SecA1 in the export of some proteins via the Sec pathway. However, SecA2 is not the functional equivalent of SecA1. This finding, in combination with the fact that SecA2 is highly conserved throughout mycobacteria, suggests a second role for SecA2. The possibility exists that another role for SecA2 is to export a specific subset of proteins.
Mycobacterium tuberculosis is the causative agent of tuberculosis and represents a severe health threat throughout the world. Nearly three million people die each year from tuberculosis (51). M. tuberculosis is an intracellular pathogen that is able to survive and grow in macrophages. Secreted and cell surface-associated proteins are ideally positioned to act on the host macrophage and enable survival of the bacillus in this normally hostile antimicrobial environment. In support of this hypothesis, the exported repetitive protein (Erp) has been shown to participate in the pathogenesis of M. tuberculosis (2). An erp mutant of M. tuberculosis is attenuated in mice and exhibits a significantly reduced ability to grow in macrophages. However, the specific role of Erp in the intracellular survival strategy of M. tuberculosis is unknown.
Protein export is an important aspect of bacterial pathogenesis. Research on diverse bacterial pathogens has demonstrated that the majority of virulence factors are extracytoplasmic proteins (20, 29). Recently, the importance of protein export pathways to pathogenicity has been underscored by the identification of specialized secretion pathways (types I, II, III, and IV) in numerous bacterial pathogens. There are many examples of these types of export pathways being encoded in pathogenicity islands and being required for the proper localization of specific virulence factors (28).
Surprisingly little is known about the protein export pathways of mycobacteria (4). In contrast, the protein export pathways of other bacteria are better characterized, most notably in Escherichia coli (11, 15). The Sec-dependent protein export pathway is responsible for the transport of the majority of extracytoplasmic proteins in E. coli; consequently, this export system represents an essential function of the cell (16). The proteins translocated across the cytoplasmic membrane by the Sec pathway are initially synthesized as precursor proteins containing conserved amino-terminal signal sequences. These precursor proteins are routed to the translocase, the channel through which translocating proteins travel across the membrane. During translocation, the signal sequence is cleaved off by a signal peptidase to generate the mature exported protein. The translocase core is composed of integral membrane proteins SecY and SecE and the peripheral membrane protein SecA. SecA plays a central and essential role in this export pathway. It is an ATPase that provides energy for protein translocation. In addition, SecA binds to nearly all of the components of the Sec pathway, including precursor proteins, chaperones such as SecB, acidic phospholipids in the membrane, and integral membrane components of the translocase (15, 33, 43). Through cycles of ATP binding and hydrolysis, SecA delivers bound precursor proteins to the translocase and undergoes cycles of membrane insertion and deinsertion that lead to stepwise export of the protein (18, 42, 50). Additional proteins, SecG and the complex of SecD, SecF, and YajC, are not essential to Sec-dependent export but serve to increase the overall efficiency of the process (14). The Sec-dependent export pathway is highly conserved among different bacteria (39, 43).
In order to begin understanding the protein export pathways that operate in mycobacteria, we set out to identify and characterize SecA. To our surprise, there are two homologues of secA in mycobacteria, referred to as secA1 and secA2. This was an unexpected finding, as no other fully sequenced genome, among those available at the time, had revealed the existence of two secA genes in a single organism. In this report, we present a genetic analysis in Mycobacterium smegmatis, a fast-growing and nonpathogenic mycobacteria, that demonstrates that the two mycobacterial secA genes are not redundant and that both function in the cell.
Very recently, the Staphylococcus aureus and Streptococcus pneumoniae genomes provided two additional examples of bacteria that possess two secA genes (24, 27, 48). The presence of two SecA proteins may represent a new type of specialized protein export pathway.
MATERIALS AND METHODS
Bacterial strains and culture methods.
All of the strains used in this work are described in Table 1. E. coli strain DH5α was used for DNA cloning procedures. For culturing of E. coli, Luria-Bertani (LB) broth or agar (Difco) was used. When required, the following antibiotics were used for E. coli strains: kanamycin at 40 μg/ml and carbenicillin at 50 μg/ml. A variety of media were employed for the growth of M. smegmatis and included all of the following: Middlebrook 7H9 broth (Difco) with 0.2% glycerol, 0.5% dextrose, and 0.1% Tween 80; the same medium supplemented with 0.2% glycerol, 1× ADS (0.5% bovine serum albumin, fraction V [Boehringer Mannheim], 0.2% dextrose, 0.85% NaCl), and 0.1% Tween 80; LB broth with 0.1% Tween 80; 7H10 agar with 0.2% dextrose and 0.05% Tween 80; LB agar with 0.2% dextrose; and Mueller-Hinton agar (Difco). When appropriate, sucrose was incorporated into 7H10 plates at a concentration of 4.5%. When required, the following antibiotics were used to grow M. smegmatis strains: kanamycin at 20 μg/ml and hygromycin B at 50 μg/ml. When appropriate, cultures were grown overnight in Middlebrook 7H9 with 0.2% glycerol, 1× ADS, 0.1% Tween 80, and 100 μM sodium azide.
TABLE 1.
Strains used in this study
| Strain | Description | Reference |
|---|---|---|
| E. coli K-12 DH5α | F− [φ80dΔlacZM15] Δ(lacZYA-argF) U169 deoR recA1 endA1 hsdR17 glnV44 thi-1 gyrA96 relA1 | Gibco Life Technologies |
| M. smegmatis | ||
| mc2155 | ept-1 | 45 |
| MB526 | mc2155::pMB128, secA1 single-crossover strain | This work |
| MB573 | mc2155::pMB160, secA2 single-crossover strain | This work |
| mc22522 | mc2155, ΔsecA2 | This work |
| mc22718 | mc2155, attB::fbpB-Tn552 ′phoA′ oriE1 kan | 5 |
| mc22724 | mc2155, attB::pepA-Tn552 ′ phoAl oriE1 hyg | 5 |
| mc22725 | mc2155, attB::rv1566c-Tn552 ′phoA-oriE1-kan | 5 |
| mc22757 | mc2155 (pMB174), expresses ′phoA | 5 |
| MB509 | mc2155(pMV261) | This work |
| MB624 | mc2155(pMB196), expresses a pepA-′phoA fusion | This work |
| MB625 | mc22522(pMB196), expresses a pepA-′phoA fusion | This work |
| MB634 | mc2155(pMB202), expresses a rv1566c-′phoA fusion | This work |
| MB635 | mc22522(pMB202), expresses a rv1566c-′phoA fusion | This work |
| MB648 | mc2155(pMB206), expresses a fbpB-HA-′phoA fusion | This work |
| MB649 | mc22522(pMB206), expresses a fbpB-HA-′phoA fusion | This work |
DNA methodologies.
Standard molecular biology techniques for cloning were employed as previously described (41). Restriction enzymes and Vent polymerase were obtained from New England Biolabs, Inc. The Expand high fidelity PCR system was obtained from Boehringer Mannheim. Dimethyl sulfoxide (1.0 to 10.0%) was included in selected PCRs. DNA sequencing was performed by conventional and automated methods. Automated sequencing employed the Applied Biosystems Big Dye Terminator Cycle Sequencing kit (Perkin-Elmer) and an Applied Biosystems 377 sequencer. Sequence assembly was carried out with the MacVector and Assembly Align software (Genetics Computer Group).
Plasmid construction.
The plasmids used in this study are described in Table 2.
TABLE 2.
Plasmids used in this study
| Plasmid | Phenotype/genotype | Description | Source |
|---|---|---|---|
| pKSII+ | bla ColE1 | Stratagene | |
| pSKII+ | bla ColE1 | Stratagene | |
| pCR2.1 | bla aph ColE1 | Invitrogen | |
| pMV261.kan | aph Phsp60 oriM ColE1 | Multicopy mycobacterial shuttle plasmid | 46 |
| pMV306.kan | aph int attP ColE1 | Single-copy mycobacterial shuttle plasmid | 46 |
| pMV361.hyg | hyg Phsp60int attP ColE1 | Single-copy mycobacterial shuttle plasmid | 46 |
| pYUB412 | bla hyg int attP ColE1 cosλ | Single-copy mycobacterial shuttle plasmid | S. Bardarov and W. R. Jacobs |
| pYUB282 | bla ColE1 cosλ | V. Balasubramanian and W. R. Jacobs | |
| pMD31 | aph oriM ColE1 | Multicopy mycobacterial shuttle plasmid | 12 |
| pMB198 | hyg int attP ColE1 | Phsp60 deleted from pMV361.hyg | This work |
| pYUB657 | bla hyg Phsp60-sacB ColE1 | Counterselectable suicide vector for mycobacteria | 37 |
| pYUB495 | bla secA1 (M. smegmatis) ColE1 cosλy | pYUB282 containing M. smegmatis secA1 | This work |
| pYUB536 | bla secA1 (M. smegmatis) ColE1 | 4.0-kb BamHI fragment containing M. smegmatis secA1 from pYUB495 cloned in pKSII+ | This work |
| pYUB544 | aph Phsp60-secA1 oriM ColE1 | 4.0-kb BamHI fragment from pYUB536 cloned into pMV261; M. smegmatis secA1 under control of hsp60 promoter | This work |
| pH.3 | bla secA1 (M. bovis BCG) ColE1 | pKSII+ containing an 8.0-kb fragment with M. bovis BCG secA1 | This work |
| pYUB499 | aph secA1 (M. bovis BCG) oriM ColE1 | 3.0-kb HindIII-KpnI fragment of M. bovis BCG secA1 cloned into pMD31 | This work |
| pYUB538 | aph secA1AzR (M. bovis BCG) oriM ColE1 | pYUB499 with secA1 azide resistance allele | This work |
| pMB117 | bla secA1 (M. smegmatis) ColE1 | 12.0-kb SacI fragment containing M. smegmatis secA1 from pYUB495 cloned into pKSII+ | This work |
| pMB120 | bla ΔsecA1 (M. smegmatis) ColE1 | In-frame deletion mutant form of secA1 derived from pMB117 | This work |
| pMB125 | bla ΔsecA1 (M. smegmatis) ColE1 | SacI fragment of pMB120 subcloned into pSKII+ | This work |
| pMB128 | bla hyg Phsp60-sacB ΔsecA1 (M. smegmatis) ColE1 | ΔsecA1 from pMB125 cloned into pYUB657 | This work |
| p6-1 | bla secA2 (M. smegmatis) ColE1 | pYUB412 cosmid containing M. smegmatis secA2 | This work |
| pMB144 | bla aph secA2 (M. tuberculosis) ColE1 | M. tuberculosis secA2 cloned into pCR2.1 | This work |
| pMB147 | aph Phsp60-secA2 (M. tuberculosis) oriM ColE1 | M. tuberculosis secA2 from pMB144 cloned into pMV261 under control of Phsp60 | This work |
| pMB148 | bla secA2 (M. smegmatis) ColE1 | 6.0-kb BamHI fragment from p6-1 containing M. smegmatis secA2 cloned into pSKII+ | This work |
| pMB155 | bla secA2 (M. smegmatis) ColE1 | 3.2-kb BamHI-ScaI fragment of pMB148 carrying M. smegmatis secA2 cloned into pKSII+ | This work |
| pMB156 | bla ΔsecA2 (M. smegmatis) ColE1 | In-frame deletion of secA2 derived from pMB155 | This work |
| pMB160 | bla hyg Phsp60-sacB ΔsecA2 (M. smegmatis) ColE1 | ΔsecA2 cloned from pMB156 into pYUB657 | This work |
| pMB162 | aph Phsp60-secA2 (M. tuberculosis) int attP ColE1 | HindIII-XbaI fragment carrying Phsp60-secA2 from pMB147 subcloned into pMV306.kan | This work |
| pMB110 | aph Phsp60-′phoA oriM ColE1 | ′phoA PCR product cloned under control of Phsp60 | This work |
| pMB174 | aph Phsp60-′phoA int attP ColE1 | NotI fragment of pMB110 carrying Phsp60-′phoA cloned into pMV306.kan | This work |
| pMB175 | aph secA1 (M. bovis BCG) int attP ColE1 | HindIII/Asp718 M. bovis BCG secA1 containing fragment cloned into pMV306.kan | This work |
| pMB196 | aph pepA::Tn552 ′phoA oriE1 hyg int attP ColE1 | pepA::′phoA fusion recovered as NheI fragment from mc22724 cloned into pMB198 | This work |
| pMB202 | hyg rv1566c::Tn552 ′phoA oriE1 kan int attP ColE1 | rv1566c::′phoA fusion recovered as NarI fragment from mc22725 cloned into pMB198 | This work |
| pMB203 | hyg fbpB::Tn552 ′phoA oriE kan int attP ColE1 | fbpB::′phoA fusion recovered as NarI fragment from mc22718 cloned into pMB198 | This work |
| pMB206 | hyg fbpB-HA::Tn552 ′phoA oriE1 kan int attP ColE1 | HA tag cloned in frame into fbpB::′phoA fusion in pMB203 | This work |
| pMB207 | bla aph secA2 (M. smegmatis) ColE1 | M. smegmatis secA2 cloned into pCR2.1 | This work |
| pMB208 | aph Phsp60-secA2 (M. smegmatis) oriM ColE1 | M. smegmatis secA2 from pMB207 cloned into pMV261 under control of Phsp60 | This work |
Cloning and sequencing of secA1 homologues.
Degenerate oligonucleotide primers were designed to regions of identity in the secA genes of E. coli, Bacillus subtilis, and Pavlova lutherii. The 5′ primer (5′-ACN-GGN-GAX-GGN-AAX-ACN-YT-3′) was designed from the peptide TGEGKT (amino acids 104 to 109 of the E. coli sequence), and the 3′ primer (5′-AXX-TAX-TCX-AAN-CC-3′) was designed from the peptide GFDYL (amino acids 183 to 187 of the E. coli sequence), where N = GAT or C, X = G or A, and Y = C or T. These primers were employed in a touchdown PCR (40) to amplify 250-bp products from M. bovis BCG and M. smegmatis genomic DNAs.
To clone the full-length genes, the resulting PCR products were used as probes to screen the appropriate libraries. The M. bovis BCG product was used to screen a pKSII+::M. bovis BCG library (7). One hybridizing clone, pH.3, was subjected to exonuclease digestion to generate a collection of nested deletions, by using the Erase-A-Base system (Promega), that were then sequenced. The M. smegmatis product was used to screen a pYUB282::mc2155 cosmid library (kind gift of V. Balasubramanian). A 4.0-kb fragment from one hybridizing clone, pYUB495, was subcloned into pKSII+ to generate pYUB536. Nested deletions of pYUB536 were constructed with the Erase-A-Base system and sequenced.
Cloning and sequencing of secA2 homologues.
Full-length M. tuberculosis secA2 was amplified by PCR from M. tuberculosis H37Rv genomic DNA by using primers SecAS14 (5′ TCGGATC-CAGCGGAACACCCCGGGCAGACT) and SecAS15 (5′GCGGATCCAGTGCACGGTTGTCCACGAATTGC). The PCR product was cloned into the pCR2.1 vector by using the TA cloning kit (Invitrogen) to generate pMB144. This PCR product was used to probe a pYUB412::M. smegmatis genomic cosmid library (kind gift of F. C. Bange) under low-stringency conditions. A 6.0-kb fragment from one hybridizing clone, p6-1, was subcloned into pSKII+ to generate pMB148. Nested deletions of pMB148 were constructed with the Erase-A-Base system and used to generate the sequence of secA2 (M. smegmatis).
To clone M. smegmatis secA2 under the control of the constitutive hsp60 promoter, the gene was amplified by PCR from M. smegmatis mc2155 genomic DNA. The primers used were SmSecA2-14 (5′ACGGATCCA-GTGGCGAATGAGTCCTGGCGAAC) and SmSecA2-13 (5′CGTCTTCCATGCCTAGAACCTAATG). The resulting PCR product was cloned in pCR2.1 to create pMB207. The BamHI fragment containing secA2 was then subcloned into pMV261 to create pMB208.
Construction of ΔsecA suicide plasmids. (i) ΔsecA1 suicide plasmid pMB128.
An approximately 12.0-kb SacI fragment of pYUB495 containing secA1 of M. smegmatis was identified by Southern analysis. Restriction digest analysis showed that secA1 is located near the middle of this fragment. This fragment was cloned into pKSII+ to generate plasmid pMB117. pMB117 was linearized with BglII (a unique site near the middle of secA1) and then subjected to exonuclease III digestion in both directions outward from the BglII-cut site by using the Erase-A-Base system and self-ligated. One of the resulting deletion constructs, pMB120, was analyzed by PCR and sequencing analysis and shown to contain a 1,278-bp in-frame deletion of the secA1 gene. The large SacI fragment of pMB120 was subcloned into pSKII+ to create pMB125. The in-frame unmarked secA1 deletion fragment was then cloned into counterselectable suicide vector pYUB657 (37). This was achieved by cutting pMB125 with SpeI and ScaI and cloning the resulting secA1 fragment into SpeI- and ScaI-cut pYUB657. The final vector produced was pMB128.
(ii) ΔsecA2 suicide plasmid pMB160.
A 3.2-kb BamHI-ScaI fragment of pMB148 was cloned into BamHI-EcoRV-cut pKSII+ to generate pMB155. pMB155 was used as the template in an inverse PCR using primers SmSecA2-5 (5′-ACTGATATCGCGCAGCACGTCGAAGCCGAT-3′) and SmSecA2-6 (5′-TGAGATATCGTCGAGCGCCGCGAGACCCT-3′) (underlined residues denote the EcoRV site). The resulting PCR product was gel purified, cut with EcoRV, and self-ligated. The resulting vector was pMB156. Due to primer locations in secA2, pMB156 contains an in-frame deletion of 1,296 bp in secA2. A SpeI-ScaI secA2-containing fragment of pMB156 was subcloned into SpeI-ScaI-cut pYUB657. The final vector was pMB160.
Electroporation of M. smegmatis.
M. smegmatis strain mc2155 (45) was electroporated as previously described (36).
Two step allelic exchange to create ΔsecA mutants of M. smegmatis.
To construct ΔsecA1 mutants, pMB128 was electroporated into mc2155 and hygromycin-resistant transformants were selected. Individual transformants were subjected to Southern analysis, and 14 out of the 16 strains evaluated contained the suicide vector integrated into the secA1 region of the chromosome by means of a single-crossover event. One of these strains, MB526, was employed in the subsequent steps. For the second homologous recombination event, MB526 was first grown to saturation in 7H9 medium with hygromycin at 50 μg/ml. For MB526 strains that additionally carry kanamycin resistance element-containing plasmids, kanamycin was included in this medium. This culture was subcultured at a 1:100 dilution into the same medium lacking hygromycin and incubated overnight. Dilutions were plated onto 7H10 plates containing 4.5% sucrose to select for sucrose-resistant colonies. To obtain ΔsecA2 mutants, pMB160 was electroporated into mc2155 and hygromycin-resistant transformants were obtained. Three out of three transformants were confirmed to be the result of a single-crossover event by Southern analysis. One of these clones, MB573, was used for the subsequent work presented in this report. The second homologous recombination event was selected by using the protocol described above for MB526.
Southern analysis.
To analyze secA1 recombinants, genomic DNA was isolated from M. smegmatis clones as previously described (10) and digested with BglII. The probe used was a 669-bp MscI fragment of pYUB536 containing secA1 of M. smegmatis. To analyze secA2 recombinants, genomic DNA was isolated and digested with BamHI. The probe used was a 1.9-kb BamHI-HindIII fragment obtained from pMB156 that contains secA2 of M. smegmatis. Southern analysis was performed as previously described (41), and probes were labeled with [32P]dCTP using the Ready-to-Go Labeling kit (Pharmacia).
Construction of ′phoA fusion vectors.
fbpB-′phoA, pepA-′phoA, and rv1566c-′phoA fusions were rescued from strains mc22718, mc22724, and mc22725, respectively. These strains contain M. tuberculosis DNA-′phoA fusions that encode active PhoA fusion proteins in M. smegmatis and were recently obtained from transposon libraries (5). These phoA fusions are present on cosmids integrated into the chromosome. To rescue the fusions, genomic DNA was isolated from each strain and digested with NarI (for mc22718 and mc22725) or NheI (for mc22724), self-ligated, and transformed into DH5α. Due to the presence of an E. coli origin of replication on the transposon, these self-ligated molecules are replicative plasmids in E. coli. These restriction enzymes cut outside of the phoA fusion and promoter element. Once isolated, the fusions were subcloned onto a mycobacterial shuttle vector, pMB198, that contains the attP/int L5 mycobacteriophage integration attachment system, which promotes integration into the chromosome in single copy (25).
Isolation of azide-resistant alleles of M. bovis BCG SecA.
Plasmid pYUB499 was subjected to N-methyl-N′-nitro-N-nitrosoguanidine (Sigma) mutagenesis (30). Mutagenized DNA was electroporated into mc2155, and azide-resistant cells were selected on 7H10 agar plates containing sodium azide at 25 μg/ml. This concentration of sodium azide was chosen on the basis of azide sensitivity disk assays that determined the minimum amount of sodium azide capable of inhibiting growth of M. smegmatis on 7H10 agar plates.
Azide sensitivity disk assays.
Cells were grown in LB to saturation. A 0.1-ml volume of saturated culture was mixed with molten LB top agar and plated on an LB plate. Seven-millimeter-diameter paper filter disks (Schleicher & Schuell) were then placed on the hardened top agar. To each disk, 10 μl of 0.3 M sodium azide was added. The zone of sensitivity was calculated as the diameter of clearing around the disk minus the diameter of the disk.
Preparation of whole-cell protein extracts from M. smegmatis and Western analysis.
Strains were grown to an A600 of 0.5 to 1.0 in 7H9–1× ADS–glycerol–0.1% Tween 80–kanamycin at 20 μg/ml. The same number of cells, 1.2 × 109, was harvested by centrifugation. The cells were washed twice in phosphate-buffered saline–0.02% Tween 80, pelleted by centrifugation, quick-frozen in a dry-ice –ethanol bath, and stored at −20°C. At a later time, the pellets were resuspended in 200 μl of extraction buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 0.6% sodium dodecyl sulfate [SDS], 10 μg/ml aprotinin, E-64 at 10 μg/ml, leupeptin at 10 μg/ml, Pefabloc SC at 500 μg/ml, pepstatin A at 10 μg/ml) to which 200 μl of 106-μm glass beads was added. The cells and beads were then vortexed twice for 5 min at 4°C with a 5-min rest on ice. A 200-μl volume of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer was added to each sample. The samples were denatured by boiling, and 20 μl was loaded onto an SDS-PAGE gel. The proteins were transferred to nitrocellulose, and Western analysis to detect PhoA or hemagglutinin (HA) was carried out with anti-PhoA polyclonal antibodies obtained from 5 Prime-3 Prime, Inc., or anti-HA monoclonal antibodies obtained from Covance, Inc.
Nucleotide sequence accession numbers.
The nucleotide sequences reported here have been submitted to the GenBank database and assigned the following accession numbers: M. smegmatis secA1, U66081; M. smegmatis secA2, AF287049; M. bovis BCG, U66080.
RESULTS
Cloning of mycobacterial secA homologues.
At the start of this study, a minimal number of secA genes had been cloned. By using available sequences from E. coli, B. subtilis, and P. lutherii, degenerate oligonucleotide primers were designed to a region of identity in the amino termini of these proteins (see Materials and Methods). These primers were used to amplify an approximately 250-bp PCR product from both M. bovis BCG and M. smegmatis genomic DNAs. The resulting PCR products were used to probe appropriate M. bovis BCG and M. smegmatis genomic libraries and led to the cloning and sequencing of secA homologues from these two mycobacterial species. These first secA genes we identified are named secA1. M. bovis BCG secA1 and M. smegmatis secA1 encode proteins with predicted sizes of 106 and 107 kDa, respectively. Subsequently, a secA1 homologue (rv3240c) was identified in the complete genome sequence of M. tuberculosis (8). Each of these secA1 genes encodes a protein with significant sequence similarity to SecA1 in other mycobacterial species and to the SecA proteins of other bacteria (Table 3). Southern analysis with secA1 probes revealed hybridizing restriction fragments in the genomic DNAs of all of the mycobacterial species tested. This Southern analysis was consistent with secA1 being a single-copy gene; there was no indication that another secA gene existed in the mycobacterial genome (data not shown).
TABLE 3.
Amino acid similarities between M. tuberculosis SecA proteins and selected SecA homologues
| Protein | Accession no. | % Similarity to M.
tuberculosisa
|
|
|---|---|---|---|
| SecA1 | SecA2 | ||
| Mycobacterium tuberculosis SecA1 | Z95121 | 100.0 | 50.2 |
| Mycobacterium bovis BCG SecA1 | U66080 | 98.6 | 50.8 |
| Mycobacterium smegmatis SecA1 | U66081 | 88.0 | 50.2 |
| Mycobacterium leprae SecA1 | P57996 | 90.4 | 48.5 |
| Mycobacterium tuberculosis SecA2 | Z78020 | 50.2 | 100.0 |
| Mycobacterium smegmatis SecA2 | AF287049 | 50.2 | 86.4 |
| Mycobacterium leprae SecA2 | O32922 | 50.1 | 90.8 |
| Corynebacterium glutamicum SecA | D17428 | 74.9 | 46.1 |
| Streptomyces coelicolor SecA | U21192 | 73.4 | 49.1 |
| Synechococcus sp. strain PCC7942 SecA | Q55357 | 63.6 | 44.8 |
| Staphylococcus aureus SecA-1 | BAB41941 | 63.3 | 51.2 |
| Staphylococcus aureus SecA-2 | BAB43747 | 49.9 | 46.5 |
| Listeria monocytogenes SecA | P47847 | 61.1 | 46.5 |
| Streptococcus pneumoniae SecA-1 | AE005672 | 60.2 | 50.8 |
| Streptococcus pneumoniae SecA-2 | AE005672 | 52.3 | 47.8 |
| Escherichia coli SecA | P10408 | 60.9 | 49.2 |
| Bacillus subtilis SecA | P28366 | 60.6 | 49.0 |
| Pisum sativum SecA | Q41062 | 59.5 | 46.8 |
| Caulobacter crescentus SecA | P38380 | 59.4 | 50.1 |
| Actinobacillus actinomycetemcomitans SecA | AF116183 | 59.2 | 48.4 |
| Haemophilus influenzae SecA | U32772 | 58.9 | 48.4 |
| Neisseria meningitidis MC58 SecA | AAF41891 | 58.2 | 47.7 |
| Helicobacter pylori SecA | O25475 | 57.3 | 47.7 |
| Pavlova lutherii SecA | QO1570 | 55.5 | 46.1 |
| Borrelia burgdorferi SecA | OO7497 | 54.6 | 47.6 |
| Mycoplasma genitalium SecA | P47318 | 50.9 | 44.1 |
Percent similarity was determined by Gap Comparison with GCG.
Based on the above-described work and the fact that the other fully sequenced bacterial genomes that were available at the time contained only one secA homologue, we were surprised to see a second and distinct secA homologue present in the genome sequence of M. tuberculosis (8). This second secA homologue has been named secA2. M. tuberculosis secA2 corresponds to open reading frame rv1821 and encodes a protein with a predicted size of 89 kDa. By Southern analysis, we identified secA2-hybridizing fragments in the H37Rv and Erdman strains of M. tuberculosis, M. bovis BCG, and M. smegmatis (data not shown). We used the M. tuberculosis secA2 gene as a probe with which to screen an M. smegmatis genomic library to clone and sequence M. smegmatis secA2. The predicted size of M. smegmatis SecA2 is 87 kDa.
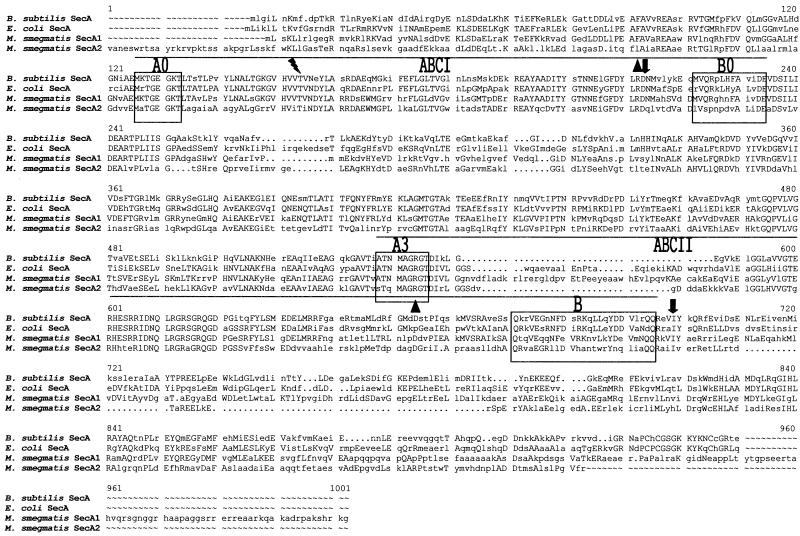
Reproducibly, when a secA1 or secA2 probe was used for Southern analyses (high and low stringency in nature) only a single secA gene, corresponding to the probe used, was identified (data not shown). This is consistent with the difference in nucleotide sequence (50%) between the two secA genes. At the amino acid level, SecA1 and SecA2 are only 50% similar. An alignment of the two predicted proteins reveals that SecA2 is a smaller protein with a shorter carboxy terminus (Fig. 1). E. coli SecA contains two ATP-binding sites (ABCI and ABCII) that are important to SecA function (31). Both SecA1 and SecA2 exhibit strong homology to the proposed Walker A and B motifs present in these sites (Fig. 1), suggesting that both might have physiological functions similar to those of E. coli SecA.
FIG. 1.
Alignment of SecA proteins from B. subtilis, E. coli, and M. smegmatis (SecA1 and SecA2). ABCI (high-affinity ATP-binding site) and ABCII (low-affinity ATP-binding site) are indicated. The lightning bolt indicates the threonine residue that can be mutated to produce azide resistance. Triangles represent the deletion junction points in the ΔsecA1 mutation. The arrows pointing down identify the deletion junction points in the ΔsecA2 mutation.
Determination of the essentiality of secA1 and secA2.
In other bacteria in which SecA has been studied, SecA is essential and encoded by a single-copy gene (17, 34, 43). One explanation for the presence of two secA genes in mycobacteria is that they encode redundant functions. In this scenario, deletion of either secA gene alone would not be lethal because the remaining homologue would compensate for the absence of the other. To determine the essential nature of each SecA protein in mycobacteria, we tested whether it is possible to delete either secA gene.
We used a two-step allelic-exchange strategy to determine the essentiality of each secA gene. This approach has previously been used in M. smegmatis (22, 36). In the first step, a suicide vector containing an in-frame deletion of a given secA gene, a selectable hyg (encoding hygromycin resistance) marker, and a counterselectable sacB marker is integrated into the chromosome by means of a single homologous recombination event between the secA allele on the vector and the wild-type secA allele in the chromosome. The resulting recombinant strain is referred to as a single-crossover strain, and it contains a tandem duplication of the secA region (both a mutated and a wild-type allele are present in the chromosome) separated by vector sequences including sacB and hyg (diagrams of such strains are shown in Fig. 2B and 3B). The second step is to select recombinants that have undergone a second homologous recombination event between the two secA alleles. Depending on the site of this second recombination event, it leaves either a mutant or a wild-type allele in the chromosome. This second recombination event also eliminates the intervening vector sequences, including sacB and hyg. Expression of sacB results in sensitivity to sucrose; therefore, these secondary recombinants are selected as sucrose-resistant colonies (38). The failure to obtain recombinants in which the secA gene is deleted strongly suggests that it is an essential gene. If the inability to delete the chromosomal secA gene is overcome in merodiploid strains that contain an extra copy of secA, it demonstrates the absolute requirement of that secA gene for viability.
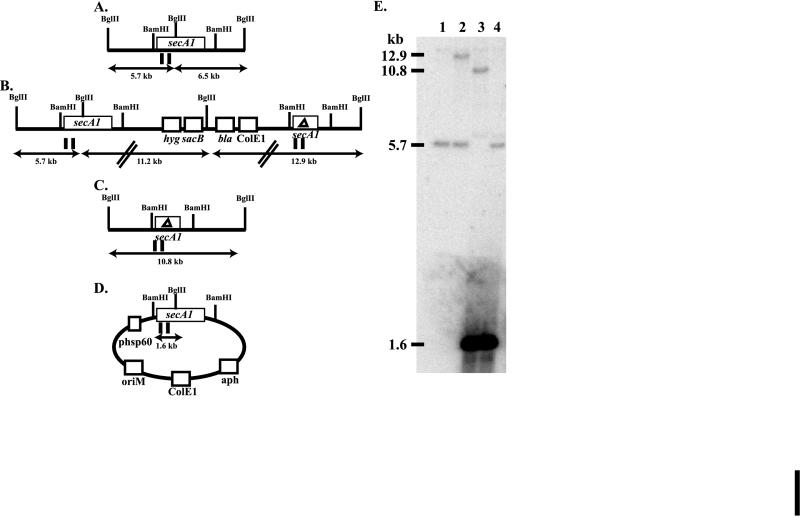
FIG. 2.
Southern analysis of secA1 recombinants in M. smegmatis. (A to D) The relevant BglII and BamHI restriction endonuclease sites are denoted. The MscI probe used in this Southern analysis is represented by vertical lines below the diagram. Panels: A, diagram of the chromosomal wild-type secA1 allele; B, diagram of secA1 single-crossover strain MB526; C, diagram of a chromosomal ΔsecA1 allele; D, diagram of pYUB544, a multicopy plasmid present in merodiploid strains; E, Southern blot of BglII-digested genomic DNAs probed with the secA1 probe. Sizes of fragments are indicated on the left. Lanes: 1, mc2155; 2, MB526; 3, ΔsecA1 recombinant with pYUB544; 4, wild-type recombinant with pYUB544.
secA1 is essential in M. smegmatis.
We first applied this approach to the secA1 gene of M. smegmatis. Suicide plasmid pMB128 contains a 1,278-bp in-frame deletion within M. smegmatis secA1. This deletion will generate a mutated protein comprising 531 amino acids (in comparison to the wild-type protein of 957 amino acids). Importantly, the deletion involves both ATP-binding sites (Fig. 1). This plasmid was electroporated into M. smegmatis, and hygromycin-resistant transformants were obtained. One of these transformants, MB526, was used in the subsequent experiments. MB526 is the product of a single-crossover event between the secA1 deletion allele on pMB128 and the wild-type secA1 allele on the chromosome, as demonstrated by Southern analysis (Fig. 2B and E).
Following the growth of MB526 in the absence of hygromycin, sucrose-resistant colonies were selected and then screened for hygromycin sensitivity. Sucrose-resistant and hygromycin-sensitive colonies have undergone a second recombination event between the two secA1 alleles. The individual secA1 allele remaining in the resulting recombinants was assayed by PCR and Southern analysis. All of the recombinants assayed contained the wild-type allele (Table 4). This failure to obtain secA1 deletion mutants strongly suggests that deletion of secA1 in M. smegmatis is a lethal event.
TABLE 4.
Secondary recombination products produced from single-crossover strains
| Strain (plasmid) | % secA allele present
among secondary recombinants
|
na | |
|---|---|---|---|
| Deletion | Wild type | ||
| secA1 single crossover | |||
| MB526 | 0 | 100 | 13 |
| MB526(pMV261) | 0 | 100 | 9 |
| MB526(pYUB544) | 62 | 38 | 16 |
| MB526(pYUB499) | 59 | 41 | 39 |
| MB526(pMB208) | 0 | 100 | 20 |
| MB526(pMB147) | 0 | 100 | 24 |
| MB526(pMB162) | 0 | 100 | 19 |
| secA2 single crossover | |||
| MB573 | 31 | 69 | 13 |
| MB575 | 50 | 50 | 18 |
Number of sucrose-resistant and hygromycin-sensitive recombinants analyzed.
To confirm this interpretation, we created secA1 merodiploid strains by transformation of MB526 with plasmids expressing secA1 of M. smegmatis and M. bovis BCG (pYUB544 and pYUB499, respectively). From these merodiploid single-crossover strains, sucrose-resistant and hygromycin-sensitive recombinants were obtained. In contrast to the results obtained with starting strain MB526 or MB526 transformed with control vector pMV261, these merodiploid strains produced two classes of recombinants: ΔsecA1 recombinants and wild-type secA1 recombinants (Table 4; Fig. 2). From these results, we concluded that secA1 is an essential gene in M. smegmatis.
secA2 cannot functionally replace secA1.
The essentiality of secA1 in M. smegmatis suggested that secA1 and secA2 are not redundant genes. However, if secA2 is not expressed under these experimental conditions, secA1 and secA2 might still have the same function. To address this possibility, we tested whether a ΔsecA1 strain can be rescued by secA2 expression. Single-crossover strain MB526 was independently electroporated with the plasmids pMB208, pMB147, and pMB162, which all carry the secA2 gene under control of the constitutive hsp60 promoter. Vector pMB208 is a multicopy vector expressing M. smegmatis secA2, while pMB147 and pMB162 are multicopy and single-copy vectors, respectively, that express M. tuberculosis secA2. Secondary recombination products were selected from these recombinant strains. None of these secA2 expression constructs enabled the production of ΔsecA1 mutants (Table 4).
secA2 is not essential in M. smegmatis.
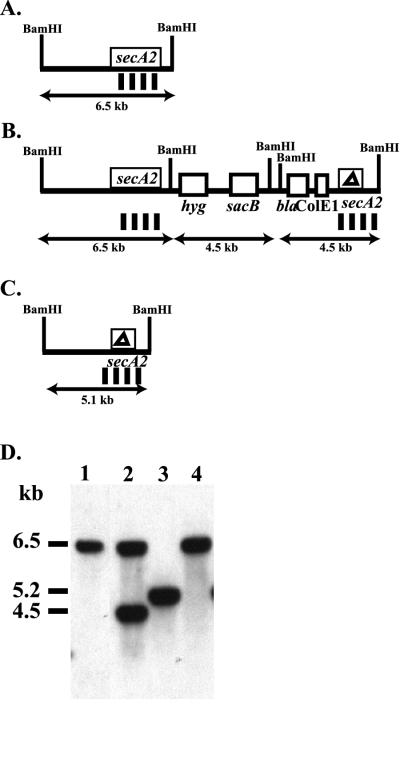
The essential nature of secA2 in M. smegmatis was also evaluated. Suicide plasmid pMB160 contains an in-frame deletion of 1,296 bp within M. smegmatis secA2. This deletion produces a mutated protein of 366 amino acids (in comparison to the wild-type protein of 798 amino acids). Once again, the ATP-binding sites are included in the deletion (Fig. 1). By using pMB160, single-crossover strain MB573 was constructed (Fig. 3). From MB573, sucrose-resistant and hygromycin-sensitive secondary recombinants were identified. Some of these recombinants contained the ΔsecA2 deletion. One of these ΔsecA2 mutants, mc22522, was used in all of the subsequent analyses presented in this report. A separate secA2 single-crossover strain, MB575, similarly led to the production of ΔsecA2 mutants. The ability to delete secA2 demonstrates that it is not an essential gene. To our knowledge, this is the first secA that has been shown to be nonessential.
FIG. 3.
Southern analysis of secA2 recombinants in M. smegmatis. (A to C) BamHI restriction endonuclease sites are denoted. The BamHI-HindIII probe used in this Southern analysis is indicated by vertical bars below the diagram. Panels: A, diagram of the chromosomal wild-type secA2 allele; B, diagram of secA2 single-crossover strain MB573; C, diagram of chromosomal ΔsecA2 allele; D, Southern blot of BamHI-digested genomic DNAs probed with the secA2 probe. Sizes of fragments are indicated on the left. Lanes: 1, mc2155; 2, MB573; 3, ΔsecA2 (mc22522 produced from MB573); 4, wild-type recombinant produced from MB573.
The ΔsecA2 mutant exhibits a growth defect on rich agar plates.
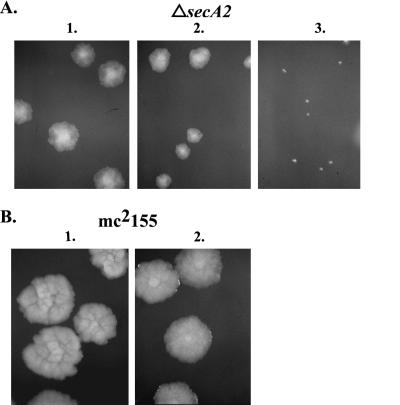
Shortly after generating mc22522, we observed that when grown on rich medium (LB or Mueller-Hinton) agar plates, it produces smaller single colonies than those of wild-type M. smegmatis strain mc2155 (Fig. 4). This small-colony phenotype is medium dependent; it is not seen on minimal agar plates (7H10). Furthermore, there is no observable growth defect associated with mc22522 when it is grown in rich liquid medium.
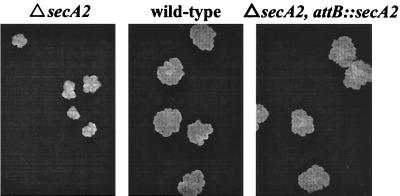
FIG. 4.
Growth defect of the ΔsecA2 mutant on rich medium agar plates. Single colonies of ΔsecA2 mutant mc22522, wild-type mc2155, and complemented ΔsecA2 attB::secA2 strain mc22522/pMB162 are shown on Mueller-Hinton plates grown at 37°C.
We do not understand the basis of this phenotype. However, it is clear that it is due to the deletion mutation in secA2. Even though strain mc22522 contains an in-frame deletion in secA2, which should avoid any polar effects on other genes, we performed complementation analysis. We transformed mc22522 with pMB162, which integrates into the mycobacterial chromosome and expresses the secA2 gene of M. tuberculosis from the constitutive hsp60 promoter. The introduction of this secA2 expression construct successfully rescued the small-colony phenotype of mc22522, demonstrating that this phenotype is the result of the secA2 mutation.
Since SecA1 and SecA2 both share significant homology to other SecA proteins, we tested whether increased expression of secA1 could suppress the small-colony phenotype of mc22522. To our surprise, not only did overexpression of SecA1 not suppress the phenotype, it exacerbated the growth defect (Fig. 5). An extra copy of secA1 integrated into the chromosome of mc22522 causes a reduction in colony size, while expression of secA1 from a multicopy vector has an even more dramatic effect. This same result was obtained with the secA1 genes of both M. bovis BCG and M. smegmatis. This effect on the ΔsecA2 phenotype was seen only on rich agar plates and was specific for a ΔsecA2 background, since overexpression of secA1 in wild-type parent strain mc2155 has no obvious effect on colony size. This synthetic phenotype between ΔsecA2 and high-level expression of secA1 is suggestive of a relationship between these two genes.
FIG. 5.
The ΔsecA2 mutant is sensitive to overexpression of secA1. (A) ΔsecA2 mutant mc22522 transformed with various plasmids. Lanes: 1, pMV261; 2, pMB175 (single-copy secA1); 3, pYUB499 (multicopy secA1). (B) mc2155 transformed with various plasmids. Lanes: 1, pMV261; 2, pYUB499. Colonies were grown on Mueller-Hinton agar plates at 37°C.
The ΔsecA2 mutant is supersensitive to azide.
In E. coli, sodium azide inhibits protein export both in vivo and in vitro (35). Furthermore, SecA has been shown to be the major essential target of sodium azide, as azide-resistant mutants map to secA in E. coli (21, 35). Therefore, we investigated whether the presence of SecA2 influences the azide sensitivity of M. smegmatis.
Since azide sensitivity has not previously been studied in mycobacteria, we first set out to determine whether sodium azide similarly acts to inhibit SecA in mycobacteria. We tested whether specific mutations in secA1 can confer azide resistance on M. smegmatis. Multicopy plasmid pYUB499, which contains secA1 from M. bovis BCG, was subjected to chemical mutagenesis and introduced into mc2155. Azide-resistant transformants were selected on plates containing azide and arose at a frequency of 10−4. The azide resistance of one transformant was shown to segregate with the mutagenized plasmid, designated pYUB538, by means of retransformation experiments. Sequence analysis of the secA1 gene on pYUB538 revealed a G-to-A mutation at base pair 386. This mutation results in replacement of threonine 129 with isoleucine in ABCI (Fig. 1). The corresponding mutation in secA of B. subtilis, replacement of threonine 128 with isoleucine, has previously been reported to produce azide resistance (32). Thus, this test provided compelling evidence that sodium azide acts in a similar fashion on SecA1 in mycobacteria.
To measure the impact of SecA2 on the azide sensitivity of M. smegmatis, a disk assay was employed (see Materials and Methods). The zone of clearing around an azide-soaked disk was used as the measure of azide sensitivity. This assay clearly demonstrated that the ΔsecA2 mutant is supersensitive to azide. While wild-type mc2155 had a zone of sensitivity of 17.7 mm, mc22522 exhibited a 31.3-mm zone of sensitivity. The complemented strain, mc22522 with pMB162, exhibited an intermediate zone of sensitivity of 20.3 mm. This increased azide sensitivity of mc22522 was similarly observed in a liquid MIC analysis (data not shown). This supersensitivity phenotype is specific to azide and is not a pleiotropic effect of altered cell wall permeability. The sensitivity of mc22522 to a collection of drugs including streptomycin, isoniazid, rifampin, ampicillin, and vancomycin was unchanged. Given the relationship between azide and SecA, we believe that this supersensitivity phenotype reflects a role for SecA2 in assisting SecA1 in the essential Sec pathway.
SecA2 functions in protein export.
The azide supersensitivity phenotype suggested that SecA2 plays a role in assisting SecA1 in protein export. The strong homology of SecA2 to other SecA proteins also suggested a role in protein export. Therefore, we set out to determine whether cells lacking secA2 exhibit a defect in protein export.
To monitor protein export, we used immunoblot analysis of exported PhoA (E. coli alkaline phosphatase) fusion proteins to ascertain whether protein export occurs normally or whether unprocessed precursor proteins accumulate intracellularly (indicative of a defect in protein export). Each of the fusions analyzed had an exported M. tuberculosis protein (FbpB [antigen 85B, Rv1886c], Rv1566c, or PepA [Rv0125]) fused at the amino terminus of a truncated PhoA protein lacking its endogenous signal sequence. These fusions were recently isolated in a genetic screen to identify M. tuberculosis proteins that are exported in the host strain of M. smegmatis (5). We, and others, have observed that often when exported PhoA fusion proteins are expressed in M. smegmatis and detected by Western analysis of whole-cell extracts, the products identified do not migrate at the expected molecular weight of the fusion protein (6, 49). Rather, different fusion proteins lead to the production of similar, smaller PhoA-reactive products that are near the size expected for native PhoA. Although the size of these products is unexpected, we believe that they reflect exported PhoA fusions. This is based on the findings that these PhoA fusion proteins exhibit phosphatase activity (indicating export out of the cytoplasm) and that they contain recognizable signal sequences. We propose that these smaller PhoA-reactive products arise by proteolysis that occurs following export to the cell wall. In E. coli, a similar proteolytic degradation near the PhoA fusion junction of some exported PhoA fusion proteins has been observed (23). Given this property of PhoA fusions in M. smegmatis, we predicted that a defect in the export of these proteins would result in a decrease in the degradation of the fusions, yielding an increase in the amount of full-length fusion inside the cells.
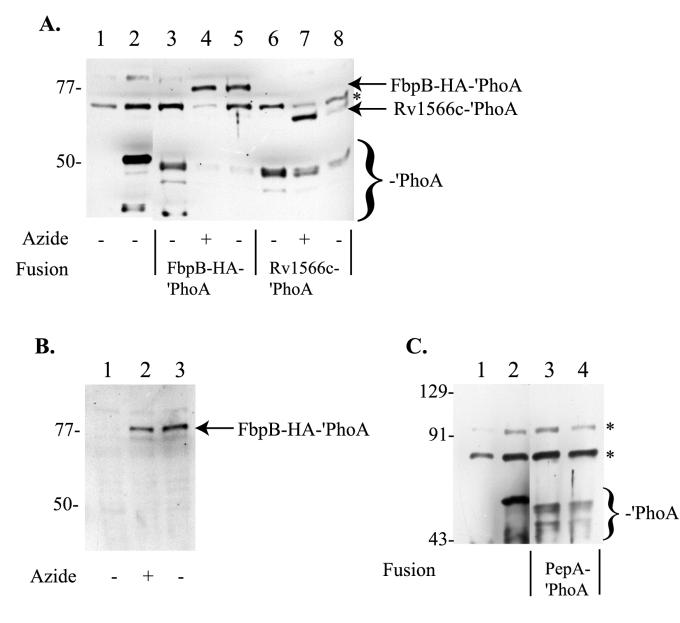
When either the FbpB-HA-′PhoA or the Rv1566c-′PhoA fusion is expressed in wild-type mc2155, bands between 45 and 50 kDa are observed but no full-length fusion product is observed (Fig. 6A, lanes 3 and 6). In contrast, when these same fusion proteins are expressed in the ΔsecA2 mutant (mc22522), the correct-sized full-length fusion product (76.4 kDa for FbpB-HA-′PhoA or 64.7 kDa for Rv1566c-′PhoA) is observed, along with a reduced amount of the proteolytic PhoA products (Fig. 6A, lanes 5 and 8). We interpret this change in size as reflecting the accumulation of the full-length fusion product in the cytoplasm, where it is protected from cell wall proteases, and showing that mc22522 exhibits a defect in the export of these two fusion proteins.
FIG. 6.
Immunoblot analysis of PhoA fusions in wild-type and ΔsecA2 (mc22522) strains. Whole-cell extracts from individual strains were prepared, and 20-μl volumes were loaded per lane for SDS-PAGE and Western analysis. (A) Anti-PhoA antibodies were used for Western analysis of whole-cell extracts. Lanes: 1, MB509 (mc2155/pMV261); 2, mc22757 (mc2155/pMB174); 3, MB648 (mc2155/pMB206); 4, MB648 with azide treatment; 5, MB649 (mc22522/pMB206); 6, MB634 (mc2155/pMB202); 7, MB634 with azide treatment; 8, MB635 (mc22522/pMB202). (B) Anti-HA Western blot. Lanes: 1, MB648; 2, MB648 with azide treatment; 3, MB649. (C) Anti-PhoA Western blot. Lanes: 1, MB509; 2, mc22757; 3, MB624 (mc2155/pMB196); 4, MB625 (mc22522/pMB196). Marker sizes are indicated to the left in kilodaltons. Full-length FbpB-HA-′PhoA and Rv1566c′-′PhoA fusions are indicated by arrows. ′PhoA breakdown products are identified by a bracket near the bottom of the blots. The asterisks indicate cross-reacting products. Strains grown overnight in the presence of sodium azide are indicated below the blot. Plasmid pMB174 expresses ′phoA, pMB206 expresses an fbpB-HA-′phoA fusion, pMB202 expresses an rv1566c-′phoA fusion, and pMB196 expresses a pepA-′phoA fusion.
In support of this interpretation, we found that when strains MB648 (mc2155 expressing FbpB-HA-′PhoA) and MB634 (mc2155 expressing Rv1566c-′PhoA) were treated with azide, the same full-length fusion protein appeared (Fig. 6A, lanes 4 and 7). Since this azide treatment should cripple the export pathway by inactivating the essential SecA1 protein, and possibly SecA2 as well, we believe that the large product that appears is a result of accumulation of the full-length fusion protein in the cytoplasm. Finally, we confirmed that the product accumulating in mc22522 is the FbpB-HA-′PhoA fusion protein by taking advantage of an in-frame HA tag present in the FbpB portion of the fusion. Western analysis with anti-HA antibodies was carried out on MB648 (mc2155 expressing FbpB-HA-′PhoA), MB648 following treatment with azide, and MB649 (mc22522 expressing FbpB-HA-′PhoA) (Fig. 6B). This analysis confirmed that the large product that appears in mc22522 is the FbpB-HA-′PhoA full-length fusion product.
In contrast, a PepA-′PhoA fusion was not similarly affected. When this fusion protein was expressed in either mc2155 or mc22522, the same PhoA proteolytic products were observed (Fig 6C). Neither strain led to production of the full-length 67.5-kDa fusion protein. From our studies so far, we conclude that the export of some, but not all, proteins involves SecA2.
DISCUSSION
Mycobacteria have two different SecA proteins.
The proper localization of cell surface and secreted proteins is an important aspect of mycobacterial physiology and pathogenesis. The cell envelope of mycobacteria is an unusual structure that presents unique barriers to protein secretion. Yet, the protein export pathways of mycobacteria have remained uncharacterized (5). The genome of M. tuberculosis contains homologues of all of the Sec pathway components (5, 8), with the exception of SecB, a protein that is not found in all Sec systems (19). In addition, mycobacteria are known to secrete proteins that are initially synthesized as precursors containing recognizable signal sequences at their amino termini. Simply based on this information, it might appear that mycobacteria contain a typical Sec pathway for protein export beyond the cytoplasm. However, as presented in this report, the mycobacterial Sec pathway is unusual in that it has two SecA proteins. Although SecA is ubiquitously present and highly conserved in bacteria, the identification of organisms containing two secA genes is new.
Both the mycobacterial SecA1 and SecA2 proteins exhibit significant sequence similarity to other bacterial and plant (plastid) SecA homologues, and both contain the hallmark ATP-binding motifs (Table 3; Fig. 1). All of the mycobacteria evaluated so far have both secA1 and secA2, including M. leprae (9, 26). This is significant, given the reductive evolution that has been documented in M. leprae—a smaller genome size and 44% of the genes being recognizable pseudogenes (9). The evolutionary basis for two SecA proteins in mycobacteria is unknown. If the two genes arose by a duplication event, it likely took place a long time ago since the two SecA proteins are not extremely similar to each other, but each is highly conserved in different mycobacterial species. SecA1 exhibits higher sequence similarity with the other SecA homologues, including that of the distantly related bacteria E. coli and B. subtilis, than it does with mycobacterial SecA2 (Table 3).
SecA1 is a housekeeping SecA protein, and SecA2 is an extra SecA protein.
As a starting point for defining the roles played by SecA1 and SecA2, we used allelic exchange to determine whether each secA gene is essential. These experiments showed that while secA1 is essential in M. smegmatis, secA2 is not. In other bacteria, SecA is essential.
The essentiality of SecA1 leads us to believe that it represents the mycobacterial equivalent of E. coli SecA or the housekeeping SecA protein. Our isolation of azide-resistant mutations in SecA1 also supports this idea. We believe that SecA1 acts together with the other mycobacterial Sec family homologues to transport signal sequence-containing proteins across the cytoplasmic membrane. However, definitive evidence that SecA1 functions in this manner requires further investigation and the construction of a conditional secA1 allele.
Our ability to generate a ΔsecA2 mutant demonstrates that SecA2 is not essential. Although this mutation is not a full deletion, we do not believe that the truncated protein retains its function. Of the two ATP-binding sites, all but the A motif of ABCI is deleted, which should eliminate all ATP-binding and hydrolysis activity (Fig. 1). Furthermore, we have subsequently constructed a mutant of M. tuberculosis with a complete deletion of secA2 that is also viable (M. Braunstein and W. R. Jacobs, unpublished data). To our knowledge, this is the first SecA protein that has been shown to be nonessential.
The role of SecA2 in mycobacteria.
To begin to understand the role of SecA2 in the cell, we examined whether SecA2 is capable of carrying out the same functions as SecA1. The fact that a mutant with a deletion of secA1 is inviable suggested that SecA2 is not simply an equivalent copy of SecA1. However, it remained a possibility that this result reflected a lack of expression of SecA2 under these experimental conditions. To address this directly, we expressed secA2 from the constitutive mycobacterial hsp60 promoter. By Western analysis, we saw that the SecA2 protein was produced (data not shown); however, the requirement for SecA1 was not relieved by this expression of SecA2. This finding that SecA2 cannot functionally replace SecA1 is consistent with the fact that the homology shared between the two SecA proteins is only 50% (Table 3).
If SecA2 is not simply an extra copy of SecA1, what is the role of SecA2? Based on the significant sequence homology between SecA2 and other SecA homologues, we believed it would be involved in protein export—perhaps the export of a specific subset of proteins. Our subsequent analysis supports this idea.
A phenotypic analysis of ΔsecA2 mutant mc22522 revealed phenotypes that include a growth defect on rich agar plates. The source of the phenotype is unclear. This growth phenotype of mc22522 is exacerbated when secA1 is overexpressed, but overexpression of secA1 in a wild-type background does not have the same effect. This synthetic relationship between high levels of SecA1 and the absence of SecA2 suggests that these two SecA proteins interact with some of the same proteins or each other. This is consistent with a role for SecA2 in protein export. A possible explanation for this synthetic phenotype is that when SecA2 is absent and SecA1 is overexpressed, nonproductive complexes between SecA1 and other essential Sec factors arise, leading to an overall decrease in the efficiency of protein export. This possibility will be explored in future experiments.
The azide supersensitivity phenotype of mc22522 also suggests an involvement of SecA2 in protein export. Azide poisons ATPases, and SecA1 appears to be the most sensitive essential ATPase in mycobacteria. For this reason, we interpret this phenotype as indicative that azide has a greater effect on the Sec pathway when SecA2 is not present to assist in the process.
The most direct experiment we undertook to identify a role for SecA2 in the export of proteins was to monitor protein processing as a measure of protein translocation across the cytoplasmic membrane. In looking at the steady-state levels of three PhoA fusion proteins, we found that an FbpB-HA-′PhoA fusion and an Rv1566c-′PhoA fusion are not as efficiently exported in the ΔsecA2 mutant. It is important to emphasize that this export defect of mc22522 is not complete. The immunoblots reveal a reduced amount of export (PhoA proteolytic products), and the mc22522 strains expressing these fusions still exhibit phosphatase activity (which is associated with transport of the fusion protein out of the cytoplasm). In contrast, we did not see any evidence of a defect in the export of a PepA-′PhoA fusion protein in mc22522. This finding reveals that SecA2 participates in the protein export of some, perhaps not all, signal sequence-containing proteins.
We interpret our results as indicating a role for SecA2 in protein export. An alternative explanation could be that SecA2 is a regulator of the level of SecA1. This idea is supported by the demonstration in E. coli that SecA can regulate its own expression (44). In examining Western analyses with the currently available reagents, there is no indication that the levels of SecA1 are altered by the presence or absence of SecA2 in the cell; therefore, we think this explanation for the role of SecA2 unlikely (data not shown).
Two roles for SecA2 in mycobacteria.
We propose that one role for SecA2 is as a facilitator of protein export via the primary Sec pathway involving SecA1. We believe that the azide supersensitivity phenotype and export defect of some signal sequence-containing PhoA fusion proteins reflect this role of SecA2. There may be a collection of signal sequence-containing presecretory proteins that are substrates for both SecA1 and SecA2. Thus, when SecA2 is absent, there is an impact on the overall efficiency of the pathway. We propose that FbpB-′PhoA and Rv1566c-′PhoA are two such proteins, since SecA2 participates, but dos not exclusively act, in their export. In E. coli, B. subtilis, and Streptomyces lividans, SecA has been shown to exist as a dimer (1, 3, 13, 47). Therefore, it is also possible that SecA1 and SecA2 form mixed dimers and that this species is important in the recognition of certain substrates.
The findings that SecA2 cannot functionally replace SecA1 and that SecA2 is highly conserved throughout mycobacteria lead us to believe that SecA2 has a second role in the cell.
This second role may be in the export of a distinct subset of proteins that are exclusive or preferential substrates of SecA2. The identities of those proteins have not been established, and their identification awaits a more complete analysis of the multitude of exported proteins. In order to identify these proteins, it will be important to know the conditions under which SecA2-specific protein export functions.
Research on numerous bacterial pathogens has repeatedly demonstrated the importance of specialized protein secretion systems to virulence. Many of the specialized export pathways are present in pathogenicity islands. The genome of M. tuberculosis does not appear to have specialized export systems homologous to those previously identified in other pathogens (4). However, the recent identification of two secA genes in the gram-positive pathogens S. aureus and S. pneumoniae raises the interesting possibility that SecA2 is a component of a new type of specialized protein export pathway that is important to pathogenesis (24, 27, 48). SecA2 may represent a protein that has maintained some of its abilities to function with SecA1 and the other Sec family homologues in the primary Sec pathway but has evolved to have a second function that is utilized under a certain set of conditions to export a specific subset of proteins.
ACKNOWLEDGMENTS
We gratefully acknowledge F. C. Bange and V. Balasubramanian for genomic libraries, T. Weisbrod for assistance with DNA sequencing, and A. Flower and M. Pavelka for critical reading of the manuscript.
This work was supported by NIH grant AI21670 to W.R.J. M.B. was a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation during the course of this work.
REFERENCES
- 1.Akita M, Shinkai A, Matsuyama S, Mizushima S. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem Biophys Res Commun. 1991;174:211–216. doi: 10.1016/0006-291x(91)90507-4. [DOI] [PubMed] [Google Scholar]
- 2.Berthet F X, Lagranderie M, Gounon P, Laurent-Winter C, Ensergueix D, Chavarot P, Thouron F, Maranghi E, Pelicic V, Portnoi D, Marchal G, Gicquel B. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erpgene. Science. 1998;282:759–762. doi: 10.1126/science.282.5389.759. [DOI] [PubMed] [Google Scholar]
- 3.Blanco J, Driessen A J, Coque J J, Martin J F. Biochemical characterization of the SecA protein of Streptomyces lividans—interaction with nucleotides, binding to membrane vesicles and in vitro translocation of proAmy protein. Eur J Biochem. 1998;257:472–478. doi: 10.1046/j.1432-1327.1998.2570472.x. [DOI] [PubMed] [Google Scholar]
- 4.Braunstein M, Belisle J T. Genetics of protein secretion. In: Hatfull G F, Jacobs W R J, editors. Molecular genetics of mycobacteria. Washington, D.C.: ASM Press; 2000. pp. 203–220. [Google Scholar]
- 5.Braunstein M, Griffin T I, Kriakov J I, Friedman S T, Grindley N D, Jacobs W R., Jr Identification of genes encoding exported Mycobacterium tuberculosis proteins using a Tn552′phoAin vitro transposition system. J Bacteriol. 2000;182:2732–2740. doi: 10.1128/jb.182.10.2732-2740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll J D, Wallace R C, Keane J, Remold H G, Arbeit R D. Identification of Mycobacterium avium DNA sequences that encode exported proteins by using phoAgene fusions. Tuber Lung Dis. 2000;80:117–130. doi: 10.1054/tuld.2000.0239. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo J D, Weisbrod T R, Banerjee A, Bloom B R, Jacobs W R., Jr Genetic determination of the meso-diaminopimelate biosynthetic pathway of mycobacteria. J Bacteriol. 1994;176:4424–4429. doi: 10.1128/jb.176.14.4424-4429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Cole S T, Eiglmeier K, Parkhill J, James K D, Thomson N R, Wheeler P R, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies R M, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail M A, Rajandream M A, Rutherford K M, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward J R, Barrell B G. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 10.Connell N D. Mycobacterium: isolation, maintenance, transformation, and mutant selection. Methods Cell Biol. 1994;45:108–125. doi: 10.1016/s0091-679x(08)61848-8. [DOI] [PubMed] [Google Scholar]
- 11.Danese P N, Silhavy T J. Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli. Annu Rev Genet. 1998;32:59–94. doi: 10.1146/annurev.genet.32.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly-Wu M K, Jacobs W R, Jr, Hatfull G F. Superinfection immunity of mycobacteriophage L5: applications for genetic transformation of mycobacteria. Mol Microbiol. 1993;7:407–417. doi: 10.1111/j.1365-2958.1993.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 13.Driessen A J. SecA, the peripheral subunit of the Escherichia coliprecursor protein translocase, is functional as a dimer. Biochemistry. 1993;32:13190–13197. doi: 10.1021/bi00211a030. [DOI] [PubMed] [Google Scholar]
- 14.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16:2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Economou A. Bacterial preprotein translocase: mechanism and conformational dynamics of a processive enzyme. Mol Microbiol. 1998;27:511–518. doi: 10.1046/j.1365-2958.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- 16.Economou A. Bacterial protein translocase: a unique molecular machine with an army of substrates. FEBS Lett. 2000;476:18–21. doi: 10.1016/s0014-5793(00)01662-8. [DOI] [PubMed] [Google Scholar]
- 17.Economou A. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 1999;7:315–320. doi: 10.1016/s0966-842x(99)01555-3. [DOI] [PubMed] [Google Scholar]
- 18.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 19.Fekkes P, Driessen A J. Protein targeting to the bacterial cytoplasmic membrane. Microbiol Mol Biol Rev. 1999;63:161–173. doi: 10.1128/mmbr.63.1.161-173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortin Y, Phoenix P, Drapeau G R. Mutations conferring resistance to azide in Escherichia coli occur primarily in the secAgene. J Bacteriol. 1990;172:6607–6610. doi: 10.1128/jb.172.11.6607-6610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez M, Doukhan L, Nair G, Smith I. sigA is an essential gene in Mycobacterium smegmatis. Mol Microbiol. 1998;29:617–628. doi: 10.1046/j.1365-2958.1998.00960.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman M R, Taylor R K. Identification of bacterial cell-surface virulence determinants with TnphoA. Methods Enzymol. 1994;235:426–448. doi: 10.1016/0076-6879(94)35159-7. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi N K, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of methicillin-resistant Staphylococcus aureus. Lancet. 2001;357:1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 25.Lee M H, Pascopella L, Jacobs W R, Jr, Hatfull G F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guerin. Proc Natl Acad Sci USA. 1991;88:3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limia A, Sangari F J, Wagner D, Bermudez L E. Characterization and expression of secA in Mycobacterium avium. FEMS Microbiol Lett. 2001;197:151–157. doi: 10.1111/j.1574-6968.2001.tb10597.x. [DOI] [PubMed] [Google Scholar]
- 27.Mazmanian S K, Ton-That H, Schneewind O. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol Microbiol. 2001;40:1049–1057. doi: 10.1046/j.1365-2958.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 28.Mecsas J J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:270–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J F, Cossart P. Bacterial pathogenesis: before the post-genomic era. Curr Opin Microbiol. 1999;2:15–17. [Google Scholar]
- 30.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 31.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coliSecA ATPase. Mol Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakane A, Takamatsu H, Oguro A, Sadaie Y, Nakamura K, Yamane K. Acquisition of azide-resistance by elevated SecA ATPase activity confers azide-resistance upon cell growth and protein translocation in Bacillus subtilis. Microbiology. 1995;141:113–121. doi: 10.1099/00221287-141-1-113. [DOI] [PubMed] [Google Scholar]
- 33.Oliver D B. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across the Escherichia coliinner membrane. Mol Microbiol. 1993;7:159–165. doi: 10.1111/j.1365-2958.1993.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 34.Oliver D B, Beckwith J. E. colimutant pleiotropically defective in the export of secreted proteins. Cell. 1981;25:765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- 35.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia colialter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavelka M S, Jr, Jacobs W R., Jr Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavelka M S, Jr, Jacobs W R., Jr Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis, bacillus Calmette-Guerin, and Mycobacterium tuberculosisH37Rv by allelic exchange. J Bacteriol. 1999;181:4780–4789. doi: 10.1128/jb.181.16.4780-4789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelicic V, Reyrat J M, Gicquel B. Expression of the Bacillus subtilis sacBgene confers sucrose sensitivity on mycobacteria. J Bacteriol. 1996;178:1197–1199. doi: 10.1128/jb.178.4.1197-1199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohlschroder M, Prinz W A, Hartmann E, Beckwith J. Protein translocation in the three domains of life: variations on a theme. Cell. 1997;91:563–566. doi: 10.1016/s0092-8674(00)80443-2. [DOI] [PubMed] [Google Scholar]
- 40.Roux K H. Using mismatched primer-template pairs in touchdown PCR. BioTechniques. 1994;16:812–814. [PubMed] [Google Scholar]
- 41.Sambrook J E, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Schiebel E, Driessen A J, Hartl F U, Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt M G, Kiser K B. SecA: the ubiquitous component of preprotein translocase in prokaryotes. Microbes Infect. 1999;1:993–1004. doi: 10.1016/s1286-4579(99)80517-6. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt M G, Oliver D B. SecA protein autogenously represses its own translation during normal protein secretion in Escherichia coli. J Bacteriol. 1989;171:643–649. doi: 10.1128/jb.171.2.643-649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 46.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 47.Takamatsu H, Nakane A, Oguro A, Sadaie Y, Nakamura K, Yamane K. A truncated Bacillus subtilis SecA protein consisting of the N-terminal 234 amino acid residues forms a complex with Escherichia coliSecA51(ts) protein and complements the protein translocation defect of the secA51 mutant. J Biochem (Tokyo) 1994;116:1287–1294. doi: 10.1093/oxfordjournals.jbchem.a124677. [DOI] [PubMed] [Google Scholar]
- 48.Tettelin H, Nelson K E, Paulsen I T, Eisen J A, Read T D, Peterson S, Heidelberg J, DeBoy R T, Haft D H, Dodson R J, Durkin A S, Gwinn M, Kolonay J F, Nelson W C, Peterson J D, Umayam L A, White O, Salzberg S L, Lewis M R, Radune D, Holtzapple E, Khouri H, Wolf A M, Utterback T R, Hansen C L, McDonald L A, Feldblyum T V, Angiuoli S, Dickinson T, Hickey E K, Holt I E, Loftus B J, Yang F, Smith H O, Venter J C, Dougherty B A, Morrison D A, Hollingshead S K, Fraser C M. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 49.Timm J, Perilli M G, Duez C, Trias J, Orefici G, Fattorini L, Amicosante G, Oratore A, Joris B, Frere J M, et al. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitumbeta-lactamase genes cloned from a natural isolate and a high-level beta-lactamase producer. Mol Microbiol. 1994;12:491–504. doi: 10.1111/j.1365-2958.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 50.van der Wolk J P, de Wit J G, Driessen A J. The catalytic cycle of the Escherichia coliSecA ATPase comprises two distinct preprotein translocation events. EMBO J. 1997;16:7297–7304. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. The world health report 1998: life in the 21st century—a vision for all. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]