Abstract
Background
Fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been proven to be valuable in guiding the diagnosis and management of sarcoidosis. However, its differential value for sarcoidosis is unclear. The objective of this study was to explore the value of 18F-FDG PET/CT in differentiation sarcoidosis from lung cancer with lymph node metastasis.
Methods
A total of 361 consecutively diagnosed sarcoidosis patients and 1,944 consecutively diagnosed lung cancer patients at Shanghai Pulmonary Hospital were retrospectively reviewed. Among them, 85 patients diagnosed with sarcoidosis and 94 lung cancer patients with lymph node metastasis were enrolled. Demographic data and 18F-FDG PET/CT parameters were analyzed by the chi-square test or independent sample Student’s t-test. Receiver operating characteristic (ROC) curves were generated to identify cut-off values. Multivariate logistic regression was performed to identify independent predictors of sarcoidosis on 18F-FDG PET/CT, and those with P<0.1 were included in a regression model using the forward log rank (LR) method to generate a ROC curve.
Results
The ratio of extrapulmonary lymph node involvement in sarcoidosis patients was significantly higher than that in lung cancer patients (64.7% vs. 29.8%, P<0.001). After adjusting for gender and age, extrapulmonary lymph node involvement [odds ratio (OR): 3.160; 95% confidence interval (CI): 1.105–9.035], maximum standardized uptake value (SUVmax) of mediastinum/hilar lymph nodes >13.86 (OR: 3.245; 95% CI: 1.045–10.083), and short axis of the corresponding lymph node >11.5 mm (OR: 5.470; 95% CI: 1.149–26.037) on 18F-FDG PET/CT were independent predictors of sarcoidosis, with a sensitivity and specificity of 77.5% and 69.3%, respectively. The area under the curve was 0.769.
Conclusions
18F-FDG PET/CT could be helpful to distinguish sarcoidosis from lung cancer patients with lymph node metastasis.
Keywords: Sarcoidosis, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), lung cancer, standardized uptake value, short axis
Introduction
Sarcoidosis is a multisystem non-caseating granulomatous autoimmune disorder of unknown etiology (1). Although fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is not included in the standard workup for sarcoidosis, it has been proven to be valuable in guiding diagnosis and management (2-5). 18F-FDG PET/CT enables detailed functional and anatomic evaluation of the entire body that is not possible with other imaging modalities. It may result in a better understanding of sarcoidosis disease behavior and phenotypes expression (6). It can help to assess cardiac involvement and response to therapy (7-9). It may also be useful to evaluate reversible granulomas and confirm the most suitable target site for biopsy (2).
However, the differential diagnostic value of 18F-FDG PET/CT for sarcoidosis is controversial. In some studies, 18F-FDG PET/CT has been reported to play a role in differentiating sarcoidosis from lymphoma and IgG4-related disease (10,11). In another study, it was reported that the uptake of 18F-FDG increased both in the sarcoid lesions and malignant lesion, but 18F-FDG PET/CT was not considered helpful for differentiating sarcoidosis from lung cancer (12). The maximum standardized uptake value (SUVmax), a commonly used semiquantitative measurement in 18F-FDG PET/CT, has also been shown not to be helpful for differentiation between benign and malignant lesions in patients with lymphadenopathy, but mostly in a small sample of cases (13,14). We must highlight that the role of 18F-FDG PET/CT in the staging of lung cancer (per guidelines) is not mainly related to mediastinum but to exclude distant metastasis. A study has shown that cut-off levels of SUVmax ≥2.5 can predict malignancy with a sensitivity, specificity, PPV, NPV of 80%, 88%, 75%, and 91% respectively (15). To explore the diagnostic value of 18F-FDG PET/CT in sarcoidosis, we conducted this retrospective cohort study with more cases, hoping to provide more evidence for clinical practice. We present the following article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-611/rc).
Methods
Patient screening
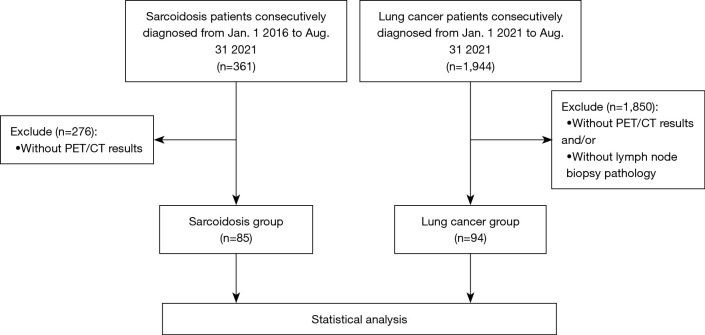
This is a retrospective cohort study. A total of 361 sarcoidosis patients consecutively diagnosed in Shanghai Pulmonary Hospital between Jan. 1 2016 to Aug. 31 2021 were eligible for this study, and their electronic medical records were retrospectively reviewed. Of these, 276 patients without 18F-FDG PET/CT examination were excluded, and a total of 85 patients with sarcoidosis were finally enrolled. The diagnosis of sarcoidosis was based on symptoms, radiological manifestations, and evidence of non-caseating epithelioid cell granulomas after exclusion of other known causes of granulomatosis, as defined by the American Thoracic Society (ATS)/European Respiratory Society consensus statement (16). Organ involvement was determined for each sarcoidosis patient using the revised World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) instrument described in 2014 (17). Meanwhile, we reviewed a total of 1944 consecutively diagnosed patients with lung cancer from Jan. 1 2021 to Aug. 31 2021 in Shanghai Pulmonary Hospital. After excluding 1850 cases without 18F-FDG PET/CT and/or lymph node biopsy pathological results, 94 cases were enrolled in the lung cancer group. The flow chart of enrollment is shown in Figure 1. The study conformed to the principles outlined in the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. k21-390). Informed consent was obtained from all individual participants included in the study.
Figure 1.
Study flow diagram. PET/CT, positron emission tomography/computed tomography.
Variables recorded
Baseline characteristics of recruited patients, including age, gender, and smoking history, were recorded. The X-ray stage (18) and level of 24-hour urinary calcium of sarcoidosis patients were also recorded. The TNM classification and final pathological diagnosis of each lung cancer patient was checked.
A dual probe multi-slice scanner (GE Millennium VG with Hawkeye) was used to perform 18F-FDG PET/CT examination. Scans were obtained 60 min after the intravenous administration of 185–370 MBq of 18F-FDG based on body-weight. All patients were required to have a low-carbohydrate dinner before the examination. In addition, overnight fasting (>12 h) was required before the examination. Fasting blood glucose was controlled below 7 mmol/L. Whole-body PET studies were evaluated by 2 experienced nuclear medicine physicians. We focused on the manifestations of mediastinal/hilar lymph nodes in 18F-FDG PET/CT. The SUVmax of mediastinal/hilar lymph nodes, the long and short axes of corresponding lymph nodes, and the distribution of lymph node involvement were recorded for all patients.
Statistical analysis
GraphPad Prism (Version 8.0) and SPSS (Version 26.0, IBM) were used for figure drawing and statistical analysis. Quantitative data were presented as mean ± standard deviation (SD). The independent sample Student’s t-test was used for comparisons between the sarcoidosis and lung cancer groups. The chi-square test was used for constituent ratio comparisons. If the theoretical frequency was greater than or equal to one and less than five, the continuous correction chi-square test was used. If the theoretical frequency was less than one, Fisher’s exact test was used. The sample size was estimated at least 10 times the number of positive predictors. Receiver operating characteristic (ROC) curves were generated to identify cut-off values for each predictor. Multivariate logistic regression was performed to identify independent predictors of sarcoidosis on 18F-FDG PET/CT, and those with P<0.1 were included in a regression model using the forward log rank (LR) method to generate an ROC curve. Values of P<0.05 (two-sided) were considered statistically significant.
Results
Clinical characteristics
A total of 85 patients with sarcoidosis and 94 lung cancer patients with lymph node metastases were enrolled in this retrospective study. The clinical characteristics are shown in Table 1. The ratios of females and non-smokers in the sarcoidosis group were both higher than those in the lung cancer group (P<0.001 and P=0.003), and the average age in the sarcoidosis group was lower (53.1±10.5 vs. 62.7±9.0 years, P<0.001). The majority of sarcoidosis patients exhibited a stage II chest X-ray (62/85), and the majority of lung cancer patients had non-small cell lung cancer (NSCLC) (73/94). In the lung cancer group, according to TNM staging, there were 9 cases in stage II, 48 cases in stage III, and 37 cases in stage IV.
Table 1. Characteristics of patients with sarcoidosis and lung cancer at the onset of disease.
| Characteristics | Sarcoidosis (n=85) | Lung cancer (n=94) | P value |
|---|---|---|---|
| Age at diagnosis, years | 53.1±10.5 | 62.7±9.0 | <0.001 |
| Gender (female/male), n | 57/28 | 18/76 | <0.001 |
| Smoking history, n (%) | 14 (16.5) | 34 (36.2) | 0.003 |
| X stage (0/I/II/III/IV) of sarcoidosis, n | 0/15/62/1/7 | – | – |
| Pathology of lung cancer, n | |||
| NSCLC$ | – | 73 | – |
| SCLC | – | 16 | – |
| Othersa | – | 5 | – |
| 18F-FDG PET/CT parameters of mediastinum/hilar lymph nodes | |||
| SUVmax | 13.38±7.68 | 9.19±5.00 | <0.001 |
| Long axis, mm | 25.5±8.3 | 20.4±8.6 | <0.001 |
| Short axis, mm | 18.3±6.5 | 15.5±7.3 | 0.042 |
| Diagnostic accuracy of 18F-FDG PET/CT, % | 76.5 | 98.9 | <0.001 |
Quantitative data are presented as mean ± standard deviation. $, including 42 cases of adenocarcinoma, 19 cases of squamous cell carcinomas and 12 cases of unclassified NSCLC; a, including 2 cases of neuroendocrine carcinoma, 2 cases of non-classification, and 1 case of lymphoepitheliomatoid carcinoma. NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
Organ involvement of sarcoidosis is illustrated in Figure 2. All patients had lung involvement (100%), including infiltration of the lung and/or mediastinal/hilar lymph node involvement. Non-thoracic lymph node involvement ranked second, which was present in 63.5% of patients, followed by hypercalciuria (20.0%). Spleen and skin involvement were present in 8.2% and 5.9% of patients, respectively. Liver and bone involvement were present in 4.7% of patients. Muscle and eye involvement were present in 3.5% and 2.3% of patients, respectively. Parotid gland and bone marrow involvement both accounted for 1.2% of sarcoidosis patients.
Figure 2.
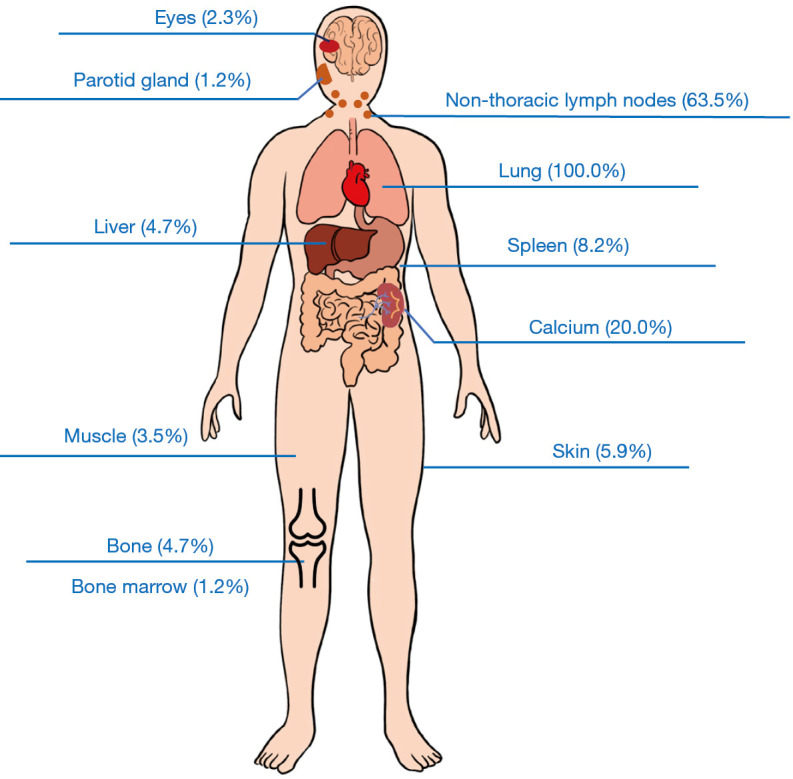
Organ involvement of sarcoidosis patients.
Comparison of sarcoidosis and lung cancer on 18F-FDG PET/CT
The distribution of lymph node involvement on PET/CT in the 2 groups is shown in Table 2. Thoracic lymph nodes were involved in all patients, and the extrapulmonary lymph nodes involved were mainly distributed in the cervical, pelvic, abdominal, axilla, and groin regions. The ratio of extrapulmonary lymph node involvement in sarcoidosis patients was significantly higher than that in lung cancer patients (64.7% vs. 29.8%, P<0.001).
Table 2. Distribution of lymph node involvement on 18F-FDG PET/CT.
| Lymph nodes | Sarcoidosis (n=85) | Lung cancer (n=94) | P value |
|---|---|---|---|
| Thoracic lymph nodes | 85 (100) | 94 (100) | – |
| Cervical lymph nodes | 49 (57.6) | 20 (21.3) | <0.001 |
| Pelvic and celiac lymph nodes | 41 (48.2) | 12 (12.8) | <0.001 |
| Groin | 12 (14.1) | 0 (0) | – |
| Axillary lymph nodes | 10 (11.8) | 3 (3.2) | 0.027 |
| Extrapulmonary lymph node involvement | 55 (64.7) | 28 (29.8) | <0.001 |
Data are presented as number of patients with percentage of total in parentheses. 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography.
By quantitative measurement of 18F-FDG PET/CT parameters, we found that the SUVmax of mediastinum/hilar lymph nodes and the lengths of the long and short axes of the corresponding lymph nodes in sarcoidosis patients were all significantly higher than those in lung cancer patients [13.38±7.68 vs. 9.19±5.00 (P<0.001), 25.5±8.3 vs. 20.4±8.6 mm (P<0.001), and 18.3±6.5 vs. 15.5±7.3 mm (P=0.042), respectively], as shown in Table 1. Furthermore, the 3 factors were used to draw ROC curves to predict sarcoidosis. The areas under the curves (AUCs) were 0.702 for SUVmax, 0.676 for the long axis, and 0.632 for the short axis, while the optimal cut-off values were 13.86, 21.5, and 11.5 mm, respectively (Figure 3).
Figure 3.
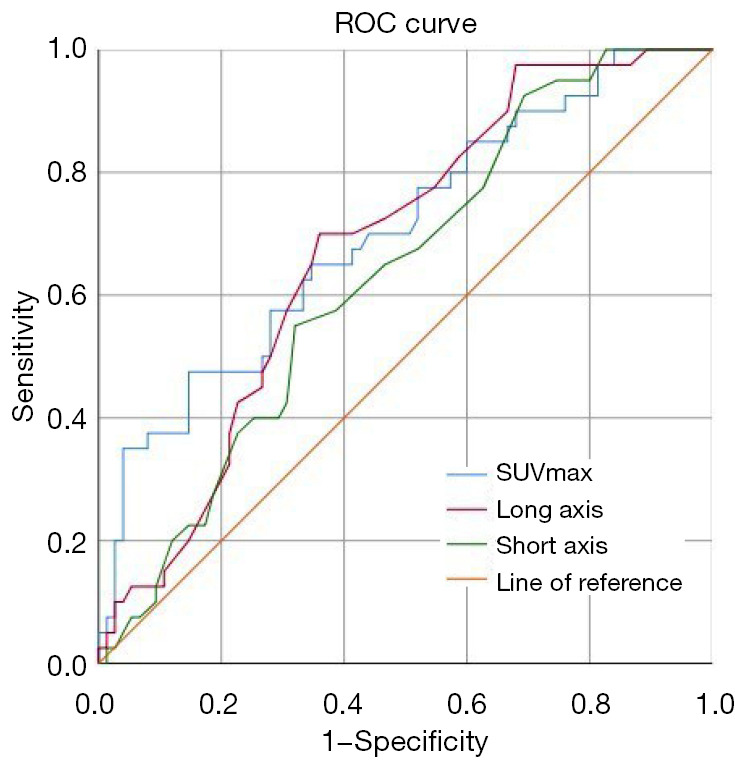
ROC curves of SUVmax, long axis, and short axis of mediastinum/hilar lymph nodes on 18F-FDG PET/CT to predict sarcoidosis. The areas under the curves were 0.702 for SUVmax, 0.676 for the long axis, and 0.632 for the short axis. The optimal cut-off values were 13.86, 21.5, and 11.5 mm, respectively. The sensitivities and specificities were 47.5% and 85.3% for SUVmax, 70.0% and 64.0% for long axis, 92.5% and 30.7% for short axis. ROC, receiver operating characteristic; SUVmax, maximum standardized uptake value; 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography.
Independent predictors of sarcoidosis on 18F-FDG PET/CT
Combined with all factors that were different between the sarcoidosis and lung cancer groups (P<0.1), the independent predictors of sarcoidosis were obtained by multivariate regression analysis, as illustrated in Figure 4. After adjusting for gender and age, extrapulmonary lymph node involvement [odds ratio (OR): 3.160; 95% confidence interval (CI): 1.105–9.035], SUVmax of mediastinum/hilar lymph nodes >13.86 (OR: 3.245; 95% CI: 1.045–10.083), and short axis of the corresponding lymph node >11.5 mm (OR: 5.470; 95% CI: 1.149–26.037) on 18F-FDG PET/CT were independent predictors of sarcoidosis. A ROC curve to predict sarcoidosis combining the 3 parameters showed an AUC of 0.769, with a sensitivity and specificity of 77.5% and 69.3%, respectively (Figure 5).
Figure 4.
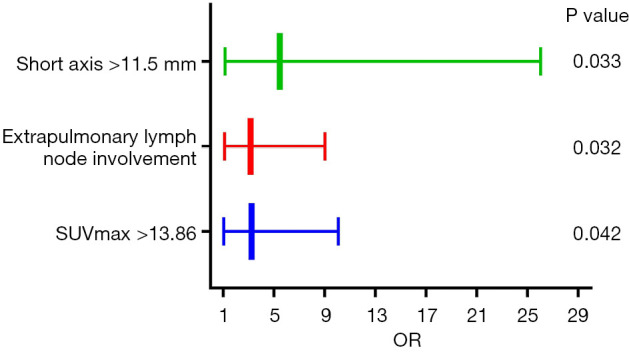
Independent predictors of sarcoidosis on 18F-FDG PET/CT. After adjusting for gender and age, extrapulmonary lymph node involvement (OR: 3.160; 95% CI: 1.105–9.035), SUVmax >13.86 (OR: 3.245; 95% CI: 1.045–10.083), and short axis >11.5 mm (OR: 5.470; 95% CI: 1.149–26.037) of mediastinum/hilar lymph nodes on 18F-FDG PET/CT were independent predictors of sarcoidosis as determined by logistic regression. SUVmax, maximum standardized uptake value; OR, odds ratio; 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography; CI, confidence interval.
Figure 5.
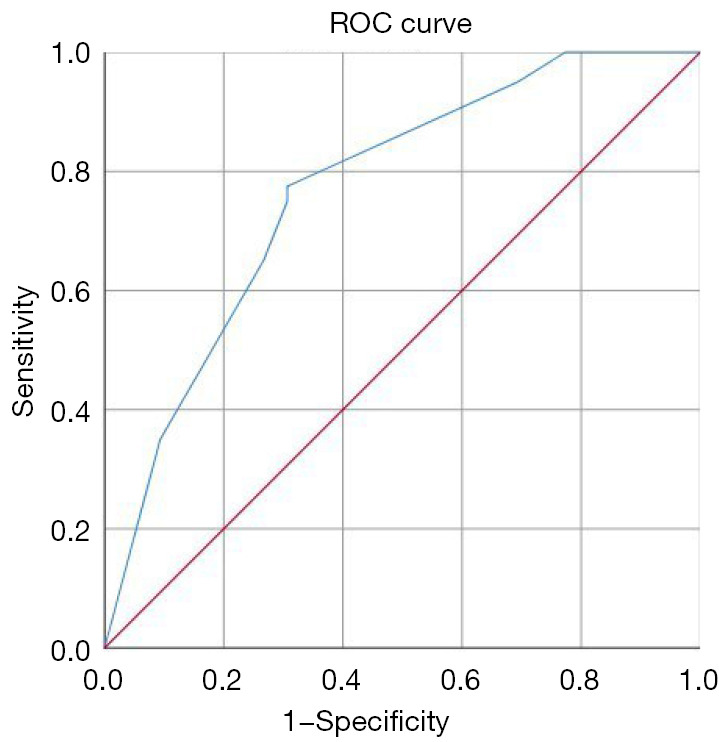
A ROC curve to predict sarcoidosis using parameters of 18F-FDG PET/CT. A combination of extrapulmonary lymph node involvement, SUVmax >13.86, and short axis >11.5 mm yielded the ROC curve, with a sensitivity and specificity of 77.5% and 69.3%, respectively. The area under the curve was 0.769. ROC, receiver operating characteristic; 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
Representative cases
Figure 6 displays part of the 18F-FDG PET/CT scan of a 47-year-old man, which shows hypermetabolic lesions in the mediastinal and hilar lymph nodes (Figure 6A). The largest lymph node with the highest glucose metabolism activity was the right hilar lymph node (Figure 6B), with a size of 37×25 mm and SUVmax of 25.21. 18F-FDG PET/CT examination indicated the diagnosis of sarcoidosis. The pathology of cervical lymph node biopsy was proliferative granuloma. Combined with special staining and clinical features, the patient was eventually diagnosed with sarcoidosis.
Figure 6.
18F-FDG PET/CT scan of a 47-year-old man diagnosed with sarcoidosis. (A) Hypermetabolic lesions in the mediastinal and hilar lymph nodes (red arrows). (B) Hypermetabolic lesions in the mediastinal and hilar lymph nodes (red arrows). The highest metabolism activity was shown in the right hilar lymph node (blue arrow), with a size of 37 mm × 25 mm and SUVmax of 25.21. 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
Figure 7 shows part of the 18F-FDG PET/CT scan of a 69-year-old man, which displays a nodule with abnormally increased glucose metabolism in the right upper lobe of the lung, with a diameter of about 19 mm and SUVmax of 13.26 (Figure 7A). Lymph nodes with abnormally increased glucose metabolism were observed in groups 4R and 5, and the larger ones were located in group 4R, with a size of about 15 mm × 11 mm and SUVmax of 8.96 (Figure 7B). Biopsy of 4R lymph nodes through ultrasonographic tracheoscopy confirmed the diagnosis of poorly differentiated small cell lung cancer.
Figure 7.
18F-FDG PET/CT scan of a 69-year-old man diagnosed with lung cancer. (A) A nodule with abnormally increased glucose metabolism in the right upper lobe of the lung, with a diameter of about 19 mm and SUVmax of 13.26. (B) Lymph nodes with abnormally increased glucose metabolism were observed in groups 4R and 5, and the larger ones were located in group 4R, with a size of about 15 mm × 11 mm and SUVmax of 8.96. 18F-FDG PET/CT, 18-fluorodeoxyglucose positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value.
Discussion
18F-FDG PET/CT, combined functional and anatomic imaging modality, is widely applied to assess, stage, and monitor malignancy (19). The uptake of FDG in malignant tumor cells is significantly higher than that in normal tissues and benign lesions because of the significantly enhanced glycolysis in malignant tumor cells. However, increased 18F-FDG uptake is not always limited to malignant lesions (20). Although sarcoidosis is a benign lesion, the glucose metabolism of inflammatory cells (such as lymphocytes and macrophages) in the lesion tissue also increases, so both sarcoidosis and lung cancer have increased uptake of FDG (21). Previously, it seemed impossible to differentiate the sarcoidosis and lung cancer on PET/CT. SUVmax, a commonly used semiquantitative measurement in PET/CT, was reported to have no significant relationship with the benignity or malignancy of lesions in a retrospective study, as it was observed to be elevated in the 2 groups (13). However, diseases such as sarcoidosis and lung cancer were not studied separately from benign and malignant diseases. In our study, the differential diagnosis between sarcoidosis and lung cancer lymph nodes metastasis was studied specifically. We found that the SUVmax of mediastinal/hilar lymph nodes was much higher in the sarcoidosis group compared with the lung cancer group. In other words, increased SUVmax is not only limited to malignant diseases as reported, but also to sarcoidosis, which could be at a much higher level. It may draw attention and play a certain role in the diagnosis of sarcoidosis. Furthermore, it may help locate the suitable site for biopsy to obtain the final diagnosis.
In Yu et al.’s study, the long axis and the short axis of lymph nodes were helpful in distinguishing between benign and malignant mediastinal lymph nodes, especially the short axis (13). However, in our study, the long axis and the short axis of enlarged lymph nodes in sarcoidosis were both significantly longer than those in the lung cancer group. Furthermore, the regression analysis showed that only the short axis of >11.5 mm on 18F-FDG PET/CT was an independent predictor of sarcoidosis. This suggests that the short axis not only has a certain value in the differentiation of malignant diseases (22,23), but may also play a certain role in the differentiation of sarcoidosis and lung cancer.
As a systemic examination, 18F-FDG PET/CT can show the involvement of various organs. We found that the ratio of extrapulmonary lymph node involvement in sarcoidosis patients was significantly higher than that in lung cancer patients (64.7% vs. 29.8%, P<0.001), and was proven to be an independent predictor. By combining extrapulmonary lymph node involvement, SUVmax >13.86, and short axis >11.5 mm, we plotted the ROC curve for predicting sarcoidosis, indicating a sensitivity and specificity of 77.5% and 69.3%, respectively, and the AUC was 0.769. We hope our findings could provide some clues in clinical practice. However, our findings can only provide some clues for differential diagnosis. Tissue biopsy remains the gold standard and key element to adequately identify indeterminate lesions in mediastinal/hilar lymph nodes.
There are some limitations in this study. First, this was a retrospective, single-center study. Second, other diseases such as lymphoma and mediastinal lymph node tuberculosis were not included in the study. According to our statistics, only a few cases of lymphoma were diagnosed in this center, and only a few patients with tuberculosis had 18F-FDG PET/CT examinations, which was insufficient to be included in the study. Due to the differences in 18F-FDG PET/CT equipment and operators, the cut-off values drawn from this study may not be applicable to other institutions. However, our study for the first time suggests that sarcoidosis and lung cancer can be distinguished by 18F-FDG PET/CT parameters. In the future, more multi-center, larger sample and more patients with mediastinal lymphadenopathy will be needed to validate and explore the diagnostic value of 18F-FDG PET/CT in sarcoidosis.
Conclusions
This study implies that 18F-FDG PET/CT may be helpful to differentiate sarcoidosis from lung cancer. Extrapulmonary lymph node involvement, SUVmax of mediastinal/hilar lymph nodes >13.86, and short axis of the corresponding lymph node >11.5 mm on 18F-FDG PET/CT were independent predictors of sarcoidosis.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from AME Respiratory Medicine Collaborative Group. The authors also appreciate the great support from Dr. Tine Nøhr Christensen (Copenhagen University Hospital, Denmark) in improving the quality of this paper.
Funding: The authors wish to acknowledge grants from Dream Mentor-Freshman Training Program of Shanghai Pulmonary Hospital (No. fkxr1901), National Science Foundation of Shanghai, China (No. 18ZR1431400), Science and Technology Innovation Research Project of Shanghai Science and Technology Commission, China (No. 20Y11902700), Clinical Research Plan of SHDC (No. SHDC20CR40011C), and National Science Foundation of China (No. 81200046).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the principles outlined in the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Shanghai Pulmonary Hospital (No. k21-390). Informed consent was obtained from all individual participants included in the study.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-611/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-611/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-611/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Ungprasert P, Ryu JH, Matteson EL. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin Proc Innov Qual Outcomes 2019;3:358-75. 10.1016/j.mayocpiqo.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike G, Itani M, Shah H, et al. PET/CT in the Diagnosis and Workup of Sarcoidosis: Focus on Atypical Manifestations. Radiographics 2018;38:1536-49. 10.1148/rg.2018180053 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Lower EE, Li H, et al. Clinical management of pulmonary sarcoidosis. Expert Rev Respir Med 2016;10:577-91. 10.1586/17476348.2016.1164602 [DOI] [PubMed] [Google Scholar]
- 4.Belperio JA, Shaikh F, Abtin FG, et al. Diagnosis and Treatment of Pulmonary Sarcoidosis: A Review. JAMA 2022;327:856-67. 10.1001/jama.2022.1570 [DOI] [PubMed] [Google Scholar]
- 5.Mangas Losada M, Cabrera Villegas A, García Megías I, et al. 18F-FDG PET / CT as a tool for the assessment of therapeutic response in a patient with systemic sarcoidosis. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2021. [Epub ahead of print]. pii: S2253-654X(21)00145-1. doi: 10.1016/j.remn.2021.06.007. 10.1016/j.remn.2021.06.007 [DOI] [Google Scholar]
- 6.Papiris SA, Georgakopoulos A, Papaioannou AI, et al. Emerging phenotypes of sarcoidosis based on 18F-FDG PET/CT: a hierarchical cluster analysis. Expert Rev Respir Med 2020;14:229-38. 10.1080/17476348.2020.1684902 [DOI] [PubMed] [Google Scholar]
- 7.Higashi H, Inaba S, Iio C, et al. Features and clinical impact of extra-cardiac lesions with 18F-fluorodeoxyglucose positron emission tomography in patients with suspected cardiac sarcoidosis. Int J Cardiol Heart Vasc 2020;30:100587. 10.1016/j.ijcha.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindler TH, Anderson A, El Ghannudi S, et al. Identification and characterization of cardiac sarcoidosis with positron emission tomography. Eur J Clin Invest 2022;52:e13722. 10.1111/eci.13722 [DOI] [PubMed] [Google Scholar]
- 9.Aghayev A, Cheezum MK, Steigner ML, et al. Multimodality imaging to distinguish between benign and malignant cardiac masses. J Nucl Cardiol 2022;29:1504-17. 10.1007/s12350-021-02790-9 [DOI] [PubMed] [Google Scholar]
- 10.Ozawa Y, Yamamoto H, Yasuo M, et al. A comparison of the features of fluorine-18 fluorodeoxyglucose-positron emission tomography (FDG-PET) between IgG4-related disease with bilateral hilar lymphadenopathy and sarcoidosis. Nagoya J Med Sci 2020;82:101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovinfosse P, Ferreira M, Withofs N, et al. Distinction of lymphoma from sarcoidosis at FDG PET/CT - evaluation of radiomic-feature guided machine learning versus human reader performance. J Nucl Med 2022. [Epub ahead of print]. doi: . 10.2967/jnumed.121.263598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaira K, Oriuchi N, Otani Y, et al. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest 2007;131:1019-27. 10.1378/chest.06-2160 [DOI] [PubMed] [Google Scholar]
- 13.Yu C, Xia X, Qin C, et al. Is SUVmax Helpful in the Differential Diagnosis of Enlarged Mediastinal Lymph Nodes? A Pilot Study. Contrast Media Mol Imaging 2018;2018:3417190. 10.1155/2018/3417190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krüger S, Buck AK, Mottaghy FM, et al. Use of integrated FDG-PET/CT in sarcoidosis. Clin Imaging 2008;32:269-73. 10.1016/j.clinimag.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 15.Chrysikos S, Gkiozos I, Dimakou K, et al. Clinical utility of thoracic endosonography (EBUS/EUS-b) in mediastinal staging of patients with non-small cell lung cancer: comparison with integrated PET/CT-a real-life prospective study in Greece. J Thorac Dis 2020;12:5657-66. 10.21037/jtd-20-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [DOI] [PubMed] [Google Scholar]
- 17.Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19-27. [PubMed] [Google Scholar]
- 18.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149-73. [PubMed] [Google Scholar]
- 19.Truong MT, Pan T, Erasmus JJ. Pitfalls in integrated CT-PET of the thorax: implications in oncologic imaging. J Thorac Imaging 2006;21:111-22. 10.1097/00005382-200605000-00003 [DOI] [PubMed] [Google Scholar]
- 20.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. 10.1007/s00259-014-2961-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bois JP, Muser D, Chareonthaitawee P. PET/CT Evaluation of Cardiac Sarcoidosis. PET Clin 2019;14:223-32. 10.1016/j.cpet.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 22.Yonetsu K, Sumi M, Izumi M, et al. Contribution of doppler sonography blood flow information to the diagnosis of metastatic cervical nodes in patients with head and neck cancer: assessment in relation to anatomic levels of the neck. AJNR Am J Neuroradiol 2001;22:163-9. [PMC free article] [PubMed] [Google Scholar]
- 23.Sumi M, Ohki M, Nakamura T. Comparison of sonography and CT for differentiating benign from malignant cervical lymph nodes in patients with squamous cell carcinoma of the head and neck. AJR Am J Roentgenol 2001;176:1019-24. 10.2214/ajr.176.4.1761019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as