Abstract
Introduction:
Olfactory neuroblastoma (ONB) is a rare cancer of the sinonasal region. We provide a comprehensive analysis of this malignancy with molecular and clinical trial data on a subset of our cohort to report on the potential efficacy of SSTR2-targeting imaging and therapy.
Methods:
We conducted a retrospective analysis of 404 primary, locally recurrent, and metastatic ONB patients from twelve institutions in the US, UK and Europe. Clinicopathological characteristics and treatment approach were evaluated. SSTR2 expression, SSTR2-targeted imaging and the efficacy of peptide receptor radionuclide therapy (177Lu-dotatate) were reported in a subset of our cohort (LUTHREE trial; NCT03454763).
Results:
Dural infiltration at presentation was a significant predictor of OS and DFS in primary cases (n=278). Kadish-Morita staging and Dulguerov T-stage both had limitations regarding their prognostic value. Multivariable survival analysis demonstrated improved outcomes with lower stage and receipt of adjuvant radiotherapy. Prophylactic neck irradiation significantly reduces the rate of nodal recurrence. 82.4% of the cohort were positive for SSTR2; treatment of three metastatic cases with SSTR2-targeted PRRT in the LUTHREE trial was well-tolerated and resulted in stable disease.
Conclusions:
This study presents pertinent clinical data from the largest dataset, to date, on ONB. We identify key prognostic markers and integrate these into an updated staging system, highlight the importance of adjuvant radiotherapy across all disease stages, the utility of prophylactic neck irradiation and the potential efficacy of targeting SSTR2 to manage disease.
Keywords: esthesioneuroblastoma, olfactory, nose neoplasms, prognosis, radiotherapy, adjuvant, SSTR2 protein, human, receptors, somatostatin, retrospective studies, diagnostic imaging
Introduction
Olfactory neuroblastoma (ONB) is a rare sinonasal malignancy with an incidence of 0.4 per one million, accounting for approximately 6% of sinonasal malignancies.1,2 5-year survival is stage-dependent with exceptionally high survival for early stage disease; however, survival declines substantially with increasing stage as treatment modalities become less effective.3
In view of the fact that this is such a rare disease, most analyses have been limited by sample size and have only been possible through the analysis of cancer databases, such as the Surveillance Epidemiology and End Results (SEER) and National Cancer databases, or through meta-analyses. These include the recent investigation of age-related outcomes by Yin et al, the role of chemotherapy in ONB by Cranmer et al, a comparison of staging systems by Joshi et al, an evaluation of the Hyams grading system by Goshtabi et al. as well as an investigation of differences in outcome between the endoscopic and open surgical approaches.4-9
Historically, the most commonly used prognosticator is the Kadish staging system.10 This staging system is based on the analysis of 17 patients and was published in 1976. This was later re-evaluated by Morita et al. who performed a retrospective analysis on 49 patients treated at the Mayo Clinic between 1951 and 1990.11 A new staging system was proposed by Dulguerov et al. in 1992 and a modified version of this is commonly used.12,13 While the Dulguerov system has been shown to be superior to the Kadish-Morita system in a recent individual patient data (IPD) meta-analysis of publicly available data, a recent analysis of the National Cancer Database determined that, in general, current clinical staging systems do not adequately predict survival over ten years (Supplemental Table 1).14,15
The only grading system based on histologic maturation and differentiation was developed by Hyams and has been shown to be of prognostic value, particularly in complementing current staging systems.16,17 Across four reports, it has been noted that Hyams score allows for the identification of aggressive locoregional disease and subsequent prediction of poor DFS, and may enable stratification for adjuvant therapy.18-21 Previously, Kane et al demonstrated the independent prognostic utility of Hyams grading; the ability to predict metastasis and overall survival was further confirmed in a recent meta-analysis.6
Here we assess prognostic factors and disease staging, and also investigate the role of novel biomarkers and targets for therapies. Somatostatin receptors (SSTRs) are G protein-coupled cell surface receptors expressed on various normal tissue as well as in several human malignancies, the most notable being neuroendocrine tumours (NETs). SSTR expression has been previously reported in ONB; as early as 1996, 111ln-Octreotate PET/CT has been used for the detection of recurrent or metastatic disease, albeit not routinely.22 More recently, the use of 68Ga-DOTATE PET/CT was found to be superior to 18F-FDG PET/CT for the detection of tumours in areas with high background noise.23 Nevertheless, SSTR-based imaging has yet to enter routine clinical care.
SSTR-targeting therapies have also proven to be efficacious in the treatment of NETs. These include peptide-receptor radionuclide therapies (PRRT) as well as the use of somatostatin analogues (SSA). Whether this can be extended to ONB remains poorly described and no clinical trials have been published to date. Indeed, current molecular understanding of SSTR expression in ONB is extremely limited. A recent study conducted by Czapiewski et al demonstrated a high prevalence of SSTR2 expression in a cohort of 40 ONBs, which was not seen in the comparative sinonasal carcinoma nor sinonasal small round blue cell neoplasm cohorts.24
Importantly, advances in the diagnosis, treatment and prognosis of ONB remain extremely challenging due to the paucity of large-scale studies as a result of the rarity of this malignancy. Few studies are sufficiently powered to assess clinically meaningful results. Here, we present data from a large, multi-center and international ONB cohort, comprehensively assessing the role of SSTR2 in this malignancy and describe the use of SSTR2 targeted radionuclide therapy in a subgroup of patients with metastatic disease from a clinical trial. To the best of our knowledge, this is the largest cohort of ONBs investigated to date and provides updated evidence for the use of SSTR2 targeted radionuclide therapy in metastatic ONB.
Materials and Methods
Patients
De-identified data on 404 ONB patients was obtained from 5 US institutions (The University of Texas MD Anderson Cancer Center, USA; Johns Hopkins University School of Medicine, USA; Stanford University School of Medicine, USA; Emory University, USA; Yale University School of Medicine, USA) and 7 European institutions (University College London/University College London Hospital, UK; Beaumont Hospital, Ireland; University of Insubria, Italy; Universita degli Studi di Brescia, Italy; Ludwig-Maximilians University, Germany; King’s College/Guy’s Hospital, UK; Instituto de Investigacion Sanitaria del Principado de Asturias, Spain). Inclusion criteria required confirmed histopathological diagnosis of ONB with histological characterization and sample/cohort selection performed by head and neck pathologists experienced in the evaluation of ONB. Clinical data were obtained retrospectively and reviewed by the lead team. Data collected include patient demographics, tumour status at presentation (i.e. expression of immunohistochemical markers including SSTR2, clinical stage and grade), treatment details and survival outcome. IRB approval was obtained from all institutions with further approval for multi-center data analysis from University College London IRB/Research Ethics Committee (UCL REC no. 9609/002; ML/VJL).
Diagnosis and Treatment of ONB
The date of diagnosis was defined as the date of tissue extraction for histological determination of the diagnosis. Patients were treated per their respective institution’s standard-of-care and all institutions involved are tertiary level centers of excellence with longstanding experience in the diagnosis and management of this disease. In general, surgical resection with curative intent was conducted in the first instance, with or without adjuvant chemoradio- or radiotherapy. Surgery was conducted with either an endoscopic, open or combined approach.
Immunohistochemical analysis of expression of SSTR2
Immunohistochemistry was performed in different institutions, all using a standardized Ventana automated staining protocol, shared by the lead team. The rabbit monoclonal antibody UMB1 (Abcam, Cambridge, UK) was used to detect SSTR2. The slides were evaluated under the guidance of head and neck pathologists. The evaluators of the immunohistochemical stains were blinded to the clinical outcomes (Figure 1). The slides were dichotomously scored as being positive or negative, based on the extent of staining and intensity. The extent was scored on a continuous scale from 0%-100%. The intensity was scored as 3 categories (1: weak staining not easily seen via the low power objective; 2: moderate staining still seen on a low power objective; 3: strong staining easily visible via a low power objective), as per M. Lechner et al..25
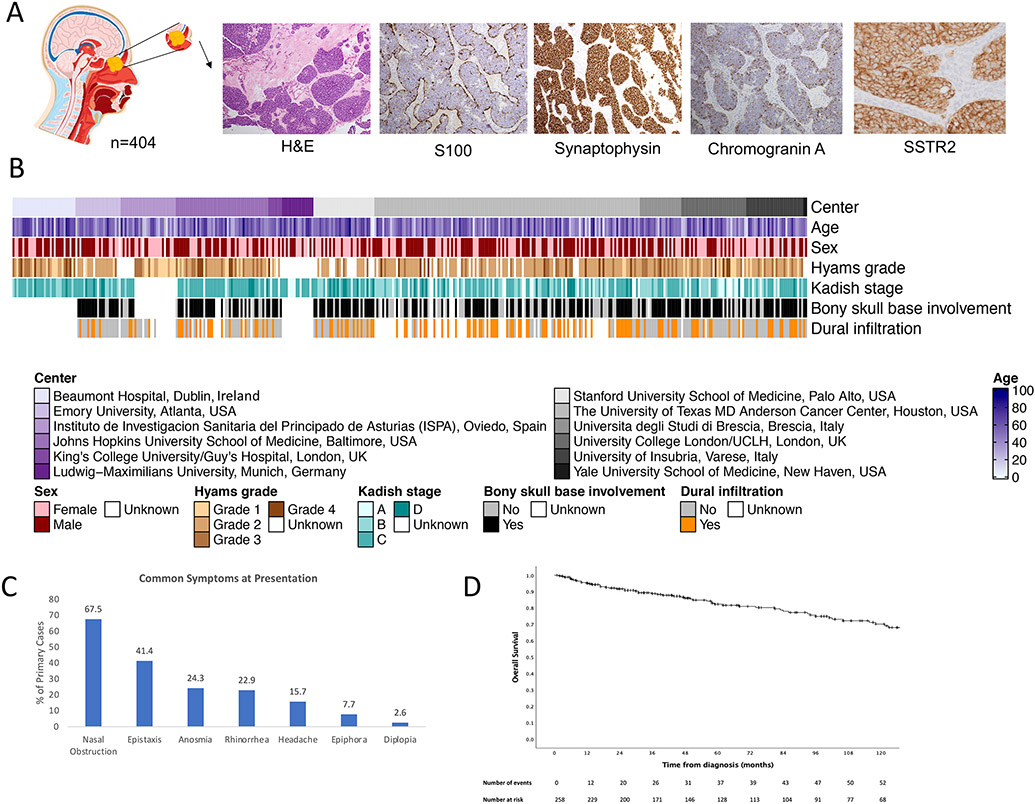
Figure 1: Clinical characteristics of olfactory neuroblastoma.
A) Anatomical localization and representative images of histology (H&E staining), expression of common markers (S100, chromogranin A, synaptophysin) and SSTR2, which were assessed by immunohistochemistry; B) Heatmap representation of clinical annotations; C) Bar graph representation of common symptoms at presentation; D) Kaplan-Meier overall survival of primary cases.
SSTR2-based PET Imaging and Peptide-Receptor Radionuclide Therapy
A subgroup of our patients with recurrent disease unsuitable for further surgery and/or radiation were recruited prospectively under the LUTHREE randomized phase II comparative study of 177 Lu-DOTATATE PRRT in SSTR2-positive tumours (clinicaltrials.gov: NCT03454763) and treated every 5 and 8-10 weeks. Informed consent was obtained from all patients and ethical approval was obtained (EudraCT number: 2015-004727-31). Recruitment and treatment took place at the ISRT (Istituto Scientifico Romagnolo per lo Studio e la curadei Tumori, Meldola, FC, Italy). 450 patients diagnosed with SSTR2-positive neuroendocrine tumours have been recruited to this trial in total and our subset of patients with olfactory neuroblastoma was enrolled prospectively. A diagnostic OctreoScan and 68Ga-DOTA-peptide PET imaging were performed for each patient. Only patients with a tumour uptake scores of grade 2 or 3 were considered for therapy (the Tumour Uptake score is based on planar scinitigrams obtained 24-hours post-administration of imaging and is composed of a 3-grade scale, where 1 = liver uptake, 2 > liver uptake and < kidney uptake and 3 > kidney uptake). In every experimental arm, patients received 5 cycles of 177lu-DOTATATE PRRT at 5.5 or 3.7 GBq dose. Lower dosages were administered in cases of kidney or bone marrow risk factors. 68Ga-DOTATOC PET and MRI imaging were performed at baseline, after the third therapeutic cycle and every three months after therapy for the first two years, then every six months thereafter. Response to treatment was determined through follow-up imaging per RECIST 1.1 criteria.
Statistical analysis
The primary aim of this study was to investigate prognostic factors of ONB patients in terms of disease-free (DFS) and overall survival (OS), calculated from the date of diagnosis and censored at the date the patient was last known to be alive if no event had occurred. DSF and OS are described using the Kaplan-Meier method and log rank tests. Univariable and multivariable Cox regression analyses were used to derive hazard ratios, 95% confidence and corresponding p-values, both unadjusted and after accounting for other factors. Associations with the following factors were explored: age, sex, tumor grade, stage, extent of disease at presentation including bony skull base involvement, dural infiltration, orbital and intracranial involvement, and treatment approach. The data analysis was generated using IBM SPSS Statistics for Windows version 27.0 (IBM Corp., Armonk, NY, USA).
A nested log-likelihood ratio test was conducted between the univariate cox regression model with Kadish-INSICA stage and the bivariate model with both Kadish-INSICA stage and Hyams grade (dichotomized into 1/2 vs. 3/4). The cox regression was implemented with survival R package (V3.2, R version 4.0) and a chi-squared test for the log-likelihood ratio of the two models was conducted.
To further investigate if including Hyams grade can improve the Kadish-INSCIA system, a Boolean logic random forest model was also implemented. The details of the procedure:
Data splitting and binarizing: There are 177 samples in total which have the survival/grade/stage information (28 events and 149 censoring) available. The training set contains 125 samples (20 events and 105 censoring). The test set contains 52 samples (8 events and 44 censoring). And the training set was split into 5-fold. For each fold, there are 4 events and 21 censoring. Each of the Kadish-INSICA stage and Hyams grade was binarized. So, there are KI1(0,1), KI2(0,1), KI3(0,1), KI4(0,1), H1(0,1), H2(0,1), H3(0,1), H4(0,1) for Boolean logic tree.
Cross-validation to determine the optimal number of trees (nt): For each nt=(1,2,3,4,5), 5-fold cross validation with 100 trees generated for each fold were implemented. The final predicted score was defined as the average predicted score from 100 runs for each sample. Then for all the samples the score was correlated with survival function. It indicates nt=2 is the best parameter, although not significant.
Testing: (nl=number of leaves) 500 models with (nt=2, nl=4) generated during cross validation step were used to predict the scores with the testing set(n=52), and did cox regression to test the correlation between survival function and the predicted scores.
The Boolean logic random forest model was implemented with LogicReg R package (V1.6.4, R version 4.0).
Results
Clinical characteristics and presentation of patients with olfactory neuroblastoma
404 cases of histologically confirmed ONB from 12 institutions in the United States and Europe were analyzed (Figure 1A and 1B, Supplemental Table 2). 54.1% of patients were male and 70.4% presented with primary disease (Supplemental Figure 1A). 18.0% and 8.9% presented with recurrent or persistent disease, respectively; 30.6% of patients had received prior treatment. The mean age at diagnosis for primary cases was 50.9 (range 2-91) years and, contrary to previous reports, we did not observe a bimodal age distribution, rather a single peak was observed (Supplemental Figure 1B). Clinical characteristics and outcome were similar between pediatric (< 18 years) and adult cases (Supplemental Figure 1C). For patients who had not received prior treatment, typical symptoms at presentation were nasal obstruction, epistaxis, anosmia, rhinorrhea, headache, epiphora and diplopia; these were present in 67.5%, 41.4%, 24.3%, 22.9%, 15.7%, 7.7% and 2.6% of patients, respectively (Figure 1C).
Clinical predictors of outcome in primary cases
Five-year and ten-year disease-free survival (DFS) were 67.6% (95% CI: 60.7 – 73.6%) and 51.9% (95% CI: 43.8 – 59.4%), and overall survival (OS) 82.3% (95% CI: 76.3% – 86.9%) and 70.2% (95% CI: 62.3% - 76.8%), respectively (Figure 1D). The main prognostic parameters routinely used in clinic are Hyams grade, Kadish-Morita stage and Dulguerov T-stage; presented in Figures 3A, 3B and 3C, respectively. There was evidence that all systems were prognostic to some extent, but with notable limitations. For example, Kadish-Morita staging only identified a small proportion of patients in groups A and D, and outcomes in groups A and B were not well separated. Similarly, substantial overlap was observed across Dulgueorv T1-3 stage groups. Better performance for all systems was observed when dichotomized: Hyams grades 1 and 2 vs. 3 and 4, Dulguerov T-stage T1-3 versus T4 as well as for Kadish A and B versus C and D (Supplemental Figures 3A-C).
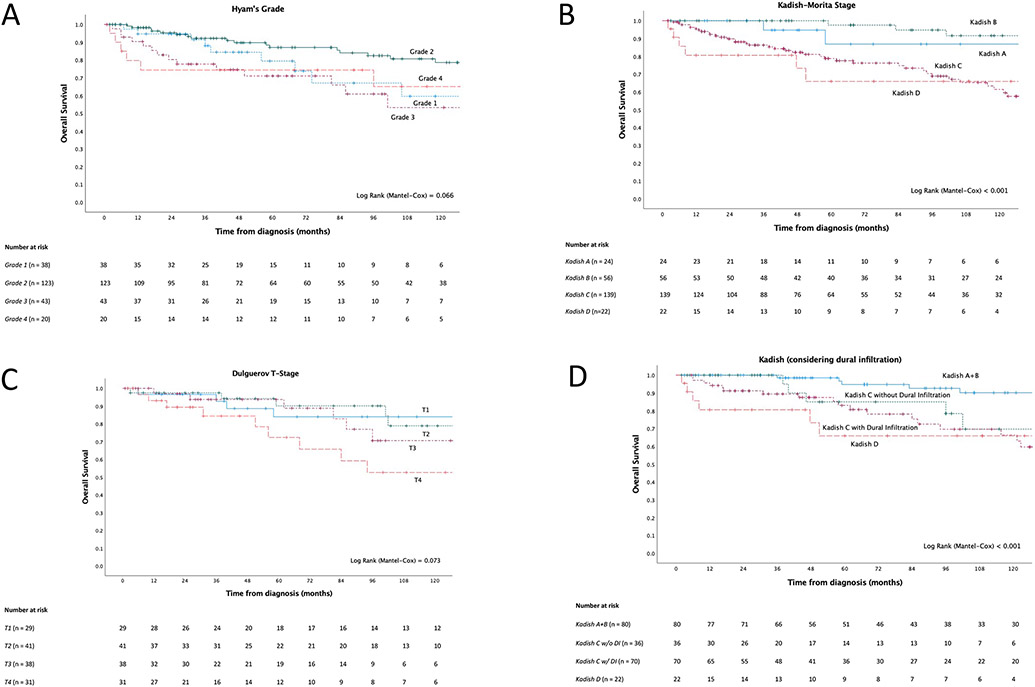
Figure 3. Survival outcomes of primary cases by clinicopathological characteristic.
A) Kaplan-Meier survival of Hyams Grade. B) Kaplan-Meier survival curve of Kadish-Morita stage. C) Kaplan-Meier survival curve of Dulguerov T-stage. D) Kaplan-Meier survival curve of a modification of the Kadish-Morita staging, which stratifies the Kadish C group into those who present with or without dural infiltration.
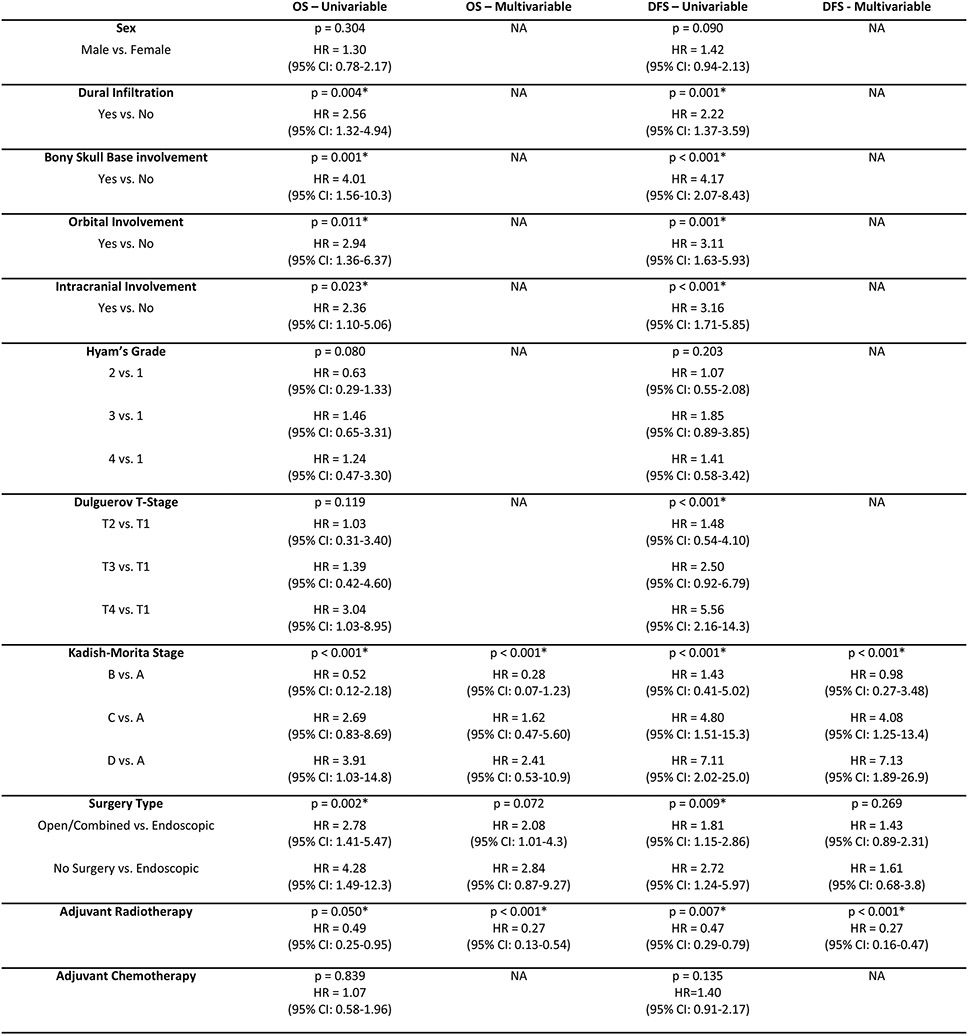
Univariable Cox regression analysis showed strong evidence of an association between the presence of dural infiltration, bony skull base involvement, orbital involvement, and intracranial involvement, as well as Kadish-Morita stage with overall and disease-free survival (Figure 2). With this, we sought to determine whether any of the aforementioned factors could improve the prognostic utility of Kadish-Morita stage. This was done in light of advances in treatment, which will greatly benefit from improved patient stratification and subsequent treatment allocation.
Figure 2. Survival outcomes of primary cases by clinicopathological characteristics and treatment approach.
Univariable and multivariable Cox regression survival analysis of clinicopathological characteristics and treatment approach.
Based on the finding that the Kadish A and B groups are prognostically similar and the separation observed with dichotomized Kadish, these groups were combined for subsequent investigations toward a modified staging system. We hypothesized that further prognostic information could be achieved by further stratifying the largest Kadish C group based on either of orbital involvement, intracranial involvement or dural infiltration at presentation based on the prognostic significance observed with these factors. Bony skull base involvement was not explored as the vast majority (105/113, 92.9%) of Kadish C patients presented with it (Supplemental Figure 5A).
Kaplan-Meier survival analysis on Kadish stage was conducted with further stratification of the Kadish C group by presence of each of dural infiltration (Figure 3D), intracranial and orbital involvement (Supplemental Figures 3A and 3B). Each of these modified Kadish systems showed improved performance. Dural infiltration appeared to best delineate the Kadish C group. Further explorations of combined dural infiltration and orbital involvement were performed, however this did not provide additional prognostic information (Supplemental Figures 3C and 3D).
Patients, who presented with Kadish D disease, i.e. with neck and/or distant metastases, had the poorest prognoses. Distant metastasis, in particular, substantially shortens survival. Whilst there were only 3 patients with distant metastases, its significant detrimental effect on survival is evident (Supplemental Figure 3E). Therefore, an additional analysis of the above modified staging system with the inclusion of dural infiltration was conducted with further subdivision of Kadish D by neck or distant metastases (Supplemental Figure 3F). This system also demonstrated statistical significance.
Lastly, to further refine our novel prognosticator, we sought to take into account Hyams grade due to the clinical utility observed when the system was dichotomised. However, no further improvements were observed in a multivariable model, including the revised Kadish staging system, mentioned above, and Hyams grade. To determine if the Kadish-INSICA model can be improved further, we ran a Boolean Logic Regression Random Forest Model (BLRRF) including Hyams grading in addition to the staging as specified by Kadish-INSICA. Although the BLRRF model could predict outcome in the blinded test set (HR = 2.19 (1.15-4.17) Wald test P=0.02), it did not outperform the simpler model based on Kadish-INSICA (HR = 3.31 (1.49-7.36) Wald test P=0.003), suggesting that further improvements to Kadish-INSICA will require either other clinical covariates or further increase in sample size.
Management of ONB
Induction chemotherapy was conducted in 30/213 (14.1%) of primary cases and, generally, for patients with late-stage disease (1/19, Kadish A, 4/54 Kadish B, 13/112 Kadish C, 10/21 Kadish D; data missing for two patients). Furthermore, more patients presenting with dural infiltration received induction chemotherapy, compared to those without dural infiltration (16/77, 20.8% vs. 8/84, 9.5%, p = 0.050) (Supplemental Figure 4B).
Regarding surgical modalities used in primary treatments, 120/238 (50.4%) patients underwent endoscopic resection, while 85/238 (35.7%) and 15 (6.3%) underwent open or combined resection, respectively. Adjuvant radiotherapy was typically given (211/244, 86.5%), with or without chemotherapy (65/211, 30.8% and 146/211, 69.2%, respectively). For those who received adjuvant radiotherapy, roughly one-quarter also underwent prophylactic neck irradiation (36/130, 27.7%). Of these, 36.1% received irradiation unilaterally, 50.0% bilaterally, with the remainder unspecified. Adjuvant chemotherapy was rarely given alone, in only two cases for the present cohort (Supplemental Table 3).
Less than one-third of patients (64/222, 28.8%) presenting with primary disease experienced disease recurrence. Of the sixty-four recurrences, 29 (45.3%) occurred locally, 16 (25.0%) in cervical lymph nodes, 8 (12.5%) occurred intracranially, 3(4.7%) in the parotid, 2 (3.1%) locoregionally and 6 (9.4%) were distant recurrences (Supplemental Table 3). The vast majority of patients, who did relapse, did so within the initial ten years post-treatment of the primary (56/63, 88.9%), but late recurrence still occurred in some patients.
Surgical approach and outcome
Exploring the associations between surgical approach and outcomes, worse outcome were observed for those who underwent a combined/open approach compared to an endoscopic approach (DFS HR=1.81, 95%CI: 1.15-2.86, p=0.009; OS HR=2.78, 95%CI: 1.41-5.47, p=0.002). However, this effect was smaller after adjusting for Kadish stage and receipt of adjuvant radiotherapy (DFS HR=1.43, 95%CI: 0.89-2.31, p=0.269; OS HR=2.08, 95%CI: 1.01-4.28, p=0.072) (Figure 2). Indeed, it is important to note several potential confounders. Firstly, the majority of patients with Kadish A+B disease underwent endoscopic surgery compared to only half of those with Kadish C+D disease (52/72, 72.2% vs. 64/127, 50.4%, p=003). In addition, patients who presented with dural infiltration were more likely to undergo open/combined resection, compared to those who did not (39/83, 47.0% vs. 24/87, 27.6%, p = 0.011). Similarly, those with bony skull base involvement were more likely to undergo an open/combined approach, compared to those without (61/136, 44.9% vs. 12/51, 23.5%, p = 0.011).
Due to the heterogenous clinical presentation of Kadish C cases, we sought to further understand the role of surgical approach on survival in a subgroup analysis of these cases. Through a Kaplan-Meier survival analysis, endoscopic surgery appeared to be superior to the open/combined approach (Supplemental Figure 4C).
Adjuvant treatment and outcome
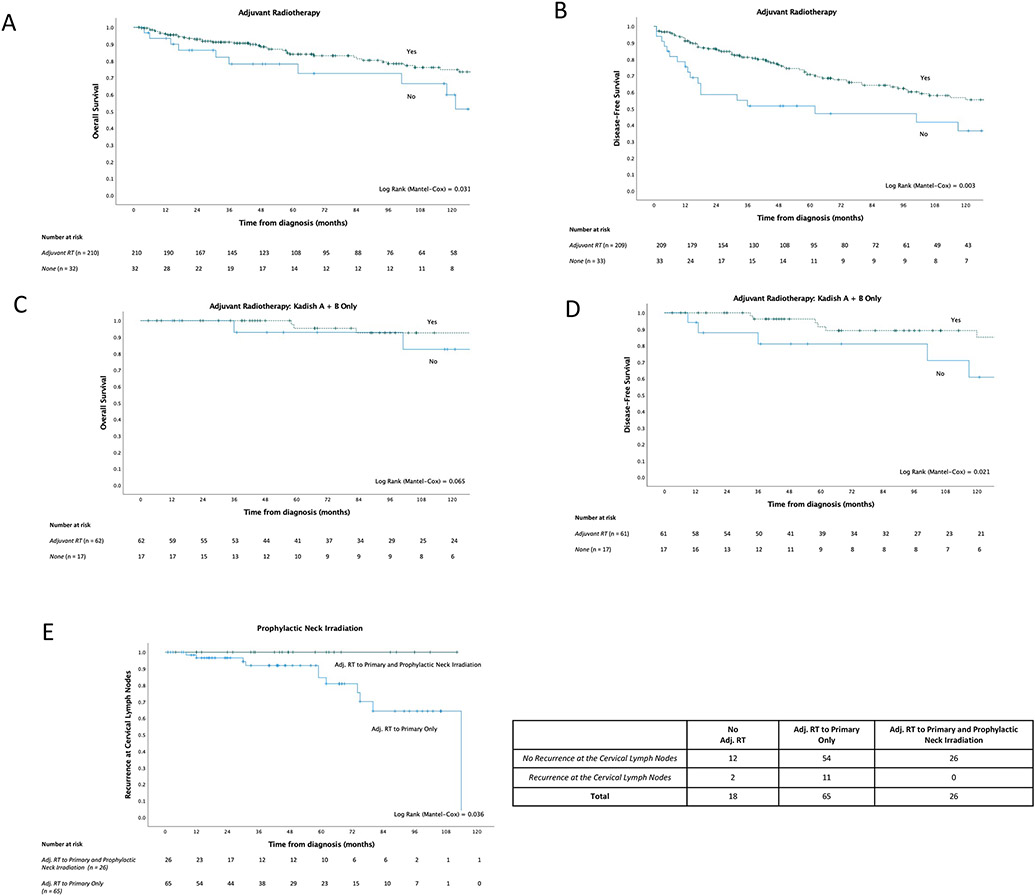
Adjuvant radiotherapy was associated with superior outcomes (DFS HR=0.47, 95% CI: 0.26-0.79, p=0.007; OS HR=0.49, 95% CI: 0.25-0.95, p=0.050). This strong association remained, and the observed effect was larger, after adjusting for Kadish stage and surgical modality (DFS HR=0.27, 95% CI: 0.13-0.47, p<0.001; OS HR=0.27, 95% CI: 0.16-0.54, p<0.001) (Figure 2, Figures 4A and 4B). Furthermore, there was evidence that adjuvant radiotherapy was similarly beneficial for those with earlier stage disease (subgroup analyses of Kadish A and B patients), for which complete surgical resection is typically achieved (DFS HR=0.30, 95% CI: 0.10-0.88, p=0.029; OS HR=0.26, 95% CI: 0.06-1.21, p=0.085), and remained adjusting for Kadish stage and surgical approach (OS HR = 0.17, 95% CI: 0.03-1.00) (Figures 4C and 4D).
Figure 4. Survival outcomes of primary cases by clinicopathological characteristics and treatment approach.
A)-D) Kaplan-Meier survival analyses demonstrating survival differences between patients who receive adjuvant radiotherapy and those who do not; further analyses of early-stage patients also demonstrated. E) Number of recurrences (occurring 10 years post-initial diagnosis of the primary or earlier) at the cervical lymph nodes, considering receipt of adjuvant radiotherapy to the primary tumor only, compared to additional prophylactic neck irradiation. Kaplan-Meier event-free (where event is cervical lymph node recurrence) survival analysis demonstrating the difference in the incidence of cervical lymph node recurrence between patients who receive adjuvant radiotherapy to the primary tumor only, compared to the ones who receive additional prophylactic neck irradiation.
Prophylactic neck irradiation appears to play a major role with regard to risk of recurrence within the initial ten years post-diagnosis, particularly regarding recurrences at the cervical lymph nodes. Kaplan-Meier event-free survival analysis was conducted on Kadish A-C patients, considering the first ten years following initial diagnosis, where the event-of-interest was recurrence at the cervical lymph nodes. Only patients who received adjuvant radiotherapy to the primary and did not receive prophylactic neck irradiation recurred at the cervical lymph nodes (11/65, 16.9% vs. 0/26, 0.0% of patients who received both, p = 0.036) (Figure 4E). Nine of the eleven cases of lymph node recurrences (81.8%) occurred in patients who presented with Kadish C disease. The single recurrence in the neck nodes in our entire cohort occurred in a patient thirteen years after initial diagnosis of the primary.
Comparing those who received adjuvant radiotherapy to the primary only versus those who additionally received prophylactic neck irradiation, a greater proportion of patients recurred at ten years or earlier, at any site, in the former (26/89, 29.2% vs. 5/33, 15.2%, p=0.160). However, this difference is not statistically significant. Lastly, prophylactic neck irradiation does not appear to impact overall or disease-free survival (Supplemental Figures 5A and 5B).
Lastly, there was no strong evidence of a benefit with adjuvant chemotherapy across all patients, who had not received prior treatment (DFS HR=1.40, 95% CI: 0.91-2.17, p=0.135; OS HR = 1.07, 95% CI: 0.58-1.96, p=0.839) (Figure 3A). When comparing adjuvant chemoradiotherapy to radiotherapy alone, no significant difference in overall survival was observed; there was weak evidence that chemoradiotherapy appeared to confer worse disease-free survival than radiotherapy alone, but this is likely confounded by disease stage/grade (Supplemental Figure 5C and 5D).
Clinical characteristics and outcome of recurrent cases at presentation
For patients who presented at the participating institution with recurrent disease (n=52, considering first recurrences only), 5-year overall survival was 89.4% (95% CI: 76.3%-95.5%) (Supplementary Figure 5E). However, disease-free survival was 44.2% (95% CI: 29.7%-57.7%) (Supplementary Figure 5F). The majority of patients experienced a second relapse (40/44, 90.9%) with 21/44 (47.7%) recurring locally, and a median time to second relapse of 63.5 months. This reiterates the fact that these patients need to be followed-up life-long.
Clinical Translation of SSTR2 Upregulation
82.4% of the one hundred forty-two primary tumours, for which staining was available, expressed SSTR2 by immunohistochemical assessment (Supplemental Figure 6). However, there was no evidence of an association between SSTR2 expression and survival (Supplemental Figure 7). Furthermore, SSTR2 positive and negative cases were clinically similar with regards to stage, grade and extent of disease at presentation (Supplemental Table 4) Representative images of SSTR2 expression in recurrent and metastatic disease are demonstrated in Figure 5A.
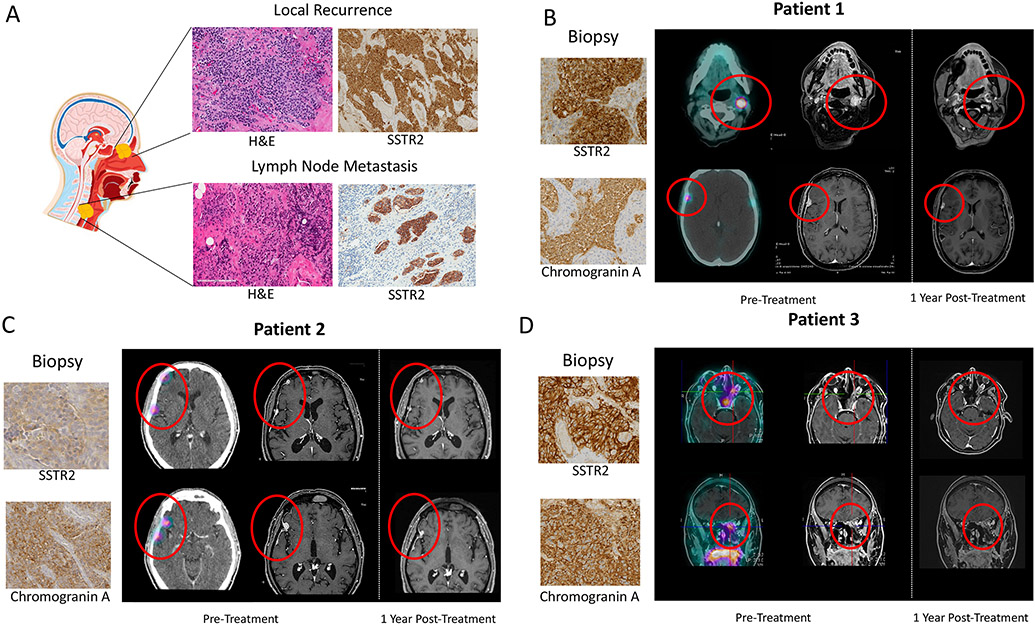
Figure 5: Confirmation of SSTR2 expression in local recurrences and metastases and clinical trial on Somatostatin receptor (SSTR) 2-positive olfactory neuroblastoma.
A) Representative images of SSTR2 expression, with corresponding H&E, in local recurrence and lymph node metastasis, determined by IHC. B)-D) Immunohistochemical characterization of tumor biopsies (SSTR2 and Chromogranin correlation of SSTR2 IHC with in vivo uptake of 68Ga-DOTATATE in PET MRI imaging of 3 patients who were enrolled in the LUTHREE trial (NCT03454763) and underwent SSTR2-targeted peptide-radionuclide receptor therapy (PRRT). Pre-treatment 68Ga-DOTATATE PET MRI with corresponding MRI and subsequent MRI 1-year post-treatment.
From our cohort, three patients with histologically confirmed ONB were enrolled in the LUTHREE trial (NCT03454763) (Figures 5B-D). Protein expression of SSTR2 and 68Ga-DOTA-peptide imaging demonstrate the utility of SSTR2-based imaging in the diagnosis and monitoring of disease. All clinical trial data on olfactory neuroblastoma are included in this manuscript and no more patients with olfactory neuroblastoma were enrolled. Hence, the data on olfactory neuroblastoma is reported here.
In these three cases, 177lu-DOTATATE PRRT was used for metastatic or persistent disease, after all other treatment options had been exhausted and surgery was not deemed an option. PRRT was well-tolerated with two cases of grade 1 neutrophils, which were self-limiting and did not interfere with the normal prosecution of therapy performed according to the trial protocol. For all three cases, PRRT stopped disease progression in the first instance. Two patients experienced relapse, 12 and 62 months after initial PRRT. Of these, treatment for one is ongoing while the second has since completed re-PRRT and has stable disease. All patients are currently alive with disease.
Discussion
This study considers the largest collection of ONB tumours and associated clinical data reported to date. The multi-center and international design of the study improves the generalizability of the following findings.
Establishment of the Kadish-INSICA (The International Network for Sinonasal Cancers and Skull Base Tumours; www.insica.org) Staging System
In our analysis, the Kadish-Morita staging system appeared to be superior to the alternative Dulguerov T-stage system. Indeed, better delineation between stage groups was observed in the former, in comparison with the substantial overlap between Dulguerov T1, T2 and T3. However, Kadish A and B appeared to have similar outcomes. In their analysis of the SEER database, Joshi et al did not observe a statistically significant difference between Kadish A and B.5 Therefore, we combined these two stage groups since separating them does not appear to provide significant prognostic information.
Dural infiltration/invasion was found to be a significant prognostic indicator in our cohort, which confirms early findings in craniofacial surgery and expands on recent work.31-34 In a recent report, Marinelli et al further demonstrated a significant relationship between dural infiltration and neck metastases where patients with dural infiltration more frequently presented with or developed neck disease following treatment, with an observed survival difference between Kadish C patients with or without dural infiltration.
Taking this into account, we sought to devise a modified Kadish staging system, combining the A and B groups and separating the C group into those with and without dural infiltration. We further explored subdividing the Kadish D group into those with neck metastases only and those with distant metastases. Strikingly, Kadish D cases with distant metastases did significantly worse than those with positive neck nodes only. However, in view of the fact that very few patients present with Kadish D with distant metastases, we did not feel that a further separation of the Kadish D group would be appropriate and conferred to the original Kadish staging system for this group. However, it is clear that Kadish D with distant metastasis indicates very poor prognosis and treatments need to be allocated accordingly.
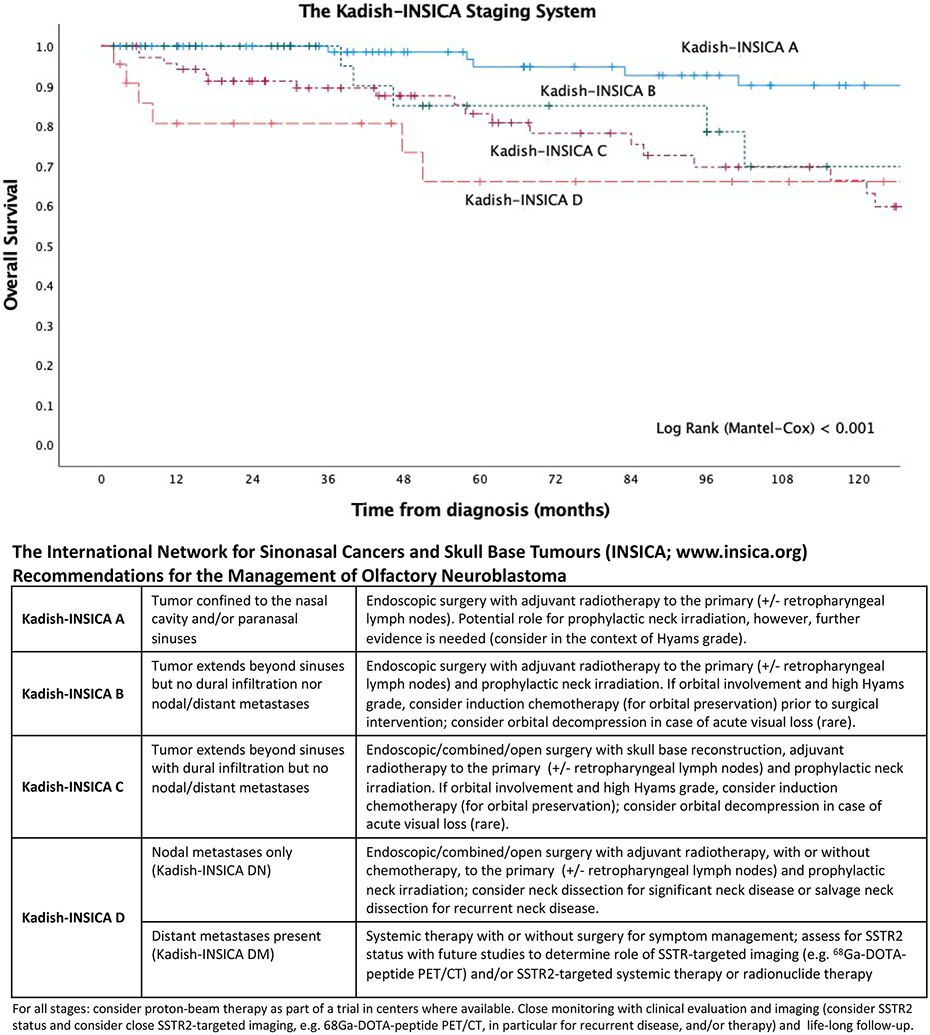
Importantly, the initial Kadish staging system was created based on a relatively small cohort of cases (n = 17) and has recently been shown to be an inadequate prognosticator of ten-year survival (discussed above). With the excellent outcome observed with modern endoscopic surgery, the efficacy of adjuvant radiotherapy, and the potential role of targeted therapy for metastatic disease, an update to current staging systems is much needed. Based on our findings, we propose an updated system, the Kadish-INSICA Staging System, which consolidates the Kadish A and B groups and further stratifies the Kadish C group into those with or without dural infiltration. We have shown that this system better predicts survival and provides a framework for the establishment of updated management guidelines (Figure 6). Moreover, to further refine our novel prognosticator, we ran a Boolean Logic Regression Random Forest Model (BLRRF) including Hyams grading in addition to the staging as specified by Kadish-INSICA. The BLRRF model did not outperform the simpler model based on Kadish-INSICA (HR = 3.31 (1.49-7.36) Wald test P=0.003), suggesting that further improvements to Kadish-INSICA will require either other clinical covariates or a further increase in sample size.
Figure 6: Kadish-INSICA staging system.
Definitions and proposed management guidelines.
Endoscopic surgery and adjuvant radiotherapy confer excellent outcomes with reduced nodal recurrence after prophylactic neck irradiation; perioperative chemotherapy may be helpful
Endoscopic surgery has emerged as a standard-of-care with comparable outcome to an open approach and the potential for reduced perioperative complications and improved long-term quality of life.9,35-38 While previous reports have demonstrated comparable or even superior outcomes with an endoscopic approach, it is important to note that stage of disease was generally not adequately taken into account. As demonstrated by our data and others, early stage is more commonly treated with an endoscopic approach. In light of this, it was not unexpected to observe a smaller effect of surgical approach on survival after adjusting for Kadish stage. Altogether, the question of undertaking the appropriate surgical approach, ultimately, appears to be one of the extent of disease, risk of perioperative complications as well as overall patient quality of life, due to the significant morbidity associated with open surgical resection.
Significantly, we observed a survival benefit with adjuvant radiotherapy in the entire cohort. Even in cases of early-stage disease (i.e. Kadish A or B), adjuvant radiotherapy improved outcomes. In these cases, where complete surgical resection is typically achieved, the balance between eliminating residual disease through adjuvant treatment and consequent treatment-associated morbidities needs to be carefully determined. Nevertheless, as established by Lund et al and confirmed in our data, adjuvant radiotherapy to the tumour primary significantly reduces the risk of recurrence and should be considered even for early-stage disease.34
With regards to the emergence of proton beam therapy (PBT) as a viable treatment option for ONB, this is a subject of ongoing investigation. Efficacy and safety of PBT has been reported retrospectively, however, no randomised, controlled clinical trials have been performed.39,40 In view of the improved side effect profile of PBT compared to more traditional forms of irradiation, this could offer a very valuable future option and further refine the management of ONB - if efficacy can be shown in a prospective trial. However, since the vast majority of the patients in the present cohort did not receive PBT, as it is not readily available in all regions, and due to limitations with existing studies, a conclusion for its adoption to standard-of-care cannot be drawn. Future studies are much needed.
Whilst prophylactic neck irradiation did not appear to significantly impact overall and disease-free survival, it does appear to prevent cervical lymph node recurrence in our cohort. Indeed, none of the patients who received neck irradiation recurred in the neck nodes in the initial ten years post-treatment of primary disease, compared to 12.2% of those who received radiotherapy to the primary tumour only. These results complement a recent report by Song et al, who demonstrated a significant reduction in regional recurrence in those with cervical lymph nodes metastasis, who underwent prophylactic neck irradiation.41 Importantly, several of the recurrences we observed occurred even after the initial ten years post-treatment of the primary. Therefore, long-term follow-up is imperative.
Lastly, the vast majority of cervical lymph node recurrences occurred in patients who presented with Kadish C disease; nevertheless, a handful of Kadish A and B patients also experienced neck recurrence. Therefore, the use of neck irradiation across early stage disease may be warranted, however, further studies investigating these and other predictive markers of recurrence are much needed.
The majority of patients who received perioperative chemotherapy had later stage disease, so any potential benefit may be masked by the aggressiveness of their disease. In a recent retrospective analysis, patients who received adjuvant chemoradiotherapy tended to have higher Hyams grade disease, dural infiltration and positive surgical margins.42 Thus, the addition of chemotherapy in the adjuvant setting may be warranted in more advanced or for more aggressive disease, particularly as higher Hyams grade has been associated with increased sensitivity to systemic therapy. This was demonstrated in cases of ONB with distant metastasis where chemotherapy combined with surgery and/or radiotherapy improved survival outcomes.43 In contrast, a recent analysis of the Surveillance Epidemiology and End Results (SEER) database, consisting of over seven hundred eligible cases, found that perioperative chemotherapy treatment was associated with worse disease-specific (p < 0.001) and overall survival (p < 0.001). Nevertheless, a formal clinical trial is much needed to inform future recommendations on the use of chemotherapy for the management of ONB.
SSTR-based imaging may guide diagnosis and treatment allocation
Preliminary studies on the expression of SSTR2 have been published as early as 1996 and in 2018 Czapiewski et al. published on a series of 40 ONB cases.24 Here we present the largest cohort ever published with a validated grading system of SSTR2 staining (ready for clinical application) and correlation of uptake in Dota-peptide imaging. Intriguingly, expression is maintained in neck and distant metastases.
The use of SSTR2 imaging has become the standard of care for NETs, allowing for improved diagnostic sensitivity and specificity. Over the course of thirty years, this area has progressed toward the routine use of 68Ga labelled SSAs PET/CT. For thoracic and abdominal NETs, 93% sensitivity and 96% specificity of SSTR2 PET/CT has been observed.44 Our data indicate that the vast majority of ONBs overexpress SSTR2 and that this is associated with 68Ga-DOTATOC uptake, which warrant more extensive prospective trials on the usage of SSTR2 PET/CT for diagnosis and surveillance in this disease type. Preliminary studies on this have been published as early as 1996 by Ramsay et al., who demonstrated the clinical utility of 111In-Octreotate PET/CT in the detection of recurrent disease and extensive neck and chest metastases.22 More recently, the use of 68Ga-DOTATE PET/CT and 18F-FDG PET/CT was shown to be useful for the detection of tumors in areas with high background noise.23 Furthermore, due to the variability of FDG uptake in ONB, particularly in well differentiated tumors or those with low metabolic rate, the exploitation of the high expression of SSTR2 appears to be very useful, enabling detection of recurrent disease and metastases.45,46
A potential role for PRRT in metastatic disease
Importantly, overexpression of SSTR2 in ONB opens the door for the implementation of peptide receptor radionuclide therapy (PRRT) as treatment, particularly in cases of aggressive relapse and persistent disease. Three patients in the present cohort were enrolled in the LUTHREE trial and underwent PRRT. PRRT was well-tolerated and successfully stabilized disease. In cases where patients experienced progression, re-PRRT was subsequently able to stop further progression. These findings align with previous case series. In a case report, Savelli et al. have demonstrated the feasibility of this treatment modality, demonstrating successful detection of brain lesions upon recurrence and treatment of a patient with PRRT.45 Schneider et al. similarly applied PRRT for the palliative treatment of one case of refractory ONB of high Hyams grade with metastases to the lymph nodes.47 Four cycles of PRRT resulted in partial response from all lesions and improved symptom management. More recently, another retrospective study similarly demonstrated feasibility of PRRT with partial response in four of seven patients, two had disease stabilization and one experienced disease progression.48 Ultimately, our findings add some clinical trial evidence to further support the use of PRRT in otherwise untreatable cases, as we also demonstrate sustained expression in local recurrence and metastatic disease.
Limitations
The main limitation of this study is its retrospective design (apart from the presented prospective clinical trial data on the translational findings and the molecular data). Therefore, statistical analyses are limited to those of an exploratory nature and results should be considered in this context. Furthermore, with twelve institutions collaborating across the US, UK and Europe, heterogeneity in the data collected as well as missing data were unavoidable, even though incredible effort was made to mitigate these.
Conclusions
This study presents pertinent clinical data from the largest international ONB cohort to date. We identify key prognostic factors and integrate these into an updated staging system, highlight the importance of adjuvant radiotherapy across all disease stages, the utility of prophylactic neck irradiation for the prevention of neck recurrence and the potential efficacy of targeting SSTR2 in the management of disease.
Supplementary Material
Acknowledgements
We would like to acknowledge the invaluable support from Prof. Tariq Enver and the UCL Cancer Institute. We would also like to thank Josep Linares, Dr. Naomi Guppy and David Allan from HSL Labs/UCL Advanced Diagnostics.
Funding
This work was supported by the Rhinology and Laryngology Research Fund, Royal College of Surgeons (Lionel Colledge Family Research Grant) and the UCL/UCLH Biomedical Research Centre (BRC). Additional support was provided by the Intramural Research Program of the National Institutes of Health/National Institute on Deafness and Other Communication Disorders.
Footnotes
Conflicts of Interest
NL receives research funding from Merck Inc., not related to this manuscript, and was a consultant for CoolTech Inc. and holds stock in Navigen Pharmaceuticals, both of which are unrelated to this manuscript. SW is on the advisory board of ALK, Genentech, OptiNose, SinopSys and a Consultant to NeurENT, Stryker, all of which are unrelated to this manuscript. All other authors declare no potential relevant conflicts of interest.
References
- 1.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cnacer: a historical analysis of population-based data. Head Neck. 2012;34:877–885. doi: 10.1002/HED [DOI] [PubMed] [Google Scholar]
- 2.Thompson LDR. Olfactory Neuroblastoma. Head Neck Pathol. 2009;3:252–259. doi: 10.1007/s12105-009-0125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song X, Wang J, Wang S, Yan L, Li Y. Prognostic factors and outcomes of multimodality treatment in olfactory neuroblastoma. Oral Oncol. 2020;103(February):1–7. doi: 10.1016/j.oraloncology.2020.104618 [DOI] [PubMed] [Google Scholar]
- 4.Cranmer LD, Chau B, Rockhill JK, Ferreira M, Liao JJ. Chemotherapy in Esthesioneuroblastoma/Olfactory Neuroblastoma: An Analysis of the Surveillance Epidemiology and End Results (SEER) 1973-2015 Database. Am J Clin Oncol Cancer Clin Trials. 2020;43(3):203–209. doi: 10.1097/COC.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 5.Joshi RR, Husain Q, Roman BR, et al. Comparing Kadish, TNM, and the modified Dulguerov staging systems for esthesioneuroblastoma. J Surg Oncol. 2019;119(1):130–142. doi: 10.1002/jso.25293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goshtasbi K, Abiri A, Abouzari M, et al. Hyams grading as a predictor of metastasis and overall survival in esthesioneuroblastoma: a meta-analysis. Int Forum Allergy Rhinol. 2019;9(9):1054–1062. doi: 10.1002/alr.22373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu TS, Monteiro E, Muhanna N, Goldstein DP, de Almeida JR. Comparison of outcomes for open versus endoscopic approaches for olfactory neuroblastoma: A systematic review and individual participant meta-analysis. Head Neck. 2016;38:E2306–E2316. doi: 10.1002/HED [DOI] [PubMed] [Google Scholar]
- 8.Yin Z, Wang Y, Wu Y, et al. Age distribution and age-related outcomes of olfactory neuroblastoma: a population-based analysis. Cancer Manag Res. 2018;10:1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimmer J, Lund VJ, Beale T, Wei WI, Howard D. Olfactory neuroblastoma: A 35-year experience and suggested follow-up protocol. Laryngoscope. 2014;124(7):1542–1549. doi: 10.1002/lary.24562 [DOI] [PubMed] [Google Scholar]
- 10.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma—A clinical analysis of 17 cases. Cancer. 1976;37(3):1571–1576. doi: [DOI] [PubMed] [Google Scholar]
- 11.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: Prognosis and Management. Neurosurgery. 1993;32(5):706–715. [DOI] [PubMed] [Google Scholar]
- 12.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: The UCLA Experience 1970-1990. Laryngoscope. 1992;102(843-849). [DOI] [PubMed] [Google Scholar]
- 13.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001;2(11):683–690. doi: 10.1016/S1470-2045(01)00558-7 [DOI] [PubMed] [Google Scholar]
- 14.Joshi RR, Husain Q, Roman BR, et al. Comparing Kadish, TNM, and the modified Dulguerov staging systems for esthesioneuroblastoma. J Surg Oncol. 2019;119(1):130–142. doi: 10.1002/jso.25293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold MA, Farnoosh S, Gore MR. Comparing Kadish and Modified Dulguerov Staging Systems for Olfactory Neuroblastoma: An Individual Participant Data Meta-analysis. Otolaryngol - Head Neck Surg (United States). 2020;163(3):418–427. doi: 10.1177/0194599820915487 [DOI] [PubMed] [Google Scholar]
- 16.Saade RE, Hanna EY, Bell D. Prognosis and Biology in Esthesioneuroblastoma: the Emerging Role of Hyams Grading System. Curr Oncol Rep. 2015;17(1):1–5. doi: 10.1007/s11912-014-0423-z [DOI] [PubMed] [Google Scholar]
- 17.Bell D, Saade R, Roberts D, et al. Prognostic Utility of Hyams Histological Grading and Kadish-Morita Staging Systems for Esthesioneuroblastoma Outcomes. Head Neck Pathol. 2015;9(1):51–59. doi: 10.1007/s12105-014-0547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Gompel JJ, Giannini C, Olsen KD, et al. Long-term outcome of esthesioneuroblastoma: Hyams grade predicts patient survival. J Neurol Surgery, Part B Skull Base. 2012;73(5):331–336. doi: 10.1055/s-0032-1321512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur G, Kane AJ, Sughrue ME, et al. The prognostic implications of Hyam’s subtype for patients with Kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013;20(2):281–286. doi: 10.1016/j.jocn.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malouf GG, Casiraghi O, Deutsch E, Guigay J, Temam S, Bourhis J. Low- and high-grade esthesioneuroblastomas display a distinct natural history and outcome. Eur J Cancer. 2013;49(6):1324–1334. doi: 10.1016/j.ejca.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 21.Su SY, Bell D, Hanna EY. Esthesioneuroblastoma, neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma: Differentiation in diagnosis and treatment. Int Arch Otorhinolaryngol. 2014;18:S149–S156. doi: 10.1055/s-0034-1390014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsay HA, Kairemo KJA, Jekunen AP. Somatostatin receptor imaging of olfactory neuroblastoma. J Laryngol Otol. 1996;110(12):1161–1163. doi: 10.1017/S0022215100136023 [DOI] [PubMed] [Google Scholar]
- 23.Dadgar H, Norouzbeigi N, Ahmadzadehfar H, Assadi M. 68Ga-DOTATATE and 18F-FDG PET/CT for the Management of Esthesioneuroblastoma of the Sphenoclival Region. Clin Nucl Med. 2020;45(8):e363–e364. doi: 10.1097/RLU.0000000000003133 [DOI] [PubMed] [Google Scholar]
- 24.Czapiewski P, Kunc M, Gorczyński A, et al. Frequent expression of somatostatin receptor 2a in olfactory neuroblastomas: a new and distinctive feature. Hum Pathol. 2018;79:144–150. doi: 10.1016/j.humpath.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 25.Lechner M, Schartinger VH, Steele CD, et al. Somatostatin receptor 2 expression in nasopharyngeal cancer is induced by Epstein Barr virus infection: impact on prognosis, imaging and therapy. Nat Commun. 2021;12(117). doi: 10.1038/s41467-020-20308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. doi: 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pipinikas CP, Dibra H, Karpathakis A, et al. Epigenetic dysregulation and poorer prognosis in DAXX-deficient pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2015;22(3):L13–L18. doi: 10.1530/ERC-15-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capper D, Engel NW, Stichel D, et al. DNA methylation-based reclassification of olfactory neuroblastoma. Acta Neuropathol. 2018;136(2):255–271. doi: 10.1007/s00401-018-1854-7 [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Liu N, Chen XZ, et al. Genome-wide identification of a methylation gene panel as a prognostic biomarker in nasopharyngeal carcinoma. Mol Cancer Ther. 2015;14(12):2864–2873. doi: 10.1158/1535-7163.MCT-15-0260 [DOI] [PubMed] [Google Scholar]
- 30.The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1126/science.1249098.Sleep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turri-Zanoni M, Maragliano R, Battaglia P, et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: Refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74(May):21–29. doi: 10.1016/j.oraloncology.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 32.Marinelli JP, Janus JR, Van Gompel JJ, et al. Dural Invasion Predicts the Laterality and Development of Neck Metastases in Esthesioneuroblastoma. J Neurol Surgery, Part B Skull Base. 2018;79(5):495–500. doi: 10.1055/s-0038-1625977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimmer J, Lund VJ, Beale T, Wei WI, Howard D. Olfactory neuroblastoma: A 35-year experience and suggested follow-up protocol. Laryngoscope. 2014;124(7):1542–1549. doi: 10.1002/lary.24562 [DOI] [PubMed] [Google Scholar]
- 34.Lund VJ, Howard D, Wei W, Spittle M. Olfactory neuroblastoma: Past, present, and future? Laryngoscope. 2003;113(3):502–507. doi: 10.1097/00005537-200303000-00020 [DOI] [PubMed] [Google Scholar]
- 35.Su SY, Kupferman ME, Demonte F, Levine NB, Raza SM, Hanna EY. Endoscopic resection of sinonasal cancers. Curr Oncol Rep. 2014;16(2). doi: 10.1007/s11912-013-0369-6 [DOI] [PubMed] [Google Scholar]
- 36.Gallia GL, Asemota AO, Blitz AM, et al. Endonasal endoscopic resection of olfactory neuroblastoma: An 11-year experience. J Neurosurg. 2019;131(1):238–244. doi: 10.3171/2018.2.JNS171424 [DOI] [PubMed] [Google Scholar]
- 37.Abuzeid WM, Song C, Fastenberg JH, et al. Correlations between cystic fibrosis genotype and sinus disease severity in chronic rhinosinusitis. Laryngoscope. 2018;128(8):1752–1758. doi: 10.1002/lary.27019 [DOI] [PubMed] [Google Scholar]
- 38.Nicolai P, Battaglia P, Bignami M, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: A 10-year experience. Am J Rhinol. 2008;22(3):308–316. doi: 10.2500/ajr.2008.22.3170 [DOI] [PubMed] [Google Scholar]
- 39.Nishimura H, Ogino T, Kawashima M, et al. Proton-Beam Therapy for Olfactory Neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68(3):758–762. doi: 10.1016/j.ijrobp.2006.12.071 [DOI] [PubMed] [Google Scholar]
- 40.Nakamura N, Zenda S, Tahara M, et al. Proton beam therapy for olfactory neuroblastoma. Radiother Oncol. 2017;122(3):368–372. doi: 10.1016/j.radonc.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 41.Song X, Huang C, Wang S, Yan L, Wang J, Li Y. Neck management in patients with olfactory neuroblastoma. Oral Oncol. 2020;101(December 2019):1–6. doi: 10.1016/j.oraloncology.2019.104505 [DOI] [PubMed] [Google Scholar]
- 42.Miller KC, Marinelli JP, Van Gompel JJ, et al. Utility of adjuvant chemotherapy in patients receiving surgery and adjuvant radiotherapy for primary treatment of esthesioneuroblastoma. Head Neck. 2019;41(5):1335–1341. doi: 10.1002/hed.25558 [DOI] [PubMed] [Google Scholar]
- 43.Marinelli JP, Janus JR, Van Gompel JJ, et al. Esthesioneuroblastoma with distant metastases: Systematic review & meta-analysis. Head Neck. 2018;40(10):2295–2303. doi: 10.1002/hed.25209 [DOI] [PubMed] [Google Scholar]
- 44.Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40(11):1770–1780. doi: 10.1007/s00259-013-2482-z [DOI] [PubMed] [Google Scholar]
- 45.Savelli G, Bartolomei M, Bignardi M. Somatostatin receptors imaging and therapy in a patient affected by esthesioneuroblastoma with meningeal metastases. A classic example of theranostic approach. J Neurooncol. 2016;127(3):617–619. doi: 10.1007/s11060-016-2067-3 [DOI] [PubMed] [Google Scholar]
- 46.Thavarool SB, Muttath G, Nayanar S, et al. Improved survival among oral cancer patients: Findings from a retrospective study at a tertiary care cancer centre in rural Kerala, India. World J Surg Oncol. 2019;17(1):1–7. doi: 10.1186/s12957-018-1550-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider JR, Shatzkes DR, Scharf SC, et al. Neuroradiological and Neuropathological Changes After 177Lu-Octreotate Peptide Receptor Radionuclide Therapy of Refractory Esthesioneuroblastoma. Oper Neurosurg. 2018;15(6):E100–E109. doi: 10.1093/ons/opy028 [DOI] [PubMed] [Google Scholar]
- 48.Hasan OK, Ravi Kumar AS, Kong G, et al. Efficacy of Peptide Receptor Radionuclide Therapy for Esthesioneuroblastoma. J Nucl Med. 2020;61(9):1326–1330. doi: 10.2967/jnumed.119.237990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.