Abstract
Anthropogenic ozone depletion has led to a 2–5% increase in ultraviolet B radiation (UVBR) levels reaching the earth's surface. Exposure to UVBR causes harmful DNA damage in amphibians, but this is minimized by DNA repair enzymes such as thermally sensitive cyclobutane pyrimidine dimer (CPD)-photolyase, with cool temperatures slowing repair rates. It is unknown whether amphibian species differ in the repair response to a given dose of UVBR across temperatures. We reared larvae of three species (Limnodynastes peronii, Limnodynastes tasmaniensis and Platyplectrum ornatum) at 25°C and acutely exposed them to 80 µW cm−2 UVBR for 2 h at either 20°C or 30°C. UVBR-mediated DNA damage was measured as larvae repaired damage in photoreactive light at their exposure temperatures. Cool temperatures increased DNA damage in two species and slowed DNA repair rate in P. ornatum. The magnitude of DNA damage incurred from UVBR was species-specific. Platyplectrum ornatum had the lowest CPDs and DNA repair rates, and the depressive effects of low temperature on photorepair were greater in L. tasmaniensis. Considering the susceptibility of most aquatic organisms to UVBR, this research highlighted a need to consider the complexity of species-specific physiology when forecasting the influence of changing UVBR and temperature in aquatic ecosystems.
Keywords: amphibian declines, DNA repair rates, temperature, photolyase, climate change, ultraviolet radiation
1. Introduction
Solar ultraviolet (UV) radiation has wide-ranging effects on organisms and biological processes. Ultraviolet B radiation (UVBR) is largely absorbed by the stratospheric ozone column; however, significant amounts of UVBR reach the Earth's surface and penetrate aquatic ecosystems [1]. Anthropogenic O3 depletion led to a 2–5% increase in UVBR levels in some areas [2,3] and will likely remain high [4] or even possibly increase as a result of climate change [5–7]. UVBR can directly damage nucleic acids by forming dimers between nucleotide bases that disrupt gene expression patterns, cause mutations and trigger apoptosis [8,9]. To avoid the toxic effects of UV-induced DNA damage, organisms employ DNA repair mechanisms including nucleotide excision repair (NER) [10] and photoenzymatic repair (PER) [11]. During PER, the enzyme photolyase uses energy from photoreactive light to break UVBR-induced nucleotide bonds [12]. However, if DNA damage occurs at rates exceeding DNA repair, deleterious photoproducts may accumulate, resulting in whole-organism fitness consequences [13,14]. Aquatic organisms are susceptible to UVBR damage at the cellular level, leading to population impacts [15].
Globally, amphibians face a high extinction risk [16–18]. Although a significant number of recent population declines have been linked to the emergence and spread of novel pathogenic amphibian chytrid fungi [19–22], global decline patterns point to co-occurring environmental changes, such as increasing UVBR, as proximate and interactive stressors with the pathogen [23–25]. Increased UVBR is hypothesized to influence amphibian populations through direct impacts on eggs and larvae as these life stages are often diurnal and typically laid during spring and summer when UVBR levels are highest [26]. UVBR exposure causes a range of sublethal and lethal effects in amphibian embryos and larvae [27] which occur primarily through the formation of cyclobutane pyrimidine dimer (CPD) photoproducts in DNA, which are repaired largely via PER in amphibians [28].
The negative effects of UVBR on amphibians are significantly compounded when UVBR exposure occurs at low temperatures [29–33], reflecting the thermal sensitivity of PER [34–39]. Morison et al. [37] proposed that the thermal sensitivity of UVBR-associated DNA repair may partially explain why a disproportionately high number of amphibian declines have occurred at higher altitudes [25,40–46]. However, species from high UV or cool environments may differ in their tolerance to UVBR, by compensating for the depressive effect of temperature on DNA repair or by employing more efficient or effective DNA repair. In this study, we investigate the thermal sensitivity of UVBR effects on DNA in the larvae of three closely related amphibian species: Limnodynastes peronii, Limnodynastes tasmaniensis and Platyplectrum ornatum. Limnodynastes peronii larvae are known to be UVBR-sensitive [29,32] while P. ornatum larvae are considerably more UVBR tolerant and likely experience higher UVBR doses in nature [47]; the UVBR sensitivity of L. tasmaniensis larvae is unknown, however, L. tasmaniensis are found in cooler habitats compared with L. peronii. It was hypothesized that there would be species-specific differences in the amount of incurred DNA damage, in DNA repair rates and in the thermal dependence of DNA repair rates which reflect the differing thermal and UV environments inhabited by the three species.
2. Methods
(a) . Animal collection and maintenance
Freshly laid L. peronii, L. tasmaniensis and P. ornatum spawn was collected near Meeanjin (greater Brisbane, QLD, Australia). Spawn was immediately transported to the University of Queensland, separated into small pieces and left to hatch in 2 l ice-cream containers half-filled with carbon-filtered Brisbane tap water at 25°C. Partial water changes were conducted every second day and larvae were fed thawed spinach ad libitum daily. Larvae were reared for two to three weeks to Gosner stage 25 [48] under a 12L : 12D photoperiod generated by standard room fluorescent lights.
(b) . UVR lighting and heating
UVR was generated using 40 W, full spectrum fluorescent light sources which emit visible light, UVAR and UVBR (Repti-Glo 10.0, 1200 mm, Exo Terra, Montreal, Canada). Light heights were adjusted to achieve an absolute UVBR irradiance of approximately 80 µW cm−2 (UVBR irradiance: mean ± s.d. = 79.8 ± 4.9; UVAR irradiance: mean ± s.d. = 81.3 ± 54.5 µW cm−2) at the water surface. Light intensities for UVAR and UVBR were measured using a calibrated radiometer (IL1400BL, International Light Inc., Newburyport, USA).
(c) . Experimental design
Upon reaching Gosner stage 25, larvae were placed into individual wells of a six-well plate (34 plates per species) containing 10 ml of filtered water. The plates were evenly allocated across four water baths at 20°C or 30°C test temperatures (two per temperature). The temperature of experimental water baths was controlled using 300 W heaters (AquaOne, Kongs Pty Ltd, Ingleburn, NSW, Australia) and water was circulated by small pumps. Larvae were left for 1 h at the experimental temperature. Larvae (n = 72 in total, n = 24 per species) were randomly removed from water baths (n = 12 per temperature treatment) rapidly euthanized with buffered MS222 (0.25 mg l−1) and then snap frozen at −80°C. UVR lights were switched on and all remaining larvae were exposed to 80 µW cm−2 of UVBR for 2 h. Water baths were then covered with UVBR blocking film (Melinex 516, 100 µm, Archival Survival, Doncaster, Victoria, Australia) and larvae were allowed to photorepair for up to 24 h. Larvae (n = 72) were removed at the following time points post-UVBR exposure: 0, 0.25, 0.5, 0.75, 1, 1.5, 3, 6, 12 and 24 h), then euthanized and snap frozen. Larval wet mass was recorded (L. peronii mean ± s.d. = 5.7 ± 2; L. tasmaniensis mean ± s.d. = 9.5 ± 7.5; P. ornatum mean ± s.d. = 6.2 ± 3.1).
(d) . DNA damage
Genomic DNA was extracted and purified from whole-animal homogenates using PureLink Genomic DNA Minikits (ThermoFisher Scientific Inc., Waltham, MA, USA) and quantified using a Qubit dsDNA High-Range Assay Kit (ThermoFisher Scientific Inc.). CPD concentrations were determined using an anti-CPD ELISA assay following the primary antibody manufacturers protocol [49]. Briefly, DNA (0.4 ng µl−1) was loaded into triplicate wells of a protamine sulfate-coated 96-well plate and detected using an anti-CPD monoclonal primary antibody (NM-ND-D001, clone TDM-2, Cosmo Bio Co., Ltd). The primary antibody was detected using a biotinylated goat anti-mouse IgG (QD209886, Life Technologies, USA), then an HRP-conjugated streptavidin (ab7403, Abcam, Cambridge, UK). Colour development was achieved with TMB substrate (416 mM; Sigma-Aldrich, Saint Louis, MO, USA) following the manufacturers guidelines. Colour development was stopped with H2SO4, and absorbance determined at 450 nm (Beckman Coulter DTX880 multimode detector, MN, USA) using the SoftMax Pro program (v.7.1.0, Molecular Devices LLC, CA, USA). CPD concentrations were calculated from a standard dose–response curve of UVC-irradiated calf thymus (NM-MA-R010, Cosmo Bio Co., Ltd, Tokyo, Japan) on each plate. CPD concentration is reported as units of UVCR-dose equivalent per 20 ng of DNA.
(e) . Statistical analyses
All analyses were conducted in the R statistical environment [50] from the resulting dataset [51]. CPD levels and wet body masses were log transformed to meet the assumptions of statistical tests. The decline in CPD abundance over time was interpreted as the repair rate of DNA damage. CPD abundance values >10 J m2 in a small number of larvae (n = 11) were changed to 10 J m2 to ensure that these data fit within the dynamic range of the standard curve (0–10 J m2). ELISA Plate ID was recorded as a random effect. CPDs were fitted in a linear mixed effects model with the lme4 package [52] as
Where [CPD] was the UVCR-dose equivalent at three levels of species across 14 experimental ELISA 96-well plates, mass was the wet body mass of the tadpole in milligrams, and time was the hours post-UVBR exposure. The fitted model was analysed using omnibus Type II χ2 tests from the car package [53]. Specific contrasts between groups were generated from the lme4 package. Mean larval masses were compared between species using two-sample Wilcoxon rank sum tests with continuity correction. One-sample t-tests were used to confirm that pre-UVBR exposure (i.e. baseline) levels of CPDs were not significantly different from zero and whether CPD levels in the 24 h post-exposure groups had returned to baseline levels in each species.
3. Results
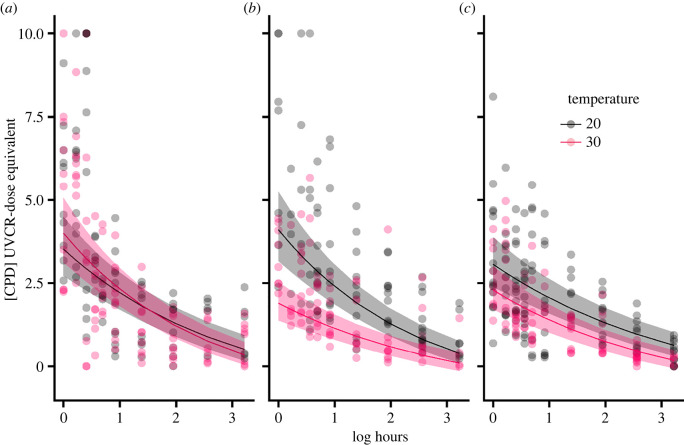
Larvae that received no UVBR exposure had no CPDs (L. peronii: t11 = 1.48, p = 0.17; L. tasmaniensis: t13 = 1.89, p = 0.08; P. ornatum: t14 = 1.84, p = 0.09). For all species and temperatures, acute exposure to UVBR resulted in the formation of CPDs (figure 1; electronic supplementary material, tables S1 and S2) which decreased over the 24 h recovery period but did not return to baseline (electronic supplementary material, figure S1; L. peronii: t13 = 4.68, p < 0.001; L. tasmaniensis: t10 = 3.51, p < 0.01; P. ornatum: t15 = 4.35, p < 0.001). Specific contrasts between regression coefficients were compared across the three reference species in the full model (electronic supplementary material, table S2). There was no overall effect of temperature on DNA repair rates, but L. tasmaniensis at 20°C had higher rates of repair than P. ornatum at 20°C (figure 1). There was a species-specific effect of temperature on CPD abundance (figure 1). Larvae held at 20°C accumulated more CPDs than larvae held at 30°C in both L. tasmaniensis and P. ornatum, but not L. peronii (max CPD abundance measured as [CPD] UVCR-dose equivalent (J m2): L. peronii: 20°C = 10, 30°C = 10; L. tasmaniensis: 20°C = 10, 30°C = 4.57; P. ornatum: 20°C = 8.11, 30°C = 4.97). There were significant interspecific differences between CPD levels, dependent on the exposure temperature. At 20°C, L. tasmaniensis had higher CPDs than P. ornatum. At 30°C, L. tasmaniensis had lower CPDs than L. peronii, and L. peronii had higher CPDs than P. ornatum. Mean larval masses were significantly higher in L. tasmaniensis compared with L. peronii (W = 20 537, p < 0.001) and P. ornatum (W = 14 012, p < 0.001). Platyplectrum ornatum larval mass was not significantly different to L. peronii (W = 20 263, p = 0.78).
Figure 1.
The effect of water temperature on CPD repair rates (measured as [CPD] UVCR-dose equivalent (J m2)) following 2 h of acute UVBR exposure (80 µw cm−2) and 24 h of blue light-assisted photorepair in (a) L. peronii; (b) L. tasmaniensis and (c) P. ornatum larvae. Points represent individual values. Curves represent the fitted parameters extracted from the linear mixed effects model, predicted for an average-sized tadpole of all species (6.08 mg). Ribbons show the upper and lower s.d. for the fitted line.
4. Discussion
This study demonstrated a complex interplay between temperature and time on the amount of UV-induced DNA damage among three species of amphibian larvae. UVBR exposure caused significant DNA damage in all larvae, but there were considerable interspecific differences in the magnitude and thermal sensitivity of the damage incurred. At cool temperatures, P. ornatum accumulated less damage during UVBR exposure but had a lower rate of repair compared to L. tasmaniensis. Considering P. ornatum larvae likely experience greater natural UVBR levels than L. peronii and L. tasmaniensis, our data suggest that this species may use alternate strategies to prevent DNA damage from occurring; however, any that does occur is repaired more slowly than in the other two species. P. ornatum larvae can maintain performance over a relatively large range of environmental temperatures and in the presence of elevated UVBR [47]. Preventing DNA damage may reduce metabolic costs associated with repair mechanisms [54–58]. Metabolism underlies important fitness traits by providing energy for growth, development and performance [59–61]. A lower DNA repair rate and lower amount of initial DNA damage following UVR exposure in P. ornatum may mean that more energy is available for other metabolic activities such as performance traits. However, the lower initial CPDs in P. ornatum does not necessarily mean the species is UV tolerant. Slower rates of DNA photorepair in P. ornatum may be problematic if larvae were to incur significant CPD concentrations in nature unless it also stimulated an increase in DNA repair rates.
Our study shows that cool temperatures did not lower UVBR-associated repair rates in any species, which contrasted with earlier work showing a thermal dependence of DNA repair rates in L. peronii [37]. While photolyase activity decreases at low temperatures, it also increases with substrate concentration [62], possibly explaining why we observed no net changes in DNA repair rates at low temperatures. In the current experiment, larvae were subject to 80 µW cm−2 UVBR for 2 h which resulted in substantially greater DNA damage levels compared to the 100 µW cm−2 for 1 h exposure used by Morison et al. [37]. The additional hour of exposure time in the present study may be where differential rates of repair are occurring, rather than during the period of photorepair where larvae were sampled. This could also explain why CPD levels were higher in L. peronii and L. tasmaniensis larvae held at cool temperatures compared with warm temperatures, even though no difference in DNA repair rate were reported between temperatures. Comparing DNA damage abundance and repair across UV dose, duration and intensity treatments may help to elucidate these different responses [63].
The finding that L. tasmaniensis accumulated a greater abundance of CPDs than P. ornatum at 20°C suggests that L. tasmaniensis larvae are more susceptible to UVR exposure at cool temperatures. This result was surprising given that L. tasmaniensis can occur in cooler habitats than P. ornatum and L. peronii and might be expected to be more resilient against the depressive effects of temperature on physiological rate processes. However, the L. tasmaniensis populations sampled in this study were not from particularly cool climates and may possess a thermal phenotype that more closely resembles that of L. peronii and P. ornatum. Population-level differences in thermal sensitivity can be underpinned by genotypic differences that may moderate the effects of temperature on the DNA photorepair response [64]. Alternatively, species-specific variations in cutaneous melanin concentration may explain differences in the degree of DNA damage experienced by larvae. Melanin pigments can migrate rapidly to the outer epithelium with UV insult to guard against DNA damage [65–67], and in response to low temperatures [68,69]. Although no obvious differences in melanization were observed between species or treatments in the current study, small differences in melanization could have reduced DNA damage in exposed animals. Differences in inherent melanin levels, or the capacity to rapidly mobilize melanin stores to minimize DNA damage could vary across species and with temperature. Similarly, sampling across a larger range of species would elucidate the contribution of phylogeny to the DNA damage response. While the genotoxic impacts of UVR on amphibian larvae are directly linked to the DNA photorepair response, caution must be taken in interpreting greater DNA damage as worse for larval fitness. For example, some species may have greater ‘DNA damage tolerance’ [70].
Amphibians are among the world's most threatened taxa, with global declines linked with co-occurring and interacting stressors such as disease and climate. Therefore, knowledge of how amphibians respond to environmental change is vital to their conservation. Our results show that UVR tolerance in amphibian larvae may depend upon the thermal context of their environment, but this influence depends on the species. Species and populations in which larvae can be more at risk of the interplay between harmful UVR exposure and cool temperatures may possess physiological adaptations enabling them to persist. However, these physiological differences could contribute to the differential susceptibility of species to decline, which may explain why a disproportionately high number of amphibian declines have occurred at high altitude. We argue that when attempting to predict how changing UVR and temperature levels in aquatic ecosystems has and will continue to influence amphibian larvae, it is critical to consider species-specific physiological responses.
Ethics
Spawn was collected under the Queensland Department of Environment and Heritage Protection Scientific Purposes Permit (WA0017092) and procedures were approved by The University of Queensland's Animal Ethics Unit (SBS/428/19).
Data accessibility
The complete dataset, a description of the dataset and R scripts used for analysing the data are publicly available at UQ eSpace https://doi.org/10.48610/4fdfc26 [51].
The data are provided in electronic supplementary material [71].
Authors' contributions
C.H.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; R.L.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, software, supervision, validation, visualization, writing—review and editing; C.E.F.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was funded by an Australian Research Council Discovery grant awarded to C.E.F. (DP190102152).
References
- 1.Xenopoulos MA, Schindler DW. 2001. Physical factors determining ultraviolet radiation flux into ecosystems. In Ecosystems, evolution, and ultraviolet radiation (eds Cockell CS, Blaustein AR), pp. 36-62. New York, NY: Springer. [Google Scholar]
- 2.Madronich S, McKenzie RL, Caldwell MM, Bjorn LO. 1995. Changes in ultraviolet radiation reaching the earth's surface. Ambio 24, 143-152. ( 10.2307/4314320) [DOI] [PubMed] [Google Scholar]
- 3.Lemus-Deschamps L, Makin JK. 2012. Fifty years of changes in UV index and implications for skin cancer in Australia. Int. J. Biometeorol. 56, 727-735. ( 10.1007/s00484-011-0474-x) [DOI] [PubMed] [Google Scholar]
- 4.Montzka SA, et al. 2018. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature 557, 413-417. ( 10.1038/s41586-018-0106-2) [DOI] [PubMed] [Google Scholar]
- 5.McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S. 2011. Ozone depletion and climate change: impacts on UV radiation. Photochem. Photobiol. Sci. 10, 182-198. ( 10.1039/c0pp90034f) [DOI] [PubMed] [Google Scholar]
- 6.Williamson CE, et al. 2014. Solar ultraviolet radiation in a changing climate. Nat. Clim. Change 4, 434-441. ( 10.1038/nclimate2225) [DOI] [Google Scholar]
- 7.Bais AF, McKenzie RL, Bernhard G, Aucamp PJ, Ilyas M, Madronich S, Tourpali K. 2015. Ozone depletion and climate change: impacts on UV radiation. Photochem. Photobiol. Sci. 14, 19-52. ( 10.1039/c4pp90032d) [DOI] [PubMed] [Google Scholar]
- 8.Tevini M. 1993. Molecular biological effects of ultraviolet radiation. In UV-B radiation and ozone depletion: effects on humans, animals, plants, microorganisms, and materials (ed. Tevini M), pp. 1-16. Boca Raton, FL: Lewis Publishers. [Google Scholar]
- 9.Goodsell DS. 2001. The molecular perspective: ultraviolet light and pyrimidine dimers. Stem Cells 19, 348-349. [DOI] [PubMed] [Google Scholar]
- 10.Kusakabe M, et al. 2019. Mechanism and regulation of DNA damage recognition in nucleotide excision repair. Genes Environ. 41, 1-6. ( 10.1186/s41021-019-0119-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei Q, Dvornyk V. 2015. Evolutionary history of the photolyase/cryptochrome superfamily in eukaryotes. PLoS One 10, e0135940. ( 10.1371/journal.pone.0135940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies RJH. 1995. Ultraviolet radiation damage in DNA. Biochem. Soc. Trans. 23, 407-418. ( 10.1042/bst0230407) [DOI] [PubMed] [Google Scholar]
- 13.Pandelova I, Hewitt SR, Rollins-Smith LA, Hays JB. 2006. UVB dose-toxicity thresholds and steady-state DNA-photoproduct levels during chronic irradiation of inbred Xenopus laevis tadpoles. Photochem. Photobiol. 82, 1080. ( 10.1562/2004-05-14-ra-169) [DOI] [PubMed] [Google Scholar]
- 14.Schuch AP, Lipinski VM, Santos MB, Santos CP, Jardim SS, Cechin SZ, Loreto ELS. 2015. Molecular and sensory mechanisms to mitigate sunlight-induced DNA damage in treefrog tadpoles. J. Exp. Biol. 218, 3059-3067. ( 10.1242/jeb.126672) [DOI] [PubMed] [Google Scholar]
- 15.Peng S, Liao H, Zhou T, Peng S. 2017. Effects of UVB radiation on freshwater biota: a meta-analysis. Glob. Ecol. Biogeogr. 26, 500-510. ( 10.1111/GEB.12552) [DOI] [Google Scholar]
- 16.Hof C, Araújo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516-519. ( 10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
- 17.Houlahan JE, Findlay SC, Schmidt BR, Meyer AH, Kuzmin SL. 2000. Quantitative evidence for global amphibian population declines. Nature 404, 752-755. [DOI] [PubMed] [Google Scholar]
- 18.Alroy J. 2015. Current extinction rates of reptiles and amphibians. Proc. Natl Acad. Sci. USA 112, 13 003-13 008. ( 10.1073/pnas.1508681112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger L, et al. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl Acad. Sci. USA 95, 9031-9036. ( 10.1073/pnas.95.15.9031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young BE, et al. 2001. Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 15, 1213-1223. ( 10.1046/j.1523-1739.2001.00218.x) [DOI] [Google Scholar]
- 21.Gillespie GR, et al. 2020. Status and priority conservation actions for Australian frog species. Biol. Conserv. 247, 108543. ( 10.1016/j.biocon.2020.108543) [DOI] [Google Scholar]
- 22.Martel A, et al. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl Acad. Sci. USA 110, 15 325-15 329. ( 10.1073/pnas.1307356110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey C. 1993. Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conserv. Biol. 7, 355-362. ( 10.1046/j.1523-1739.1993.07020355.x) [DOI] [Google Scholar]
- 24.Broomhall SD, Osborne WS, Cunningham RB. 2000. Comparative effects of ambient ultraviolet-B radiation on two sympatric species of Australian frogs. Conserv. Biol. 14, 420-427. ( 10.1046/j.1523-1739.2000.98130.x) [DOI] [Google Scholar]
- 25.Kiesecker JM, Blaustein AR, Belden LK. 2001. Complex causes of amphibian declines. Nature 410, 681-684. [DOI] [PubMed] [Google Scholar]
- 26.Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. 2003. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 9, 123-140. ( 10.1046/j.1472-4642.2003.00015.x) [DOI] [Google Scholar]
- 27.Alton LA, Franklin CE. 2017. Drivers of amphibian declines: effects of ultraviolet radiation and interactions with other environmental factors. Clim. Change Responses 4, 1-26. ( 10.1186/s40665-017-0034-7) [DOI] [Google Scholar]
- 28.dos Santos CP, Londero JEL, dos Santos MB, Feltrin R dos S, Loebens L, Moura LB, Cechin SZ, Schuch AP. 2018. Sunlight-induced genotoxicity and damage in keratin structures decrease tadpole performance. J. Photochem. Photobiol. B 181, 134-142. ( 10.1016/J.JPHOTOBIOL.2018.03.013) [DOI] [PubMed] [Google Scholar]
- 29.Alton LA, Franklin CE. 2012. Do high temperatures enhance the negative effects of ultraviolet-B radiation in embryonic and larval amphibians? Biol. Open 1, 897-903. ( 10.1242/bio.2012950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bancroft BA, Baker NJ, Blaustein AR. 2008. A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv. Biol. 22, 987-996. ( 10.1111/j.1523-1739.2008.00966.x) [DOI] [PubMed] [Google Scholar]
- 31.Kiesecker JM, Blaustein AR.. 1995. Synergism between UV-B radiation and a pathogen magnifies amphibian embryo mortality in nature. Proc. Natl Acad. Sci. USA 92, 11 049-11 052. ( 10.1073/pnas.92.24.11049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Uitregt VO, Wilson RS, Franklin CE.. 2007. Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Glob. Change Biol. 13, 1114-1121. ( 10.1111/j.1365-2486.2007.01353.x) [DOI] [Google Scholar]
- 33.Lundsgaard NU, Cramp RL, Franklin CE, Martin L. 2020. Effects of ultraviolet-B radiation on physiology, immune function and survival is dependent on temperature: implications for amphibian declines. Conserv. Physiol. 8, coaa002. ( 10.1093/conphys/coaa002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamare MD, Barker MF, Lesser MP, Marshall C. 2006. DNA photorepair in echinoid embryos: effects of temperature on repair rate in Antarctic and non-Antarctic species. J. Exp. Biol. 209, 5017-5028. ( 10.1242/jeb.02598) [DOI] [PubMed] [Google Scholar]
- 35.Li S, Paulsson M, Björn LO. 2002. Temperature-dependent formation and photorepair of DNA damage induced by UV-B radiation in suspension-cultured tobacco cells. J. Photochem. Photobiol. B 66, 67-72. ( 10.1016/S1011-1344(01)00277-9) [DOI] [PubMed] [Google Scholar]
- 36.MacFadyen EJ, Williamson CE, Grad G, Lowery M, Jeffrey WH, Mitchell DL. 2004. Molecular response to climate change: temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria. Glob. Change Biol. 10, 408-416. ( 10.1111/j.1529-8817.2003.00750.x) [DOI] [Google Scholar]
- 37.Morison SA, Cramp RL, Alton LA, Franklin CE. 2020. Cooler temperatures slow the repair of DNA damage in tadpoles exposed to ultraviolet radiation: implications for amphibian declines at high altitude. Glob. Change Biol. 26, 1225-1234. ( 10.1111/gcb.14837) [DOI] [PubMed] [Google Scholar]
- 38.Pakker H, Martins RST, Boelen P, Buma AGJ, Nikaido O, Breeman AM. 2000. Effects of temperature on the photoreactivation of ultraviolet-B-induced DNA damage in Palmaria palmata (Rhodophyta). J. Phycol. 36, 334-341. ( 10.1046/j.1529-8817.2000.99087.x) [DOI] [Google Scholar]
- 39.Sanders RW, Macaluso AL, Sardina TJ, Mitchell DL. 2005. Photoreactivation in two freshwater ciliates: differential responses to variations in UV-B flux and temperature. Aquat. Microb. Ecol. 40, 283-292. ( 10.3354/ame040283) [DOI] [Google Scholar]
- 40.Blaustein AR, Wake DB. 1990. Declining amphibian populations: a global phenomenom? TREE 5, 4-5. [Google Scholar]
- 41.Biodiversity Group. 1999. Declines and disappearences of Australian frogs. Canberra, ACT, Australia: Environment Australia, Department of the Environment and Heritage. [Google Scholar]
- 42.Blaustein AR, Wake DB. 1995. The puzzle of declining amphibian populations. Sci. Am. 272, 56-61. ( 10.1038/scientificamerican0495-52) [DOI] [Google Scholar]
- 43.McDonald KR. 1990. Rheobatrachus Liem and Taudactylus Straughan & Lee (Anura: Leptodactylidae) in Eungella National Park Queensland: distribution and decline. Trans. R. Soc. S. Aust. 114, 187-194. [Google Scholar]
- 44.Bradford DF. 1991. Mass mortality and extinction in a high-elevation population of Rana muscosa. J. Herpetol. 25, 174-177. [Google Scholar]
- 45.Richards SJ, McDonald KR, Alford RA. 1993. Declines in populations of Australia's endemic tropical rainforest frogs. Pac. Conserv. Biol. 1, 66-77. [Google Scholar]
- 46.Young BE, et al. 2008. Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 15, 1213-1223. ( 10.1111/j.1523-1739.2001.00218.x) [DOI] [Google Scholar]
- 47.Kern P, Cramp RL, Franklin CE. 2014. Temperature and UV-B-insensitive performance in tadpoles of the ornate burrowing frog: an ephemeral pond specialist. J. Exp. Biol. 217, 1246-1252. ( 10.1242/jeb.097006) [DOI] [PubMed] [Google Scholar]
- 48.Gosner K. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183-190. [Google Scholar]
- 49.Mori T, Nakane M, Hattori T, Matsunaga T, Ihara M, Nikaido O. 1991. Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4)photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem. Photobiol. 54, 225-232. ( 10.1111/j.1751-1097.1991.tb02010.x) [DOI] [PubMed] [Google Scholar]
- 50.R Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 51.Hird C, Cramp R, Franklin C. 2022. Data from: Temperature causes species-specific responses to UV-induced DNA damage in amphibian larvae. UQ eSpace, The University of Queensland, Australia ( 10.48610/4fdfc26) [DOI] [PMC free article] [PubMed]
- 52.Pinheiro J, Bates D, DebRoy S, Heisterkamp S, Van Willigen B, Ranke J. 2021. Package ‘nlme’. Linear and nonlinear mixed effects models. See https://cran.r-project.org/web/packages/nlme/nlme.pdf.
- 53.Fox J, Weisberg S.. 2018. An R companion to applied regression, 3rd edn. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- 54.Promislow DEL. 1994. DNA repair and the evolution of longevity: a critical analysis. J. Theor. Biol. 170, 291-300. ( 10.1006/JTBI.1994.1190) [DOI] [PubMed] [Google Scholar]
- 55.Won EJ, Han J, Lee Y, Kumar KS, Shin KH, Lee SJ, Park HG, Lee JS.. 2015. In vivo effects of UV radiation on multiple endpoints and expression profiles of DNA repair and heat shock protein (Hsp) genes in the cycloid copepod Paracyclopina nana. Aquat. Toxicol. 165, 1-8. ( 10.1016/J.AQUATOX.2015.05.002) [DOI] [PubMed] [Google Scholar]
- 56.Cortopassi GA, Wang E. 1996. There is substantial agreement among interspecies estimates of DNA repair activity. Mech. Ageing Dev. 91, 211-218. ( 10.1016/S0047-6374(96)01788-5) [DOI] [PubMed] [Google Scholar]
- 57.Augustyniak M, Orzechowska H, Kedziorski A, Sawczyn T, Dolezych B. 2014. DNA damage in grasshoppers' larvae—comet assay in environmental approach. Chemosphere 96, 180-187. ( 10.1016/J.CHEMOSPHERE.2013.10.033) [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni R, Thomas RA, Tucker JD. 2011. Expression of DNA repair and apoptosis genes in mitochondrial mutant and normal cells following exposure to ionizing radiation. Environ. Mol. Mutagen 52, 229-237. ( 10.1002/EM.20605) [DOI] [PubMed] [Google Scholar]
- 59.Angilletta MJ, Bennett AF, Guderley H, Navas CA, Seebacher F, Wilson RS.. 2006. Coadaptation: a unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 79, 282-294. ( 10.1086/499990) [DOI] [PubMed] [Google Scholar]
- 60.Wilson RS, Hammill E, Johnston IA.. 2007. Competition moderates the benefits of thermal acclimation to reproductive performance in male eastern mosquitofish. Proc. R. Soc. B 274, 1199-1204. ( 10.1098/rspb.2006.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seebacher F. 2009. Responses to temperature variation: integration of thermoregulation and metabolism in vertebrates. J. Exp. Biol. 212, 2885-2891. ( 10.1242/jeb.024430) [DOI] [PubMed] [Google Scholar]
- 62.Sancar A. 2008. Structure and function of photolyase and in vivo enzymology: 50th anniversary. J. Biol. Chem. 283, 32153. ( 10.1074/JBC.R800052200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundsgaard NU, Cramp RL, Franklin CE. 2021. Ultraviolet-B irradiance and cumulative dose combine to determine performance and survival. J. Photochem. Photobiol. B 222, 112276. ( 10.1016/j.jphotobiol.2021.112276) [DOI] [PubMed] [Google Scholar]
- 64.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. ( 10.1093/acprof:oso/9780198570875.001.1) [DOI] [Google Scholar]
- 65.Franco-Belussi L, Sköld HN, De Oliveira C.. 2016. Internal pigment cells respond to external UV radiation in frogs. J. Exp. Biol. 219, 1378-1383. ( 10.1242/jeb.134973) [DOI] [PubMed] [Google Scholar]
- 66.Agar N, Young AR. 2005. Melanogenesis: a photoprotective response to DNA damage? Mutat. Res. 571, 121-132. ( 10.1016/j.mrfmmm.2004.11.016) [DOI] [PubMed] [Google Scholar]
- 67.Nilsson Sköld H, Aspengren S, Wallin M. 2013. Rapid color change in fish and amphibians—function, regulation, and emerging applications. Pigment Cell Melanoma Res. 26, 29-38. ( 10.1111/pcmr.12040) [DOI] [PubMed] [Google Scholar]
- 68.Rodríguez-Rodríguez EJ, Beltrán JF, Márquez R. 2020. Melanophore metachrosis response in amphibian tadpoles: effect of background colour, light and temperature. Amphib.-Reptil. 42, 133-140. ( 10.1163/15685381-BJA10032) [DOI] [Google Scholar]
- 69.Moriya T, Miyashita Y. 1989. Melanophore dispersion in northern amphibian larva induced by exposure to cold. J. Exp. Zool. 250, 349-353. ( 10.1002/JEZ.1402500317) [DOI] [Google Scholar]
- 70.Friedberg EC. 2003. DNA damage and repair. Nature 421, 436-440. ( 10.1038/nature01408) [DOI] [PubMed] [Google Scholar]
- 71.Hird C, Franklin CE, Cramp RL. 2022. Data from: Temperature causes species-specific responses to UV-induced DNA damage in amphibian larvae. Figshare. ( 10.6084/m9.figshare.c.6238176) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hird C, Cramp R, Franklin C. 2022. Data from: Temperature causes species-specific responses to UV-induced DNA damage in amphibian larvae. UQ eSpace, The University of Queensland, Australia ( 10.48610/4fdfc26) [DOI] [PMC free article] [PubMed]
- Hird C, Franklin CE, Cramp RL. 2022. Data from: Temperature causes species-specific responses to UV-induced DNA damage in amphibian larvae. Figshare. ( 10.6084/m9.figshare.c.6238176) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The complete dataset, a description of the dataset and R scripts used for analysing the data are publicly available at UQ eSpace https://doi.org/10.48610/4fdfc26 [51].
The data are provided in electronic supplementary material [71].