Abstract
Objectives:
The association between inadequate sleep and type 2 diabetes has garnered much attention, but little is known about sleep and type 1 diabetes (T1D). Our objectives were to conduct a systematic review and meta-analysis comparing sleep in persons with and without T1D, and to explore relationships between sleep and glycemic control in T1D.
Methods:
Studies were identified from Medline and Scopus. Studies reporting measures of sleep in T1D patients and controls, and/or associations between sleep and glycemic control, were selected.
Results:
A total of 22 studies were eligible for the meta-analysis. Children with T1D had shorter sleep duration (mean difference [MD] = −26.4 minutes; 95% confidence interval [CI] = −35.4, −17.7) than controls. Adults with T1D reported poorer sleep quality (MD in standardized sleep quality score = 0.51; 95% CI = 0.33, 0.70), with higher scores reflecting worse sleep quality) than controls, but there was no difference in self-reported sleep duration. Adults with TID who reported sleeping >6 hours had lower hemoglobin A1c (HbA1c) levels than those sleeping ≤6 hours (MD = −0.24%; 95% CI = −0.47, −0.02), and participants reporting good sleep quality had lower HbA1c than those with poor sleep quality (MD = −0.19%; 95% CI = −0.30, −0.08). The estimated prevalence of obstructive sleep apnea (OSA) in adults with TID was 51.9% (95% CI = 31.2, 72.6). Patients with moderate-to-severe OSA had a trend toward higher HbA1c (MD = 0.39%, 95% CI = −0.08, 0.87).
Conclusion:
T1D was associated with poorer sleep and high prevalence of OSA. Poor sleep quality, shorter sleep duration, and OSA were associated with suboptimal glycemic control in T1D patients.
Keywords: Sleep quality, Sleep duration, Obstructive sleep apnea, Type 1 diabetes, Glycemic control, Meta-analysis
1. Introduction
Insufficient sleep duration and poor sleep quality are associated with insulin resistance, impaired glucose metabolism, and type 2 diabetes (T2D) in both experimental and epidemiological studies [1,2]. Obstructive sleep apnea (OSA) is also common in patients with T2D [2], and a greater severity of OSA is associated with greater insulin resistance [2]. Furthermore, insufficient sleep, poor sleep quality, and OSA have been associated with poorer glycemic control among people with T2D [1].
Type 1 diabetes (T1D), although less prevalent than T2D, has been estimated to affect three million people in the United States [3]. The incidence varies significantly among countries worldwide, with the lowest among East Asians and American Indians and the highest among Finnish people [3]. Poor glycemic control in T1D patients can lead to microvascular complications (ie, nephropathy, retinopathy, and neuropathy), cardiovascular disease, and mortality [4–6]. Despite the abundant evidence linking sleep deficiencies and T2D, little attention has been paid to patients with type 1 diabetes (T1D). In contrast to T2D, T1D is an autoimmune disorder that results in destruction of pancreatic β cells and insulin deficiency, necessitating exogenous insulin administration to regulate blood sugars. Nonetheless, research on T2D may be relevant, as sleep deficiencies have been found to be associated with insulin resistance and, if present in T1D, may result in poorer metabolic control. We hypothesized that sleep deficiencies would also be associated with T1D and suboptimal glycemic control. Therefore, the purpose of this study was to conduct a systematic review to identify studies in order to perform meta-analyses comparing sleep characteristics, including sleep stages, sleep duration, sleep quality, and OSA, between persons with T1D and healthy controls. In addition, the relationship between these sleep characteristics and glycemic control in T1D patients was examined using meta-analyses.
2. Methods
2.1. Data sources and searches
We searched studies published in English from Medline and Scopus since their inception until May 2015. The search terms and search strategy were “sleep OR insomnia OR apnea” AND “type 1 diabetes OR autoimmune diabetes OR insulin dependent diabetes”. Reference lists of included studies were examined to identify additional relevant studies.
2.2. Study selection
Studies published in English were eligible if they met one or both of the following criteria: compared sleep characteristics (ie, sleep stages, duration, quality, or OSA) in patients with T1D and nondiabetes (herein referred to as controls); or assessed the relationship between sleep characteristics and glycemic control, as evaluated by hemoglobin A1c (HbA1c), in patients with T1D. HbA1c is an indicator of glucose control in the preceding 90 days and regarded as a gold standard of glycemic measurement. We excluded studies in pregnant women and studies that induced hypoglycemia. Study selection was performed by two reviewers (S.R. and T.A.). Disagreements were resolved by a consultation with senior authors (A.T. and K.L.K.).
Because of the relatively small numbers of studies in some sleep categories, authors were contacted for additional data. Studies measuring sleep quality via questionnaires had to provide a score in the same direction to be included in the meta-analyses (ie, studies with higher score reflecting worse sleep were grouped together).
2.3. Sleep characteristics
Sleep stages, expressed as percentage of total sleep time, were obtained using polysomnography (PSG) in most studies, with the exception of one study [7] that used a wireless sleep monitor that recorded electroencephalograms (Zeo Inc, Newton MA). Stages 1 and 2 were combined into “light non-rapid eye movement (NREM) sleep,” and stages 3 and 4 (if used) into “deep NREM sleep.” Sleep duration was obtained either by objective measurements (ie, polysomnography [PSG], actigraphy, wireless sleep monitor use) or self-report. Sleep duration was examined as a continuous variable as well as categorized as shorter (≤6 hours in adults, <9 hours in children aged 6–13 years, or <8 hours in children aged >13–17 years) or longer (>6 hours in adults, ≥9 hours in children aged 6−13 years, or ≥8 hours in children aged >13–17 years) [8]. Objective and subjective assessments of sleep quality were included. Objective measurements were based on sleep efficiency (percentage of time in bed spent sleeping) obtained from PSG or actigraphy. Good sleep quality was defined as sleep efficiency of ≥85%. Self-reported sleep quality was assessed by standardized questionnaires, such as Pittsburgh Sleep Quality Index (PSQI) [9], Patient Health Questionnaire (PHQ-9) [10], the Autonomic System Profile (APS) [11], or insomnia symptoms [12,13]. Self-reported sleep quality was categorized as good or poor according to the cutoff of the original questionnaire (eg, PSQI score >5, sleeping difficulties per PHQ-9, or insomnia symptoms). In addition, a total score was used to compare sleep quality between groups of participants in the studies using PSQI or APS as described in the data analysis below (higher scores on these questionnaires reflected poorer sleep quality).
The presence of OSA in adults was defined as an apnea–hypopnea index (AHI) of ≥5 events per hour from PSG or pulse oximetry with airflow measurement that provided AHI values [14], or as having a pathological oximetry (defined as repetitive desaturation–reoxygenation sequences) result. Severity of OSA in adults was categorized as mild for AHI ≥5 to <15, and moderate to severe for AHI ≥15. In children and adolescents, OSA was defined as AHI ≥1.5[15]. Studies evaluating OSA risk using a screening questionnaire (low vs high risk of OSA) [16–18] were also included.
2.4. Glycemic measurements
In addition to using actual HbA1c values, glycemic control was categorized as optimal (HbA1c <7% in adults, or <7.5% in children) or suboptimal (HbA1c ≥7% in adults, or ≥7.5% in children) [19].
2.5. Data extraction
Data were extracted following a standardized data extraction form (see Supplemental material). Characteristics of the studies that were extracted included the age group (children/adolescents, adults), mean body mass index (BMI), HbA1c, method of sleep measurements, sleep characteristics, and glycemic control. The data pooled for analyses included the number of participants, mean and standard deviation (SD) for continuous data, and frequency for dichotomous data. Most authors (88%) of selected articles for which additional data were not available in publications responded to the communication [16,17,20–33], and 75% of these authors were able to provide additional data and were therefore included in the analyses [16,17,20–29].
2.6. Quality assessment
Quality assessment was performed using the Newcastle–Ottawa Scale [34]. For case–control studies, three domains were considered: selection of study groups (four items), comparability of groups (one item), and ascertainment of exposure (three items). The cohort assessment forms were modified to be applicable for cross-sectional studies. These consisted of three domains: selection (two items), comparability (one item), and outcome (one item). Each item was given one star or no star for all domains except comparability, for which two stars could be awarded.
2.7. Data synthesis and analysis
The meta-analyses were performed if there were three or more studies with sufficient data for pooling in each planned analysis. If the number of studies was less than three, they were included in description in Table A1 and the relevant discussion.
For eligible studies, data were pooled separately by the two analyses of interest: (1) sleep differences between T1D patients and controls; and (2) the relationship between sleep and glycemic control in T1D patients. Analyses were stratified by age (adolescents/children vs adults). When the age range in a study overlapped between adolescents and adults, we categorized the study according to the mean age of the participants. In addition, objective and subjective assessments of sleep were analyzed separately.
To compare sleep in T1D patients and controls, mean differences (MDs) of the sleep measures, including sleep duration and sleep quality (sleep efficiency and sleep questionnaire score), between T1D patients and controls were estimated across studies. Nonstandardized mean differences were applied for pooling these MDs for objective sleep measures, whereas standardized mean differences were applied for pooling MDs of the sleep questionnaire score. If heterogeneity was not present, the fixed-effect model was applied; otherwise, the random-effect model was applied.
To analyze the relationship between sleep and glycemic control in T1D patients, MDs and variances of the sleep measures were estimated across studies between optimal and suboptimal glycemic control groups, or the MDs of the HbA1c values were estimated between sleep groups (ie, good vs poor sleep quality, shorter vs longer sleep duration, OSA vs non-OSA, and moderate to severe OSA vs non-OSA). These were then pooled using nonstandardized MDs as described previously.
Finally, OSA prevalence was estimated from studies of glycemic control in T1D patients. A meta-analysis was then applied to pool the OSA prevalence across studies using a random-effect model.
Heterogeneity was explored using the Q statistic, and a degree of heterogeneity was quantified using the I2 statistic. Heterogeneity was considered to be present if the p value from the Q statistic was <0.1 or the I2 was ≥25%. Publication bias was assessed using funnel plots and Egger tests. All analyses were performed using STATA version 13.1 software. A p value of <0.05 was considered to be statistically significant.
3. Results
A total of 741 studies were identified from searching Medline and Scopus, and one study was identified from the reference lists (Fig. 1). In all, 32 studies met the inclusion criteria and were eligible for review. Of these, 22 were eligible for meta-analysis. The remaining ten studies are described in Table A1 because there were fewer than three studies in each pooling category. In addition, some sleep measures included in the 22 studies were not eligible for meta-analysis for the same reason and are therefore described in Table A1.
Fig. 1.
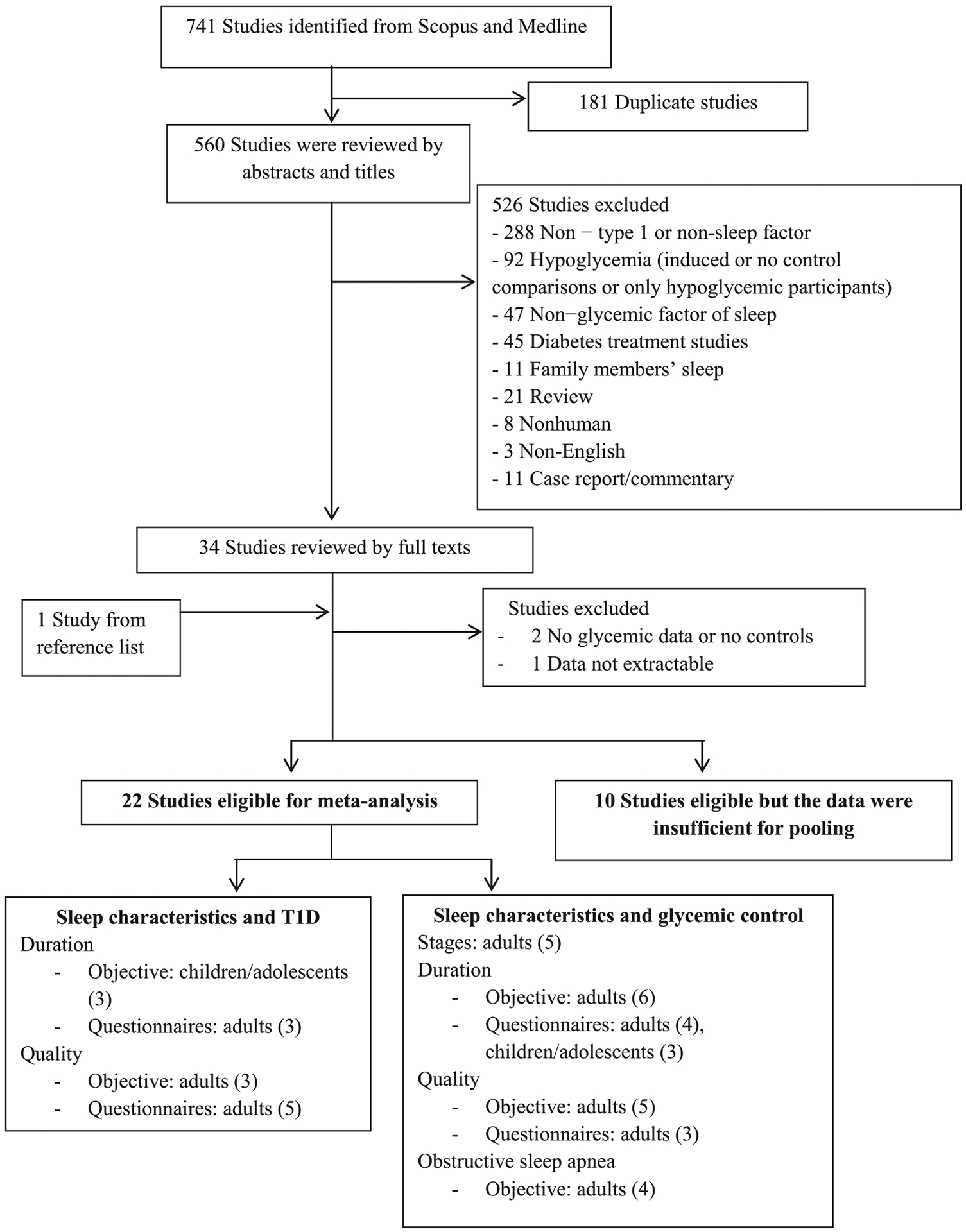
Flow chart of study selection. Poolings were performed when there were three or more studies in the same category.
Participants’ characteristics, including those of matched controls (if available), and methods of sleep measurements are listed in Table 1. Of the studies, ten were case–control, 11 were cross-sectional, and one was a prospective cohort study.
Table 1.
Characteristics of the studies and their sleep variables included in the meta-analyses.
| Study | Setting | T1D participants | Control participants | Study design | Sleep measurement | Sleep characteristics in meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age(y) | BMI(kg/m2) | HbA1c(%) | N | Age(y) | BMI(kg/m2) | |||||
| Studies comparing sleep in type 1 diabetes vs control participants | |||||||||||
| Barone et al. [35] | Brazil | 18 | 26.3 | 23.0 | 7.8 | 9 | 28.8 | 22.0 | Matched case–control (age, BMI) | Sleep diary, actimeter, PSGc | Duration, quality |
| Janovsky et al. [20] | Brazil | 20 | 28.6 | 22.9 | 7.2 | 22 | 23.2 | 21.8 | Matched case–control (age, BMI) | PSGa | Stages, duration, quality, OSA |
| Jauch-Chara et al. [21] | Germany | 14 | 31.3 | 24.2 | 7.7 | 14 | 28.9 | 23.1 | Matched case–control (age, BMI, sex) | PSGb | Stages, duration, quality |
| Mandl et al. [43] | Sweden | 31 | 52.0 | 23.9 | NA | 200 | 45.0 | 24.4 | Unmatched case-control | Questionnaire (8 sleep questions from Autonomic Symptom Profile) | Quality |
| Matyka et al. [40] | UK | 15 | 9.4 | 8.8 | 15 | 9.2 | Matched case–control (age, sex) | PSGa | Duration | ||
| Olsson et al. [13] | Norway | 138 | 54.3 | 29.2 | NA | 51050 | 43.0 | 24.8 | Prospective study, mean follow up 15.3 y | Questionnaire of insomnia symptoms | Quality |
| Palladino et al. [44] | USA | 117 | 18.5 | 25.7 | 8.9 | 122 | 18.0 | Case–control | Questionnaire (first five questions of PSQI) | Quality | |
| Perfect et al. [23] | USA | 50 | 13.4 | 67.6 percentile | 9.1 | 40 | 13.5 | 65.8 percentile | Matched case–control (age, BMI, sex) | PSGc (40 matched pairs) Actigraphyc (diabetes cohort) |
Duration |
| Pillar et al. [41] | Israel | 15 | 12.6 | 18.5 | 8.5 | 15 | 13.3 | 19.3 | Matched case–control (age, BMI) | PSGc | Stages, duration, quality |
| Sivertsen et al. [12] | Norway | 40 | 19.9 | 233.2 | NA | 9843 | 19.9 | 22.2 | Unmatched case–control | Questionnaire (sleep duration, insomnia symptoms, snoring) | Duration, quality |
| van Dijk et al. [17] | The Netherlands | 99 | 43.9 | 24.5 | 7.8 | 99 | 44.1 | 24.5 | Matched case–control (age, BMI, sex) | Questionnaires (PSQI and OSA risk) | Duration, quality |
| Studies exploring the relationship between sleep and glycemic control in type 1 diabetes patients | |||||||||||
| Bachle et al. [45] | Germany | 202 | 19.4 | 23.7 | 8.3 | Cross-sectional | Questionnaire (Patient Health Questionnaire, PHQ-9, sleeping difficulties) | Quality | |||
| Borel et al. [24] | France | 37 | 43.0 | 24.9 | 7.8 | Cross-sectional | Oximetrya (N = 37) PSGa (N = 18) |
Stages, duration, quality, OSA | |||
| Borel et al. [16] | France | 79 | 39.5 | 24.5 | 7.9 | Cross-sectional | Actigraphya Questionnaire evaluating OSA risk | Duration, quality, OSA | |||
| Bot et al. [10] | The Netherlands | 277 | 43.9 | 25.4 | 7.8 | Cross-sectional | Questionnaire (Patient Health Questionnaire, PHQ-9, sleeping difficulties) | Quality | |||
| Bouhassira et al. [25] | France | 297 | 48.3 | 25.4 | 7.9 | Cross-sectional | Questionnaire (Medical Outcome Sleep Scale assessing sleep quantity and disturbances) | Duration | |||
| Estrada et al. [26], ages 6–13 | USA | 36 | 9.8 | BMI z-score −1.11 | 8.3 | Cross-sectional | Questionnaire | Duration | |||
| Estrada et al. [26], ages >13–17 | USA | 50 | 15.1 | BMI z-score −0.40 | 9.5 | Cross-sectional | Questionnaire | Duration | |||
| Estrada et al. [26], adults | USA | 20 | 25.9 | 29.7 | 8.6 | Cross-sectional | Questionnaire | Duration | |||
| Feupe et al. [7] | USA | 17 | 19–26 | NA | 7.3 | Cross-sectional | Wireless sleep monitorsc | Stages, duration | |||
| Manin et al. [29] | France | 67 | 54.0 | 25.8 | 7.6 | Cross-sectional | PSGa (N = 54) PSGa (N = 13) |
Stages, duration, quality, OSA | |||
| Matejko et al. [27] | Poland | 148 | 26.3 | 23.3 | 7.2 | Cross-sectional | Questionnaire (Self-reported sleep duration) | Duration | |||
| Perfect [22], ages 10–13 | USA | 24 | 11.5 | BMI z-score 0.36 | 8.2 | Cross-sectional | Questionnaire (Self-reported sleep duration) | Duration | |||
| Perfect [22], ages >13–17 | USA | 26 | 15.2 | BMI z-score 0.88 | 9.7 | Cross-sectional | Questionnaire (Self-reported sleep duration) | Duration | |||
| Schober et al. [28] | Germany | 62 | 41.7 | 25.5 | 8.1 | Cross-sectional | Apnea linka (pulse oximetry and air flow measurement) | OSA | |||
Abbreviations: BMI, body mass index; OSA, obstructive sleep apnea; PSG, polysomnography; T1D, type 1 diabetes.
Recordings performed without glucose measurements.
Recordings performed under nonhypoglycemic condition.
Recordings performed with glucose measurements. Some participants had hypoglycemia.
The quality of the studies included in the meta-analysis was assessed. For case–controls and prospective studies, nine of 11 studies provided clear definitions of cases and controls, and seven had good representativeness of case and controls. All had good comparability between case and controls for their matched study designs, and seven of 11 studies had good ascertainment of exposure. All cross-sectional studies had good representativeness of subjects and good ascertainment of outcomes. However, only half had good ascertainment of exposure, and four of 11 had good comparability.
3.1. Sleep in T1D patients and controls
Results of the meta-analyses comparing sleep measures between T1D patients and controls are shown in Table 2.
Table 2.
Meta-analyses of mean difference (MD) of sleep characteristics between type 1 diabetes (T1D) patients and control participants.
| Sleep characteristics | Sleep measurements | Population | No. of studies | T1D (n) | Controls (n) | Resultsa |
|---|---|---|---|---|---|---|
| Sleep duration (min) | Questionnaire | Adults | 3 | 157 | 9,951 | No differences in sleep duration (MD = −0.73 min, 95% CI = −14.35, 12.89)a |
| PSG | Adolescents/children | 3 | 70 | 70 | T1D patients had shorter sleep duration by −26.55 min (95% CI = −35.39, −17.70). | |
| Sleep efficiency (%)b | PSG | Adults | 3 | 52 | 45 | No differences in sleep efficiency, MD = 0.70% (95% CI = −1.28, 2.68) |
| Sleep quality | Questionnaire (questionnaire score)c | Adults | 3 | 416 | 669 | T1D patients had poorer sleep quality (standardized MD = 0.51, 95% CI = 0.33, 0.70) |
| Questionnaire (dichotomized good vs poor sleep quality) | Adults | 3 | 277 | 61,269 | No differences in self-reported good sleep quality between T1Dand controls (OR = 0.79, 95% CI = 0.41, 1.52) |
Abbreviations: CI, confidence interval; OR, odds ratio; PSG, polysomnography.
Calculated by sleep variables of T1D patients minus those of control participants unless otherwise noted.
Higher number reflecting better sleep quality.
Higher number reflecting poorer sleep quality.
3.1.1. Sleep duration
Only self-reported sleep duration was available for meta-analyses in adult samples, and there was no difference in self-reported sleep duration between T1D patients and controls [12,17,35] (n = 157 patients and 9951 controls; Fig. 2A). In adolescents/children [23,40,41], sleep duration from PSG was significantly shorter in T1D patients (n = 70) than in controls (n = 70) (MD = −26.6 minutes, 95% CI = −35.4, −17.7; Fig. 2B).
Fig. 2.
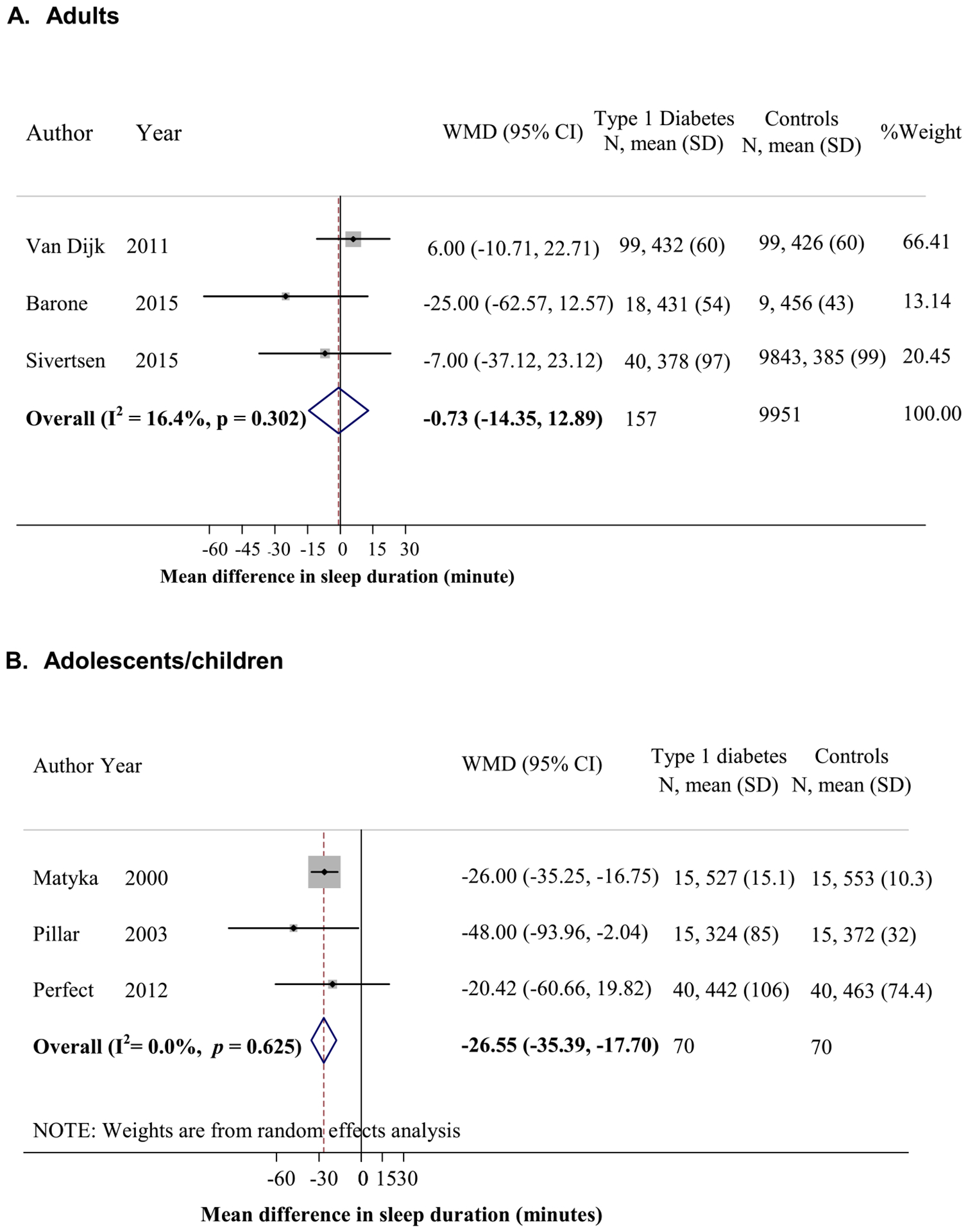
Mean difference in sleep duration between patients with type 1 diabetes (T1D) and controls (calculated by sleep duration in minutes of T1D patients minus that of controls). (A) Adults by questionnaire. (B) Adolescents/children by polysomnography.
3.1.2. Sleep quality
In adults, sleep quality based on sleep efficiency from PSG did not differ between T1D patients and controls [20,21,35] (n = 52 patients and 45 controls; Fig. 3A); however, when sleep was assessed using questionnaires, sleep quality (continuous score) was significantly worse in T1D patients compared to controls [17,43,44] (MD in standardized sleep quality score was 0.51, 95% CI 0.33, 0.70; n = 416 patients and 669 controls; Fig. 3B). However, self-reported good sleep quality did not differ significantly in T1D patients (odds ratio [OR]0.79, 95% CI 0.41, 1.52) compared to control participants [12,13,17] (n = 277 patients, 61,269 controls; Fig. 3C).
Fig. 3.
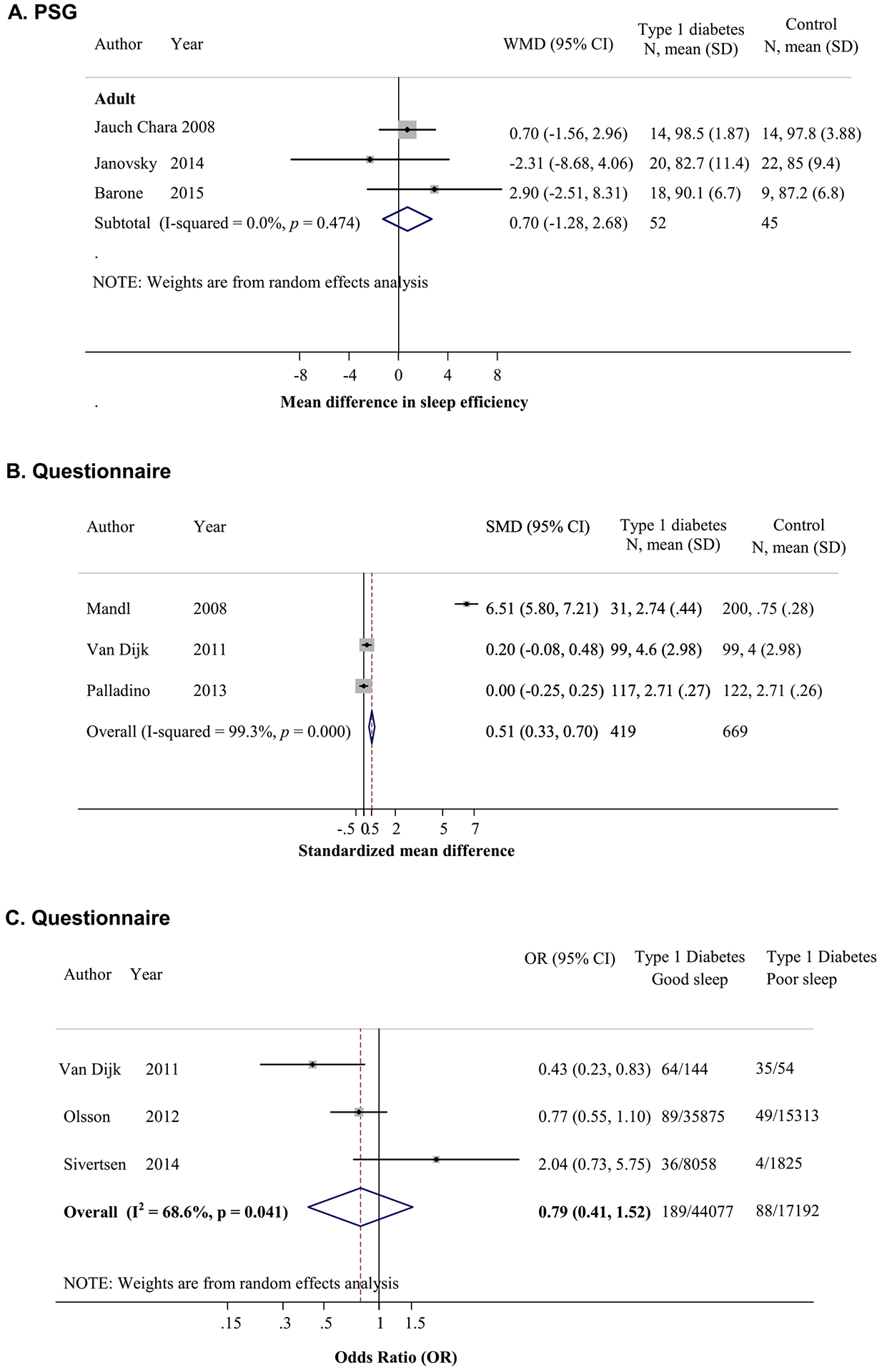
Comparisons of sleep quality between patients with type 1 diabetes (T1D) and controls. (A) Mean difference in sleep efficiency by polysomnography (PSG) (sleep efficiency of T1D patients minus that of controls). (B) Standardized mean difference in sleep quality score by questionnaire with higher score reflecting worse quality (T1D patient score minus that of controls). (C) Association between T1D and good sleep quality.
3.2. Sleep and glycemic control
A summary of the analyses of the association between sleep and glycemic control in T1D patients is presented in Table 3.
Table 3.
Meta-analyses of the relationship between sleep and glycemic control in patients with type 1 diabetes (T1D).
| Sleep variables | Analysis | Sleep measurements | Studies (n) | N | Type of participants | Resultsa |
|---|---|---|---|---|---|---|
| Sleep stages | MD in percentages of sleep stages between those with optimal and suboptimal glycemic controlsb | PSG, wireless sleep monitor | 5 | 36 vs 81 | Adults | No differences in light sleep, MD = −2.90%, (95% CI = −6.96, 1.16). |
| 36 vs 81 | No differences in deep sleep, MD = 2.95%, 95% CI = −1.98, 7.88 | |||||
| Sleep duration | MD in HbA1c levels between those with longer and shorter sleep durationsc | PSG, wireless sleep monitor or actigraphy | 6 | 127 vs 68 | Adults | No differences in HbA1c, MD = 0.03%, −0.43, 0.49 |
| Questionnaire | 4 | 381 vs 152 | Adults | Those with longer sleep duration had lower HbA1c, MD −0.24%, 95% CI = −0.47, −0.02. | ||
| Questionnaire | 4 | 96 vs 35 | Adolescents/children | No differences in HbA1c in all combined age groups, MD = −0.07%, 95% CI =−0.52, 0.39; age 6–13, MD = 0.07%, 95% CI = −0.42, 0.55; and age >13–17, MD = −0.97%, 95% CI = −2.22, 0.29 | ||
| MD in sleep duration between those with optimal and suboptimal glycemic controlsb | PSG, wireless sleep monitor, or actigraphy | 6 | 54 vs 142 | Adults | No differences in sleep duration, MD = −2.88 min, 95% CI = −18.09, 12.34 | |
| Questionnaire | 4 | 138 vs 397 | Adults | Those with optimal glycemic control had longer sleep duration, MD = 17.28 min, 95% CI = 4.13, 30.370 | ||
| Questionnaire | 4 | 35 vs 104 | Adolescents/children | No difference in sleep duration in all combined age groups, MD = 18.6 min, 95% CI = −12.6, 49.8; age 6–13, MD = 0.6 min, 95% CI = −39.0, 40.2; and age >13–17, MD = 48 min, 95% CI = −3.0, 99.0 | ||
| Sleep qualityd | MD in HbA1c levels between those with good and poor sleep qualitye | PSG or actigraphy | 4 | 86 vs 80 | Adults | No differences in HbA1c, MD = 0.01%, 95% CI = −0.35, 0.36 |
| Questionnaire | 3 | 442 vs 136 | Adults | Those with good sleep quality had lower HbA1c, MD 95% CI = −0.19%, −0.30, −0.08. | ||
| MD in sleep qualityd between those with optimal and suboptimal glycemic controlb | PSG or actigraphy | 5 | 48 vs 133 | Adults | No differences in sleep efficiency, MD = −0.11%, 95% CI = −1.69, 1.47 | |
| OSA | MD in HbA1c levels between those with and without OSAf | PSG or oximetry | 4 | 96 vs 81 | Adults | No difference in HbA1c, MD = 0.17%, 95% CI = −0.22, 0.57 |
| MD in HbA1c levels between those with moderate-severe OSA and without OSAf | PSG | 3 | 47 vs 69 | Adults | No statistically significant differences in HbA1c, MD = 0.39%, −0.08, 0.87 | |
| MD in AHI between those with optimal and suboptimal glycemic controlsb | PSG | 4 | 53 vs 114 | Adults | Those with optimal glycemic control had lower AHI, MD = −2.95 events/h, 95% CI = −5.69, −0.21. |
Abbreviations: AHI, apnea–hypopnea index; MD, mean difference; OSA, obstructive sleep apnea; PSG, polysomnography.
Calculated by sleep variables of patients with optimal glycemic control minus those of patients with suboptimal glycemic control, or HbA1c of patients with good sleep minus HbA1c of patients with poor sleep, unless otherwise noted.
Optimal glycemic control is defined as HbA1c <7% in adults or <7.5% in children, and suboptimal glycemic control is defined as HbA1c ≥7% in adults or ≥7.5% in children, with the exception of the study by Villa et al. [42], in which optimal glycemic control was defined as HbA1c <8%.
Longer sleep duration is defined as sleep duration of >6 hours in adults or >8 hours in children, and shorter sleep duration is defined as sleep duration ≤6 hours in adults or ≤8 hours in children.
Measured as sleep efficiency by PSG or actigraphy, or sleep quality score per the sleep questionnaires.
Good sleep quality is defined as sleep efficiency ≥85% as measured by PSG or actigraphy or per the cutoff of the sleep questionnaire; poor sleep quality is defined as sleep efficiency <85% as measured by PSG or actigraphy or per the cutoff of the original sleep questionnaire.
Obstructive sleep apnea (OSA) is defined as AHI ≥5 as measured by PSG or oximetry or having pathological oximetry readings; moderate to severe OSA is defined as AHI ≥15.
3.2.1. Sleep stages
Five adult studies were included in the analysis of sleep stages [7,20,21,24,29] (n = 36 vs 81 for optimal vs suboptimal glycemic control). In adults, those with optimal glycemic control (HbA1c <7%) spent less time in light NREM sleep (pooled MD = −2.90%, 95% CI = −6.96, 1.16) and more time in deep NREM sleep (pooled MD = 2.95%, 95% CI = −1.98, 7.88) than those with suboptimal glycemic control (HbA1c ≥ 7%), but it was not statistically significant (Fig. A1).
3.2.2. Sleep duration
In adults, HbA1c levels did not differ significantly between those who slept >6 hours compared to ≤6 hours based on objective sleep measurements in six studies [7,16,20,21,24,29] (n = 127 vs 68; Fig. A2A). However, in four adult studies [17,25–27] those who reported sleeping >6 hours had a significantly lower HbA1c level (−0.24%, 95% CI = −0.47, −0.02) compared to those reporting sleeping for ≤6 hours (n = 381 vs 152). In four adult studies [17,25−27], patients with optimal glycemic control (<7%) reported sleeping an average of 17.3 minutes more (95% CI = 4.13, 30.37) compared to those with suboptimal glycemic control (≥7%; n = 138 vs 397), but the objective sleep duration analyzed in six adult studies [7,16,20,21,24,29] (n = 54 vs 142) did not differ based on optimal (<7%) vs suboptimal control (≥7%), with a pooled MD of −2.88 minutes (95% CI = −18.09, 12.34) (Fig. A2B).
Meta-analysis of two child studies with four cohorts [22,26] revealed no significant differences in HbA1c levels in combined age groups between those who reported longer vs shorter sleep duration (n = 96 vs 35; Fig. A3A). The subanalysis by age groups revealed no significant difference in HbA1c levels between those reported sleeping ≥9 vs <9 hours in children aged 6–13 years. There was a trend toward lower HbA1c, albeit not statistically significant, in those reported sleeping ≥8 vs <8 hours in the age group >13–17 years (MD = −0.97%, 95% CI = −2.22, 0.29). In addition, mean sleep duration by questionnaire in combined age groups [22,26] also did not differ significantly between those with optimal and suboptimal glycemic control (pooled MD = 18.6 minutes, 95% CI = −12.6, 49.8; n = 32 vs 99; Fig. A3B). The subanalysis by age groups revealed no significant differences in self-reported sleep duration between those with optimal vs suboptimal glycemic control in children aged 6–13 years. Among children aged >13–17 years, those with optimal glycemic control tended to report longer sleep duration, but this was not statistically significant (MD = 48 minutes; −3.99).
3.2.3. Sleep quality
In four adult studies, HbA1c levels did not differ between those with good (≥85%) and poor (<85%) sleep quality, based on objective measurements [16,20,24,29] (n = 86 vs 80; Fig. A4A). Similarly, there were no differences in sleep efficiency between participants with optimal and suboptimal glycemic control in five adult studies [16,20,21,24,29] (n = 48 vs 133; Fig. A4B). However, in three adult studies, participants with good self-reported sleep quality had significantly lower HbA1c levels than those with poor sleep quality [10,17] (MD = −0.19%, 95% CI = −0.30, −0.08; n = 442 vs 136; Fig. A4A).
3.2.4. OSA
Among adult T1D patients, the prevalence of OSA (defined as AHI ≥5 or pathological oximetry findings) was 51.9% (95% CI = 31.2, 72.6) and moderate to severe OSA (AHI ≥15) was 16.7% (95% CI = 1.1, 34.5) in four studies (n = 186) [20,24,28,29]. The mean difference in HbA1c levels between adult T1D patients with and without objectively determined OSA was not different in four studies [20,24,28,29] (n = 96 vs 81, Fig. A5A). However, there was a trend toward higher HbA1c levels when comparing those with moderate–severe OSA (AHI ≥15) to those without OSA (AHI <5) in three studies [24,28,29] (n = 47 vs 69), with a pooled MD of 0.39% (95% CI = −0.08, 0.87; Fig. A5B). In addition, the AHI in T1D patients was compared between those with optimal and suboptimal glycemic controls in four adult studies [20,24,28,29] (n = 53 vs 114). Participants with optimal glycemic control had significantly lower AHI than those with suboptimal glycemic control (MD = −2.95 events per hour, 95% CI = −5.69, −0.21; Fig. A5C). There were not enough studies in children to examine OSA and T1D.
3.3. Publication bias
Funnel plots and Egger tests, where applicable, were used to assess asymmetry of the funnel and small-study effect for all pooling (Figs. A6 and A7 and Table A2). Of all the 19 poolings, 17 showed no evidence of asymmetry, and only two poolings showed asymmetry (association between objectively measured sleep duration and glycemic control in adults, and objectively measured sleep quality and glycemic control in adults). Egger tests indicated small-study effects (Table A2). The reason for this was further explored using contour-enhanced funnel plots. These suggested that studies with lower precision showed higher negative MDs (ie, lower sleep duration/quality in optimal than suboptimal glycemic control) than studies with higher precision (Fig. A7), suggesting a publication bias for these two poolings.
4. Discussion
The results of these meta-analyses indicate some significant differences in sleep characteristics between persons with and without T1D. In comparison to control participants, adults with T1D had worse sleep quality, especially when assessed by questionnaires. Unfortunately, there were too few studies using PSG to compare sleep architecture between T1D and controls. Although there was no difference in sleep duration in adults with and without T1D, youth with T1D slept significantly less than controls. However, we found an association between glycemic control and sleep duration or quality in adults. Shorter self-reported sleep duration and poor self-reported sleep quality were associated with suboptimal glycemic control. Finally, we found that the prevalence of OSA in adults with T1D is strikingly high (51.9%) and approaches that of type 2 diabetes (54%–86%) [1], despite average BMI values below 30 kg/m2. In addition, patients with suboptimal glycemic control had more sleep apnea as reflected by higher AHI in adults, and similar findings were reported in child studies. Overall, these results suggest an important relationship between sleep and T1D.
In the present analyses, the adult T1D patients with optimal glycemic control spent less time in light NREM sleep and more time in deep NREM sleep, suggesting that worse glycemic control might be associated with shallower sleep, although the difference did not reach statistical significance. A study of adolescents with T1D found that more time spent in N3 was associated with better glycemic control [23]. Physiologically, N3 is associated with less sympathetic nervous system activity and is thought to be a “restorative” stage of sleep, which could explain the association with better glycemic control [46].
Our analysis did not find differences in sleep duration between adult patients with T1D and controls. However, children with T1D slept an average of 26 minutes, by objective measurement, less than controls. The reason for the discrepancy between age groups is unclear, but could be due to the small number of studies analyzed or different glycemic conditions during the PSG recordings. A questionnaire study of 323 persons including patients with T1D and their first- and second-degree relatives found that 41% had insufficient sleep based on the American Academy of Sleep Medicine recommendations (<10 hours for those aged 5–11, <9 hours for those aged 12−19 years, and <7 hours for those aged 20 years), although comparisons with control subjects were not performed [26]. Having T1D itself could possibly affect time spent in bed or sleep duration due to nocturnal hypoglycemia disrupting sleep and the need for night-time diabetes care.
Among adults with T1D, the meta-analysis revealed a relationship between self-reported sleep duration and glycemic control. The average HbA1c level was 0.24% lower among those who reported sleeping for >6 hours. Although six hours of sleep may not be sufficient [47], the aggregated available data did not allow us to re-categorize sleep duration in more detail. Similarly, those with optimal glycemic control reported sleeping 17 minutes more on average than patients with suboptimal glycemic control. The trend was similar in the studies of children, especially in the age group of >13–17 years, although not statistically significant. Objectively measured sleep duration was not related to glycemic control in one child study [23] and most of the adult studies. One limitation of these analyses is that sleep duration estimated from PSG does not represent habitual behavior. One study in adults that used actigraphy, which better represents habitual sleep duration, revealed that HbA1c levels were significantly higher in those with shorter sleep duration (<6.5 hours) compared to those who slept for >6.5 hours (8.5% vs 7.7%) [16]. Collectively, these data suggest that there is an association between better glycemic control and longer sleep duration in T1D patients. Consistent with this, one-night experimental sleep restriction to four hours in bed in seven T1D patients was associated with decreased peripheral insulin sensitivity, compared to a night with normal sleep duration (average of 7.8 hours) [38]. This agrees with several experimental studies in healthy volunteers that showed impaired glucose tolerance after sleep restriction [48,49]. Whether sleep extension in T1D patients with short sleep will lead to improvement in glycemic control remains the subject of future research.
We found that sleep quality scores as assessed by questionnaire were worse in adults with T1D compared to controls, although the questionnaires used differed among studies. The proportion of participants with self-reported good sleep quality, however, did not differ between the two groups. There was only one prospective study suggesting that sleep disturbance was a risk factor for developing autoimmune diabetes [13]. Although the mechanism was not explored, the author postulated that sleeping difficulty may contribute to increased insulin resistance that could facilitate diabetes onset in susceptible individuals [13]. In the current analysis, adult patients with T1D with self-reported, but not objectively measured, good sleep quality had a significantly lower HbA1c by 0.19%. In addition, a longitudinal study in type 1 patients found that sleeping difficulties, reported in 21% of participants, were significantly related to higher HbA1c values at one-year follow-up [10]. The discrepancy between objective and subjective measure of sleep quality may be due to methodological differences. Objective sleep quality was represented by sleep efficiency from a single night of PSG, whereas subjective reports were based on the previous month. In addition, the number of participants who had PSG in the current analysis was relatively small. Sleep quality in T1D could be impaired by many factors, including neuropathic pain [25], hypoglycemia, which may result in increased carbohydrate consumption the following morning [50], disrupted sleep, and psychological factors, which are all associated with suboptimal glycemic control [51,52]. In healthy adult volunteers, experimental sleep disruption resulted in an increased insulin resistance in healthy individuals [51]. Whether poor sleep quality is associated with insulin resistance in T1D patients is unknown.
Although no differences in AHI were found between T1D patients and controls in two small studies [20,35], and although OSA symptoms were not consistently different when assessed by questionnaires [12,17], our results revealed a high prevalence of OSA in adult T1D patients from four larger studies (51.9%), as assessed by objective sleep measurements (oximetry or PSG). This is much higher than that in the general population, which is estimated to be 3%–7% and increases with age and obesity [53]. Mean BMI values of the participants in our analysis were between 22.9 and 25.8 kg/m2, so obesity alone could not explain the high prevalence. Studies have suggested that the presence of neuropathy, especially autonomic neuropathy, may compromise upper airway reflexes and control of the pharyngeal muscle, predisposing the patients to obstructive events [54]. A small previous study found that neuropathy was common in T1D patients with apnea [55]. PSG data in 20 T1D patients revealed a significantly higher prevalence of OSA in those with cardio-autonomic neuropathy than in T1D patients without this condition (67% vs 23%) [20]. These data support the role of neuropathy and an increased OSA risk in these patients. Finally, as OSA is known to be associated with disturbed sleep duration and quality, the presence of OSA may also be partly responsible for the findings on sleep duration and quality in our analyses.
The present analyses found that the presence of OSA in adults, especially moderate to severe OSA, may be associated with worse glycemic control, although the association did not reach statistical significance. In addition, adults with optimal glycemic control had significantly lower AHI than those with suboptimal glycemic control, and similar findings were reported in child studies, although there were not enough to be pooled for meta-analysis [23,42]. Although the mechanism linking OSA to suboptimal glycemic control has not been explored specifically in T1D patients, reduced insulin sensitivity may play a role as suggested by studies that experimentally induced intermittent hypoxia in healthy volunteers [56,57]. Thus, the presence of OSA, which is highly prevalent in T1D, may adversely affect glycemic control in these patients. One study also found that the presence of OSA in T1D patients was associated with cardiovascular disease and retinopathy [29], which resembles the findings in those with type 2 diabetes. There are currently no data exploring the effect of OSA treatment on glycemic control or complications in patients with T1D.
The inclusion of common sleep disturbances and exploration of their relationship with glycemic control in T1D population is the strength of this study. Additional data obtained from authors, mostly unpublished, also contributed to the strength of our analyses. Still, the primary limitation of these analyses is the small number of studies available, which limited our statistical power and increased the likelihood of type 2 error. This underscores the importance of more research on sleep in T1D patients. A second limitation is that almost all studies were cross-sectional, precluding the assumption of causality. Indeed, impaired sleep could affect glycemic control, but suboptimally controlled glucose levels could also impair sleep. Third, some patients experienced hypoglycemia during the single-night PSG recording, which could not be controlled for in our analyses [23]. Hypoglycemia has been known to affect sleep architecture [58] and sleep efficiency [41]. However, the occurrence of hypoglycemia is common in T1D, and therefore not excluding patients who experience hypoglycemia is more reflective of real-world experiences. It is also important to note that the magnitude of HbA1c differences in those with and without sleep disturbances is relatively small, although it is comparable to some of the standard and advanced therapies for T1D patients, such as carbohydrate counting [59], or the use of continuous subcutaneous insulin infusion [60]. In addition, none of the studies specifically excluded participants with anemia or certain hemoglobinopathies that could potentially affect HbA1c measurements. Finally, summary data analysis does not allow adjustments for factors related to glycemic control such as therapy adherence or assessments of hypoglycemia. Future studies should include a larger number of participants and should use consistent multi-day, multi-informant, and multi-methods to prospectively and longitudinally assess sleep.
5. Conclusion
In summary, the interactions between sleep and type 1 diabetes are complex and likely bidirectional. Type 1 diabetes is associated with poor sleep quality and a high prevalence of OSA. Sleep disturbances, including poor sleep quality, shorter sleep duration, and OSA, are associated with suboptimal glycemic control. Whether sleep optimization will improve glycemic control is a subject of future research. More research is clearly needed to understand the relationship between sleep and glycemic control in type 1 diabetes patients.
Supplementary Material
Financial disclosure
Portions of the data in the manuscript were obtained from studies supported by Pfizer, ResMed, the Father’s Day Council, University of Arizona Foundation Faculty Small Grants Program, National Institutes of Health (NIH) HL62373, and American Diabetes Association 7-13-CE-32 (co-sponsored by the Order of the Amaranth).
Appendix A
Fig. A1.
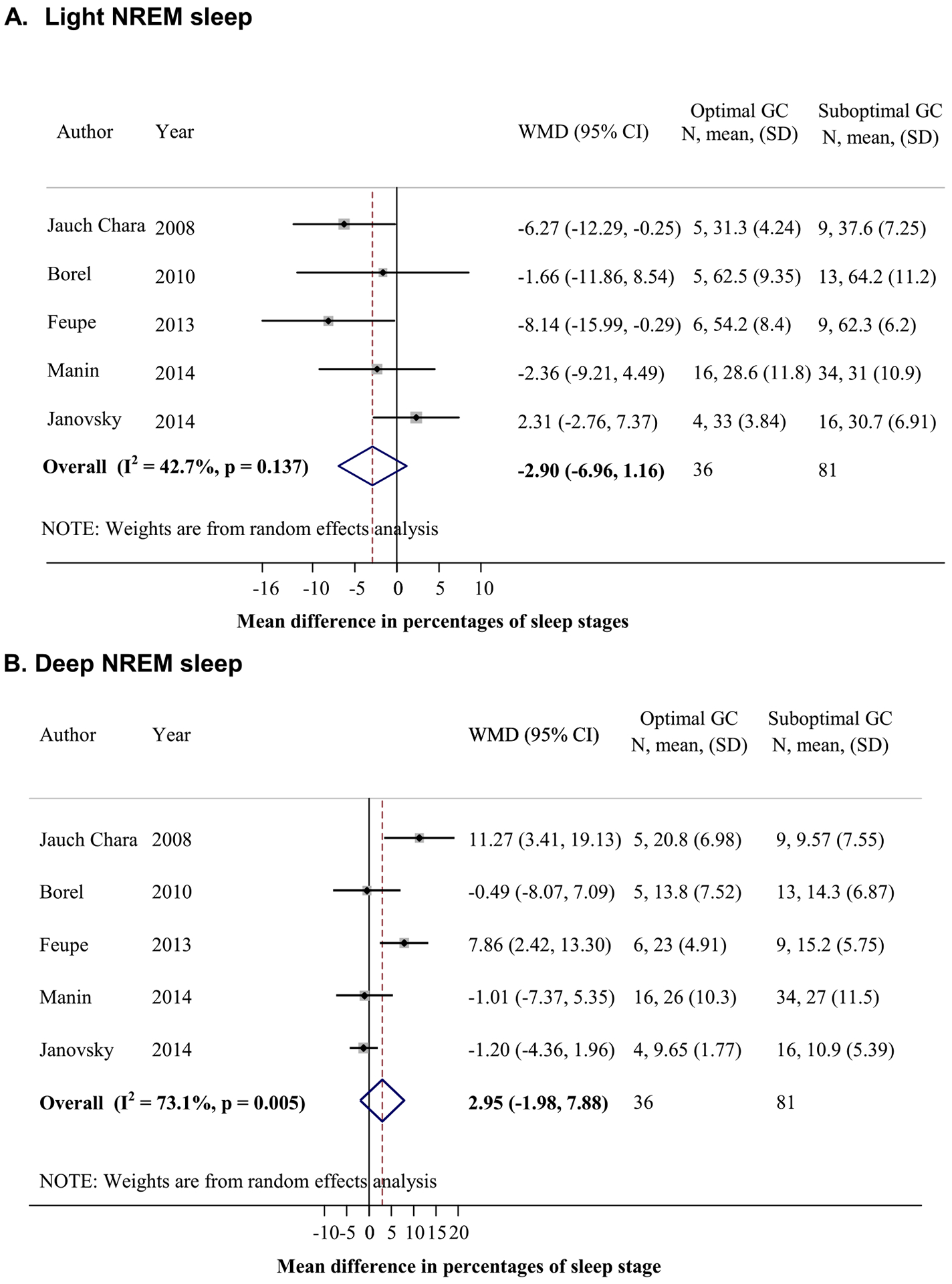
Relationship between sleep stages and glycemic control in type 1 diabetes (T1D) patients. (A) Mean difference of percentages of sleep time spent in light sleep between participants with optimal (HbA1c <7%) and suboptimal (HbA1c ≥7%) glycemic control (GC) (calculated by percentage of sleep time of participants with optimal GC minus those with suboptimal GC). (B) Mean difference of percentages of sleep time spent in deep sleep between those with optimal and suboptimal GCs. NREM, non-rapid eye movement; REM, rapid eye movement.
Fig. A2.
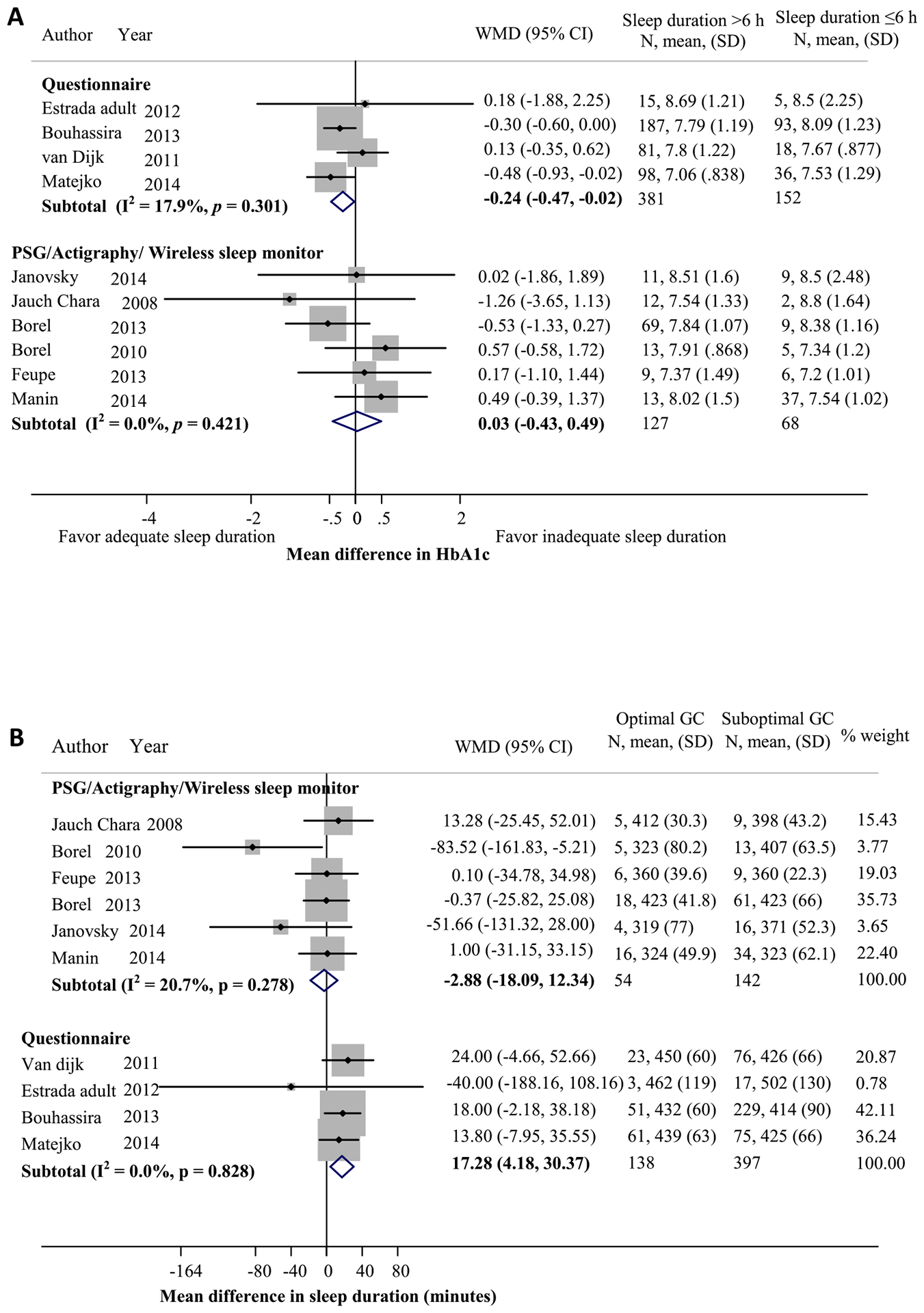
Relationship between sleep duration and glycemic control (GC) in adults with type 1 diabetes (T1D). (A) Mean difference in HbA1c levels between participants with longer sleep duration (>6 hours) and those with shorter sleep duration (≤6 hours). (B) Mean difference in sleep duration between participants with optimal (HbA1c <7%) and suboptimal (HbA1c ≥7%) GCs (calculated by sleep duration in minutes of those with optimal GC minus those with suboptimal GC). PSG, polysomnography.
Fig. A3.
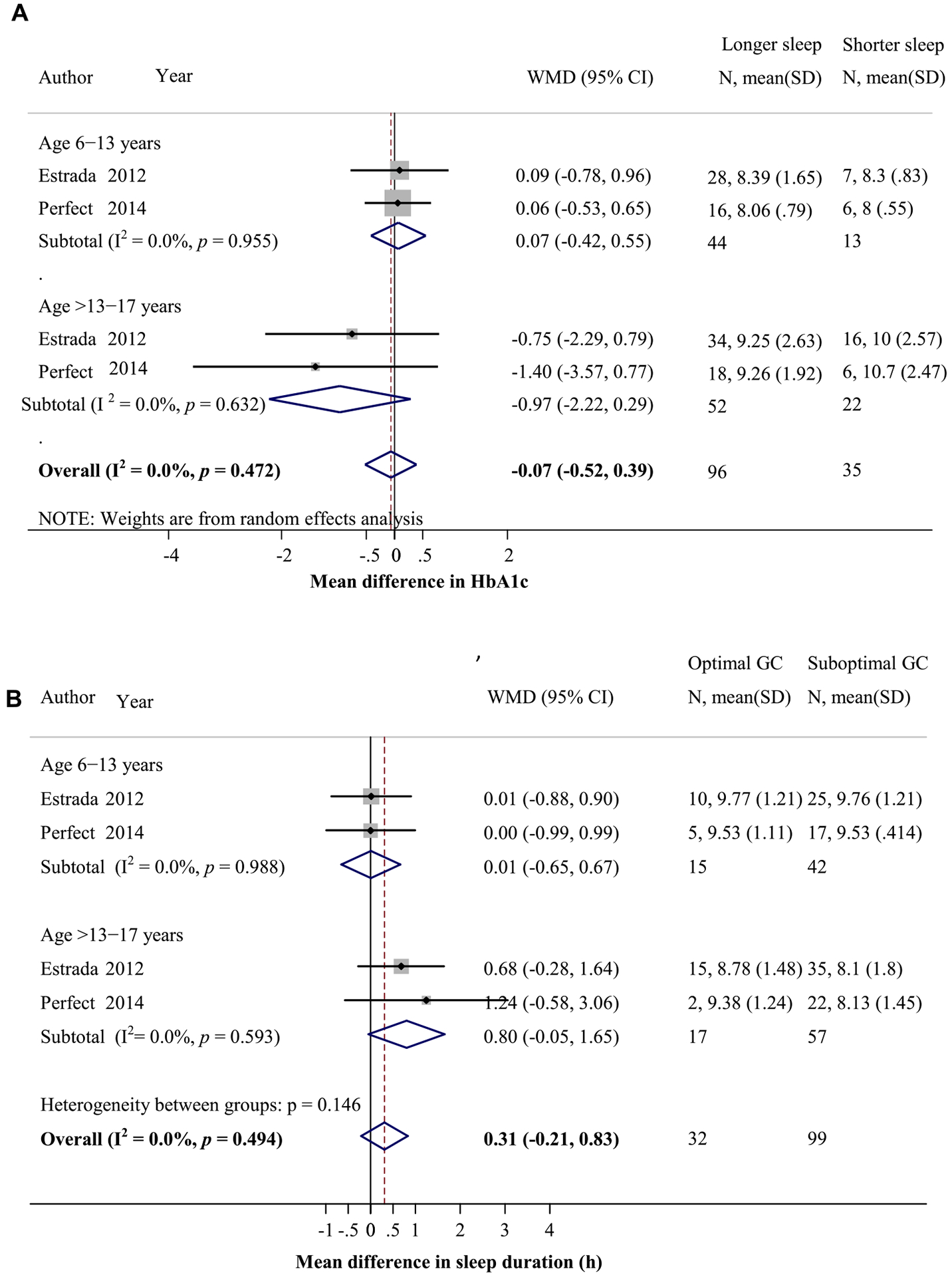
Relationship between sleep duration and glycemic control (GC) in children with type 1 diabetes (T1D). (A) Mean difference in HbA1c levels between participants with longer and shorter sleep durations, calculated by HbA1c in those with longer sleep duration minus that of those with shorter sleep duration. (B) Mean difference in sleep duration between participants with optimal (HbA1c < 7.5–8%) and suboptimal (HbA1c ≥ 7.5–8%) GCs.
Fig. A4.
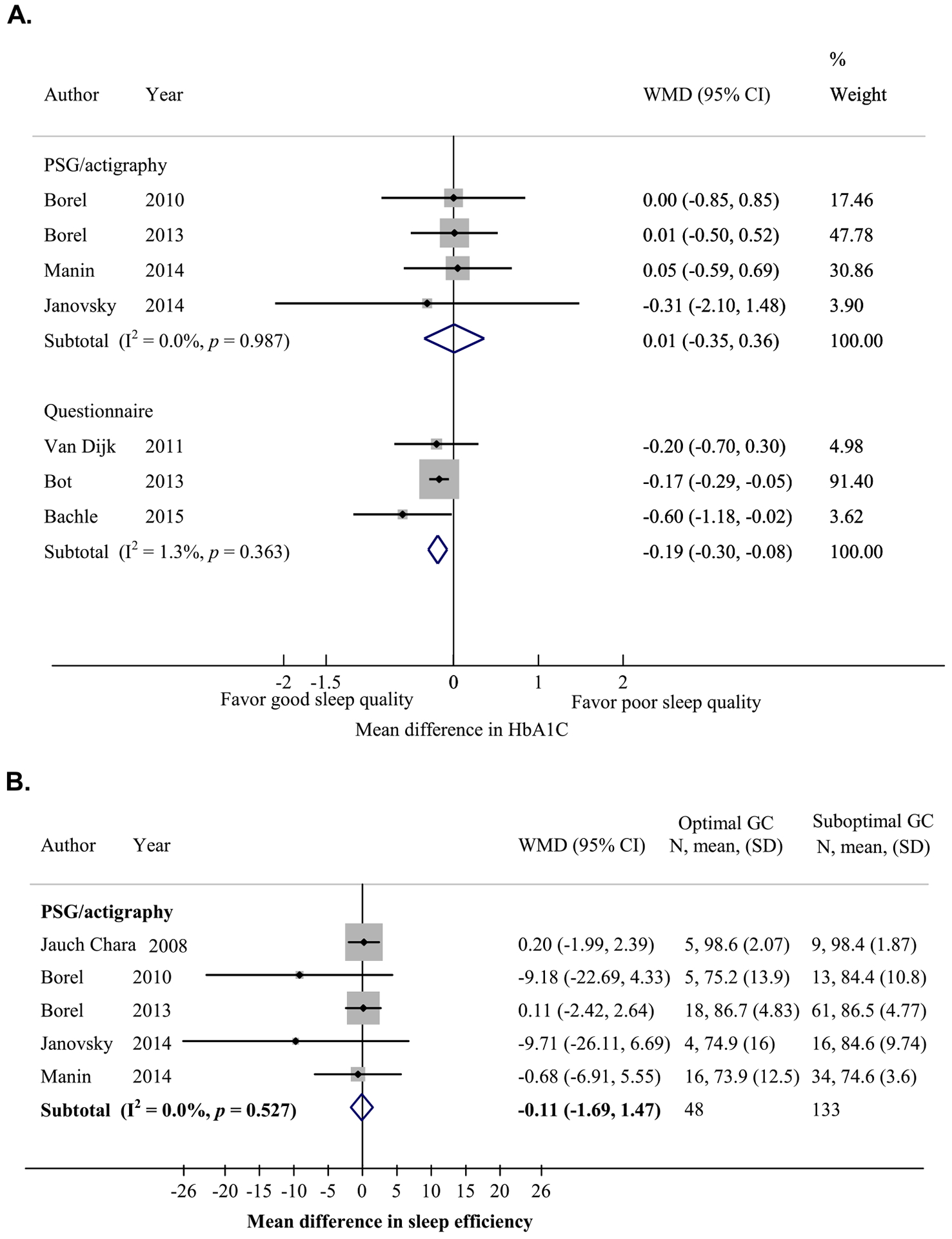
Relationship between sleep quality and glycemic control (GC) in adults with type 1 diabetes (T1D). (A) Mean difference in HbA1c levels between participants with good sleep quality (sleep efficiency ≥85% as measured by polysomnography [PSG] or actigraphy, or per sleep quality score cutoff according to the sleep questionnaire used) and those with poor sleep quality. (B) Mean difference in sleep efficiency between participants with optimal (HbA1c <7%) and suboptimal (HbA1c ≥7%) GCs.
Fig. A5.
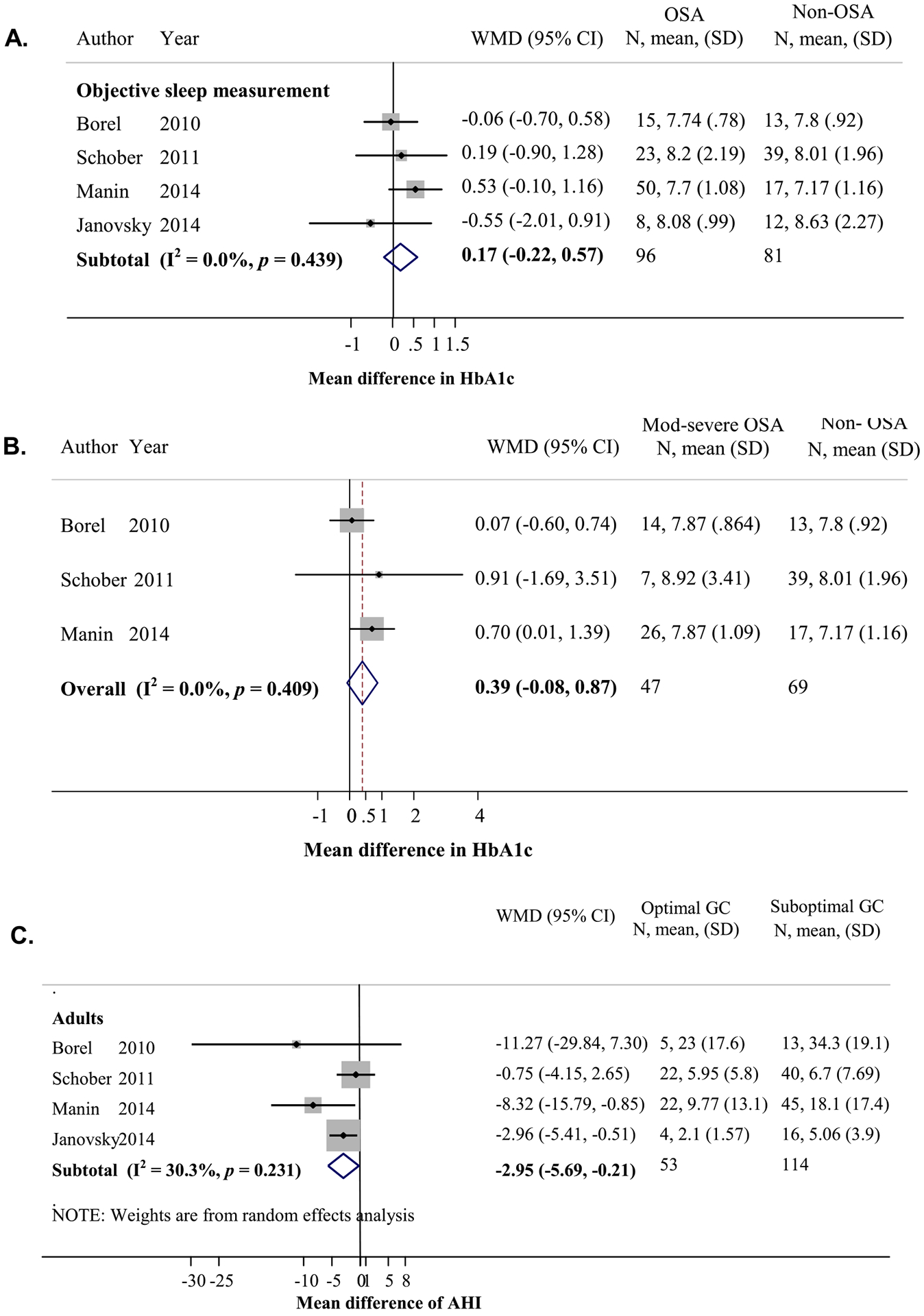
Relationship between obstructive sleep apnea (OSA) and glycemic control (GC) in patients with type 1 diabetes (TID). (A) Mean difference in HbA1c levels between participants with OSA and without OSA in adults (calculated by HbA1c in those with OSA minus those without OSA). (B) Mean difference in HbA1c levels between those with moderate to severe OSA (AHI ≥15) and those without OSA (AHI <5) in adults (calculated by HbA1c in those with moderate to severe OSA minus those without OSA). (C) Mean difference in AHI between those with optimal (HbA1c < 7%) and suboptimal (HbA1c ≥ 7%) GCs (calculated by AHI of those with optimal GC minus those with suboptimal GC).
Fig. A6.
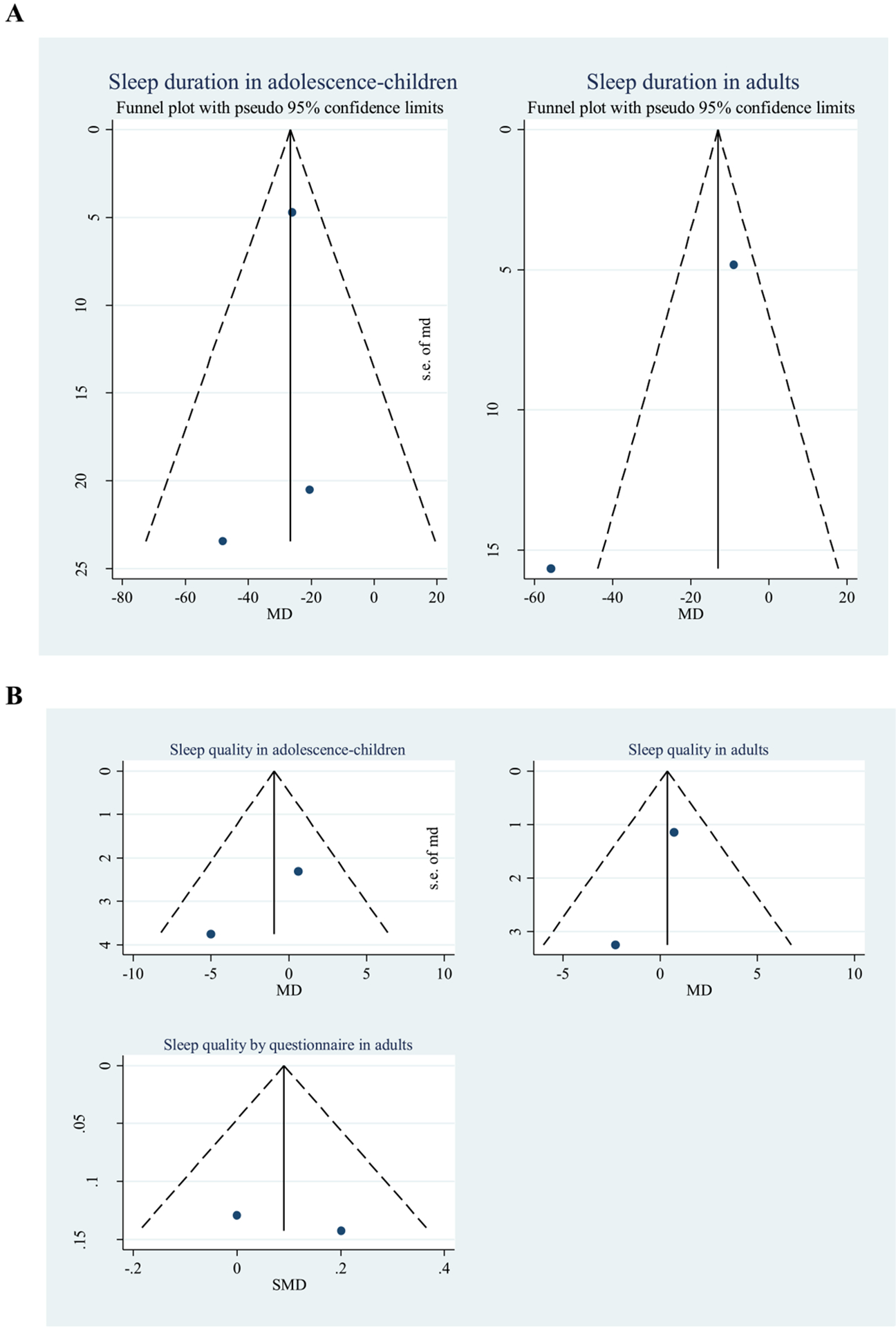
Funnel plots of the mean difference between patients with type 1 diabetes (T1D) and control participants. (A) Sleep duration. (B) Sleep quality.
Fig. A7.
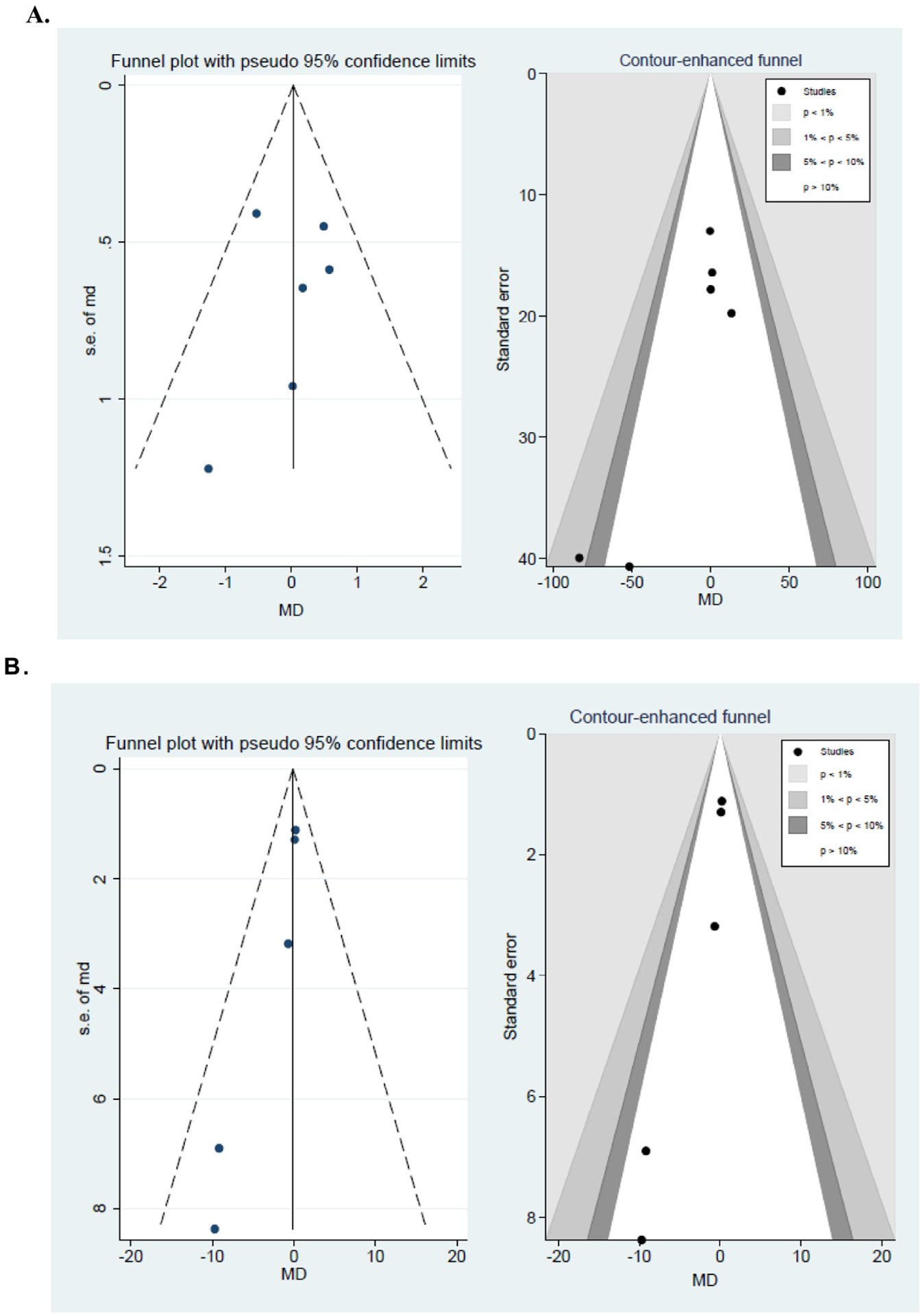
Funnel and contour-enhanced funnel plots for mean differences between adult type 1 diabetes (T1D) patients with good and poor glycemic controls. (A) Sleep duration as obtained by objective measurements. (B) Sleep quality by objective measurements.
Table A1.
Qualitative review of additional studies and sleep variables not eligible for meta-analysis.
| Study | T1D/controls (n) | Population | Study design | Sleep measurement | Sleep variables | Results |
|---|---|---|---|---|---|---|
| Barone et al. [35] | 18/9 | Adult | Matched case-control (age, BMI) | Sleep diary, actimeter, PSGd | Stages, duration, quality, OSA | No differences in percentage of sleep stages in control vs T1D participants: REM sleep (21.5% vs 19.9%, p = 0.62) or stage 3 (21.7% vs 20.6%, p = 0.76) Mean sleep duration from sleep diaries and nightly rest duration as assessed by actimeter were not correlated with mean glucose level as assessed by CGM.b However, in a subgroup of patients with low glycemia (mean glucose ≤154 mg/dL) (n = 9), nightly rest period negatively correlated with mean glycemia (r = −0.89, p = 0.03). Sleep quality as assessed by visual analogue scale did not correlate with mean glycemia as measured by CGM. In a subgroup of patients with low glycemia, sleep quality negatively correlated with mean glycemia. No differences in mean AHI (control vs T1D patients, 2.9 vs 3.4). None of the participants had OSA. In T1D, there was a correlation between AHI and mean glucose level (r = −0.55, p = 0.03), and arousal index and mean glucose level by CGM (r = 0.56, p = 0.03). |
| Blanz et al. [36] | 93/93 | Children/adolescents | Unmatched case-control | Interview as a part of psychiatric assessments (sleep disturbances) | Quality | More T1D reported sleep disturbances than control participants (χ2 test, 8.08, p < 0.01) |
| Borel et al. [16] | 79/NA | Adults | Cross-sectional | Questionnaire evaluating OSA risk | OSA | Mean HbA1c was similar between those who reported snoring and those who did not snore (7.9% ± 1.0% vs 7.9% ± 1.1%, p = 0.89) (personal communication). |
| Caruso et al. [37] | 49/36 | Children/adolescents | Unmatched case-control | Questionnaire (Sleep Disturbance Scale for Children [SDSC]) | Quality | T1D had significantly lower sleep quality than control participants (higher SDSC score). These included the total score (control vs T1D 43.8 vs 63.1, p < 0.001), disorders of initiating and maintaining sleep (55.0 vs 68.5, p < 0.001), disorders of sleep-wake transition (49.2 vs 57.1, p < 0.005) and disorders of excessive somnolence (48.5 vs 61.4, p < 0.001). No significant differences in the subscale of disorders of arousal, sleep hyperhidrosis, and sleep-disordered breathing between the two groups. |
| Donga et al. [38] | 7/NA | Adults | Intervention study | Experimental sleep restriction | Sleep duration | Sleep restriction for one night (4 h) resulted in a significantly decreased glucose disposal rate during hyperinsulinemic euglycemic clamp (reflecting decreased insulin sensitivity) compared to a night with normal sleep duration (average 7.8 h). |
| Happe et al. [39] | 46/50 | Children/adolescents | Sibling study | Questionnaire | Quality, snoring, restless legs syndrome | No differences between T1D and control participants in percentages with restless legs syndrome symptoms (2.2% vs 2.0%), sleep initiation problem (10.9% vs 4.0%), sleep maintenance problem (6.5% vs 4.0%), or snoring (13.0% vs 14.0%) |
| Janovsky et al. [20] | 20/22 | Adults | Matched case-control (age, BMI) | PSGa | Stages, duration, OSA | No differences in percentage of sleep stages in control vs T1D participants: stage 1 (3.2% vs 4.5%), stage 2 (58.5% vs 57.8%), stage 3 (21.6% vs 21.2%) (personal communication). Sleep duration was similar between control participants vs T1D patients without CAN vs T1D without CAN (416 vs 379 vs 359 min) Mean AHI was similar between control participants vs those with T1D (3.7 vs 4.5). 40% of T1D vs 4.5% of control participants had OSA.T1D patients with CAN had significantly higher AHI than T1D patients without CAN (6.4 vs 3.2). |
| Jauch-Chara et al. [21] | 14/14 | Adults | Matched case-control (age, BMI, sex) | PSGc | Stages, duration | No differences in percentage of sleep stage 1 (controls vs T1D patients 19.2% vs 14.2%, p = 0.34), slow-wave sleep (controls vs T1D patients 14.9% vs 14.7%, p = 0.75). T1D patients spent more time in stage 2 than control participants (55.2% vs 47.2%, p = 0.01). During the first half of the night, there was a trend toward less time spent in slow-wave sleep in T1D patients than in controls (21.3% vs 24.7%, p = 0.09). Sleep duration was similar between the two groups (404 min vs 395 min, p = 0.93) |
| Kilmek et al. [30] | 16,667/1,845,591 | All ages | Cross-sectional, population based | Nationwide claims data on sleep disorders diagnosis (G47) | All sleep disorders in G47 diagnosis code | Sleep disorders were more commonly comorbid in T1D patients (relative risk = 1.9, 95% CI = 1.5–2.4). |
| Low et al. [11] | 83/245 | Adults | Matched case-control, comparable age and sex | Questionnaire (eight sleep questions from Autonomic Symptom Profile) | Quality | T1D patients had poorer sleep quality than controls (mean score = 0.27 vs 0.07; higher score reflects poorer sleep), but this was not statistically significant. |
| Matyka et al. [40] | 15/15 | Children/adolescents | Matched case-control (age, sex) | PSGa | Stages, quality | No significant differences in percentage of sleep stages between controls and T1D patients (stage 1: 4.3% vs 4.9%, p = 0.1; stage 2: 24.5% vs 22.7%, p = 0.7; stage 3: 51.4% vs 50.5%, p = 0.8) or REM sleep (17.8% vs 15.7%, p = 0.2). Compared to non-hypoglycemic night, the recordings during hypoglycemia revealed more time spent in slow wave sleep (60.4% vs 38.9%, p = 0.04) and less time in REM sleep (11.5% vs 15.2%, p = 0.04). T1D spent more time in short wake (<2 min) and long wake (>2 min) than controls (median 0.8% vs 0%, and 1.2% vs 0%, respectively) |
| Perfect et al. [23] | 50/40 | Children/adolescents | Matched case-control (age, BMI, sex) | PSGd (40 matched pairs) Actigraphyd (T1D cohort) | Stages, duration, quality, OSA | Compared to controls, T1D spent more time in stage 2 (57.2% vs 52.3%, p < 0.01) and less time in stage 3 (14.5% vs 18.9%, p < 0.05). More time spent in stage 2 was associated with higher HbA1c values and mean glucose levels by 5-day CGM. Sleep duration was not related to glucose control. Sleep duration each night was not related to waking glucose levels. No differences in sleep efficiency between T1D and control participants (86.6% vs 85.9%). Sleep efficiency was not related to glucose control. OSA prevalence and mean AHI were similar in T1D and control participants (35% vs 28%, and 2.48 vs 2.20), but central apnea index was higher in T1D patients than in controls (1.44 vs 0.33, p < 0.05). T1D patients with OSA (AHI ≥1.5) had significantly higher glucose levels by 5-day CGM than those without OSA. In addition, those with optimal glycemic control (n = 6) (HbA1c <7.5%) had lower AHI than those with suboptimal glycemic control (n = 34) (0.67 ± 0.49 vs 2.79 ± 4.64) (personal communication). |
| Perfect [22] | 50/NA | Children/adolescent | Cross-sectional | Questionnaire (School Sleep Habit Survey) | Quality | Sleep quality was worse (as reflected by a lower score) in patients with suboptimal glycemic control (HbA1c ≥7.5%) than those with optimal glycemic control (7.8 ± 2.1, n = 42 vs 8.7 ± 1.3, n = 7). In addition, patients with suboptimal glycemic control had more daytime sleepiness (higher score) than those with optimal glycemic control (7.7 ± 3.5, n = 42 vs 5.2 ± 1.7, n = 7) (personal communication). |
| Pillar et al. [41] | 15/15 | Children/adolescents | Matched case-control (age, BMI) | PSGc | Stages, quality | No differences in percentage of sleep stage 3 (control vs T1D 25% vs 23%) or REM sleep (20% vs 20%). However, when analyzing only T1D with hypoglycemia during the recordings, T1D spent more time in stage 3 than controls (29% vs 25%, p < 0.05). No differences in sleep efficiency between the two groups. However, when analyzing only T1D with hypoglycemia, sleep efficiency increased compared to that in controls (95% vs 92%, p < 0.05) |
| Sivertsen et al. [12] | 40/9843 | Adults | Unmatched case-control | Questionnaire | Quality, OSA | No differences in sleep efficiency (as calculated from self-reported sleep timing and sleep latency) between control and T1D participants (85% vs 87%, p = 0.57) No differences in frequency of snoring and report of sleepiness at least three times/wk between control and T1D participants (4.1% vs 6.9%, p = 0.16) |
| Sturrock and Moriarty [31] | 300/143 | Adults | Unmatched case-control | Questionnaire (Nottingham Health Profile, sleep, NHP category) | Quality | T1D patients had worse sleep quality than control participants as reflected by a higher NHP sleep score (12.2 vs 9.3, p < 0.01) |
| van Dijk et al. [17] | 99/99 | Adults | Matched case-control (age, BMI, sex) | Questionnaires (OSA risk) | OSA | More T1D patients were at high risk for OSA compared to controls (17.2% vs 5.1%, p = 0.01). No association between OSA risk and suboptimal glycemic control (HbA1c ≥7.5%), OR = 0.50 (0.15–1.59), p = 0.24. |
| Varni et al. [33] | 83/157 | Children/adolescents | Unmatched case-control | Questionnaire (PedsQL Multidimensional Fatigue Scale) | Sleep quality | T1D had significantly worse sleep/rest fatigue score (as reflected by lower score) than control participants (69.3 vs 77.4, p < 0.05). |
| Villa et al. [42] | 25/20 | Children/adolescents | Matched case-control (age) | PSGa | OSA | Apnea index was higher in T1D than control participants (2.62 vs 1.40, p = 0.006). Central apnea index was higher in T1D with HbA1c ≥8% than control participants (2.54 vs 0.78, p < 0.0001), and tended to be higher in T1D patients with HbA1c <8% than in control participants (1.34 vs 0.78, p = 0.07). T1D patients with optimal glycemic control (n = 12) (HbA1c ≤7.9%) had a nonsignificant lower apnea index than those with suboptimal glycemic control (n = 11) (2.03 ± 1.78 vs 3.28 ± 1.64) and a significantly lower central apnea index (1.34 ± 1.29 vs 2.54 vs 1.27, p = 0.03). |
| Yeshayahu and Mahmud [32] | 75/54 | Children/adolescent | Unmatched case-control | Questionnaire | Duration | Mean sleep duration during weekdays was longer in T1D than in control participants (8.4 vs 8.0 h, p = 0.01), and both groups had longer sleep durations on weekends (an increase by 1.8 and 2.2 h in T1D and control participants, respectively). Mean sleep times or wake times in T1D patients did not differ based on HbA1c levels. |
Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; CAN, cardiac autonomic neuropathy; CGM, continuous glucose monitor; OR, odds ratio; OSA, obstructive sleep apnea; PSG, polysomnography; REM, rapid eye movement; T1D, type 1 diabetes.
Recordings performed without glucose measurements.
Recordings performed with continuous glucose monitor.
Recordings performed under non-hypoglycemic conditions.
Recordings performed with glucose measurements. Some participants had hypoglycemia.
Table A2.
Small-study effects in the relationship between sleep characteristics and glycemic control.
| Sleep variables | Analysis | Sleep measures | No. of studies | Type of subjects | Egger test |
|---|---|---|---|---|---|
| Sleep stages | MD in percentage of sleep stages between optimal and suboptimal glycemic controls | PSG, wireless sleep monitor | 5 | Adults | β = −2.45, SE = 2.70, p = 0.432 β = 2.72, SE = 2.33 , p = 0.328 |
| Sleep duration | MD in HbA1c between longer and shorter sleep durations | PSG, wireless sleep monitor or actigraphy | 6 | Adults | β = 0.53, SE = 0.59, p = 0.392 |
| Questionnaire | 4 | Adults | β = 0.74, SE = 1.42, p = 0.655 | ||
| Questionnaire | 3 | Adolescents/children | β = −3.22, SE = 5.33, p = 0.654 | ||
| MD in sleep duration between optimal and suboptimal glycemic control | PSG, wireless sleep monitor or actigraphy | 6 | Adults | β = −2.32, SE = 0.83, p = 0.048 | |
| Questionnaire | 4 | Adults | β = −0.70, SE = 0.57, p = 0.348 | ||
| Questionnaire | 4 | Adolescents/children | β = 11.674, SE = 4.605, p = 0.239 | ||
| Sleep quality | MD in HbA1c levels between good and poor sleep quality | PSG or actigraphy | 4 | Adults | β = −0.43, SE = 0.19, p = 0.149 |
| Questionnaire | 3 | Adults | β = −1.03, SE = 0.864, p = 0.445 | ||
| MD in sleep quality between optimal and suboptimal glycemic controls | PSG or actigraphy | 5 | Adults | β = −1.28, SE = 0.27, p = 0.018 | |
| OSA | MD in HbA1c levels between OSA and non-OSA | PSG or oximetry | 4 | Adults | β = −1.38, SE = 1.56, p = 0.468 |
| MD in HbA1c levels between moderate-severe OSA and non-OSA | PSG | 3 | Adults | β = 0.59, SE = 1.75, p = 0.791 | |
| MD in AHI between optimal and suboptimal glycemic controls | PSG | 4 | Adults | β = −1.32, SE = 1.03, p = 0.330 |
Abbreviations: AHI, apnea−hypopnea index; MD, mean difference; OSA, obstructive sleep apnea; PSG, polysomnography.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2016.03.019.
Appendix B: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.sleep.2016.03.019.
References
- [1].Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci 2014;1311:151–73. [DOI] [PubMed] [Google Scholar]
- [2].Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol 2012;3:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chiang JL, Kirkman MS, Laffel LM, et al. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Orchard TJ, Nathan DM, Zinman B, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Feupe SF, Frias PF, Mednick SC, et al. Nocturnal continuous glucose and sleep stage data in adults with type 1 diabetes in real-world conditions. J Diabetes Sci Technol 2013;7:1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hirschkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1:40–3. [DOI] [PubMed] [Google Scholar]
- [9].Buysse DJ, Reynolds CF III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [10].Bot M, Pouwer F, de Jonge P, et al. Differential associations between depressive symptoms and glycaemic control in outpatients with diabetes. Diabet Med 2013;30:e115–22. [DOI] [PubMed] [Google Scholar]
- [11].Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 2004;27:2942–7. [DOI] [PubMed] [Google Scholar]
- [12].Sivertsen B, Petrie KJ, Wilhelmsen-Langeland A, et al. Mental health in adolescents with type 1 diabetes: results from a large population-based study. BMC Endocr Disord 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Olsson L, Ahlbom A, Grill V, et al. Sleep disturbances and low psychological well-being are associated with an increased risk of autoimmune diabetes in adults. Results from the Nord-Trondelag Health Study. Diabetes Res Clin Pract 2012;98:302–11. [DOI] [PubMed] [Google Scholar]
- [14].American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien (IL): American Academy of Sleep Medicine; 2014. [Google Scholar]
- [15].Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714–55. [DOI] [PubMed] [Google Scholar]
- [16].Borel AL, Pepin JL, Nasse L, et al. Short sleep duration measured by wrist actimetry is associated with deteriorated glycemic control in type 1 diabetes. Diabetes Care 2013;36:2902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Dijk M, Donga E, van Dijk JG, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia 2011;54:1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–91. [DOI] [PubMed] [Google Scholar]
- [19].American Diabetes Association. Standards of Medical Care in Diabetes – 2015. Diabetes Care 2015;38. [Google Scholar]
- [20].Janovsky CC, Rolim LC, de Sa JR, et al. Cardiovascular autonomic neuropathy contributes to sleep apnea in young and lean type 1 diabetes mellitus patients. Front Endocrinol (Lausanne) 2014;5(119):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jauch-Chara K, Schmid SM, Hallschmid M, et al. Altered neuroendocrine sleep architecture in patients with type 1 diabetes. Diabetes Care 2008;31:1183–8. [DOI] [PubMed] [Google Scholar]
- [22].Perfect MM. The relations of sleep and quality of life to school performance in youth with type 1 diabetes. J Appl Sch Psychol 2014;30:7–28. [Google Scholar]
- [23].Perfect MM, Patel PG, Scott RE, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep 2012;35:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Borel AL, Benhamou PY, Baguet JP, et al. High prevalence of obstructive sleep apnoea syndrome in a type 1 diabetic adult population: a pilot study. Diabet Med 2010;27:1328–9. [DOI] [PubMed] [Google Scholar]
- [25].Bouhassira D, Letanoux M, Hartemann A. Chronic pain with neuropathic characteristics in diabetic patients: a French cross-sectional study. PLoS ONE 2013;8:e74195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Estrada CL, Danielson KK, Drum ML, et al. Insufficient sleep in young patients with diabetes and their families. Biol Res Nurs 2012;14:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Matejko B, Kiec-Wilk B, Szopa M, et al. Are late-night eating habits and sleep duration associated with glycemic control in adult type 1 diabetes patients treated with insulin pumps? J Diabetes Investig 2015;6:460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J 2011;5:165–72. [DOI] [PubMed] [Google Scholar]
- [29].Manin G, Pons A, Baltzinger P, et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabet Med 2015;32:90–6. [DOI] [PubMed] [Google Scholar]
- [30].Kilmek P, Kautzky-Willer A, Chmiel A, et al. Quantification of diabetes comorbidity risks across life using nation-wide big claims data. PLoS Comput Biol 2015;11:e1004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sturrock NDC, Moriarty KT. An assessment of perceived wellbeing in a diabetic population. Pract Diabetes Int 1995;12:260–2. [Google Scholar]
- [32].Yeshayahu Y, Mahmud FH. Altered sleep patterns in adolescents with type 1 diabetes: implications for insulin regimen. Diabetes Care 2010;33:e142. [DOI] [PubMed] [Google Scholar]
- [33].Varni JW, Limbers CA, Bryant WP, et al. The PedsQL Multidimensional Fatigue Scale in type 1 diabetes: feasibility, reliability, and validity. Pediatr Diabetes 2009;10:321–8. [DOI] [PubMed] [Google Scholar]
- [34].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. <http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp>; [accessed 24.03.14>.
- [35].Barone MT, Wey D, Schorr F, et al. Sleep and glycemic control in type 1 diabetes. Arch Endocrinol Metab 2015;59:71–8. [DOI] [PubMed] [Google Scholar]
- [36].Blanz BJ, Rensch-Riemann BS, Fritz-Sigmund DI, et al. IDDM is a risk factor for adolescent psychiatric disorders. Diabetes Care 1993;16:1579–87. [DOI] [PubMed] [Google Scholar]
- [37].Caruso NC, Radovanovic B, Kennedy JD, et al. Sleep, executive functioning and behaviour in children and adolescents with type 1 diabetes. Sleep Med 2014;15:1490–9. [DOI] [PubMed] [Google Scholar]
- [38].Donga E, van Dijk M, van Dijk JG, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care 2010;33:1573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Happe S, Treptau N, Ziegler R, et al. Restless legs syndrome and sleep problems in children and adolescents with insulin-dependent diabetes mellitus type 1. Neuropediatrics 2005;36:98–103. [DOI] [PubMed] [Google Scholar]
- [40].Matyka KA, Crawford C, Wiggs L, et al. Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: relationship to nocturnal hypoglycemia. J Pediatr 2000;137:233–8. [DOI] [PubMed] [Google Scholar]
- [41].Pillar G, Schuscheim G, Weiss R, et al. Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. J Pediatr 2003;142:163–8. [DOI] [PubMed] [Google Scholar]
- [42].Villa MP, Multari G, Montesano M, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia 2000;43:696–702. [DOI] [PubMed] [Google Scholar]
- [43].Mandl T, Granberg V, Apelqvist J, et al. Assessment of autonomic symptoms in diabetics: the Swedish version of the Autonomic Symptom Profile. Clin Physiol Funct Imaging 2008;28:312–17. [DOI] [PubMed] [Google Scholar]
- [44].Palladino DK, Helgeson VS, Reynolds KA, et al. Emerging adults with type 1 diabetes: a comparison to peers without diabetes. J Pediatr Psychol 2013;38:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bachle C, Lange K, Stahl-Pehe A, et al. Associations between HbA1c and depressive symptoms in young adults with early-onset type 1 diabetes. Psychoneuroendocrinology 2015;55:48–58. [DOI] [PubMed] [Google Scholar]
- [46].Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci USA 2008;105:1107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38:843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9. [DOI] [PubMed] [Google Scholar]
- [49].Buxton OM, Pavlova M, Reid EW, et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmid SM, Jauch-Chara K, Hallschmid M, et al. Short-term nocturnal hypoglycaemia increases morning food intake in healthy humans. Diabet Med 2008;25:232–5. [DOI] [PubMed] [Google Scholar]
- [51].Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008;105:1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–42. [DOI] [PubMed] [Google Scholar]
- [53].Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Guilleminault C, Briskin JG, Greenfield MS, et al. The impact of autonomic nervous system dysfunction on breathing during sleep. Sleep 1981;4:263–78. [DOI] [PubMed] [Google Scholar]
- [55].Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol 1985;17:391–5. [DOI] [PubMed] [Google Scholar]
- [56].Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol 2009;106:1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Oltmanns KM, Gehring H, Rudolf S, et al. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med 2004;169:1231–7. [DOI] [PubMed] [Google Scholar]
- [58].Jauch-Chara K, Schultes B. Sleep and the response to hypoglycaemia. Best Pract Res Clin Endocrinol Metab 2010;24:801–15. [DOI] [PubMed] [Google Scholar]
- [59].Bell KJ, Barclay AW, Petocz P, et al. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2014;2:133–40. [DOI] [PubMed] [Google Scholar]
- [60].Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012;157:336–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


