Graphical abstract
Natural products are one of the major sources of small-molecule pharmaceutical drugs [1,2]. However, discovery of novel natural products to expand the pool of potential drug candidates is becoming more and more challenging. Most of the traditional molecule discovery methods are based on direct separation of natural product from its natural hosts or homology search of biosynthetic pathways. However, there are currently two main obstacles in discovery of natural products from their native hosts: 1) the difficulty in cultivation of the native hosts under laboratory conditions, 2) no or not enough production of target natural products due to the silence of the biosynthetic pathway genes in the native hosts. Therefore, novel platforms are required for efficient discovery of new natural products.
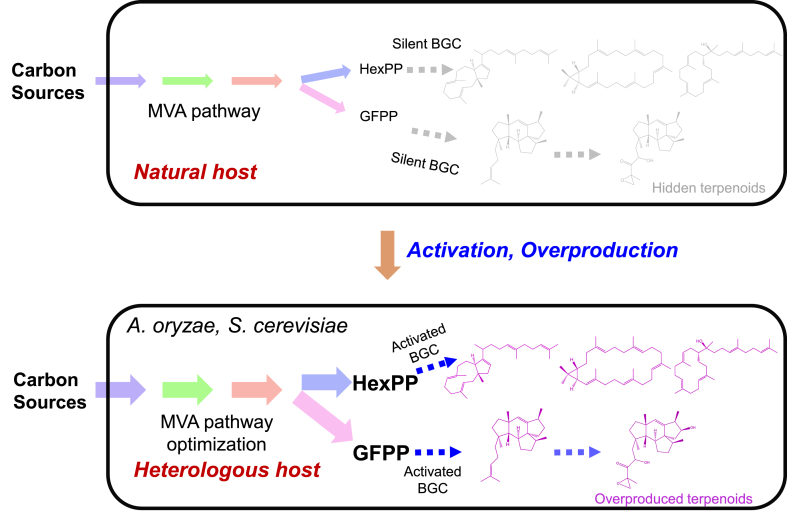
Terpenoids are the most diverse natural products with more the one hundred thousand structures been identified [3]. There are still a large number of “hidden” terpenoids yet to be discovered due to the silence of terpenoid biosynthetic genes [4]. In recent years, several strategies for mining of natural product biosynthesis genes have been developed. The traditional “chemical first” strategies excavate new compounds through mining large databases of similar biosynthetic gene clusters (BGCs) based on known enzymatic reactions. For example, Zhang used the known biosynthetic origins of meroterpenoid family to discover new and potential bioactive meroterpenoids and their biosynthetic pathways from fungi [5]. Alternatively, mining BGCs based on genomes should be helpful for expanding the coverage of compound discovery [4]. To address the challenge in mining of silencing genes, Tiangang Liu and his colleagues built robust microbial chassis for efficient activation and overproduction of several “silent” terpenoids, which demonstrated how synthetic biology can facilitate the discovery of natural products (Fig. 1). In their studies, the filamentous fungus Aspergillus oryzae and budding yeast Saccharomyces cerevisiae were harnessed for efficient mining of terpenoid synthetic genes and discovery of new triterpene biosynthetic pathways [6,7].
Fig. 1.
Engineering heterologous chassis for terpenoid discovery and overproduction. The silence of biosynthetic gene clusters and insufficient supply of precursors (such as HexPP and GFPP) usually result in non-production of terpenoids in natural host. Engineering heterologous hosts, by enhancing the precursor supply and activating the biosynthetic genes, will enable the discovery and overproduction of hidden terpenoids. MVA, mevalonate; BGC, biosynthetic gene cluster; GFPP, geranylfarnesyl diphosphate; HexPP, hexaprenyl diphosphate.
Filamentous fungus A. oryzae has the advantage of natural terpenoid synthesis and subsequent structural modification and thus can be used as an ideal host for expression of fungal BGCs. The research team developed an automated and high-throughput (Auto-HTP) biofoundry workflow for efficient genome mining in engineered A. oryzae. The Auto-HTP includes several modules: bioinformatic analysis of fungal terpenoid BGCs, plasmid library construction, rational refactoring BGCs in A. oryzae chassis, high-throughput fermentation and product extraction, as well as structural and bioactivity characterization. Auto-HTP enabled simultaneously constructing 208 engineered strains containing 39 transformed BGCs and detecting 185 distinct terpenoids [6]. Further optimizing the mevalonate (MVA) pathway significantly improved the production of mangicdiene (87 mg/L) and mangicol J (12 mg/L) by 133-fold and 112-fold, respectively, which showed A. oryzae as an ideal chassis for terpenoid production.
The budding yeast S. cerevisiae is widely used for terpenoid production due to its convenient genetic engineering and clear genetic background [8,9]. The research team previously constructed a MVA pathway-optimized S. cerevisiae YZ141 [10], which enabled sufficient supply of the precursor farnesyl diphosphate (FPP). Based on this strain, the authors expressed a variety of terpenoid synthases after excavating genomic information from a public database and identified two novel bifunctional chimeric terpene synthases, TvTS and MpMS, which could synthesize triterpenoids by using hexaprenyl diphosphate (HexPP) as a precursor, (unlike the traditional triterpenoid biosynthesis pathway, which uses squalene or epoxy squalene as precursors) [7]. Biochemical and structural analysis revealed the unique catalytic mechanism in cyclization of HexPP.
The silence of terpenoid biosynthesis in natural hosts might suffer from limited supply of precursors and/or the silence of BGCs, and engineering native hosts is always hampered by lack of genetic tools. Therefore, engineering eukaryotic model microbial chassis is an alternative strategy to expand the terpenoid kingdom and could further improve the biosynthetic efficiency of terpenoids for industrial application [11]. The strategies described in these studies could be helpful for activating biosynthesis of more terpenoids and also other natural products.
CRediT authorship contribution statement
Xiaoxin Zhai: wrote the manuscript and prepared the graph. Lun Yao: wrote the manuscript. Yongjin J. Zhou: manuscript revision, Project administration, and funding acquisition.
Declaration of competing interest
The authors declare no conficts of interest, and all the authors approved the submission.
Acknowledgements
This work was supported by Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Park D., Swayambhu G., Lyga T., Pfeifer B.A. Complex natural product production methods and options. Synth Syst Biotechnol. 2021;6(1):1–11. doi: 10.1016/j.synbio.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R., Yang S., Zhang L., Zhou Y.J. Advanced strategies for production of natural products in yeast. iScience. 2020;23(3) doi: 10.1016/j.isci.2020.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenshole E., Herisse M., Michael M., Pidot S.J. Natural product discovery through microbial genome mining. Curr Opin Chem Biol. 2021;60:47–54. doi: 10.1016/j.cbpa.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Bauman K.D., Butler K.S., Moore B.S., Chekan J.R. Genome mining methods to discover bioactive natural products. Nat Prod Rep. 2021;38(11):2100–2129. doi: 10.1039/d1np00032b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Wang T.T., Xu Q.L., Xiong Y., Zhang L., Han H., et al. Genome mining and comparative biosynthesis of meroterpenoids from two phylogenetically distinct fungi. Angew Chem Int Ed Engl. 2018;57(27):8184–8188. doi: 10.1002/anie.201804317. [DOI] [PubMed] [Google Scholar]
- 6.Yuan Y., Cheng S., Bian G., Yan P., Ma Z., Dai W., et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nat Catal. 2022;5(4):277–287. [Google Scholar]
- 7.Tao H., Lauterbach L., Bian G., Chen R., Hou A., Mori T., et al. Discovery of non-squalene triterpenes. Nature. 2022;606(7913):414–419. doi: 10.1038/s41586-022-04773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao X., Yang S., Cao C., Zhou Y.J. Harnessing sub-organelle metabolism for biosynthesis of isoprenoids in yeast. Synth Syst Biotechnol. 2020;5(3):179–186. doi: 10.1016/j.synbio.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma R., Su P., Ma Q., Guo J., Chen S., Jin B., et al. Identification of (-)-bornyl diphosphate synthase from Blumea balsamifera and its application for (-)-borneol biosynthesis in Saccharomyces cerevisiae. Synth Syst Biotechnol. 2022;7(1):490–497. doi: 10.1016/j.synbio.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R., Jia Q., Mu X., Hu B., Sun X., Deng Z., et al. Systematic mining of fungal chimeric terpene synthases using an efficient precursor-providing yeast chassis. Proc Natl Acad Sci U S A. 2021;118(29) doi: 10.1073/pnas.2023247118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y.J. Expanding the terpenoid kingdom. Nat Chem Biol. 2018;14(12):1069–1070. doi: 10.1038/s41589-018-0167-4. [DOI] [PubMed] [Google Scholar]