Abstract
Previously, we reported finding duplicated fixNOQP operons in Rhizobium etli CFN42. One of these duplicated operons is located in the symbiotic plasmid (fixNOQPd), while the other is located in a cryptic plasmid (fixNOQPf). Although a novel FixL-FixKf regulatory cascade participates in microaerobic expression of both fixNOQP duplicated operons, we found that a mutation in fixL eliminates fixNOQPf expression but has only a moderate effect on expression of fixNOQPd. This suggests that there are differential regulatory controls. Interestingly, only the fixNOQPd operon was essential for symbiotic nitrogen fixation (L. Girard, S. Brom, A. Dávalos, O. Lopez, M. Soberón, and D. Romero, Mol. Plant-Microbe Interact. 13:1283–1292, 2000). Searching for potential candidates responsible for the differential expression, we characterized two fnrN homologs (encoding transcriptional activators of the cyclic AMP receptor protein [CRP]-Fnr family) in R. etli CFN42. One of these genes (fnrNd) is located on the symbiotic plasmid, while the other (fnrNchr) is located on the chromosome. Analysis of the expression of the fnrN genes using transcriptional fusions with lacZ showed that the two fnrN genes are differentially regulated, since only fnrNd is expressed in microaerobic cultures of the wild-type strain while fnrNchr is negatively controlled by FixL. Mutagenesis of the two fnrN genes showed that both genes participate, in conjunction with FixL-FixKf, in the microaerobic induction of the fixNOQPd operon. Participation of these genes is also seen during the symbiotic process, in which mutations in fnrNd and fnrNchr, either singly or in combination, lead to reductions in nitrogen fixation. Therefore, R. etli employs a regulatory circuit for induction of the fixNOQPd operon that involves at least three transcriptional regulators of the CRP-Fnr family. This regulatory circuit may be important for ensuring optimal production of the cbb3, terminal oxidase during symbiosis.
Bacteria belonging to the family Rhizobiaceae may establish specific symbiotic relationships with their legume host plants. The bacteria elicit formation of new organs, the root nodules, in which differentiated bacterial cells (bacteroids) reduce atmospheric nitrogen to ammonia, thus supplying the host plants with combined nitrogen. Since nitrogen fixation is an energy-consuming process, effective symbioses depend on operation of a respiratory chain with a high affinity for O2, closely coupled to ATP production. This requirement is fulfilled by a special three-subunit terminal oxidase (cytochrome terminal oxidase cbb3), which was first identified in Bradyrhizobium japonicum as the product of the fixNOQP operon (19, 24, 25). Functional duplicated genes of the fixNOQP operon have been found in the Rhizobiaceae. For instance, both Sinorhizobium meliloti (28) and Rhizobium leguminosarum bv. viciae possess two copies of the fixNOQP operon, which are regulated in similar ways; both copies are required for optimal symbiotic nitrogen fixation (30).
In S. meliloti, expression of fixNOQP is regulated mainly through an O2-sensing cascade comprised of the fixL and fixJ gene products; this cascade activates expression of the fixK gene, which leads to expression of the fixNOQP operon (2, 3, 9, 12, 18). fixK encodes a transcriptional activator belonging to the cyclic AMP receptor protein (CRP)-Fnr family (2). Interesting variations of this basic regulatory scheme have been found in other rhizobial strains. For instance, R. leguminosarum bv. viciae VF39 lacks conventional homologs of FixJ and FixL and instead has an unusual FixL homolog which combines structural features observed in both the sensor and responsive elements of a two-component regulator system. This FixL homolog is also involved in ex planta fixNOQP expression but seems to lack a significant role during symbiosis (30). R. leguminosarum bv. viciae VF39 contains FixK (30) and another transcriptional activator of the CRP-Fnr family, FnrN which activates the two fixNOQP copies (8, 15, 16, 30). In contrast to FixK, FnrN has a region with a high level of similarity to a domain in the Escherichia coli Fnr protein involved in O2 sensing, suggesting that the FnrN transcriptional activity may be negatively modulated by O2 (8). Both FixK and FnrN activate fixNOQP expression by binding to a DNA sequence located in the promoter region (TTGAT-N4-ATCAA) called the anaerobox (2, 8, 11). Furthermore, R. leguminosarum fnrN is able to complement an S. meliloti fixK mutant for fixNOQP expression (8, 16), suggesting that the two proteins activate transcription in similar ways (8, 15). Additional variations are found in R. leguminosarum bv viciae UPM791, in which two fnrN genes and no fixL,fixJ, or fixK homologs are present (15, 16). Both fnrN genes are involved in activation of the fixNOQP operon (15, 16). In this strain, expression of both fnrN genes is autoregulated, thus ensuring equilibrated expression of fnrN in response to microaerobic conditions (7).
In Rhizobium etli CFN42, there are also two fixNOQP operons; one is located in the symbiotic plasmid (fixNOQPd) (33), and the other is located in a cryptic plasmid called pCFN42f (fixNOQPf) (13). Only the fixNOQPd operon is required for establishment of an effective symbiosis (13). Possible regulators of fixNOQP expression are located in plasmid pCFN42f (an fixL gene, encoding an unusual homolog of FixL, as well as the fixKf gene) and in the symbiotic plasmid (fixKd) (13, 33). Mutagenesis of these genes showed that both FixL and FixKf are needed for microaerobic induction of the fixNOQPf operon. Differential regulatory requirements were observed for microaerobic expression of the fixNOQPd operon; expression of this operon is completely dependent on FixKf but is only moderately affected by a mutation in fixL. A mutation in fixKd did not affect expression of the fixNOQP operons (13). None of the regulatory genes identified so far are indispensable for symbiotic nitrogen fixation (13).
To explain the differential control of fixNOQPd by FixL and FixKf, we postulated the existence of an additional transcriptional activator for fixNOQPd expression, whose expression should be negatively controlled by FixL (13). Here we describe finding two R. etli fnrN duplicated genes. We determined by mutagenesis and analysis of appropriate transcriptional fusions that these genes, together with FixL, are involved in induction of fixNOQPd expression and in symbiotic nitrogen fixation. Our work also revealed some of the features inherent in regulation of both fnrN genes; these features involve autoregulation and differential responses to the other regulatory genes identified previously.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used are listed in Table 1. R. etli and R. leguminosarum bv. viciae were cultured at 30°C in peptone yeast extract medium (PY) (23) or in yeast extract succinate medium (32). E. coli was grown at 37°C in Luria broth. Plasmids were transferred to R. etli strains by biparental mating using E. coli S17-1 as the donor strain. Antibiotics were used at the following concentrations: ampicillin, 100 μg ml−1; gentamicin, 10 μg ml−1; kanamycin, 30 μg ml−1; nalidixic acid, 20 μg ml−1; rifampin, 50 μg ml−1; spectinomycin, 100 μg ml−1; and tetracycline, 5 μg ml−1. When needed, sucrose was added at a concentration of 20% (wt/vol).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference |
|---|---|---|
| Rhizobium etli strains | ||
| CFN42 | Wild-type strain | 27 |
| CE3 | Strr derivative of CFN42 | 23 |
| CFNX89 | Derivative of CE3 cured of plasmid pCFN42d | 4 |
| CFNX636 | CE3 derivative, fixL::loxP Sp | 13 |
| CFNX637 | CE3 derivative, fixKf::ΩKm | 13 |
| CFNX642 | CFNX636 derivative, fixL::loxP | This study |
| IBTOL12 | CE3 derivative, fnrNd::ΩKm | This study |
| IBTOL14 | CE3 derivative, fnrNchr::ΩSp | This study |
| IBTOL15 | IBTOL12 derivative, fnrNd::ΩKm fnrNchr::ΩSp | This study |
| IBTOL16 | CFNX642 derivative, fixL::loxP fnrNchr::ΩSp | This study |
| IBTOL17 | CFNX642 derivative, fixL::loxP fnrNd::ΩKm | This study |
| IBTOL18 | CFNX642 derivative, fixL::loxP fnrNchr::ΩSp fnrNd::ΩKm | This study |
| Rhizobium leguminosarum bv. viciae VF39 | Smr | 8 |
| Escherichia coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 hsdR17 supE44 lac [F′ proAB lacIqlacZΔM15 Tn10 (Tcr)] thi | 6 |
| S17-1 | F−pro-82 thi-1 endA1 hsdR17 supE44 recA13, chromosomally integrated RP-4-2 (Tc::Mu) (Km::Tn7) | 31 |
| Plasmids | ||
| pBluescript SK+ | Apr, sequencing vector | Stratagene |
| pRK2013 | ColE1 mob+ Tra+ (RK2) Kmr | 10 |
| pMP220 | Transcriptional lacZ fusion vector, Tcr | 34 |
| pJQ200 SK+ | GmrsacB, suicide vector | 26 |
| pOL17 | pJQ200 derivative, fnrNd::ΩKm | This study |
| pOL18 | pJQ200 derivative, fnrNchr::ΩSp | This study |
| pJMS8 | pRK7813::Cre, Tcr | Martínez-Salazar, unpublished data |
| pOLfix10 | pMP220 derived, fixNd-lacZ gene fusion | 33 |
| pOL15 | pMP220 derived, fnrNd-lacZ gene fusion | This study |
| pOL16 | pMP220 derived, fnrNchr-lacZ gene fusion | This study |
Growth conditions and β-galactosidase measurements.
R. etli strains were cultured on PY plates for 4 days. Cells scraped from these plates were used to inoculate 250-ml Erlenmeyer flasks containing 50 ml of PY, and the cultures were incubated at 30°C for 24 h. Microaerobic cultures of R. etli were prepared by diluting the active inoculum to an initial optical density at 540 nm of 0.05 in 50 ml of yeast extract succinate medium in 150-ml serum stopper bottles; the cultures were flushed with a continuous stream (1,200 ml min−1) of a sterile gas mixture (98% N2, 2% O2) for 5 min, sealed, and then incubated for 8 h at 30°C with shaking (200 rpm). β-Galactosidase activity was determined by measuring o-nitrophenol production as described previously (20); activities were expressed in micromoles of o-nitrophenol produced per minute per milligram of protein.
DNA manipulations.
Cloning, restriction mapping, transformation, plasmid isolation, random priming, Southern blotting, and hybridization were performed by using standard protocols (20). Both DNA strands were sequenced either by employing appropriate subclones or by primer walking. The initial phases of sequencing were done at an automated DNA sequencing facility at the Institute of Biotechnology, Cuernavaca, Mexico; sequencing needed for gap filling was performed by the dideoxynucleotide chain termination method (29), using Sequenase 2.0 (Amersham Ltd.). Computer-assisted sequence analysis was performed with the Gene Works 2.5.1 program suite from Oxford Molecular Group Inc. Searches for homology with sequences in the GenBank database were done with the BLAST programs (1, 14) running at the National Center for Biotechnology Information server.
PCR cloning of the R. leguminosarum bv. viciae fnrN gene.
The fnrN gene of R. leguminosarum bv. viciae VF39 was obtained by PCR amplification. To do this, we designed a 27-mer forward primer complementary to a region located 418 bp upstream from the ATG of fnrN (7) (nucleotides 136 to 154; GenBank accession no. X55788), to facilitate additional cloning steps, a 9-bp extension, containing a built-in EcoRI restriction site (underlined) at the 5′ end was added, as follows: 5′-GGAATTCCATCGAATGTAGCGGTCACG-3′. The following reverse primer (27 bp) also contained an EcoRI site (underlined) and was complementary to a region 380 bp downstream of the stop codon of fnrN (nucleotides 1675 to 1692): 5′-GGAATTCCATCAGCATCGGCAAGCAGA-3′. The amplification reaction mixtures (total volume, 50 μl) typically contained each primer at a final concentration of 250 nM, 50 ng of total DNA of R. leguminosarum bv. viciae VF39, each deoxynucleoside triphosphate (dNTP) at a concentration of 200 μM, and 2 U of Taq polymerase. PCR amplifications were done with a Perkin-Elmer 480 DNA thermal cycler by using the following cycling regimen: a single denaturation step (2 min at 94°C), followed by 20 cycles consisting of 1 min at 60°C (annealing), 2 min at 73°C (extension), and 1 min at 95°C (denaturation), and then a final extension step (3 min at 73°C). The resulting 1,556-bp PCR product was digested with EcoRI and cloned into plasmid pBluescript SK+ previously digested with EcoRI. This fragment was mapped and was shown to correspond to R. leguminosarum bv. viciae fnrN on the basis of its sequence (data not shown).
Construction of lacZ gene fusions.
To generate an fnrNchr-lacZ transcriptional fusion, plasmid pMP220 (containing a promoterless lacZ gene) was digested with EcoRI and PstI, and then the EcoRI end was filled in with Klenow polymerase and dNTPs as described previously (20). The resulting fragment was ligated to a 683-bp EcoRV-PstI fragment (containing the fnrNchr promoter), resulting in pOL16. A plasmid harboring an fnrNd-lacZ fusion (pOL15) was constructed similarly by inserting a 548-bp EcoRI-SalI fragment (containing the fnrNd promoter) into pMP220 previously digested with EcoRI and PstI. Before ligation, fragments were made compatible by filling in both the SalI end in fnrNd and the PstI end in pMP220.
Construction of fnrNchr and fnrNd mutants.
To introduce a mutation into fnrNchr, a 1.3-kb BamHI-EcoRI fragment containing fnrNchr was cloned into plasmid pSK Bluescript. In the resulting plasmid, an fnrNchr::ΩSp deletion-substitution allele was generated by removing a 141-bp PstI fragment from fnrNchr (codons 90 to 132) and then filling in the PstI ends and inserting a 2-kb HindIII-HindIII ΩSpr interposon (previously treated with Klenow polymerase and dNTPs to fill in the restriction sites). A suicide plasmid derivative useful for homogenotization was constructed by excising a BamHI-EcoRI fragment containing the fnrNchr::ΩSp allele, treating it with Klenow polymerase to fill in the restriction sites, and then ligating it into SmaI-digested pJQ200SK+ (26), which resulted in plasmid pOL18. Homogenotization of the fnrNchr::ΩSp allele was carried out by mobilizing this construct into R. etli CE3; double recombinants were selected on PY containing sucrose and spectinomycin, which generated strain IBTOL14.
An fnrNd::ΩKm allele was generated by inserting an HindIII-HindIII ΩKm cartridge into the SalI site of fnrNd (codon 47); this was accomplished by filling in both fragments with Klenow polymerase, followed by ligation. The fragment containing the fnrNd::ΩKm allele was then removed by EcoRI digestion, filled in by treatment with Klenow polymerase, and ligated with SmaI-restricted pJQ200SK+, which resulted in plasmid pOL17. This construct was mobilized into R. etli CE3, and double recombinants were selected in the presence of sucrose and kanamycin, which generated strain IBTOL12.
A derivative carrying the fnrNd::ΩKm-fnrNchr::ΩSp allelic combination (strain IBTOL15) was constructed by performing a biparental cross, using E. coli S17-1/pOL17 as the donor and R. etli IBTOL12 (fnrNd::ΩKm) as the recipient; double recombinants were selected as Nalr Kmr Spr sucrose-resistant transconjugants.
To construct a derivative carrying an unmarked fixL mutant allele, we took advantage of the special characteristics of the previously described fixL::loxP Sp mutant (13). In this mutant, the spectinomycin resistance determinant is flanked by two synthetic loxP sites. In vivo site-specific recombination between the loxP sites, catalyzed by the Cre recombinase, leads to high-frequency excision of the spectinomycin resistance determinant (J. Martínez-Salazar, unpublished data), leaving an unmarked 189-bp insertion in fixL (fixL::loxP). To generate such an allele, a broad-host-range plasmid encoding the Cre recombinase (pJMS8) was introduced by conjugation into R. etli CFNX636 (fixL::lox Sp). Transconjugants resulting from the cross displayed high-frequency loss of the spectinomycin resistance determinant. Removal of pJMS8 from these Sps derivatives was accomplished by screening for Tcs segregants, which resulted in strain CFNX642 (fixL::loxP).
Homogenotization of appropriate fnrN mutant alleles was accomplished by transferring the corresponding plasmids into R. etli CFNX642 (fixL::loxP), which resulted in double mutants IBTOL16 (fixL::loxP fnrNd::ΩKm) and IBTOL17 (fixL::loxP fnrNchr::ΩSp) and triple mutant IBTOL18 (fixL::loxP fnrNd::ΩKm fnrNchr::ΩSp).
To verify that the desired gene replacements had occurred, DNA blots of all of the derivatives were analyzed by Southern hybridization with the appropriate fnrN and cassette probes.
Nitrogen fixation determination.
To measure acetylene reduction, Phaseolus vulgaris cv. Negro Jamapa seeds were surface sterilized with diluted sodium hypochlorite and germinated on moist sterile filter paper. Three-day-old seedlings were transferred to plastic pots filled with sterile vermiculite and inoculated with 1 ml of the appropriate bacterial strain (grown in PY); plants were grown in a greenhouse under irrigation with a nitrogen-free nutrient solution (33). For nitrogenase determinations, excised root systems were incubated for 40 min at room temperature in sealed glass vials containing acetylene at a final concentration of 10% in the gas phase. Ethylene production was measured with a Varian 3300 gas chromatograph fitted with a Varian 4290 integrator (33).
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study for the fnrNchr and fnrNd loci have been deposited in the GenBank database under accession numbers AF083916 and AF083917, respectively.
RESULTS
Duplication of the fnrN genes in R. etli CFN42.
To ascertain it an Fnr-like protein participates in differential regulation of the fixN duplicated genes, we decided to search for fnrN homologs in R. etli. To do this, blotted plasmid profiles from a CFN42 streptomycin-resistant derivative (CE3) and a strain lacking the symbiotic plasmid (CFNX89) (4) were subjected to high-stringency hybridization by using an R. leguminosarum fnrN gene as the probe (see Materials and Methods). Two hybridization sequences, one corresponding to the chromosome and the other corresponding to the symbiotic plasmid, were observed in the wild-type strain, while the strain cured of plasmid pCFN42d hybridized only to chromosomal DNA (data not shown). These data suggested that there are two fnrN homologs in R. etli, one located in the symbiotic plasmid (pCFN42d) and the other located on the chromosome.
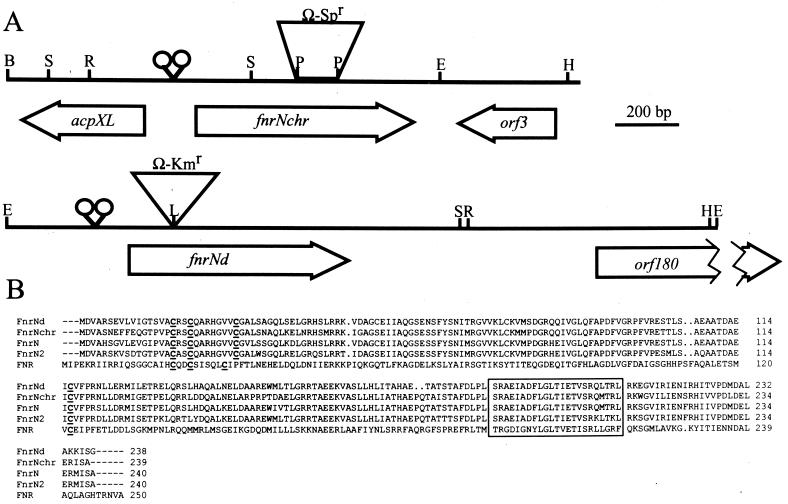
To isolate both fnrN homologs, a CFN42 cosmid library (17) was screened by hybridization with an R. leguminosarum fnrN probe. Two nonoverlapping cosmids were identified by this procedure. One cosmid carried the chromosomal homolog (fnrNchr), and the other harbored the pCFN42d homolog (fnrNd), as determined by hybridization. A 1.9-kb BamHI- HindIII fragment carrying the chromosomal fnrN homolog (fnrNchr) was cloned, as was a 2.3-kb EcoRI fragment carrying fnrN from pCFN42d (fnrNd). These fragments were completely sequenced. Figure 1A shows physical maps of the two regions sequenced.
FIG. 1.
fnrN genes and proteins of R. etli. (A) Physical and genetic map of the R. etli fnrN genes. The positions of the potential Fnr target site sequences are indicated by open circles. The open arrows represent genes discussed in the text. The restriction sites used in subcloning for sequencing are shown, as follows: E, EcoRI; S, SacI; L, SalI; H, HindIII; R, EcoRV. (B) Alignment of the predicted amino acid sequences encoded by the fnrNchr and fnrNd genes from R. etli with the amino acid sequences of FnrN and FnrN2 from R. leguminosarum bv. viciae (GenBank accession no. AA86478 and AAB58263, respectively) and Fnr from E. coli (GenBank accession no. P03019). The four cysteine residues probably involved in oxygen sensing are indicated by boldface type and underlining. The helix-turn-helix motif is enclosed in a box.
In the chromosomal region, three open reading frames (ORFs) were identified. The middle ORF (fnrNchr) encoded a polypeptide that was 239 amino acids long and exhibited extensive similarity with FnrN proteins from R. leguminosarum. FnrNchr exhibited 83 and 79% identity with R. leguminosarum bv. viciae VF39 FnrN and FnrN2, respectively, and 25% identity with Fnr from E. coli. Upstream of fnrNchr there was another ORF, which encoded a polypeptide that was 92 amino acids long and which was is transcribed in a divergent fashion. This polypeptide was very similar (98% identity) to AcpXL (5) from R. leguminosarum (previously designated ORF∗) (8), which is an acyl carrier protein involved in the synthesis of lipid A in R. leguminosarum. Downstream of fnrNchr and also in a divergent orientation, there was a third ORF (orf3), which was 106 residues long and exhibited significant similarity (92% identity) with ORF114 of R. leguminosarum (8). The overall organization of the fnrNchr region is similar to the organization found in R. leguminosarum (8).
Nucleotide sequence analysis of the region corresponding to pCFN42d revealed the presence of an ORF (fnrNd) that encoded a polypeptide which was 240 amino acids long and was very similar to several members of the CRP-Fnr family. FnrNd exhibited 85 and 81% identity with R. leguminosarum bv. viciae VF39 FnrN and FnrN2, respectively, and 27% identity with Fnr from E. coli. Downstream of fnrNd there was a partial ORF that was 108 residues long and encoded a polypeptide which was similar (96% identity) to the amino-terminal end of ORF180 from R. etli CNPAF512 (22). orf180 is also located on the symbiotic plasmid of R. etli CNPAF512 and is cotranscribed with rpoN2 (21). Both fnrN genes are preceded by two conserved anaerobox sequences (Fig. 1A), suggesting that transcription of these genes is activated in response to low oxygen concentrations.
Figure 1B shows an amino acid alignment for R. etli FnrNchr and FnrNd, R. leguminosarum FnrN1 and FnrN2, and E. coli Fnr. As expected for bona fide members of the Fnr family, the FnrNchr and FnrNd polypeptides contained the redox-sensitive module involved in oxygen sensing, formed by four conserved cysteine residues at positions 17, 20, 28, and 116, as well as the carboxy-terminal helix-turn-helix motif involved in DNA binding (Fig. 1B).
Regulatory genes controlling fnrNd and fnrNchr expression.
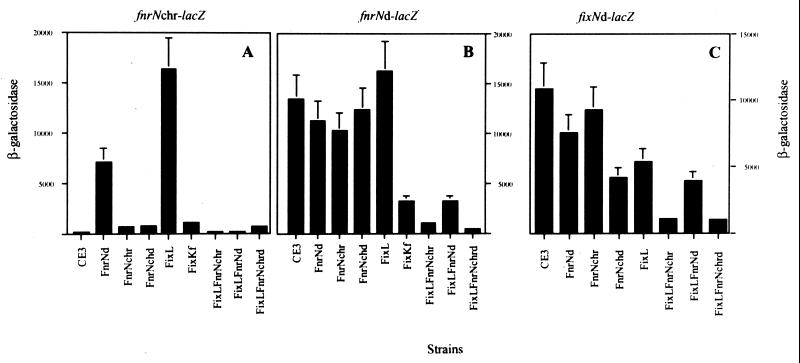
Previously, it was shown that the fixL mutant was able to moderately induce fixNOQPd expression in microaerobic cultures, in contrast to a mutant with a mutation in the fixK gene located in plasmid pf (fixKf), which exhibited no fixNOQPd expression (13). This result was previously explained by arguing that FixL, through a regulatory branch independent of FixKf, repressed another transcriptional activator for fixNOQPd (13). To evaluate the participation of different regulatory genes (including fixL and fixKf) in expression of the fnrNchr and fnrNd genes, the promoter regions of these genes were fused with a promoterless lacZ gene, as described in Materials and Methods. Plasmids pOL15 (fnrNd lacZ) and pOL16 (fnrNchr lacZ) were then introduced separately into R. etli wild-type and appropriate mutant strains, and β-galactosidase activities were determined in microaerobic cultures as described in Materials and Methods.
Only background levels of microaerobic expression of a fnrNchr-lacZ fusion were observed in the wild-type strain or in a strain harboring the fnrNchr::ΩSp mutation (Fig. 2A). In contrast, high levels of expression of the fnrNchr-lacZ fusion were observed in an fnrNd::ΩKm mutant background or in a strain carrying the fixL::loxP mutant allele, although twofold-higher levels of induction were observed in the fixL mutant than in the fnrNd mutant (Fig. 2A). These results show that FnrNd and, more importantly, FixL negatively control expression of fnrNchr, confirming our previous prediction about the repressive role of FixL in another transcriptional activator of the fixNOQPd operon (13).
FIG. 2.
Expression of fnrNchr, fnrNd, and fixNOQPd genes in different R. etli strains. (A) Expression of fnrNchr-lacZ fusion (pOL16) in different R. etli strains cultured microaerobically. (B) Expression of the fnrNd-lacZ fusion (pOL15) in different R. etli strains grown microaerobically. Data are means obtained with three independent cultures; the error bars indicate standard deviations. (C) Expression of the fixNd-lacZ fusion (pOL10) in different R. etli strains cultured microaerobically. β-Galactosidase activities are expressed in micromoles of o-nitrophenol produced per minute per milligram of protein. FnrNchrd, double fnrN mutant.
Further regulatory roles for both fnrNd and fnrNchr can be inferred from the behavior of multiple-mutant derivatives. For instance, in the fixL fnrNd double mutant (IBTOL17), an fnrNchr-lacZ fusion was not expressed; this unexpected result suggests that in the absence of FixL, FnrNd could also be involved in positive control of the fnrNchr gene (Fig. 2A). Similarly an fnrNd fnrNchr double mutant (IBTOL15) or an fixL fnrNchr double mutant (IBTOL16) exhibited background levels of expression, as observed for the fnrNchr-lacZ fusion (Fig. 2A). These results suggest the possibility that in the absence of either fnrNd or fixL, FnrNchr is involved in its own induction. It has been shown previously that in R. leguminosarum bv. viciae fnrN gene expression is subject to both positive and negative autoregulation (7).
Unlike fnrNchr, an fnrNd-lacZ fusion exhibited high levels of expression in a wild-type background; these high levels were not affected by the presence of single mutations in fnrNchr, fnrNd, or fixL or even by the presence of the fnrNd fnrNchr double mutation (Fig 2B). The lack of an effect of any of the single mutations on fnrNd expression may have been due to redundant positive functions for each gene, because in the fixL fnrNd double mutant the levels of expression of fnrNd lacZ were reduced fourfold, while in the fixL fnrNchr double mutant and in the fixL fnrNd fnrNchr triple mutant the levels of expression of fnrNd lacZ were reduced 14- and 36-fold, respectively (Fig. 2B). These results show that FixL, FnrNchr, and FnrNd are all involved in positive regulation of fnrNd expression under microaerobic conditions.
Expression of fnrNchr is controlled by FixL through a regulatory branch independent of FixKf.
Previously, we reported that a mutation in fixL results in a significant reduction in the level of transcription of fixKf (13) Thus, it is conceivable that the loss of a repressive effect on fnrNchr expression observed in a fixL mutant might be attributable to a loss of fixKf, which should act as a negative regulator of fnrNchr expression. According to this hypothesis, a mutation in fixKf should result in induction of fnrNchr at levels as high as those seen in the fixL mutant. To ascertain if this was the case, the levels of expression of the fnrNchr-lacZ transcriptional fusion were determined for an fixKf mutant (CFNX637) that was characterized previously (13). Figure 2A shows that contrary to the hypothesis, expression of the fnrNchr-lacZ fusion in the fixKf mutant was not significantly induced compared with the levels observed for the wild-type strain. This result suggests that the negative effect of FixL on fnrNchr expression is not exerted through FixKf but operates through a separate regulatory branch.
To find out if the positive regulation of FixL on fnrNd was exerted through FixKf, we also determined the levels of expression of the fnrNd-lacZ transcriptional fusion in an fixKf mutant background. The levels of induction of this fusion were fivefold lower in the fixKf mutant than in the wild-type strain (Fig. 2B), indicating that the positive regulation of FixL on fnrNd is exerted through FixKf. A mutation in fixKd had no effect on expression of the two fnrN genes (data not shown).
FnrNchr participates in differential regulation of fixNOQPd expression.
The expression characteristics exhibited by the fnrNchr gene, namely, (i) lack of expression in a wild-type background, (ii) negative control of expression by FixL, and (iii) control through a regulatory branch independent of FixKf, make this gene a good candidate for the hypothetical regulatory gene responsible for differential regulation of the fixNOQPd operon (13). To evaluate the role of the fnrN genes in expression of the fixNOQPd operon, plasmid pOL10, containing an fixNd-lacZ transcriptional gene fusion (32), was introduced separately into different mutant backgrounds. β-Galactosidase activity was determined in cultures grown under microaerobic conditions as described in Materials and Methods.
As shown in Fig. 2C, a high level of expression of the fixNd-lacZ fusion was observed in a wild-type background. The levels of expression of this fusion were not significantly reduced by the introduction of single mutations in either fnrNd or fnrNchr; however, the fnrNd fnrNchr double mutant exhibited induction levels that were twofold lower than those of the wild-type strain. As reported previously, a mutation in fixL reduced the levels of expression of the fixNd-lacZ fusion twofold (13). The levels of expression of this fusion in a fixL fnrNd double mutant background were the same as the levels in the fixL background, thus eliminating the possibility that FnrNd is the regulator responsible for differential regulation of the fixNOQPd operon. In contrast, the fixL fnrNchr double mutant, as well as the fixL fnrNchr fnrNd triple mutant, exhibited levels of induction of the fixNd-lacZ fusion that were 10-fold lower than the levels of expression found in the wild-type strain (Fig. 2C). These results suggest that FixL-FixKf and FnrNchr are responsible for induction of fixNOQPd under microaerobic free-living culture conditions. However, FnrNd also participates in fixNOQPd expression to some extent, as suggested by the reduction in the levels of fixNOQPd expression in the double fnrN mutant.
Nitrogenase activities of R. etli strains with mutations in fnrNchr and/or fnrNd.
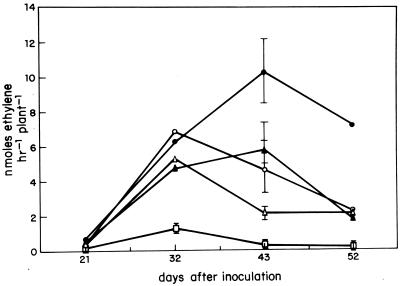
To determine the roles of both FnrN proteins in nitrogen fixation, P. vulgaris plants were inoculated separately with the wild-type strain or strains harboring mutations in fnrNchr, in fnrNd, in both fnrNchr and fnrNd, or in fnrNchr, fnrNd, anf fixL, and nitrogenase activities were determined at different times after inoculation. Figure 3 shows that all of the mutant strains except the fnrNchr fnrNd fixL triple mutant were still able to fix nitrogen during symbiosis. However, all mutations had some effect on the temporal activity of nitrogenase. Interestingly, 32 days after inoculation nitrogenase activity was greatly affected in plants inoculated with the fnrN mutants (Fig. 3). These data show that a loss of FnrN proteins has a long-term effect on nitrogenase expression in planta. Also, these data show that both FnrN proteins, in conjunction with FixL, participate in maintaining nitrogenase activity during symbiosis.
FIG. 3.
Time course of acetylene reduction activity in plants inoculated with different R. etli strains. Plants were inoculated with CE3 (●), IBTOL14 (fnrNchr) (○), IBTOL12 (fnrNd) (▴), IBTOL15 (fnrNd fnrNchr) (▵), and IBTOL18 (fixL fnrNd fnrNchr) (□). The acetylene reduction activities of four plants in each of two pots were determined on different days. The data are means based on the results for two pots (eight plants); the variations were 30% or less. The error bars indicate standard deviations.
DISCUSSION
Production of the symbiotic terminal oxidase by Rhizobium strains is a key process for achieving optimal symbiotic nitrogen fixation, since this terminal oxidase has a high affinity for oxygen and is efficiently coupled to the synthesis of ATP. We have shown previously that overexpression of the fixNOQP genes, which code for the cbb3 type of symbiotic terminal oxidase, can enhance symbiotic nitrogen fixation in certain genetic backgrounds (33). Since nitrogen fixation is a microaerobic process, oxygen is a key enviromental signal determining expression of fixNOQP. Our previous studies of microaerobic control of the two fixNOQP operons (fixNOQPd and fixNOQPf) in R. etli CFN42 showed that both set of genes are controlled by an fixL-fixKf cascade without participation of an fixJ gene (13). Interestingly, expression of the operon most important for symbiotic nitrogen fixation, fixNOQPd, is completely dependent on FixKf but can proceed at adequate levels in the absence of FixL. This unexpected result was explained previously by proposing the existence of an additional transcriptional activator for fixNOQPd expression, whose expression should be under negative control by FixL in a regulatory branch independent of FixKf (13).
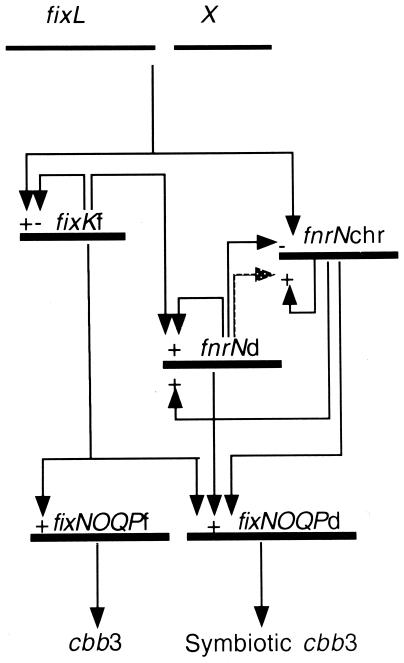
In this paper, we describe an analysis of expression of two fnrN genes (fnrNd and fnrNchr) in R. etli CFN42. In this study we also explored the role of these fnrN genes in controlling expression of the fixNOQPd operon. Figure 4 shows our current view of the circuit used for regulation of the fixNOQP genes under microaerobic conditions. In this model X represents the functional homolog of FixJ that has not been identified yet. Our results show that FnrNchr is a possible additional transcriptional activator for fixNOQPd because (i) it is not expressed in a wild-type background, (ii) it is negatively controlled by FixL through a regulatory branch independent of FixKf, and (iii) it is required for microaerobic expression of fixNOQPd in an fixL mutant background. Moreover, fnrNd also plays a role, albeit a minor one, in microaerobic expression of the fixNOQPd operon, as suggested by the behavior of an fnrNd fnrNchr double mutant (Fig. 2C). The effect of the mutations on fnrN genes, fixKf, and fixL could not be attributed to polar effects on downstream genes since in the case of the fnrN genes and fixKf (13) there are no downstream genes that could be cotranscribed, while in the case of fixL we have previously shown that fixKf is transcribed independently (13) Thus, microaerobic expression of the fixNOQPd operon in R. etli is subject to the direct regulatory input of three different activators: FixKf, FnrNchr, and FnrNd (Fig. 4). However, although the levels of expression of the fixNd-lacZ fusion in the triple mutant were 11-fold lower than the levels of expression in the wild-type strain, supporting hypothesis that these regulators have an important role, they were 5-fold higher than the background levels. Therefore, we cannot exclude the possibility that additional signal transduction pathways participate in induction of microaerobic fixNOQP expression. In this regard, an anaerobic two-component signal transduction pathway (RegAB) regulates the expression of genes encoding a cbb3 terminal oxidase in Rhodobacter capsulatus (36). However, our data indicates that the transcriptional regulators FixL, FnrNchr, and FnrNd are essential for efficient nitrogen fixation (Fig. 3). In particular, FnrN proteins have a major role in the late stage of symbiosis since single mutations in the fnrN genes have a severe effect on nitrogenase activity 32 days after plant inoculation (Fig. 3).
FIG. 4.
Regulatory circuit for fixNOQP gene expression in R. etli CE3 under microaerobic conditions. The dotted arrow indicates conditional control in an fixL background. Positive regulation and negative regulation are indicated by plus and minus signs, respectively.
Our data shows that both FnrN genes are involved in positive and negative control of gene expression. Although dual control by a regulatory protein that acts on the same target gene (as exhibited by FnrNd acting on the fnrNchr gene) is rare, it is not without precedent. Transcriptional factors of the MerR family can act both positively and negatively when they are bound at a single site (35). A more similar example occurs in R. leguminosarum UPM791, in which both positive autoregulation and negative autoregulation have been observed for the fnrN genes (7).
The model for expression of fixNOQPd in R. etli (Fig. 4) combines elements from several systems. For instance, in R. leguminosarum UPM791, microaerobic expression of fixNOQP is achieved with participation of duplicated fnrN genes and without participation of FixL, FixJ, or FixK (7, 15). In R. leguminosarum bv. viciae VF39, control is achieved through an unusual FixL protein and FnrN without participation of a conventional FixJ or FixK protein (32). Finally, B. japonicum (22, 24, 25) and S. meliloti (2, 9, 18) are similar in the sense that they control fixNOQP expression mainly through a system in which FixL, FixJ, and FixK are used. Regulation of the fixNOQPd operon in R. etli is striking because of the number of putative interactions among regulatory proteins, including control by an unusual FixL protein, FixKf, FnrNchr, and FnrNd without participation of a conventional FixJ protein. Furthermore, this model also includes regulatory interactions between the two fnrN genes, as well as control of fnrNchr by FixL. We believe that this regulatory system should allow exquisite tuning of fixNOQP expression to cope with the demands imposed by the nitrogen fixation process. Indications that this is the case came from the nitrogenase activities of plants inoculated with the fnrN mutants. Although a complete loss of nitrogenase activity was observed only with the fixL fnrNchr fnrNd triple mutant, analysis of the temporal activity of nitrogenase revealed that the two fnrN genes are more important for supporting nitrogen fixation at late stages of the symbiosis (Fig. 3).
Current efforts in our group are devoted to determining which protein acts, together with FixL, to activate expression of fixKf and to repress expresssion of fnrNchr (X in Fig. 4). Also, it is important to understand the mechanisms by which the two fnrN genes and the two fixNOQP operons are differentially regulated. As pointed out previously, two anaerobox sequences were found in front of both fnrN genes. However, in the promoter regions of R. etli genes (fnrNchr, fnrNd, fixNOQPf, fixNOQPd) that are under the control of either FixKf or FnrN or both, the anaerobox sequences are identical to the consensus anaerobox sequence. Therefore, we still have to determine the affinities of binding of the different FixK and FnrN proteins to the different anaerobox sequences. Such efforts should provide a better understanding of the molecular events involved in the differential expression of these genes.
ACKNOWLEDGMENTS
This work was supported in part by the European Communities through International Scientific Co-operation Program contract CI1∗-CT94-0042, by DGAPA contracts IN204697 and IN202599, and by CONACyT contracts 31561-N and 0028.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Batut J, Daveran-Mingot M-L, David M D, Jacobs J, Garnerone A M, Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989;8:1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batut J, Boistard P. Oxygen control in Rhizobium. Antonie Leeuwenhoek. 1994;66:129–150. doi: 10.1007/BF00871636. [DOI] [PubMed] [Google Scholar]
- 4.Brom S, García de los Santos A, Stepkowski T, Flores M, Dávila G, Romero D, Palacios R. Different plasmids of Rhizobium leguminosarum bv. phaseoliare required for optimal symbiotic performance. J Bacteriol. 1992;174:5183–5189. doi: 10.1128/jb.174.16.5183-5189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brozek K A, Carlson R W, Raetz R H. A special acyl carrier protein for transferring long hydroxylated fatty acids to lipid A in Rhizobium. J Biol Chem. 1996;271:32126–32136. doi: 10.1074/jbc.271.50.32126. [DOI] [PubMed] [Google Scholar]
- 6.Bullock J C, Fernandez J M, Short J M. XL1-Blue: a high efficiency plasmid transforming recA Escherichia colistrain with β-galactosidase selection. BioTechniques. 1987;5:376–379. [Google Scholar]
- 7.Colombo M V, Gutierrez D, Palacios J M, Imperial J, Ruiz-Argüeso T. A novel autoregulation mechanism of fnrN expression in Rhizobium leguminosarum bv. viciae. Mol Microbiol. 2000;36:477–486. doi: 10.1046/j.1365-2958.2000.01867.x. [DOI] [PubMed] [Google Scholar]
- 8.Colonna-Romano S, Arnold W, Schlüter A, Boistard P, Pühler A, Priefer U B. An Fnr-like protein encoded in Rhizobium leguminosarum bv. viciae shows structural and functional homology to Rhizobium melilotiFixK. Mol Gen Genet. 1990;223:138–147. doi: 10.1007/BF00315806. [DOI] [PubMed] [Google Scholar]
- 9.David M, Daveran M L, Batut J, Dedieu A, Domergue O, Ghai J, Hertig C, Boistard P, Khan D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988;54:671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- 10.Figurski D H, Helinski D R. Replication of an origin containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foussard M, Garnerone A-M, Ni F, Soupène E, Boistard P, Batut J. Negative autoregulation of the Rhizobium meliloti fixKgene is indirect and requires a newly identified regulator, FixT. Mol Microbiol. 1997;25:27–37. doi: 10.1046/j.1365-2958.1997.4501814.x. [DOI] [PubMed] [Google Scholar]
- 12.Gilles-Gonzalez M A, Gonzalez G, Perutz M F, Kiger L, Marden M C, Poyart C. Heme-based sensors, exemplified by the kinase FixL, are a new class of heme protein with distinctive ligand binding and autoxidation. Biochemistry. 1994;33:8067–8073. doi: 10.1021/bi00192a011. [DOI] [PubMed] [Google Scholar]
- 13.Girard L, Brom S, Dávalos A, Lopez O, Soberón M, Romero D. Differential regulation of fixN reiterated genes in Rhizobium etli by a novel fixL-fixKcascade. Mol Plant-Microbe Interact. 2000;13:1283–1292. doi: 10.1094/MPMI.2000.13.12.1283. [DOI] [PubMed] [Google Scholar]
- 14.Gish W, States D W. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez D, Hernando Y, Palacios J M, Imperial J, Ruiz- Argüeso T. FnrN controls symbiotic nitrogen fixation and hydrogenase activities in Rhizobium leguminosarum biovar viciaeUPM791. J Bacteriol. 1997;179:5264–5270. doi: 10.1128/jb.179.17.5264-5270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernando Y, Palacios J M, Imperial J, Ruiz-Argüeso T. The hypBFCDE operon from Rhizobium leguminosarum biovar viciae is expressed from an Fnr-type promoter that escapes mutagenesis of the fnrNgene. J Bacteriol. 1995;177:5661–5669. doi: 10.1128/jb.177.19.5661-5669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huerta-Zepeda A, Ortuño L, Du Pont G, Durán S, Lloret A, Merchant-Larios H, Calderón J. Isolation and characterization of Rhizobium etlimutants altered in degradation of asparagine. J Bacteriol. 1997;179:2068–2072. doi: 10.1128/jb.179.6.2068-2072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lois A F, Weinstein M, Ditta G S, Helinski D R. Autophosphorylation and phosphatase activity of the oxygen-sensing protein FixL of Rhizobium melilotiare coordinately regulated by oxygen. J Biol Chem. 1993;268:4370–4375. [PubMed] [Google Scholar]
- 19.Mandon K, Kaminski A, Elmerich C. Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J Bacteriol. 1994;176:2560–2568. doi: 10.1128/jb.176.9.2560-2568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 21.Michiels J, Moris M, Dombrecht B, Verreth C, Vanderleyden J. Differential regulation of Rhizobium etli rpoN2gene expression during symbiosis and free-living growth. J Bacteriol. 1998;180:3620–3628. doi: 10.1128/jb.180.14.3620-3628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nellen-Anthamatten D, Rossi P, Preisig O, Kullik I, Babst M, Fisher H M, Hennecke H. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J Bacteriol. 1998;180:5251–5255. doi: 10.1128/jb.180.19.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noel K D, Sánchez A, Fernández L, Leemans J, Cevallos M A. Rhizobium phaseoli symbiotic mutants with transposon Tn5insertions. J Bacteriol. 1984;158:148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicumare essential for nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preisig O, Zufferey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quandt J, Hynes M F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene. 1993;127:15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]
- 27.Quinto C, de la Vega H, Flores M, Fernández L, Ballado R, Soberón G, Palacios R. Reiteration of nitrogen fixation gene sequences in Rhizobium phaseoli. Nature (London) 1982;229:724–726. [Google Scholar]
- 28.Renalier M H, Batut J, Ghai J, Terzaghi B, Ghérardi M, David M, Garnerone A M, Vasse J, Huguet T, Boistard P. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nodlocus. J Bacteriol. 1987;169:2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlüter A, Patchkowski T, Quandt J, Selinger L B, Weidner S, Krämer M, Zhou L, Hynes M F, Priefer U B. Functional and regulatory analysis of the two copies of the fixNOQP operon of Rhizobium leguminosarumstrain VF39. Mol Plant-Microbe Interact. 1997;10:605–616. doi: 10.1094/MPMI.1997.10.5.605. [DOI] [PubMed] [Google Scholar]
- 31.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivogenetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 32.Soberón M, Lopez O, Miranda J, Tabche M L, Morera C. Genetic evidence for 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) as a negative effector of cytochrome terminal oxidase cbb3 production in Rhizobium etli. Mol Gen Genet. 1997;254:665–673. doi: 10.1007/s004380050464. [DOI] [PubMed] [Google Scholar]
- 33.Soberón M, Lopez O, Morera C, Girard M L, Tabche M L, Miranda J. Enhanced nitrogen fixation in a Rhizobium etli ntrC mutant that overproduces the Bradyrhizobium japonicum symbiotic terminal oxidase cbb3. Appl Environ Microbiol. 1999;65:2015–2019. doi: 10.1128/aem.65.5.2015-2019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarumSym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 35.Summers A O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992;174:3097–4001. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swem L R, Elsen S, Bird T H, Swem D L, Koch H-G, Myllykallio H, Daldal F, Bauer C E. The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J Mol Biol. 2001;309:121–138. doi: 10.1006/jmbi.2001.4652. [DOI] [PubMed] [Google Scholar]