Abstract
Eosinophils are important effector cells and therapeutic targets in allergic diseases. Emerging data indicate that eosinophils infiltrate a variety of solid tumor types and have pleiotropic activities by at least two non-mutually exclusive mechanisms: direct interactions with tumor cells, and intricate cross-talk with lymphocytes. In light of the immune checkpoint inhibition revolution in cancer therapy, we review eosinophil–lymphocyte interactions in the tumor microenvironment. We also analyze potential interactions between eosinophils and lymphocyte subsets, including T cells, natural killer cells and innate lymphoid cells. We provide perspectives on the consequences of these interactions and how eosinophils are accessory cells that can affect the response to various forms of T cell-mediated immunotherapies and might be therapeutically targeted to improve cancer immunotherapy.
Eosinophils differentiate from distinct CD34+ myeloid progenitor cells in the bone marrow. They have been mostly studied in the context of parasitic infections and allergic diseases1 in which CD4+ type 2 helper T cells (TH2 cells) primarily regulate eosinophils by generating the eosinophil growth, activation and survival cytokine interleukin (IL)-5 (ref.1).
Reports dating back to the late 1900s identified eosinophilia in the peripheral blood of patients with cancer2. Since then, eosinophils have been shown to infiltrate a variety of solid tumors, especially in mucosal organs such as the gastrointestinal tract and the lungs3, where they function either as participants of an integral inflammatory response or in response to therapies4. However, how eosinophils are recruited and activated in cancer has been some-what overlooked.
In this Perspective, we provide an overview of known and possible interactions between eosinophils and lymphocyte subsets, including T cells, natural killer (NK) cells and innate lymphoid cells (ILCs). Clinical and experimental data regarding the potential involvement of eosinophils in various forms of T cell-mediated immunotherapies are also presented. This focus on eosinophils should promote a better understanding of how the cellular components of the tumor microenvironment (TME) are orchestrated and might encourage the development and optimization of therapeutic strategies for cancer immunotherapy.
Interactions of eosinophils with innate lymphoid cells
ILC2–eosinophil interactions.
The ILC family includes a heterogeneous group of immune cells with distinct yet pleiotropic activities. These cells have now joined a growing list of cellular TME components that might contribute to tumorigenisis5. ILC2s can respond to many signals and mediators of type 2 immune responses6 and can secrete type 2 cytokines (such as IL-4, IL-5 and IL-13) that promote eosinophil expansion or migration via epithelial cell and macrophage production of chemotactic eotaxins6,7 (Fig. 1).
Fig. 1 |. Eosinophil–innate lymphoid cell interactions.
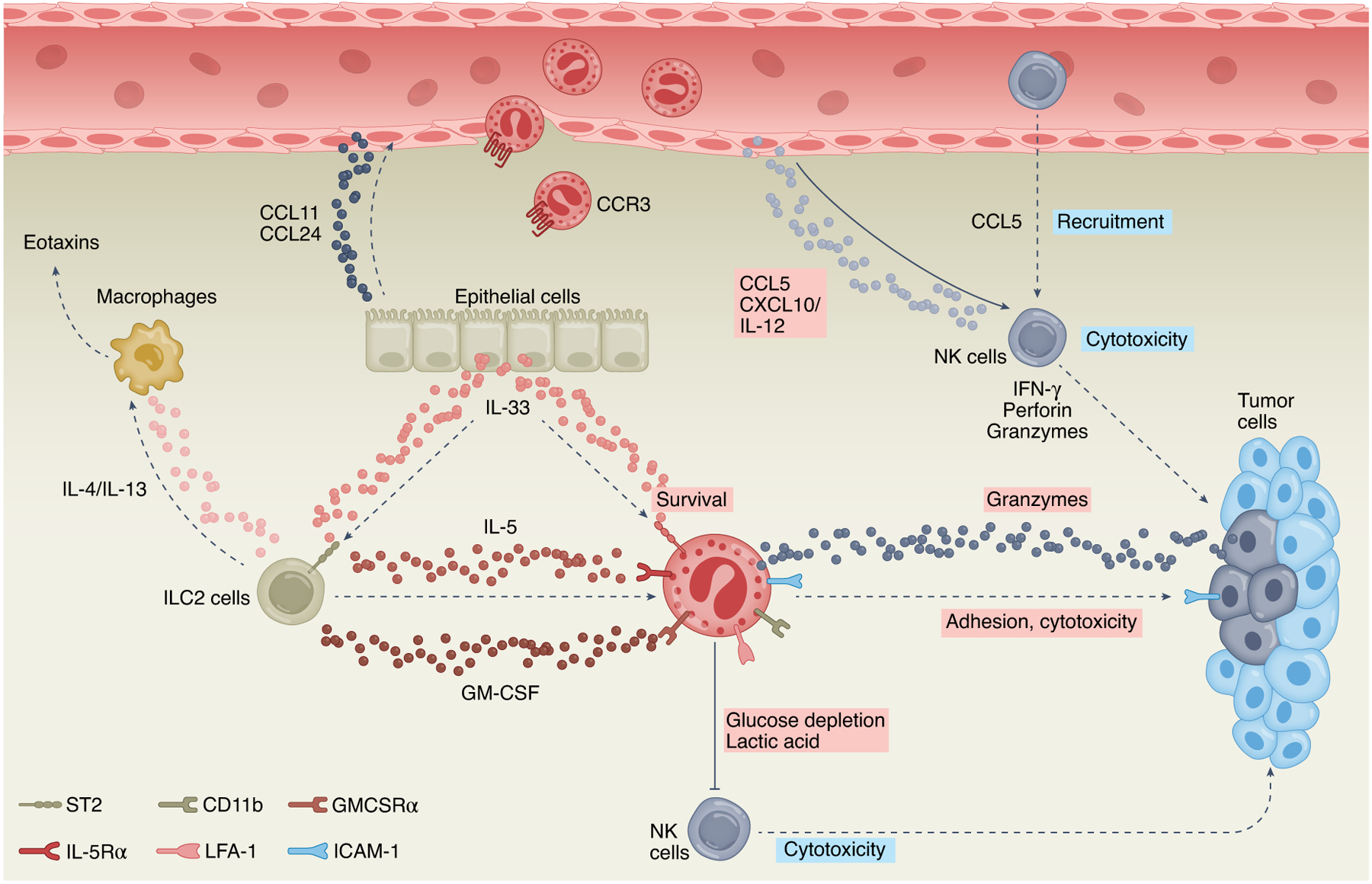
Type 2 innate lymphoid cells (ILC2s) can shape the TME by regulating eosinophil activities. Stimulating ILC2s with IL-33 induces the expression of IL-4 and/or IL-13, which can promote eosinophil migration via induction of eotaxins (for example, CCL11 and/or CCL24) in epithelial cells and macrophages. Moreover, IL-33 induces the secretion of ILC2-derived IL-5 and/or GM-CSF, which supports eosinophil survival, degranulation and cytotoxic activities. IL-33 can directly stimulate the expression of adhesion molecules (LFA-1, CD11b) in eosinophils, which mediate their binding to tumor cells via ICAM-1 and subsequent induction of eosinophil-mediated cytotoxicity. In addition to ILC2s, eosinophils have complex interactions with NK cells. Eosinophils can promote NK cell cytotoxicity via secretion of CCL5, CXCL10 and IL-12, which support NK cell migration and activation. In settings of preexisting allergic inflammation, ILC2s secrete IL-5, which binds to IL-5Rα (CD125) on eosinophils and can promote eosinophil-mediated metabolic shift in NK cells via glucose depletion and production of lactic acid, resulting in inhibition of NK cell cytotoxic activities. Pink boxes indicate direct eosinophil-derived mediators and/or activities; blue boxes indicate indirect eosinophil-mediated lymphocyte activities.
ILC2s have been shown to contribute to the TME in an eosinophil-dependent and eosinophil-independent fashion8. In one study9, intravenous injection of B16-F10 melanoma cells resulted in increased production of IL-5 by lung ILCs that share similar surface receptors and functional properties with ILC2s, including their ability to produce IL-5 in response to the epithelial cell-associated alarmins IL-33 or IL-25 (ref.9) (Fig. 1). This increase in IL-5 production was associated with increased eosinophilic infiltration into the lungs. Moreover, in mice lacking IL-5 or treated with anti-IL-5, intravenous injection of B16-F10 cells resulted in decreased eosinophil infiltration and increased lung tumor colonization in comparison with wild-type (WT) or control antibody-treated mice9. In a different study10, eosinophil–ILC2 interactions were proposed in experimental melanoma10. Induction of melanoma in BRAFCA;PTENloxp; Tyr:CreERT2 mice via topical application of 4-hydroxytamoxifen leading to primary malignant melanoma formation, which resembles human disease11, resulted in recruitment of multiple types of ILCs10. Specific depletion of ILC2s using Il7rCre/+RoraΔ/Δ mice or the Cd4Cre/+Icosfl-Dtr/+ mice increased tumor burden, which was eosinophil dependent and granulocyte-macrophage colony-stimulating factor (GM-CSF) dependent10. Indeed, single-cell RNA sequencing of tumor-infiltrating leukocytes revealed that ILC2s were enriched with IL-5 and GM-CSF10, two hallmark eosinophil survival cytokines12. Notably, GM-CSF-deficient mice displayed an increased tumor burden, and reconstitution of ILC2s into tumor-bearing GM-CSF-deficient mice resulted in a specific increase of eosinophils and decreased tumor burden10. Conditioned media of IL-33-stimulated ILC2s promoted eosinophil survival10, and induced expression of genes encoding eosinophil granule proteins, Epx, Ear1 and Ear2, all of which are associated with the cytotoxic activity of eosinophils10 (Fig. 1). In support of an anti-tumorigenic function for the ILC2–eosinophil axis, intravenous injection of melanoma cells increased colonization of tumors in the lungs of ΔdblGATA eosinophil-deficient mice compared with WT mice10.
In addition to IL-5 and GM-CSF, IL-33 (ref.13) is an important cytokine that can regulate the ILC2–eosinophil cross-talk in the TME. Transgenic expression or exogenous delivery of IL-33 in models of lung colonization following intravenous injections of B16-F10 melanoma cells or Lewis lung carcinoma increased antitumor immunity that was characterized by activation and infiltration of ILC2s and eosinophils, as well as CD8+ T cells and NK cells14,15. Furthermore, IL-33 directly activated eosinophils to enhance the expression of the adhesion molecules LFA-1 and CD11b and facilitated recognition and subsequent adhesion of eosinophils to tumor cells via tumor cell-expressed ICAM-1 (refs.14,16). In a different study17, in response to IL-33, eosinophils were transcriptionally activated17 and secreted granulate ribonucleases and granzymes, which can directly induce tumor cell death4 (Fig. 1).
NK cell–eosinophil interactions.
Eosinophils express multiple receptors that are capable of interacting with NK cells. Human eosinophils express the CD2 subfamily of receptors (including NTB-A, 2B4, CD84, CD58, IRp60 and CD48)18,19 as well as LFA-1 (ICAM-3) and ligands for the natural cytotoxicity receptors NKp30 and NKp46 (ref.20). These receptors are functional in mediating eosinophil–NK cell cross-talk as blockade of NKp46, NKp30 and LFA-1 can reduce eosinophil-mediated NK cell activation in vitro20.
Experimentally, IL-33 facilitates the recruitment of NK cells to the lungs of mice in response to intravenous injection of 4T1 tumor cells21. In this study21, NK cell recruitment was dependent on CCL5, mainly produced by eosinophils and CD8+ T cells21. Accordingly, the increase in IL-33-induced NK cell numbers in the lungs was reversed following depletion of eosinophils and CD8+ T cells21 (Fig. 1). Additionally, peritoneal injection of bone marrow-derived eosinophils that were stimulated with lipopolysaccharides or CpG DNA increased the percentage of peritoneal NK cells via a mechanism that seems to involve eosinophil secretion of CXCL10 and IL-12 (ref.22). In addition, CpG-activated mouse eosinophils increased NK cell-dependent interferon (IFN)-γ production and killing of YAC1 lymphoma cells22 (Fig. 1). A pro-cytotoxic effect was also seen following co-culture of human eosinophils with IL-12-stimulated NK cells, resulting in a contact-dependent increase in NK cell cytotoxicity toward K562 myelogenous leukemia cells20 (Fig. 1).
Interestingly, under settings of preexisting type 2 immune responses such as those that occur in allergic inflammation, eosinophils can suppress NK cell cytotoxic activities and thus increase tumor burden23. This phenomenon was observed in mice that were treated with IL-33 or had preexisting Aspergillus-induced allergic airway disease, which resulted in increased lung colonization by B16-F10 melanoma cells after intravenous injection23. In these IL-33-treated mice, eosinophilia was positively correlated with IL-33 suppression of IFN-γ production by NK cells23. Depletion of eosinophils by anti-IL-5 reversed the suppressive effect of IL-33 on IFN-γ and granzyme B production by NK cells23. Furthermore, in these settings, ILC2-derived IL-5 promotes eosinophil-mediated suppression of lung NK cell activity by modulating the metabolic environment. Eosinophils readily take up the glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) and secrete lactate, which can suppress the effector functions of NK cells via glucose depletion and production of lactic acid23 (Fig. 1).
Interactions of eosinophils with T cells
Effector T cells are the major effector cells in the adaptive immune response leading to tumor elimination24. Activated CD8+ T cells have direct cytotoxic activity against tumor cells, and optimal polarization of CD4+ T cells is essential to obtain durable systemic antitumor immunity25,26. Furthermore, CD4+ cytotoxic cells and the TH1 subset of helper T cells have antitumor activity27, whereas regulatory T (Treg) cells exert potent tumor-promoting activity24.
Eosinophils–T cell interactions might promote antitumor immunity.
Bioinformatics and experimental evidence indicate a role for eosinophil–T cell interactions in cancer. An RNA profiling algorithm of RNA signatures corresponding with multiple immune cells was used to predict that the abundance of eosinophils correlated with abundance of resting memory CD4+ T cells in a variety of bulk RNA-sequenced primary tumors3. This predicted eosinophil RNA signature also correlated with RNA signatures of CD8+ T cells in bulk RNA-sequenced pleural metastatic samples from patients with breast cancer28.
Experimentally, eosinophils respond to cytokines that can be secreted by CD4+ T cells and CD8+ T cells. For example, clearance of lung and visceral metastases after intravenous injection of chicken ovalbumin (OVA)-expressing B16-F10 melanoma cells demonstrated that tumor clearance is dependent on secretion of IL-5 by TH2 cells, expression of CCL11, and the presence of degranulating eosinophils29. Furthermore, rejection of B16-F10 cells that were engineered to secrete GM-CSF led to the concurrent induction of TH1 and TH2 responses, including the hallmark TH1 (IFN-γ) and TH2 (IL-4 and IL-5) cytokines. Both types of cytokine were required for optimal antitumor immunity, which resulted in activation of eosinophils and macrophages and their production of superoxide and nitric oxide29,30. Interestingly, IFN-γ stimulation augments direct eosinophil-mediated cytotoxicity toward colorectal cancer cells31, and IFN-γ-stimulated eosinophils acquire a transcriptional profile similar to M1 macrophages, which also typically have antitumor functions32 (Fig. 2). In addition, IFN-γ-stimulated eosinophils secrete T cell-recruiting chemokines, such as CCL5, CXCL9, CXCL10 and CXCL16 (ref.28). Furthermore, conditioned medium that was obtained from IFN-γ-activated eosinophils enhanced T cell migration in vitro28. In this study, bulk RNA sequencing of eosinophils obtained from the lungs of mice after intravenous injections of polyomavirus middle tumor antigen (PyMT) breast cancer cells, displayed a transcriptome signature that was enriched with IFN-γ-associated and STAT-1-associated pathways28. Adoptive transfer of IFN-γ/tumor necrosis factor (TNF)-activated eosinophils into the lungs facilitated the infiltration of CD4+ and CD8+ T cells and promoted antitumor immunity (Fig. 2). Depletion of eosinophils using anti-Siglec-F antibodies in Rag2−/−/Il2rg−/− mice and pharmacological depletion CD4+ T cells, CD8+ T cells and eosinophils in WT mice showed that the anti-tumorigenic functions of eosinophils in this model are lymphocyte dependent28. IFN-γ also governs the anti-tumorigenic activities of eosinophils in other experimental models. Depletion of Treg cells in a model of intradermal injection of B16-OVA melanoma cells induced pronounced tumor eosinophilia associated with tumor rejection33. Pharmacological depletion of eosinophils demonstrated an essential role for eosinophils in tumor rejection due to their ability to promote CD8+ T cell accumulation through secretion of CCL5, CXCL9 and CXCL10 (ref.33) (Fig. 2). In this study, co-adoptive transfer of OT-I CD8+ T cells with TNF and IFN-γ-activated eosinophils led to CD8+ T cell-mediated antitumor immunity, whereas adoptive transfer of CD8+ T cells alone failed to induce tumor rejection33. Also, intravenous transfer of eosinophils and CD8+ T cells triggered vessel normalization and reprogramming of tumor-associated macrophages to an M1 phenotype with increased expression of Ifng, Tnf and Cxcl10 and decreased expression of M2 macrophage markers associated with tumor-promoting activities, including Tgfb1, Chil3 (Ym1) and Arg1 (ref.33) (Fig. 2).
Fig. 2 |. Eosinophil–T cell interactions.
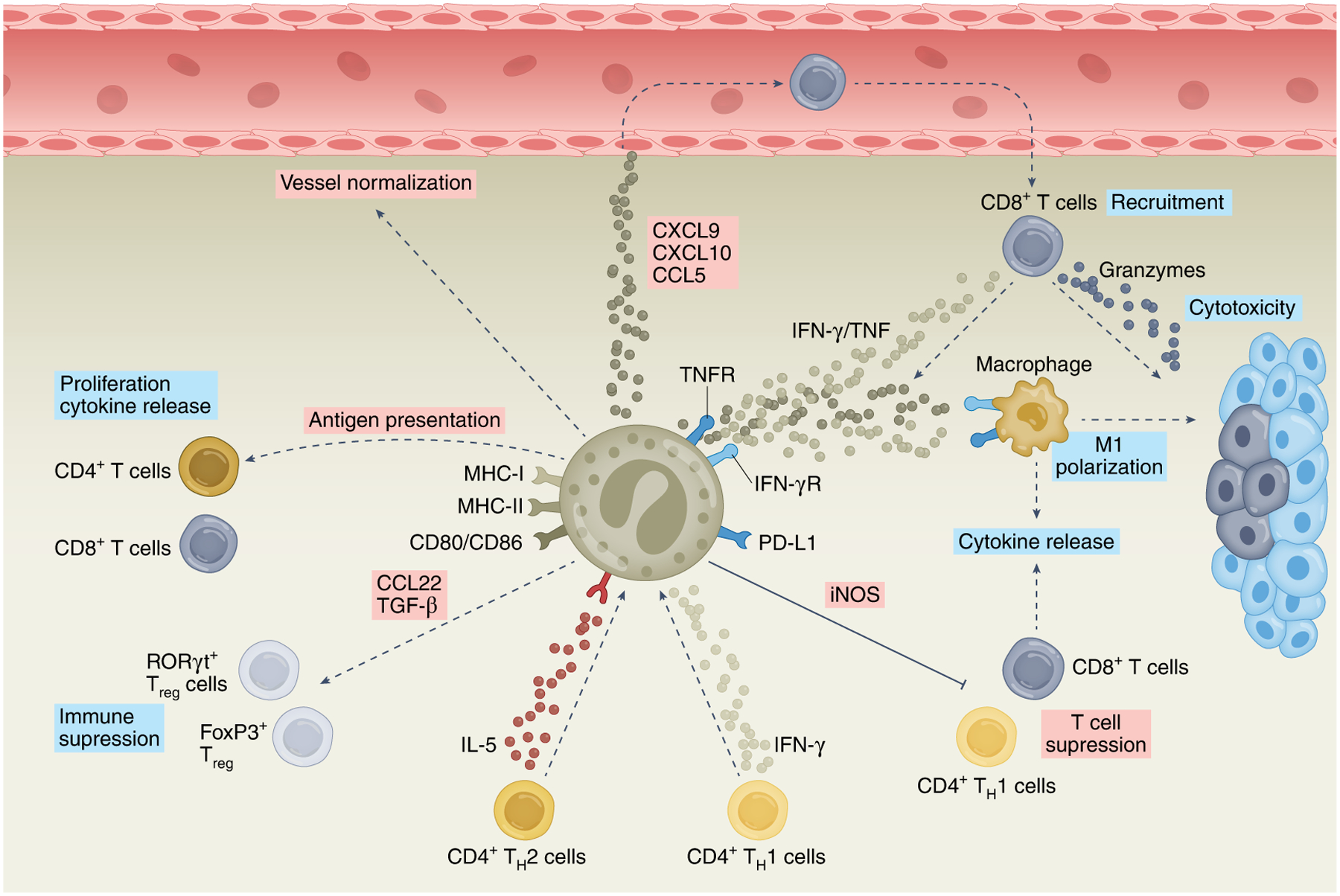
Eosinophils can interact with T cells in various ways. IL-5 derived from TH2 cells and IFN-γ derived from TH1 cells induce eosinophils to display anti-tumorigenic activities toward melanoma cells. Furthermore, activated eosinophils express multiple co-stimulatory ligands, including MHC-I, MHC-II, CD80 and CD86, which can facilitate eosinophil-dependent antigen presentation to CD4+ and CD8+ T cells and consequently support their proliferation and cytokine release. Activation of eosinophils with IFN-γ with or without TNF can induce the secretion of multiple chemokines, including CXCL9, CXCL10 and CCL5, which support the recruitment and cytotoxic activities of CD8+ T cells. In these settings, eosinophils reduce vascular leakiness and hypoxia (vessel normalization) and promote the polarization of macrophages toward an anti-tumorigenic (M1-like) phenotype. Eosinophils can also suppress T cell activities via direct and indirect mechanisms. Indirectly, eosinophil-derived TGF-β regulates the expansion of RORγt+ gastrointestinal Treg cells. In addition, eosinophils can secrete CCL22, which promotes lung metastasis through recruitment of Treg cells to the TME. Finally, eosinophils can suppress T cell responses in an iNOS-dependent manner via PD-L1. Pink boxes indicate direct eosinophil-derived mediators and/or activities; blue boxes indicate indirect eosinophil-mediated lymphocyte activities.
Eosinophils as antigen-presenting cells and amplifiers of T cell responses.
In addition to supporting T cell migration, eosinophils can amplify T cell activation via antigen presentation34. Although eosinophil-mediated antigen presentation has not been shown in the context of cancer, eosinophils can express and upregulate co-stimulatory and co-inhibitory molecules, such as major histocompatibility complex class II (MHC-II), CD80, CD86, CD40, CD48 and programmed death ligand 1 (PD-L1)32,35–40. Eosinophils also function as antigen-presenting cells (APCs) in various experimental models, where they induce CD4+ T cell proliferation and type 2 cytokine production (for example, IL-4, IL-5 and IL-13) (Fig. 2). Purification of eosinophils in the absence of ammonium chloride, which inhibits antigen processing, indicates that eosinophils can act as professional APCs and stimulate naïve T cells41. Proteomic analysis of eosinophils obtained from the lungs of PyMT-colonized mice demonstrated that eosinophils display high expression of the antigen presentation-related proteins β2-microglobulin, transporter 2 ATP-binding cassette subfamily B member (TAP2), and TAP-binding protein (TAPBP)28. Whether eosinophils can present antigen in the context of MHC class I recognition is unclear. Nonetheless, co-culture of bone marrow-derived eosinophils with OT-I CD8+ T cells resulted in robust T cell proliferation in an antigen-dependent manner, suggesting that culture-derived eosinophils can present antigen via MHC-I (ref.42) (Fig. 2).
The ability of eosinophils to amplify T cell responses has also been shown in animal models of colorectal cancer. Assessing CD4+ and CD8+ T cell numbers in eosinophil-deficient PHIL mice (transgenic mice that express diphtheria toxin under the eosinophil peroxidase promoter causing eosinophil-specific cell death)43 and depletion of eosinophils using anti-IL-5 neutralizing antibodies showed that T cell frequency and absolute numbers were unchanged in the TME. However, in the absence of eosinophils, decreased production of IFN-γ and TNF occurred upon restimulation of T cells with phorbol myristate acetate and ionomycin44. Similarly, depleting eosinophils in Apcmin/+ mice, which display spontaneous intestinal adenoma formation, resulted in impaired TH1 responses, which increased tumor burden. In this same study, specific ablation of Irf5 or Csf2rb (which mediate the signals of IL-5, GM-CSF and IL-3) in eosinophils resulted in impaired T cell responses and increased tumor burden44. These data suggest that eosinophils function through T cells to enhance antitumor immunity in colorectal cancer. Despite these findings, contrasting evidence suggests that the anti-tumorigenic function of eosinophils in colorectal cancer might be due to their responsiveness to T cell-derived cytokines, such as IFN-γ, rather than eosinophil-dependent recruitment or activation of T cells31. Certainly, in another study31, the anti-tumorigenic activities of eosinophils in inflammation-induced colorectal cancer and in Apcmin/+ mice are suggested to be driven by direct eosinophil cytotoxicity following IFN-γ stimulation, as direct cytotoxicity was not demonstrated in vivo31. Thus, the precise interaction of eosinophils with T cells and T cell-derived cytokines (especially IFN-γ) requires further research.
Eosinophil–T cell interactions in cryo-thermal therapy.
Eosinophils have also been shown to contribute to tumor-specific T cell responses induced by cryo-thermal therapy45. In this study, cryo-thermal therapy induced the infiltration of eosinophils into treated tumors. Infiltrating eosinophils had an activated phenotype as defined by increased mRNA expression of Infg, Tnfa, Il6, Cxcl10 and Cd86, as well as genes encoding the cytotoxic molecules granzyme B (Gzmb)) and perforin (Prf1). Cryo-thermal-activated eosinophils induced the functional differentiation of CD4+ T cells and development of cytotoxic CD8+ T cells and promoted the cyto-lytic activities of these two cell types in vitro45.
Mechanisms of eosinophil-mediated T cell suppression.
Eosinophils are capable of suppressing T cell responses by direct and indirect mechanisms. Directly, eosinophil-express PD-L1 suppresses T cell responses in at least two settings, namely Heliobacter pylori infection36 and in allograft transplantation38. In the latter study, eosinophils suppressed lung T cell responses in a dose-dependent, contact-dependent and nitric oxide synthase (iNOS)-dependent fashion38 (Fig. 2). Indirectly, eosinophil-derived transforming growth factor (TGF)-β regulates the expansion of RORγt+ gastrointestinal Treg cells and eosinophils are capable of inducing Foxp3 expression in naïve T cells upon co-culture46.
In cancer, eosinophils can regulate the accumulation of Treg cells. In one study, intravenous injection of tumor cells resulted in an early and transient induction of IL-5 and eosinophils in the lungs of WT mice47. Il5−/− mice displayed decreased lung tumor nodule formation compared to WT mice, which was associated with decreased CCL22 expression and Treg cell accumulation. Adoptive transfer of bone marrow-derived eosinophils into these Il5−/− mice resulted in increased lung tumor colonization. Mechanistically, this study showed that eosinophil-secreted CCL22 facilitated tumor growth through local recruitment of Treg cells.
Cancer immunotherapy
Therapeutic targeting of T cells and/or utilizing their abilities to eliminate tumor cells have become two of the most promising avenues in cancer immunotherapy24. Although only a few studies convincingly demonstrate a causative role for eosinophils in T cell-targeted immunotherapies, multiple clinical observations link eosinophils to such therapies4, proposing a paradigm in which a possible positive feedback loop exists between eosinophils and lymphocytes to augment treatment efficacy.
Immune checkpoint blockade.
Immune checkpoints are cell surface molecules that control the function of various immune cells, including lymphocytes and myeloid cells48. Antibodies that therapeutically target cytotoxic T lymphocyte-associated protein 4 (CTLA4; inhibited by ipilimumab), programmed cell death protein 1 (PD-1, inhibited by pembrolizumab, nivolumab and cemiplimab-rwlc) and PD-L1 (inhibited by atezolizumab, avelumab and dervalumab) have entered mainstream oncology therapy49,50. Nonetheless, not all tumors respond to immune checkpoint inhibition (ICI), so identifying predictive biomarkers and accessory cellular mechanisms that participate in the antitumor response has the potential to enable the development of better therapeutics.
Eosinophils have been suggested as cellular biomarkers and possibly even end-stage effector cells in cancer therapy following ICI, especially with anti-CTLA4 and anti-PD-1 antibodies51–54. Clinical reports from independent centers suggest that peripheral eosinophil counts are increased after ICI (Fig. 3 and Table 1). For example, in one study, the absolute eosinophil number was positively correlated with overall survival in patients with stage IV melanoma treated with at least one dose of ipilimumab55. In another study, increased eosinophil counts during ipilimumab treatment correlated with longer survival rates of patients with melanoma56. Although most clinical studies assess increased peripheral blood eosinophilia with ICI, degranulating eosinophils were also identified within melanoma tumors following ICI, and the presence of degranulating eosinophils correlated with CD8+ T cell numbers57,58. In that study, ICI treatment altered the activation profile of eosinophils and increased the frequency of eosinophils, which were associated with expression of the T cell-attracting cytokine IL-16 in the sera of treated patients57. Experimentally, anti-CTLA4 treatment in models of breast cancer leads to intra-tumor eosinophil accumulation that is dependent on CCL11+ CD4+ T cells and CCL5+ CD8+ T cells59. Furthermore, IFN-γ production by eosinophils was essential for anti-CTLA4-induced blood vessel normalization, which results in elevated vessel perfusion, increased pericyte coverage and decreased vessel density59, and eosinophil depletion via anti-Siglec-F attenuated the inhibition of tumor growth by anti-CTLA4 therapy (Fig. 3). Although these data imply a causative role for eosinophils in the efficacy of CTLA blockade, corroboration is required using additional forms of eosinophil depletion. This limitation is especially important given that eosinophil depletion in the latter study was not confirmed by immunohistochemistry59.
Fig. 3 |. Eosinophils as biomarkers and accessory cells in cancer immunotherapy.
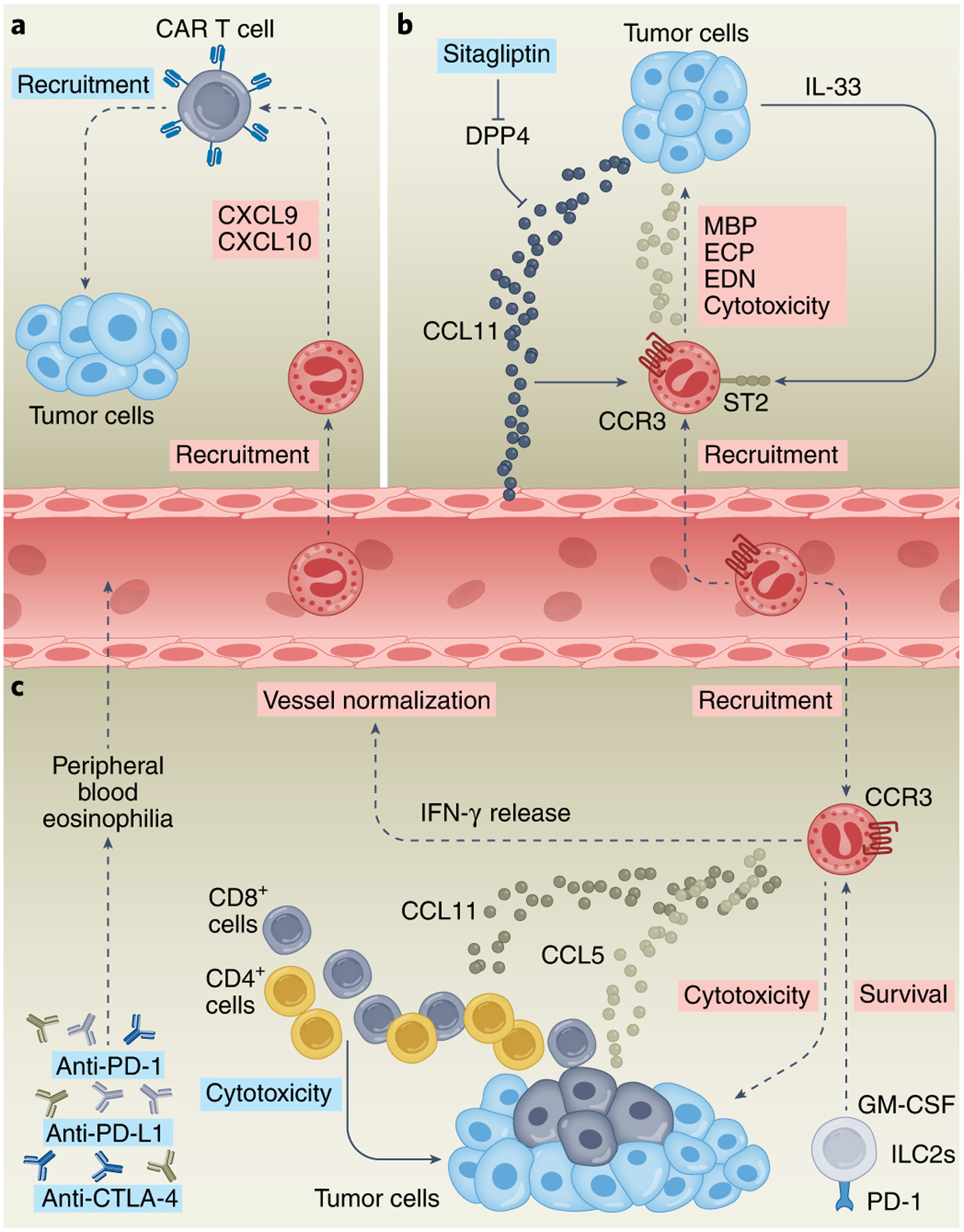
a, CAR T cell treatment resulted in intratumoral infiltration of eosinophils. Depletion of eosinophils caused downregulation of CXCL9 and CXCL10, suggesting that tumor-infiltrating eosinophils actively recruit CAR T cells. b, Therapeutic inhibition of DPP4 in mice using sitagliptin results in induction of IL-33, increased CCL11 and subsequent eosinophil infiltration, degranulation, secretion of the eosinophil cationic proteins MBP (major basic protein), ECP (eosinophil cationic protein) and EDN (eosinophil-derived neurotoxin), and cytotoxicity, which leads to reduced tumor growth. c, Treatment by immune checkpoint blockade, such as with anti-CTLA4, anti-PD-L1 and/or anti-PD-1 is associated with increased eosinophilia that is concomitant with better prognosis. Mechanistically, anti-CTLA4 therapy results in CD4+ and CD8+ T cell-dependent accumulation of eosinophils into the TME via T cell-expressing CCL11 and CCL5. IFN-γ production by eosinophils is essential for anti-CTLA4 treatment-induced vessel normalization. Blockade of PD-1 results in increased secretion of GM-CSF by ILC2s expressing PD-1, which leads to eosinophil survival and increased eosinophil-mediated cytotoxicity and antitumor immunity. Pink boxes indicate direct eosinophil-derived mediators and/or activities; blue boxes indicate indirect eosinophil-mediated lymphocyte activities.
Table 1 |.
Studies describing the effect of immune checkpoint blockade on eosinophils and clinical response
| Cancer type | Therapy | Eosinophil effect |
|---|---|---|
| Breast cancer | Durvalumab | Increase in peripheral blood eosinophil count during treatment was correlated with good response to therapy and extended PFS72. |
| Hodgkin lymphoma | Nivolumab | High relative eosinophil count was associated with a decreased risk for progression73. |
| Head and neck squamous cell carcinoma | Nivolumab | Relative eosinophil count and the ratio of eosinophil increase following treatment affected the prognostic score correlating with better OS65. |
| Melanoma | Ipilimumab | High baseline absolute eosinophil counts were positively correlated with OS55. An early increase in eosinophil count during the treatment with ipilimumab was associated with an improved clinical response54. Increase in eosinophil count during treatment correlated with long-term survival56. |
| Melanoma | Ipilimumab + nivolumab | Degranulating eosinophils were identified within the tumor mass of ICI-treated patients with melanoma and their presence was correlated with CD8+ T cells57. |
| Melanoma | Pembrolizumab | Baseline relative eosinophil count ≥ 1.5% was associated with favorable OS61. |
| Non-small-cell lung cancer | Pembrolizumab, nivolumab or atezolizumab | Patients with peripheral eosinophilia presented more frequently non-progression as best overall response to treatment and increased OS and PFS62. |
| Renal cell carcinoma | Nivolumab | An increase in eosinophils and relative eosinophil change at 6 weeks of treatment was associated with good response to immunotherapy63. |
| Urothelial carcinoma | Pembrolizumab | Decreased posttreatment relative eosinophil count was an independent prognostic factor for a worse OS74. |
PFS, progression-free survival; OS, overall survival.
Eosinophilia has been reported as a biomarker for positive outcomes following anti-PD-1 therapy in many tumors60–65 (Table 1). The functional role of eosinophils in anti-PD-1 therapy has been established in experimental models of melanoma, where increased tumor growth occurred in PHIL and ΔdblGATA eosinophil-deficient mice compared with WT mice10. In this study, ILC2-derived GM-CSF facilitated eosinophil activation and survival, and melanoma growth was decreased following in vivo treatment with IL-33 or anti-PD-1. Notably, ILC2s expressed PD-1, and the combination of IL-33 with PD-1 blockade substantially enhanced the antitumor response driven by the IL-33–ILC2–GM-CSF–eosinophil axis (Fig. 3). Finally, another study showed that therapeutic inhibition of dipeptidyl peptidase 4 (DPP4) in mice using sitagliptin results in IL-33 induction, increased levels of CCL11, and subsequent eosinophil infiltration, degranulation and cytotoxicity leading to reduced tumor growth66. Blockade of PD-1 and CTLA4 reduced the growth of Hepa1-6 and EMT6 tumor cells, and combination therapy with inhibition of DPP4 resulted in a smaller tumor. The efficacy of the treatment was reduced after pharmacological depletion of eosinophils with anti-Siglec-F, even in the presence of PD-1 and CTLA4 antibodies66 (Fig. 3). Collectively, these data suggest that eosinophils can participate in the antitumor response elicited by ICI.
CAR T cells.
Chimeric antigen receptors (CARs) are engineered synthetic receptors that function to redirect cells to recognize and eliminate other cells expressing a specific target antigen67. A major obstacle in the success of adoptive cell transfer of CAR T cells, especially in solid tumors, is an inability of CAR T cells to infiltrate the tumor. In one study, peripheral blood eosinophil counts correlated with clinical efficacy of CAR T cells recognizing CD19 in patients with B-lineage non-Hodgkin lymphoma68. In this study, depletion of eosinophils using anti-Siglec-F or anti-CCR3 in an experimental model of B-lineage non-Hodgkin lymphoma treated with anti-CD19 CAR T cells also indicated a function for eosinophils in CAR T cell antitumor efficacy. Eosinophils might support CAR T cell accumulation as fewer CAR T cells were recovered from the tumors in eosinophil-depleted mice and a decreased expression of CXCL9 and CXCL10 was observed in eosinophil-depleted tumors (Fig. 3). In support of this notion, in a different study, eosinophils facilitated the infiltration of T cells in a model of local irradiation69. NOD-Prkdcscid-Il2rg-null mice were subcutaneously and bilaterally injected with Raji lymphoma cells. Subsequently, irradiation was applied to one side and anti-CD19 CAR T cells were intravenously injected. Radiation promoted the intra-tumor infiltration of eosinophils and CD3+Fab+ CAR T cells. In this study, adoptive transfer of ex vivo-activated eosinophils improved tumor control in mice treated with irradiation and cytotoxic T cell transfer therapy. Conversely, eosinophil depletion impaired radiation-driven infiltration of CD8+ T cells and subsequent tumor control.
Finally, the potential application of eosinophils to enhance CAR T cell efficacy has been reported70. Human pluripotent stem cell-derived eosinophils had competent tumor-killing capacity in established solid tumors, such as HCT116 (human colorectal carcinoma), MDA-MB-231 (human breast adenocarcinoma) and HepG2 (human hepato-cellular carcinoma)70. In that study, treatment with CAR T cells injected into established tumor-bearing mice revealed that the combination of CAR T cells and human embryonic stem cell-derived eosinophils had superior antitumor effects compared with those of CAR T cells or human embryonic stem cell-derived eosinophils alone.
Conclusion
Progress has been made in our understanding of the TME26 and evidence is now emerging that eosinophils are important contributors, including in response to cancer immunotherapy. Nonetheless, our understanding of eosinophil functions in cancer and cancer therapy is limited. This knowledge gap should be filled by further preclinical studies of causative anti-tumorigenic or pro-tumorigenic functions for eosinophils in the TME. To date, the majority of studies in this area have focused on lung tumors, and to a lesser extent on tumors in the gastrointestinal tract. However, eosinophils infiltrate many tumor types3,4, thus future studies should look at additional anatomical sites. Although multiple single-cell-based approaches have been used to characterize the cellular diversity and plasticity in any given TME, the transcriptional landscape and heterogeneity of eosinophils are not well understood. This is especially important as eosinophils are ‘absent’ from most single-cell RNA-sequencing analyses71. This limits our ability to fully understand whether unique eosinophil subsets exist and whether eosinophils interact with specific cells in the TME or have distinct functions. Future research might require new experimental approaches to overcome these challenges. Despite these limitations, the available data suggest that eosinophils can enhance tumor immunity by direct and indirect mechanisms that involve cross-talk with various lymphocyte subsets. Whether this cross-talk can be manipulated for therapeutic purposes is a guiding question for future research.
Acknowledgements
This work was supported by grants and fellowships to A.M. from the US-Israel Bi-national Science Foundation (grant no. 2015163, to A.M. and M.E.R.), Israel Science Foundation (grant nos. 886/15 and 542/20), Israel Cancer Research Fund, Richard Eimert Research Fund on Solid Tumors, Israel Cancer Association, Dotan Hemato Oncology fund, Cancer Biology Research Center, Tel Aviv University, The Tel Aviv University Faculty of Medicine Recanati Fund and Azrieli Foundation Canada-Israel. M.E.R. was further supported by the National Institutes of Health (R37 AI045898, R01 AI124355, U19 AI070235 and P30 DK078392; Gene and Protein Expression Core), Campaign Urging Research for Eosinophilic Disease (CURED) and Sunshine Charitable Foundation and its supporters, D. A. Bunning and D. G. Bunning.
Footnotes
Competing interests
A.M. is a consultant and/or international advisory board member for GSK, AstraZeneca, Sanofi, Oravax and Sartorious and is an inventor of patents owned by the Tel Aviv University. M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex Therapeutics, Nextstone One, Bristol Myers Squibb, AstraZeneca, Ellodi Pharma, GSK, Regeneron/Sanofi, Revolo Biotherapeutics and Guidepoint; has an equity interest in the first seven companies listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust) and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital. S.G.-T. declares no competing interests.
References
- 1.Jacobsen EA et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu. Rev. Immunol 39, 719–757 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinbach G Ueber das Verhalten der Leukocyten bei malignen Tumoren. Arch. Klin. Chir. Arch. Klin. Chir 46, 486–562 (1893). [Google Scholar]
- 3.Grisaru-Tal S et al. Primary tumors from mucosal barrier organs drive unique eosinophil infiltration patterns and clinical associations. Oncoimmunology 10, 1859732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grisaru-Tal S, Itan M, Klion AD & Munitz A A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 20, 594–607 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Jacquelot N, Seillet C, Vivier E & Belz GT Innate lymphoid cells and cancer. Nat. Immunol 23, 371–379 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Rodriguez N, Gogoi M & McKenzie ANJ Group 2 innate lymphoid cells: team players in regulating asthma. Annu. Rev. Immunol 39, 167–198 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussbaum JC et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maggi E, Veneziani I, Moretta L, Cosmi L & Annunziato F Group 2 innate lymphoid cells: a double-edged sword in cancer? Cancers 12, 3452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikutani M et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol 188, 703–713 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Jacquelot N et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol 22, 851–864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dankort D et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet 41, 544–552 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougan M, Dranoff G & Dougan SK GM-CSF, IL-3 and IL-5 family of cytokines: regulators of inflammation. Immunity 50, 796–811 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Martin NT & Martin MU Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol 17, 122–131 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Lucarini V et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 6, e1317420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao K et al. Transgenic expression of IL-33 activates CD8+ T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 335, 463–471 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Andreone S et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers 11, 1664 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusilovsky M et al. Environmental allergens trigger type 2 inflammation through ripoptosome activation. Nat. Immunol 22, 1316–1326 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munitz A et al. 2B4 (CD244) is expressed and functional on human eosinophils. J. Immunol 174, 110–118 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Munitz A et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF and eotaxin on human peripheral blood eosinophils. Blood 107, 1996–2003 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Pesce S et al. The innate immune cross-talk between NK cells and eosinophils is regulated by the interaction of natural cytotoxicity receptors with eosinophil surface ligands. Front. Immunol 8, 510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi L et al. Interleukin-33 activates and recruits natural killer cells to inhibit pulmonary metastatic cancer development. Int. J. Cancer 146, 1421–1434 (2020). [DOI] [PubMed] [Google Scholar]
- 22.O’Flaherty SM et al. TLR-stimulated eosinophils mediate recruitment and activation of NK cells in vivo. Scand. J. Immunol 85, 417–424 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Schuijs MJ et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol 21, 998–1009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldman AD, Fritz JM & Lenardo MJ A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol 20, 651–668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay RE, Richardson EK & Toh HC Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binnewies M et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh DY & Fong L Cytotoxic CD4+ T cells in cancer: expanding the immune effector toolbox. Immunity 54, 2701–2711 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grisaru-Tal S et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. 81, 5555–5571 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Mattes J et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med 197, 387–393 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung K et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med 188, 2357–2368 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichman H et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol. Res 7, 388–400 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Dolitzky A et al. Transcriptional profiling of mouse eosinophils identifies distinct gene signatures following cellular activation. Front. Immunol 12, 802839 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carretero R et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol 16, 609–617 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Akuthota P, Wang HB, Spencer LA & Weller PF Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin. Exp. Allergy 38, 1254–1263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munitz A et al. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J. Immunol 177, 77–83 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Arnold IC et al. Eosinophils suppress TH1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med 215, 2055–2072 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woerly G et al. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J. Exp. Med 190, 487–495 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onyema OO et al. Eosinophils downregulate lung alloimmunity by decreasing TCR signal transduction. JCI Insight 4, e128241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucey DR, Nicholson-Weller A & Weller PF Mature human eosinophils have the capacity to express HLA-DR. Proc. Natl Acad. Sci. USA 86, 1348–1351 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansel TT et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin. Exp. Immunol 86, 271–277 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akuthota P, Wang H & Weller PF Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr. Opin. Allergy Clin. Immunol 10, 14–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurtner A et al. Single-cell RNA sequencing unveils intestinal eosinophil development and specialization. Preprint at bioRxiv 10.1101/2021.10.27.466053 (2021). [DOI] [Google Scholar]
- 43.Lee JJ et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Arnold IC et al. The GM-CSF–IRF5 signaling axis in eosinophils promotes antitumor immunity through activation of type 1 T cell responses. J. Exp. Med 217, e20190706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia S, Li W, Liu P & Xu LX A role of eosinophils in mediating the anti-tumour effect of cryo-thermal treatment. Sci. Rep 9, 13214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallegger A et al. TGF-beta production by eosinophils drives the expansion of peripherally induced neuropilin −RORγt+ regulatory T cells during bacterial and allergen challenge. Mucosal. Immunol 15, 504–514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaynagetdinov R et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 75, 1624–1634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P & Allison JP Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster RM The immune checkpoint inhibitors: where are we now? Nat. Rev. Drug Discov 13, 883–884 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Pardoll DM The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon HU et al. Interleukin-2 primes eosinophil degranulation in hypereosinophilia and Wells’ syndrome. Eur. J. Immunol 33, 834–839 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Sosman JA et al. Evidence for eosinophil activation in cancer patients receiving recombinant interleukin-4: effects of interleukin-4 alone and following interleukin-2 administration. Clin. Cancer Res 1, 805–812 (1995). [PubMed] [Google Scholar]
- 53.Ellem KA et al. A case report: immune responses and clinical course of the first human use of granulocyte/macrophage-colony-stimulating-factor-transduced autologous melanoma cells for immunotherapy. Cancer Immunol. Immunother 44, 10–20 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebhardt C et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin. Cancer Res 21, 5453–5459 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Martens A et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin. Cancer Res 22, 2908–2918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang BM et al. Long-term survival with modern therapeutic agents against metastatic melanoma—vemurafenib and ipilimumab in a daily life setting. Med. Oncol 35, 24 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Simon SCS et al. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 9, 1727116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruikshank W & Center DM Modulation of lymphocyte migration by human lymphokines. II. Purification of a lymphotactic factor (LCF). J. Immunol 128, 2569–2574 (1982). [PubMed] [Google Scholar]
- 59.Zheng X et al. CTLA4 blockade promotes vessel normalization in breast tumors via the accumulation of eosinophils. Int. J. Cancer 146, 1730–1740 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Moreira A, Leisgang W, Schuler G & Heinzerling L Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 9, 115–121 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Weide B et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res 22, 5487–5496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alves A, Dias M, Campainha S & Barroso A Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis 13, 2716–2727 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrmann T et al. Eosinophil counts as a relevant prognostic marker for response to nivolumab in the management of renal cell carcinoma: a retrospective study. Cancer Med. 10, 6705–6713 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghebeh H, Elshenawy MA, AlSayed AD & Al-Tweigeri T Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy 14, 189–199 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa D et al. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 112, 339–346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollande C et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol 20, 257–264 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Sterner RC & Sterner RM CAR T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia Q et al. Peripheral eosinophil counts predict efficacy of anti-CD19 CAR-T cell therapy against B-lineage non-Hodgkin lymphoma. Theranostics 11, 4699–4709 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng JN et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci. Adv 7, eabc7609 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai W et al. Human pluripotent stem cell-derived eosinophils reveal potent cytotoxicity against solid tumors. Stem Cell Rep. 16, 1697–1704 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li MO et al. Innate immune cells in the tumor microenvironment. Cancer Cell 39, 725–729 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Rafei-Shamsabadi D, Lehr S, Behrens M & Meiss F Additive intralesional interleukin-2 improves progression-free survival in a distinct subgroup of melanoma patients with prior progression under immunotherapy. Cancers 14, 540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hude I et al. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin lymphoma patients treated with PD1 inhibition. Br. J. Haematol 181, 837–840 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Furubayashi N et al. The association of clinical outcomes with posttreatment changes in the relative eosinophil counts and neutrophil-to-eosinophil ratio in patients with advanced urothelial carcinoma treated with pembrolizumab. Cancer Manag. Res 13, 8049–8056 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


