Abstract
With recent advances in myeloma therapy patients can achieve long-term remissions, but eventually relapses will occur. Triple-class refractory myeloma disease that is refractory to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody, and penta-refractory myeloma—disease that is refractory to 2 proteasome inhibitors, 2 immunomodulatory agents, and an anti-CD38 antibody are associated with a particularly poor prognosis and novel treatments are desperately needed to address these patients. Targeting B cell maturation antigen (BCMA), which is ubiquitously expressed on plasma cells, has emerged as a well-tolerated and highly efficacious strategy in patients with relapsed and refractory myeloma. Several mechanisms of targeting BCMA are currently under investigation including antibody drug conjugates, bispecific antibodies, and chimeric antigen receptor T and NK cells, all with unique side effect profiles. Early phase clinical trials showed unprecedented response rates in highly refractory myeloma patients leading to the recent approvals of some of these agents. Still, many questions remain with regard to this target including: how best to target it, how to treat patients who have progressed on a BCMA targeting therapy and if response rates will deepen if these agents are used in earlier lines of therapy. In this review, we examine the rationale for targeting BCMA and summarize the data for several agents across multiple classes of BCMA-targeting therapeutics paying special attention to the diverse mechanisms and unique challenges of each therapeutic class.
1. Introduction:
Multiple myeloma (MM) is a malignancy of terminally differentiated plasma cells which represents 18% of all hematologic malignancies and 1.8% of all cancers in the US [1, 2]. With the recent advent and widespread adoption of novel therapies-including proteasome inhibitors (PIs), immunomodulatory agents (IMiDs) and anti-CD38 monoclonal antibodies-in addition to high-dose chemotherapy and autologous stem cell transplant the median overall survival of newly diagnosed MM patients now approaches 10 years with some patients surviving significantly longer [3–8]. Unfortunately, all patients will eventually relapse and patients who are triple class refractory (refractory to a PI, an IMiD and an anti-CD38 antibody) and especially penta-refractory patients (refractory to 2 PIs, 2 IMiDs and an anti-CD38 antibody) have a particularly poor prognosis of < 6 months [9–12]. Additionally, duration of response with subsequent therapies after relapses typically becomes progressively shorter as MM becomes more refractory [13–15]. This underlies the urgent need for novel targets and treatment modalities in the refractory MM patient population.
2. BCMA in MM:
B cell maturation antigen (BCMA) also known as TNFRSF17 or CD269 is a type III transmembrane glycoprotein and non-tyrosine kinase receptor in the tumor necrosis factor receptor (TNFR) superfamily [16, 17]. BCMA expression is nearly absent on naïve and memory B cells but is selectively induced during plasma cell differentiation and is ubiquitously expressed on plasmablasts and plasma cells [18, 19]. Expression is rare in other tissues, with only low-level BCMA mRNA and protein expression seen in areas with endogenous plasma cell populations (i.e., the testes, GI tract, and trachea) [20–23]. BCMA, along with B-cell activation factor receptor (BAFF-R) and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), regulate B cell maturation and differentiation into plasma cells [24, 25]. BCMA −/− mice are able to generate short-lived plasma cells but are significantly deficient in long-lived plasma cells compared to wild type suggesting that BCMA is critical to maintaining a sustained humoral immune response [19, 26]. Similarly, murine models of BCMA overexpression promote in vivo MM cell growth [17]. Notably, BCMA expression is significantly higher in myeloma cells then normal plasma cells and levels also increase with progression from monoclonal gammopathy of undetermined significance (MGUS), to smoldering multiple myeloma (SMM) and active MM [20, 27, 28].
BCMA has two agonist ligands: a proliferation inducing ligand (APRIL) and B cell activation factor (BAFF) with APRIL having ~100 fold the affinity for BCMA than BAFF [29]. APRIL is primarily secreted by bone marrow stromal cells, osteoclasts, and macrophages in a paracrine fashion [17]. Serum levels of APRIL are higher in myeloma patients than healthy volunteers and correlate with ISS stage of newly diagnosed MM patients [17, 30–33]. Inhibition of APRIL with a monoclonal antibody led to decreased MM cell growth in a mouse xenograft models and the addition of APRIL to MM cell lines inhibited dexamethasone induced apoptosis [17, 32]. The downstream effects of BCMA binding to ligand are myriad and include activation of the NF-κβ, RAS/MAPK, and PI3K/AKT signaling pathways ultimately leading to the osteoclast mediated bone degradation, cell adhesion, and angiogenesis all of which promote MM proliferation and progression[17, 32, 34–36].
While BCMA is synthesized as a cell surface protein, cleavage of the extracellular domain and part of the transmembrane domain by γ-secretase results in the formation of a soluble form of BCMA (sBCMA) [37, 38]. Levels of sBCMA are significantly higher in MM patients than healthy controls and correlate with more advanced disease and poorer prognosis [28, 37, 39–44]. While smaller studies have shown that change in levels of sBCMA may serve as a surrogate of disease response to BCMA targeting therapy, this was not the case in the phase 1 DREAMM-1 study of the belantamab mafodotin (GSK2857916) a BCMA antibody-drug conjugate which is the largest study to date to report on the correlation of sBCMA levels and response to treatment [40, 43, 45]. These divergent findings may be explained by the finding of Chen et al who have shown that high levels of sBCMA may interfere with BCMA-targeted therapies by sequestering the antibody leading to less binding to surface BCMA [42]. This has led to the of investigation of administration of γ-secretase inhibitors (GSIs) to decrease surface BCMA shedding. Preclinical studies have shown that exposure to a GSI decreases sBCMA levels, increases surface BCMA expression and number of MM cells expressing surface BCMA, and increases responses to BCMA chimeric antigen receptor (CAR-T) therapy [46]. This noteworthy finding has led to early phase clinical trials evaluating the safety and efficacy of the combination of a GSI and BCMA targeting therapy in MM patients [47]. Alternatively, MEDI2228 another BCMA antibody-drug conjugate with preferential binding to surface BCMA, has shown improved cytotoxicity in preclinical models with high levels of sBCMA and has shown impressive efficacy in a phase 1 clinical trial of relapsed refractory MM patients (NCT3489525) [44, 48].
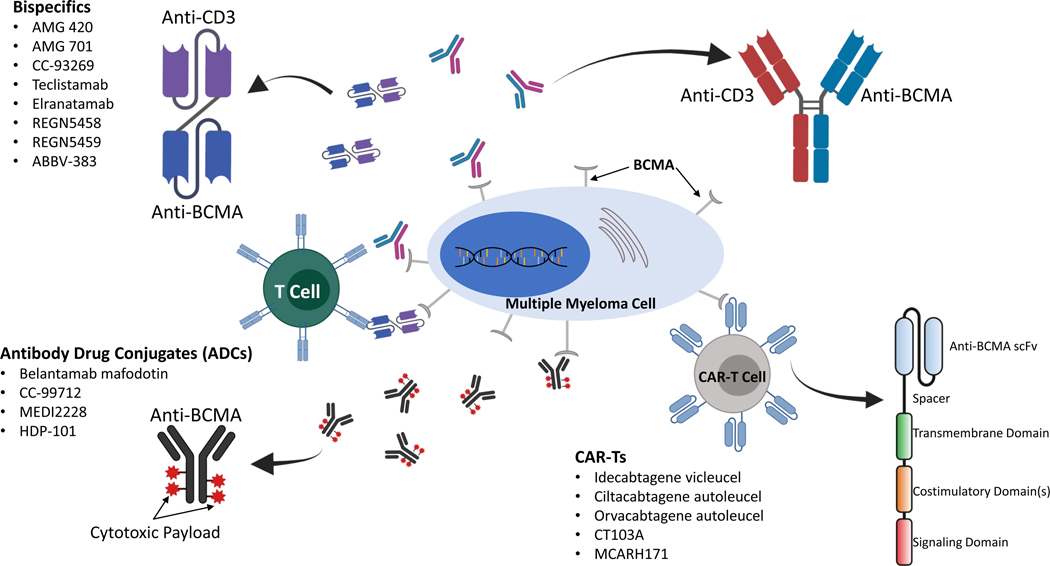
The efficacy of BCMA targeting in preclinical models and the potential for activity in MM patients who are refractory to conventional therapy has led to a wealth of research that has yielded multiple agents that target BMCA through a variety of mechanisms (Figure 1). Below we review these agents’ mechanisms of action and summarize the most recent data for relevant clinical trials.
Fig 1:
Mechanisms of action of BCMA targeting therapies (antibody-drug conjugates, bispecific antibodies, and CAR-Ts) see text for details
3. BCMA ADCs (Table 1)peer
Table 1:
BCMA ADCs
| Drug | Trial ID | Patient Population | Regimen | Phase | n | Median Prior Lines (Range) | ORR (%) | ≥VGPR (%) | PFS (months) | AE’s of Interest | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belantamab Mafadotin (GSK2857916) | NCT02064387 (DREAMM-1) | RRMM | Monotherapy | 1 | 73 (35 patients in part 2) | NR (67% ≥ 5) | 60a | 51.4a | 12a | Corneal Toxicitya: 63% grade 1 or 2 9% grade 3 | 45 |
| NCT03525678 (DREAMM-2) | 2 | 97 (2.5 mg/kg) | 7 (3–21) | 31 | 19 | 2.9 | Corneal Toxicity: 43% grade 1 or 2 27% grade 3 | 60–61 | |||
| NCT03544281 (DREAMM-6) | 99 (3.4 mg/kg) | 6 (3–21) | 34 | 20 | 4.9 | Corneal Toxicity: 54% grade 1 or 2 20% grade 3 1% grade 4 | |||||
| NCT02064387 (DREAMM-1) | ≥1 prior lines | Combination with Vd or Rd | 1/2 | 18 with BVdb | 3 (1–11) | 78 | 50 | NR | Corneal Toxicity: 44% grade 1 or 2 56% grade 3 | 66 | |
| NCT04484623 (DREAMM-8) | ≥1 prior lines | Combination with Pd or Rd | 1/2 | 60 | 3 (1–5) | 89 | 74 | NR | Corneal Toxicity: 73% grade 3 | 68 | |
| MEDI2228 | NCT03489525 | RRMM | Monotherapy | 1 | 8241 patients treated at MTD (0.14 mg/kg) | NR (2–11) | 66c | 27c | NR | Photophobiac: 41% grade 1 or 2 17% grade 3 or 4 | 48 |
At the recommended part 2 dose (3.4 mg/kg)
Only the data on the 18 patients treated with BVd (2.5mg/kg belantamab mafadotin) have been presented
Represents patients treated at the MTD (0.14mg/kg)
Abbreviations: AE: Adverse event; ORR: Overall response rate; VGPR: Very good partial response; PFS: progression-free survival; RRMM: relapsed refractory multiple myeloma; Vd: bortezomib and dexamethasone; Rd: lenalidomide and dexamethasone; Pd: Pomalidomide dexamethasone;BVd: belantamab mafadotin, bortezomib and dexamethasone; NR: not reported; MTD: maximum tolerated dose
Antibody drug conjugates (ADCs) are monoclonal antibodies (mAb) directed towards a highly expressed antigen on the cell surface of MM cells with the goal of delivering a toxic “warhead” payload bound to a linker [49–51]. When the mAb or antibody fragment binds to its target, it gets internalized and the cytotoxic drug is released by either linker cleavage or antibody dissolution, leading to cell damage and death. This modality allows for very specific drug delivery with limited off-target toxicities. Payloads must be stable to travel to the antigen site bound to the mAb and amass intracellular concentrations that are toxic to the target cells. Commonly used agents for myeloma include tubulin polymerase inhibitors like auristatin, RNA polymerase inhibitors like amanitin, or DNA-targeting agents [52–54]. While several different ADC targets are being studied for MM, compounds targeting BCMA with a multitude of cytotoxic warheads are the most mature [48, 49].
3.1. Belantamab mafodotin (GSK2857916)
The agent that spearheaded BCMA targeting ADCs in MM is belantamab mafodotin, a first in class humanized IgG1 monoclonal engineered antibody that is afucosylated and is bound to a microtubule inhibitor monomethyl auristatin-F (MMAF) through a protease resistant maleimidocaproyl linker [55]. Once the ADC is bound and internalized by the targeted cell, the payload is released and binds to tubulin leading to cell cycle arrest and eventually apoptosis [56]. More recent data also suggests an important role of immune mediated cellular killing through antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) [57, 58]. The phase I DREAMM-1 study enrolled 73 MM patients previously treated with stem cell transplant, alkyalators, proteasome inhibitors, and immunomodulators and consisted of two parts: a dose-escalation portion of 38 patients treated every three weeks, and a 35-patient dose-expansion. There was no maximum tolerated dose reached or dose limiting toxicities. The most common non-hematologic side effect was corneal toxicity which occurred in 63% of those who participated in part two (treating dose of 3.4mg/kg). Of these toxicities, 54% were grade 1 and 2 with no treatment discontinuations. Regarding grade 3 and 4 hematologic toxicities, thrombocytopenia was seen in 34% and anemia was seen in 14% of participants. Responses were seen in 60% of patients treated in the dose expansion with a median PFS of 12 months and duration of response of 14.3 months [45, 59].
The phase II registrational trial (DREAMM-2) used the recommended dose from the prior study (3.4mg/kg every 3 weeks) and added a second arm at a dose of 2.5 mg/kg to evaluate safety and activity of the drug in 196 patients in eight countries. All participants had received 3 or more prior lines of therapy and were refractory or intolerant to an IMiD, PI, and an anti-CD38 antibody therapy. Overall, response rates were 31% in the 2.5mg/kg arm and 34% in the 3.4mg/kg group. Median PFS was 2.9 months in the 2.5mg/kg arm and 4.9 months in the 3.4mg/kg arm and median duration of response was 11 months [60]. The most common grade 3 and 4 adverse events were similar to the prior study and included keratopathy in 27% of patients treated with the 2.5 mg dose and 21% of patients on the 3.4 mg arm, as well as thrombocytopenia (20% and 33% respectively) and anemia (20% and 25%) [61, 62]. These results in heavily pre-treated patients with manageable toxicities led to FDA approval on August 5, 2020 at a dose of 2.5 mg/kg every three weeks in patients with at least 4 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 antibody [56]. Given the incidence of keratopathy attributed to MMAF which was seen in 70% of all patients treated, a REMS program was established requiring ophthalmologic evaluations prior to each administration [50, 61, 63].
While the DREAMM-2 study did show that belantamab mafadotin has single-agent activity in RRMM, studies are currently evaluating using this agent in combination with other MM therapies at earlier stages of disease with the hope of improved response rates [56, 64, 65]. Preliminary data presented in 18 patients treated in with belantamab mafadotin, bortezomib, and dexamethasone showed response rates of 78% and had comparable adverse events [66, 67]. When combined (at two different dose levels) with pomalidomide and dexamethasone, response rates increased to 82–95% [68, 69]. Given the increased frequency of ocular toxicity, decreased doses at 1.92 mg/kg and frequency of every 4 weeks were performed [70]. Other combinations with agents such as lenalidomide, daratumumab, and pembrolizomab are being tested for safety and response rates in RRMM patients [71]. The interest in using BCMA-targeted therapy upfront to achieve deeper responses has led to a clinical trial in treatment-naïve transplant ineligible patients to determine if the addition of belantamab mafadotin to conventional induction therapy could induce deeper response and change the course of the disease [72].
3.2. MEDI2228
MEDI2228 is a fully human antibody with a cleavable linker containing the DNA cross-linking agent pyrrolobenzodiazepine (PBD) dimer tesirine [73]. In preclinical studies, MEDI2228 activated critical DNA damage responses (DDR) via phosphorylation of ATM/ATR kinases, checkpoint kinases (CHK)1/2, and H2AX leading to apoptotic cell death. Additionally, MEDI2228 was more efficacious than MMAF in preclinical testing regardless of amount of soluble BCMA, tumor microenvironment conditions, or presence of high risk cytogenetics [74]. Results of a first-in-human phase I study with 82 patients who had received at least 3 prior lines of therapy showed response rates of 61% and duration of response was not reached. Unlike MMAF-containing agents, no keratopathies were reported but it did cause photophobia in 53% of those who received therapeutic doses. Additional non-hematologic toxicities included rash (29%), dry eyes (20%), and pleural effusions (20%) Thrombocytopenia was the most common hematologic toxicity and was seen in 33% of patients [48]. The maximum tolerated dose was 0.14 mg/kg every three weeks. Unfortunately, development of MEDI2228 was halted given the crowded landscape of BCMA-targeted ADCs and significant toxicity seen with this agent [75].
3.3. CC-99712
This agent contains a maytansinoid toxic payload that forms part of the microtubular polymerization inhibitors and is attached to a non-cleavable linker [76, 77]. A Phase I dose escalation to determine maximum tolerated dose (MTD) and expansion arms to establish safety is ongoing.
3.4. HDP-101
HDP-101 is a BCMA ADC with a novel amanitin derivative as its payload which inhibits formation of mRNA in the targeted cell. This mechanism is unique in that it is works independently of mitotic cell division making it effective in less proliferative, more dormant clones [78, 79]. The first-in-human phase I/IIa study plans to enroll 36 patients in the dose-escalation phase and 30 patients in the dose-expansion portion [79]. There is special interest in patients with 17p deletion given the increased susceptibility seen in preclinical studies when compared to wild-type myeloma cells [80].
4. BCMA bispecifics (Table 2)
Table 2:
BCMA Bispecific Antibodies
| Drug | Trial ID | Patient Population | Regimen | Phase | n | Median Prior Lines (Range) | ORR (%) | ≥VGPR (%) | PFS (months) | CRS Rate | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMG 420 | NCT03836053 | RRMM | Monotherapy | 1 | 42 | 5 (2–14) | 31 | 24 | NR | 36% grade 1 or 2 2% grade 3 | 91 |
| AMG 701 | NCT03287908 | RRMM | Monotherapy | 1 | 75 | 6 (1–25) | 26 | 17 | NR | 53% grade 1 or 2 7% grade 3 | 94 |
| CC-93269 | NCT03486067 | RRMM | Monotherapy | 1 | 19 | 6 (3–12) | 53 (83% in patients treated with ≥6 mg dose) | 37 (58% in patients treated with ≥6 mg dose | NR | 68% grade 1 or 2 21% grade 3 | 96 |
| Teclistamab (JNJ-64007957) | NCT03145181 (MajesTEC-1) | RRMM | Monotherapy | 1 | 84 IV | 6 (2–14) | 38a | 30a | NR | 56% grade 1 or 2 0% grade 3 or 4 | 100–101 |
| 72 SC | 5 (2–14) | 63 | 58 | 60% grade 1 or 2 0% grade 3 or 4 | |||||||
| Elranatamab (PF-06863135) | NCT03269136 (MagnetisMM-1) | RRMM | Monotherapy | 1 | 50 SC | 8 (3–15) | 70b | 65b | NR | 83% grade 1 or 2 0% grade 3 or 4 | 104–105 |
| REGN5458 | NCT03761108 | RRMM | Monotherapy | 1 | 73 | 5 (2–17) | 51 | 44 | NR | 38% grade 1 or 2 0% grade 3 or 4 | 109 |
| ABBV-383 (TNB-383B) | NCT03933735 | RRMM | Monotherapy | 1 | 118 | 5 (1–15) | 47 (801% at doses ≥ 40mg) | 33 (73% at doses ≥ 40mg) | NR | 52% grade 1 or 2 3% grade 3 or 4 | 111–112 |
Only includes patients dosed ≥38.4 mg/kg
Only includes patients dosed ≥215 mg/kg
Abbreviations: ORR: Overall response rate; VGPR: Very good partial response; PFS: progression-free survival; CRS: Cytokine release syndrome; RRMM: relapsed refractory multiple myeloma; IV: Intravenous; SC: subcutaneous; NR: not reported; MTD: maximum tolerated dose.
A different modality of BCMA-directed immunotherapy is through bispecific antibodies. These constructs are engineered proteins that mimic the antibody activity but have two different binding domains, one that targets a tumor-specific antigen, and another that binds to T cells with the goal of eliciting interactions between immune-effector cells and MM cells to provoke T cell mediated cellular death [81]. The most common epitopes in immune cells are CD3 and CD16, while the most studied target in myeloma cells is BCMA, although other targets are currently under evaluation. While several different models are currently being used in immunotherapy such as those that resemble the traditional IgG structure and others that lack the Fc region, all contain at least 2 antigen-binding fragments (Fab). Early phase trials with these agents have shown excellent efficacy but have also been notable for the risk of cytokine release syndrome (CRS) and, more rarely, immune effector cell-associated neurotoxicity syndrome (ICANS). Briefly, CRS is a systemic inflammatory response thought to be secondary to activation of bystander immune and nonimmune cells leading to a significant cytokine (particularly IL-1, and IL-6) release [82, 83]. Clinical manifestations of mild CRS include, fever, fatigue, headache, arthralgias, and myalgias and can typically be managed with antipyretics, intravenous fluids, and the anti-IL-6 antibody tocilizumab. More severe cases can progress to hypotension, shock, DIC, and multiorgan system failure and require more aggressive care including vasopressors, high-dose corticosteroids, and supplemental oxygen[84–86]. It should be noted that these symptoms are often difficult to differentiate from systemic infection so empiric anti-infectives are recommended. ICANS is a form of toxic encephalopathy and a less common complication of T-cell mediated therapies. Clinical manifestations of ICANs are diverse with symptoms as varied as headache, aphasia, confusion, impaired fine motor skills and somnolence. More severe cases can progress to seizures, cerebral edema, and coma [87]. The pathophysiology of ICANS is less well understood but is thought to be related to cytokine mediated disruption of the blood-brain-barrier leading to cytokine and immune cell accumulation in the CNS resulting in direct neuronal injury [88, 89]. ICANS is typically managed with high-dose corticosteroids (and tocilizumab if coexisting CRS) [90]. Below we will focus on the constructs that target BCMA and have demonstrated clinical activity against MM.
4.1. AMG 420
The first bispecific to demonstrate positive results in MM was AMG 420. This agent, termed bi-specific T cell engager (BiTE®), consists of two single-chain variable fragments with one targeting CD3 and the other BCMA. The first in human phase I study required a continuous infusion given its very short half-life and was administered over 4 weeks with a 2-week break between cycles. Forty-two patients were enrolled in the dose-escalation phase that established 400 μg/d as the MTD. Cytokine release syndrome (CRS) was seen in 38% of treated patients and 29% developed infections. Of the 10 patients treated at the 400 μg/d dose, 70% achieved a response and 5 obtained minimal residual disease (MRD) negative status (defined as 10−4) [91]. A phase I study to confirm the MTD in multiple countries was opened but discontinued prior to completing enrollment given the risk of infections and significant patient burden of the continuous infusion. Additionally, several agents including AMG 701 (see below) had been developed with longer half-lives which allowed for a more amenable dosing schedule.
4.2. AMG 701
This BiTE® was designed to overcome the short half-life of AMG 420. It includes a tandem scFv and an Fc portion that prolongs the construct’s stability [92]. Preclinical models demonstrated dosing could be on a weekly basis as a single agent or in combination with immunomodulators with good T cell-dependent cellular toxicity (TDCC) [93]. A phase I trial to evaluate the safety and activity in humans included 75 patients. Preliminary data reported a 61% incidence of CRS with 5 cases being grade 3 that reversed with treatment [94]. Other common adverse events included anemia (43%), neutropenia (23%), and diarrhea (31%). Treatment-related neurotoxicity was seen in 6 patients. Responses to treatment were seen at doses of 3mg and higher, but final dose for the expansion portion dose has not been established yet. This study plans to open arms of combination therapy with lenalidomide and/or pomalidomide in the near future.
4.3. CC-93269
This bi-specific agent is an asymmetric bi-valent IgG compound that binds to CD3 (monovalently) and BCMA (bivalently) [95]. Data from the first 30 patients who participated in the dose escalation portion of a phase I study as a once-a-week single agent showed response rates of 43% overall, but 83% in patients treated with the 6mg dose and higher. Additionally, 92% of the responders achieved MRD negativity at 10−5. Median number of prior lines of therapy was 5. CRS was reported in 77% of cases with most being grade 1 or 2, however 1 death was associated with CRS but was also complicated by coexisting infection. Given the high rate of CRS this study has implemented prophylactic dexamethasone in all patients receiving doses ≥6 mg [96].
4.4. Teclistamab (JNJ-64007957)
Teclistamab is an IgG4 engineered antibody targeting CD3 and BCMA that showed cytotoxic activity in vitro by T cell activation and cytokine release [97]. The phase I study evaluated treatment as single agent to evaluate route of administration, safety, and dosing supported. Early data supported weekly dosing and subcutaneous (SQ) administration based on PK sampling. The most common toxicities included CRS (56%), neutropenia (26%), and anemia (23%) with two grade 4 dose-limiting toxicities: delirium and thrombocytopenia [98]. Updated data from the recommended phase II dose (1500 μg/kg weekly SQ dosing) given to 40 patients that formed part of the phase I study showed 65% response rates with a median of 5 prior lines of therapy. The study included a step-up dosing of 60 μg/kg and 300 μg/kg to reduce toxicities and reported 70% CRS with none being grade 3 or 4 [99, 100]. The longest duration on therapy reported has been 18 months. The combination of teclistamab with daratumumab +/− pomalidomide is being investigated for synergistic effects [100, 101].
4.5. Elranatamab (PF-06863135)
Elranatamab is a humanized monoclonal antibody that targets BCMA and CD3 like other agents, but unlike other agents it is paired to an IgG2a Fc backbone using hinge mutation technology [102]. Preclinical data showed a half-life of 4–6 days that led to the dosing of the first-in-human study [103]. The phase I dose-escalation study has evaluated SQ monotherapy in 50 patients and elranatmab in combination with lenalidomide or pomalidomide in an additional 8 patients. Overall response rate was for the monotherapy was 70% at doses of 215 μg/kg or higher with 30% CR/sCR. Safety profile consisted of 73% CRS, 57% anemia, 53% thrombocytopenia and injection site reaction, with neutropenia in 40% of cases. Notably, 10 patients enrolled were previously treated with a BCMA-directed therapy (either an ADC or CAR-T). The response rate among this subset was 70% suggesting that this agent has activity in the population. Duration of response and final recommended phase II dosing have not yet been established. [104, 105].
4.6. REGN5458
REGN5458 is a fully humanized bispecific antibody with an Fc region and Fab arms that bind to CD3 and BCMA [106]. REGN5458showed more rapid cellular killing than BCMA CAR-Ts in preclinical activity against myeloma cell lines, primary myeloma cells, and murine xenograft models[107]. Preliminary data of the dose-escalation portion of a phase I study with 73 patients showed a tolerable safety profile with 38% of patients having grade 1 or 2 CRS using a weekly IV dosing schema which transitioned to every other week at maintenance. Other commons side effects included anemia in 32% of cases, fatigue (45%), and fever (36%). Three patients (4%) had grade 2 ICANS. Overall response rates were 51% among all doses and 75% at doses of 200–800 mg [109]. Of those who responded, 86% achieved a VGPR or better. A follow-up registrational phase II study is currently enrolling. REGN5459 is a similar bispecific antibody therapy targeting CD3 and BCMA but contains different binding characteristics. It is currently under investigation in early phase clinical trials which are expected to report results in 2023 [106].
4.7. ABBV-383 (Formally TNB-383B)
ABBV-383 is a fully human triple chain IgG4 antibody with 2 anti-BCMA domains to favor cell surface binding [110]. The IgG4 silenced backbone confers a half-life of 18 days, making every 3-week intravenous dosing a possibility. A phase I, first-in-human study of single-agent ABBV-383 dosed every 3 weeks in 118 RRMM patients who had received a median of 5 prior lines was recently reported. Safety profile showed 69% rate of CRS with 4% grade 3 events. Other toxicities included neutropenia (32%), anemia (28%), thrombocytopenia (23%), diarrhea (27%) and nausea (27%). Overall response rate at doses of ≥ 40mg was 60% with more than 40% achieving a VGPR or better [111, 112]. The convenience of its dosing and efficacy has led to plans for a phase II study.
5. BCMA CAR-Ts (Table 3)
Table 3:
BCMA CAR-Ts
| Drug | Trial ID | Patient Population | Regimen | Phase | n | Median Prior Lines (Range) | ORR (%) | ≥VGPR (%) | PFS (months) | CRS Rate | ICANS Rate | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Idecabtagene vicleucel (bb2121, ide-cel, Abecma) | NCT03361748 (KarMMa-2) | RRMM | Monotherapy | 2 | 128 | 6 (3–16) | 73 | 53 | 8.8 | 78% grade 1 or 2 5% grade 3 or 4 | 15% grade 1 or 2 3% grade 3 or 4 | 137–139 |
| Ciltacabtagene autoleucel (JNJ-68284528, cilta-cel) | NCT03548207 (CARTITUDE-1) | RRMM | Monotherapy | 1/2 | 97 | 6 (3–18) | 98 | 95 | 61% at 24-months | 90% grade 1 or 2 5% grade 3 or 4 | 14% grade 1 or 2 2% grade 3 or 4 | 147 |
| NCT04133636 (CARTITUDE-2) | 1–3 prior lines | Monotherapy | 2 | 20 | 2 (1–3) | 95 | 95 | NR | 85% grade 1 or 2 10% grade 3 or 4 | 15% grade 1 or 2 0% grade 3 or 4 | 148 | |
| Orvacabtagene autoleucel (JCARH125, orva-cel) | NCT03430011 (EVOLVE) | RRMM | Monotherapy | 1/2 | 62 | 7 (2–24) | 92 | 68 | NR | 85% grade 1 or 2 3% grade 3 or 4 | 10% grade 1 or 2 3% grade 3 or 4 | 149–151 |
| CT053 | NCT03716856 NCT03302403 NCT03380039 | RRMM | Monotherapy | 1 | 24 | 4.5 (2–11) | 88 | NR | NR | 63% grade 1 or 2 0% grade 3 or 4 | 8% grade 1 or 2 4% grade 3 or 4 | 152–153 |
| MCARH171 | NCT03070327 | RRMM | Monotherapy | 1 | 11 | 6 (4–14) | 64 | NR | NR | 40% grade 1 or 2 20% grade 3 or 4 | 10% grade 1 or 2 0% grade 3 or 4 | 154 |
Abbreviations: ORR: Overall response rate; VGPR: Very good partial response; PFS: progression-free survival; CRS: Cytokine release syndrome; ICANS: Immune effector cell-associated neurotoxicity syndrome; RRMM: relapsed refractory multiple myeloma; NR: not reported.
Chimeric antigen receptor T cells (CAR-Ts) are T cells modified to contain an extracellular ligand binding domain made up of a fusion protein-most commonly a single-chain variable fragment (scFv) of a monoclonal antibody-designed to recognize one or more tumor-associated antigens (TAAs) on cancer cells. The ligand binding domain is fixed to extracellular spacer elements and a transmembrane domain, connected to a CD3ζ intracellular activation domain and one or more intracellular costimulatory domains (typically CD28, 4–1BB, CD27 and/or OX40) [113–117]. As they can recognize intact proteins and don’t require antigen presentation by antigen presenting cells, they are able to bypass several mechanisms of immune tolerance in contrast to HLA restricted traditional T cell receptors [118, 119]. Once bound to TAAs CAR-Ts initiate signaling through T cell activation and subsequent release of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interferon γ (IFN-γ), interleukin 2 (IL-2), and interleukin 6 (IL-6) leading to cytolysis [120, 121]. CAR-Ts are generated through leukapheresis of autologous CD3+ T cells followed by introduction of the scFv and co-stimulatory domains with a viral or retroviral vector, however, subtle differences in production are now being correlated to efficacy of specific products (described in more detail below). Prior to infusion of the CAR-T product, patients receive lymphodepleting conditioning chemotherapy (typically with fludarabine and cyclophosphamide) in hopes of decreasing endogenous T cells, especially regulatory T-cells, and increasing the levels of proliferative cytokines IL-7 and IL-15 to create a more favorable environment for the CAR-T cells to inhabit [77, 122, 123]. This technology has most notably been used to create autologous CAR-Ts directed against CD19 which have shown unprecedented results in refractory B cell malignancies and received FDA approval for B Cell ALL and relapsed refractory DLBCL in 2017 [124, 125]. Similar to the bispecific antibodies there is a risk of CRS and ICANS with CAR-T infusion. Rates of ICANS in particular tend to be higher in CAR-T trials then in similarly powered bispecific antibody trials.
BCMA is the most widely studied target of CAR-Ts for MM. Preclinical models of human myeloma demonstrated significant efficacy of autologous BCMA CAR-Ts even against myeloma cells with little expression of cellular BCMA [126, 127]. Currently, at least 15 autologous BMCA targeting CAR-Ts-each with unique antigen targets or molecular structure-are under investigation in clinical trials with other constructs targeting both BCMA and an additional antigen in early-stage trials [125, 128–133]. Recently, trials of allogenic CAR-Ts targeting BCMA have opened which have several advantages over autologous CAR-Ts which are discussed below [125]. Below we summarize the available data on specific BCMA targeting CAR-Ts.
5.1. Idecabtagene vicleucel (ide-cel; bb2121, Abecma)
Ide-cel is a second-generation CAR which uses a lentiviral vector to transduce a BCMA targeting scFv fused to a 4–1BB co-stimulatory and CD3ζ signaling domains [126, 134]. Preclinical studies of ide-cel showed potent activity in MM cell lines expressing cellular BCMA regardless of sBCMA levels [126]. Based on this data a multicenter phase I study of ide-cel in 33 relapsed/refractory multiple myeloma (RRMM) patients who had received ≥ 3 prior lines of treatment was undertaken and showed an overall response rate (ORR) of 85% with 45% of patients achieving a complete response (CR) or better. Sixteen patients were evaluated for minimal residual disease (MRD) status with 15 (94%) MRD negative at 10−5 and 3 (19%) MRD negative at 10−6. Median PFS was 11.8 months. Cytokine release syndrome (CRS) occurred in 25 (76%) patients, with 2 (6%) patients (6%) having grade 3 CRS and no patients experiencing grade 4 CRS. Median time to onset of CRS was 1 day. Immune effector cell-associated neurotoxicity syndrome (ICANS) was seen in 14 (42%) of patients, but most were grade 1–2 with only 1 patient having ≥ 3 ICANS [134].
Based on this data a multicenter phase II trial of 128 RRMM who had received ≥ 3 prior lines of therapy including an IMiD, PI, and an anti-CD38 monoclonal antibody was opened. Patients were infused with 150×106 to 450×106 CAR-T cells. ORR was 73%, with 42 (33%) patients having a CR or better. Overall MRD negative rate (at 10−5) was 26%, but it was 79% in the patients who achieved a CR or better. Notably, these response rates were relatively preserved across patients with high-risk features including penta-refractory disease, extramedullary disease, and high-risk cytogenetics. Median PFS was 8.8 months and 20.2 months in patient achieving a CR or better. CRS was seen in 84% of patients with only 7 (5%) patients having ≥ grade 3. Similarly, ICANS was seen in 18% of patients with only 4 (3%) patients having ≥ grade 3. Interestingly, 93% of patients who progressed still had detectable levels of cellular BCMA on their MM cells suggesting that antigen loss is not a dominant mechanism for resistance [135, 136]. Based on these findings, ide-cel was approved for the treatment of adults with RRMM after four or more prior lines of therapy including an IMiD, PI, and anti-CD38 monoclonal antibody by the US Food and Drug Administration (FDA) in March 2021.
Several other studies are currently evaluating the utility of ide-cel in various populations including: use in front line therapy as consolidation in place of ASCT in high-risk MM, patients with suboptimal response or early relapse after ASCT, in combination with standard of care backbones, and the phase 3 trial of ide-cel vs standard of care in patients with relapsed disease after 2–4 prior lines of therapy [137–139]. Additionally, a newer formulation termed bb21217, has recently been developed. This agent is produced when bb2121 CAR-T cells are cultured in the presence of the PI3K inhibitor bb007 to select for more memory T cells in hopes of increasing the persistence of the CAR-T product in the host [140, 141]. Results of 46 RRMM patients treated with bb21217 in a multi-center phase I clinical trial showed a 55% ORR with 18% CR or better. CRS was seen in 67% of patients with median onset at 3 days. The majority of CRS was low-grade however, with only 2 patients having ≥ grade 3. ICANS was seen in 22% of patients with 3 patients with ≥ grade 3. Notably, biomarker analysis showed that increased levels of memory T cell markers in the drug product correlated with a sustained clinical response at 6 months [142].
5.2. Ciltacabtagene autoleucel (cilta-cel; LCAR-B38M; JNJ-68284528)
Similar to ide-cel, cilta-cel uses a lentiviral vector to create a construct with a CD3ζ activation domain, and 4–1BB costimulatory domain. Cilta-cel’s antigen binding domain contains bispecifc scFvs targeting two distinct BCMA epitopes, VHH1 and VHH2 [143]. This bi-epitope binding confers higher avidity and specificity to BCMA. Initially, cilta-cel was investigated in a phase I trial of 74 RRMM patients conducted at 4 institutions in China who had progressive disease after ≥ 3 prior lines of therapy. Data presented on the first 57 patients enrolled showed an ORR of 88%, with 68% of patients achieving a CR or better. MRD negativity at 10−4 was achieved in 63% of patients. Median PFS was 15 months. CRS was seen in 90% of patients with the vast majority (82%) being grade 1 of 2 and only 4 (7%) patients with ≥ grade 3. ICANS was observed in 1 (2%) patient and was only grade 1 [144].
Based on these findings the phase Ib/II CARTITUDE-1 clinical trial was conducted in RRMM patients in the United States and Japan. Patients were required to have been treated with ≥ 3 prior lines or were double refractory to an IMiD and a PI and had previously received an anti-CD38 antibody. 97 RRMM patients (median 6 prior lines) were treated with cilta-cel (29 in the phase 1b portion, 68 in the phase 2 portion). Notably, 84% of patients were penta-exposed and 42% of patient were penta-refractory. ORR was 98%, with 95% of patients achieving a VGPR or better. Of the 61 patients evaluable, MRD negativity at 10−5 was achieved in 92% of patients. Median PFS has not been reached at 12.4 months. CRS was seen in 95% of patients with the vast majority (91%) being grade 1 of 2. ICANS was observed in 16.5% of patients and was predominately grade 1 or 2 with only 2% ≥ grade 3. Grade 3 and 4 hematologic toxicities were very common with specific rates of 92% neutropenia, 68% anemia, and 60% thrombocytopenia see across all cohorts [144–147]. Based on these data cilta-cel was granted breakthrough therapy designation from the FDA.
This has led to the multicohort, open-label, phase 2 CARTITUDE-2 trial which enrolled patients previously treated with 1–3 prior lines. Recently, data on the first 20 patients were presented. Median prior lines of therapy were 2 and all patients were previouly exposed to a PI and an IMiD. Nearly all (95%) of patient were previously treated with an alkylating agent, and 65% of patient had received prior daratumumab. ORR was 95% and responses were at least a VGPR. Of the 13 evaluable patients 92% were MRD negative at 10−5. Grade 3/4 hematologic toxicity was common (neutropenia 95%, anemia 45%, thrombocytopenia 35%), as was CRS (95%), but was predominately grade 1 or 2 (10% grade 3 or 4). ICANS was seen in 15% of patients and all were grade 1 or 2. At a median follow-up of 9.7 months PFS was not yet reached [148]. Recently additional cohorts have opened investigating the use of cilta-cel as consolidation after induction therapy and as second line therapy in patients with suboptimal response to stem cell transplant.
A phase III trial (CARTITUDE-5) comparing the efficacy of cilta-cel versus pomalidomide, bortezomib and dexamethasone (PVd) or daratumumab, pomalidomide and dexamethasone (DPd) in patients with lenalidomide-refractory MM is ongoing (NCT04181827).
5.3. Orvacabtagene Autoleucel (orva-cel; JCARH125)
Orva-cel is a BCMA-targeting CAR-T product containing a lentiviral CAR construct with a fully human scFv, an optimized spacer, and 4–1BB co-stimulatory and CD3ζ activation domains. In a multicenter phase I/II trial of patients with RRMM who received ≥ 3 prior lines including a PI, IMiD, anti-CD38 antibody and autologous stem cell transplant (ASCT). Results of 62 evaluable patients treated with the higher dose levels (300, 450, and 600 × 106 CAR-T cells) showed a 92% ORR with 36% of patients achieving a CR or better. Remarkably, 100% of patients treated at the highest dose (600 × 106) were MRD negative at 10−5. CRS was seen in 89% of patients, but only 3% experienced ≥ grade 3; ICANS was seen in 13% of patients and only 3% experienced ≥ grade 3 symptoms. Notably, all patients with high baseline sBCMA responded to the agent [149–151]. Unfortunately, commercial development of ovra-cel was recently discontinued in favor of an alternative second-generation CAR-T (CC-98633) with a more rapid production time in hopes that more patients will be able to avoid bridging therapy if they can receive their CAR-T product more quickly.
5.4. CT103A
CT103A is a second-generation chimeric antigen receptor (CAR) utilizing a fully human BCMA-specific single-chain fragment variant (25C2) with high binding affinity to BCMA bound to 4–1BB co-stimulatory and CD3ζ activation domains. A multicenter phase I trial of this agent was conducted in China and enrolled 24 RRMM patients who had received 2 more prior lines. The ORR was 88% with 79% achieving a CR or better. Median PFS was 18.8 months. Among 20 subjects who underwent evaluation for MRD status, 17 were MRD negative at 10−4. CRS was seen in 63% of patients, all of which was grade 1 or 2. Onset of CRS was 1–4 days. ICANS was seen in 3 patients and with 1 patient having grade 3 symptoms [152, 153].
5.5. MCARH171
MCARH171 is a second-generation CAR-T composed of a humanized scFv, a 4–1BB costimulatory domain, and a truncated epidermal growth factor receptor safety signal. An phase I dose escalation trial of MCARH171 is currently ongoing. Data on 11 RRMM patients had been present which showed an ORR of 64%, with a median duration of response of 106 days. Of the 10 evaluable patients 6 (60%) experienced CRS with 2 (20%) having grade 3 symptoms. One (10%) patient had grade 2 ICANS [154].
5.6. Combination and Bispecific CAR-Ts
Combinations of multiple CAR-Ts and CAR-Ts targeting more than one antigen are currently in development. The most popular combination is BCMA and CD19. This combination is based on the finding that a small component of MM cells express CD19, and these cells are considered to be less-differentiated and therefore may lack conventional MM markers such as BCMA. [155, 156].
A phase II single-center clinical trial at the Affiliated Hospital of Xuzhou Medical University in China looked at co-infusion with both a BCMA and CD19 CAR-T. A total of 21 RRMM patients received the two CAR-T products. ORR was 95% with 57% achieving a CR or better. PFS had not been reached at median follow-up of 6 months. CRS was seen in 90% of patients, but only 1 patient had grade 3 or higher. Interestingly, only 2 (10%) patients had any ICANS symptoms [132].
The University of Pennsylvania conducted a trial of a combination of BCMA and CD19 CAR-Ts as consolidation therapy in patients responding to their prior therapy. The study was conducted in two parts: Part A of the trial enrolled 7 patients treated with ≥ 3rd line (or ≥ 2nd line if previously exposed to all major agents) who had achieved a minor response or better to their last line of therapy; Part B enrolled high-risk patients responding to 1st or 2nd line therapy. The patients on part A were treated with both CAR-Ts, while the part B patients were randomized to receive the BCMA CAR-T with or without the CD19 CAR-T followed by maintenance with an IMiD. Of the 10 patients evaluable for response (6 from part A; 4 from part B-including 2 patients who received both agents), all evaluable patients achieved ≥ PR after CAR-T infusion. Of the 5 patients evaluable for MRD negativity, only one was MRD negative at 10−5. Subsequently, 5 of the 10 evaluable patients (all of whom received the combination of both CAR-Ts) had progressed suggesting that the addition of the CD19 CAR-T did not clearly prevent progression in this population compared to a BCMA CAR-T monotherapy. CRS was seen in 8 (80%) of all patients, but was all grade 1 or 2. No neurotoxicity was seen [131].
5.7. Bispecific CAR-Ts
BCMA and CD19 bispecific CAR-Ts have shown efficacy in preclinical models and have begun early phase clinical trials [157, 158]. Early results of 5 patients treated in a first in human phase I clinical trial with a BCMA and CD19 bispecific CAR-T were encouraging with all patients having a clinical response and only grade 1 CRS seen in 3 patients [158].
BM38 is bispecific CAR-T that targets both BCMA and CD38 that is currently being evaluated in a phase I clinical trial of RRMM patient who have received at least 2 prior treatment regimens, including a PI and an IMiD. Preliminary data of 16 patients showed an 88% response rate with 50% sCRs. CRS was seen in 63% of patients with 25% ≥ grade 3. At a median follow-up of 8.4 months PFS had not yet been reached [159].
5.8. Allogenic BCMA CAR-Ts
Allogeneic CAR-Ts have several advantages over autologous CAR-Ts. Specifically, they allow for immediate availability as opposed to the 2–4 week turnaround time for autologous products-ideally limiting the need for bridging therapy, improved standardization of the CAR-T cell product, redosing or combination of CAR-T cells directed against different targets, and potential cost savings due to a more scalable process [125, 160–162]. Several allogenic BCMA targeting products are currently under evaluation in early phase clinical trials for MM patients. Data for 19 RRMM patients treated with ALLO-715 an allogenic BCMA CAR-T were recently presented. Patients had received ≥3 prior lines of therapy including an IMiD, a PI, and anti-CD38 monoclonal antibody. The trial is evaluating several different lymphodepleting chemotherapy regimens prior to ALLO-715 administration. ORR in the 15 evaluable patients was 33% but was significantly higher in the higher cell doses. CRS was only reported in 24% of patients and all were grade 1 or 2. No ICANs or GVHD was appreciated [163].
5.9. BCMA CAR-NKs
NK cells are innate immune effector cells that lack antigen-specific receptors and express high levels of CD56. They recognize abnormal cells (including virally infected cells and tumor cells) without prior sensitization in a non-HLA restricted manner through a combination of surface stimulatory and inhibitory receptors that target ligands on target cells [164, 165]. Notably, mature NK cells can be transplanted into a different host without losing their function of causing graft vs host [166]. Recently BCMA directed CAR-NK cells have been developed from immortalized NK cell lines and have shown activity in preclinical models [167]. Two phase 1 trials are currently investigating these agents in RRMM patients.
9. Conclusions and Future Directions
While BCMA directed therapies have proven highly efficacious especially compared to therapies directed to alternative targets in RRMM, none of the agents currently being evaluated have proven curative and relapses are still inevitable. Additionally, each class of agents has unique toxicities and logistical challenges which may serve to further limit their widespread availability. Autologous CAR-Ts require leukapheresis and infusion at a tertiary care facility due to the risk of CRS/ICANS, logistics of leukapheresis and significant hematologic AEs. While conventional trials have looked at CAR-Ts as a single infusion without additional therapy allowing a patient an attractive treatment-free interval, it is widely thought that limited CAR-T expansion and persistence within the hostile tumor microenvironment represents significant mechanisms of CAR-T resistance and prevents durable clinical remission following CAR-T therapy [168]. To combat these challenges strategies employing maintenance regimens, addition of different therapies, and other considerations are under evaluation to help prolong the CAR-T product’s lifespan and increase its efficacy are currently under investigation. In addition, efforts are currently underway to improve the throughput of CAR-T production to decrease the “vein to vein” time (defined as the time between leukapheresis of donor and infusion of CAR-T product) in hopes to decrease the need for bridging chemotherapy and disease escape while waiting for product availability.
The concept of T cell exhaustion through multiple mechanisms is a well-known entity contributing to various malignancies including MM [169–171]. Previous studies suggest that antigen-independent tonic signaling by CARs, perhaps due to the physical interactions between CARs or scFv dimerization, limits CAR-T cells potency by induction of exhaustion pathways which is somewhat abrogated by co-stimulatory domains [172, 173]. This has led to speculation that the combination of checkpoint inhibition with CAR-Ts may be able to further abrogate T cell exhaustion, but this would need to be approached cautiously to avoid additional toxicities.
Bispecific antibodies may be more accessible but still require at least initial infusions at a tertiary care facility given the risk for CRS/ICANS seen in with initiation of therapy. Eventually, subsequent doses may able to be given locally for convenience given the improved safety profile and lower rates of toxicities after the first dose, especially with newer agents. The use of bispecific antibodies in conjunction with standard of care backbones is under investigation to evaluate for increased efficacy without additional toxicity. Ideally, utilizing T cell stimulating agents earlier in therapy when patients are more chemotherapy naïve may lead to more robust and durable responses compared to later in their treatment course when they are at higher risk for T cell exhaustion. To this end, the sequencing of when to incorporate a BCMA targeting therapy is currently being investigated with several BCMA targeting therapies are being evaluated in earlier lines of therapy including newly diagnosed MM patients. The hope is that the addition of these agents to upfront therapy backbone will induce deeper, longer-lasting responses. However, this must be weighed against the potential for additional toxicity and cost that this strategy will elicit. Additionally, the question of whether a patient who progresses on a BCMA targeting therapy would still derive benefit from an alternative BCMA directed therapy is still unanswered. While the preliminary data with elranatamab suggests activity with this agent after treatment with a BCMA CAR-T or ADC it is unclear whether this represents a class effect or is specific to this agent. Currently, trials are enrolling to assess this question. Taken together BMCA targeting therapies are an important addition to the armamentarium of myeloma physicians and provide an excellent treatment choice for RRMM patients. Time will tell if their addition to earlier lines of therapy and/or NDMM treatment will induce deeper, more durable responses and if their effects can be accentuated with adjunctive therapies. Additionally, new agents and better supportive care are helping to ameliorate the toxicities unique to each class of BCMA targeting agents which should allow their widespread use as more agents become commercially available.
Key Points.
-
−
B-cell maturation antigen (BCMA) is selectively expressed on plasmablasts and plasma cells, making it an ideal therapeutic target for treatment of multiple myeloma.
-
−
Antibody drug conjugates, bispecific antibodies and chimeric antigen receptor T cell therapies targeting BCMA have shown excellent clinical activity, but are associated with different safety profiles.
Funding:
No external funding was used in the preparation of this manuscript.
Conflicts of Interest:
BP reports personal fees from Abbvie, personal fees from Amgen, stock oownership from BMS, personal fees from Genentech, personal fees from Janssen and personal fees from Regeneron, outside the submitted work.
CR reports personal fees from Amgen, personal fees from BMS, personal fees from Janssen, personal fees from Karyopharm, personal fees from Oncopeptides, personal fees from Janssen, outside he submitted work.
SZU repots research funding from Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda; personal fees (consulting) from Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio.
Footnotes
Declarations
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2020. CA: a cancer journal for clinicians, 2020. 70(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
- 3.Barlogie B, Mitchell A, van Rhee F, Epstein J, Morgan GJ, and Crowley J, Curing myeloma at last: defining criteria and providing the evidence. Blood, The Journal of the American Society of Hematology, 2014. 124(20): p. 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, et al. , Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia, 2017. 31(9): p. 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usmani SZ, Hoering A, Cavo M, San Miguel J, Goldschimdt H, Hajek R, et al. , Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—an IMWG Research Project. Blood cancer journal, 2018. 8(12): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura KK, Barlogie B, van Rhee F, Zangari M, Walker BA, Rosenthal A, et al. , Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood advances, 2020. 4(2): p. 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph NS, Kaufman JL, Dhodapkar MV, Hofmeister CC, Almaula DK, Heffner LT, et al. , Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. Journal of Clinical Oncology, 2020. 38(17): p. 1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrot A, Lauwers-Cances V, Cazaubiel T, Facon T, Caillot D, Clement-Filliatre L, et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Followup Analysis of the IFM 2009 Trial. in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 9.Kumar S, Dimopoulos M, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. , Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia, 2017. 31(11): p. 2443–2448. [DOI] [PubMed] [Google Scholar]
- 10.Pick M, Vainstein V, Goldschmidt N, Lavie D, Libster D, Gural A, et al. , Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD 38 expression and is related to dismal prognosis. European journal of haematology, 2018. 100(5): p. 494–501. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. , Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia, 2019. 33(9): p. 2266–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa LJ, Hari P, Kumar SK, Tang S, Gandhi UH, Shah JJ, et al. , Overall survival of triple class refractory, penta-exposed multiple myeloma (MM) patients treated with selinexor plus dexamethasone or conventional care: a combined analysis of the STORM and Mammoth studies. Blood, 2019. 134: p. 3125. [Google Scholar]
- 13.Yong K, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S, et al. , Multiple myeloma: patient outcomes in real-world practice. British journal of haematology, 2016. 175(2): p. 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo AG, Iversen KF, Möller S, and Plesner T, The clinical course of multiple myeloma in the era of novel agents: a retrospective, single-center, real-world study. Clinical Hematology International, 2019. 1(4): p. 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verelst SG, Blommestein HM, De Groot S, Gonzalez-McQuire S, DeCosta L, de Raad JB, et al. , Long-term outcomes in patients with multiple myeloma: a retrospective analysis of the Dutch Population-based HAematological Registry for Observational Studies (PHAROS). HemaSphere, 2018. 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madry C, Laabi Y, Callebaut I, Roussel J.m., Hatzoglou A, Le Coniat M, et al. , The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. International immunology, 1998. 10(11): p. 1693–1702. [DOI] [PubMed] [Google Scholar]
- 17.Tai Y-T, Acharya C, An G, Moschetta M, Zhong MY, Feng X, et al. , APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood, 2016. 127(25): p. 3225–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan MC, Hering M, Peckham D, McDonagh CF, Brown L, Kim KM, et al. , Antibody targeting of B-cell maturation antigen on malignant plasma cells. Molecular cancer therapeutics, 2007. 6(11): p. 3009–3018. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. , BCMA is essential for the survival of long-lived bone marrow plasma cells. The Journal of experimental medicine, 2004. 199(1): p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. , B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clinical cancer research, 2013. 19(8): p. 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. , BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. The Journal of clinical investigation, 2003. 112(2): p. 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller-Wehr LM, et al. , Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. British journal of haematology, 2016. 174(6): p. 911–922. [DOI] [PubMed] [Google Scholar]
- 23.Frigyesi I, Adolfsson J, Ali M, Kronborg Christophersen M, Johnsson E, Turesson I, et al. , Robust isolation of malignant plasma cells in multiple myeloma. Blood, The Journal of the American Society of Hematology, 2014. 123(9): p. 1336–1340. [DOI] [PubMed] [Google Scholar]
- 24.Cho S-F, Anderson KC, and Tai Y-T, Targeting B cell maturation antigen (BCMA) in multiple myeloma: potential uses of BCMA-based immunotherapy. Frontiers in immunology, 2018. 9: p. 1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. , Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood, 2004. 103(2): p. 689–694. [DOI] [PubMed] [Google Scholar]
- 26.Xu S. and Lam K-P, B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Molecular and Cellular Biology, 2001. 21(12): p. 4067–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dogan A, Siegel D, Tran N, Fu A, Fowler J, Belani R, et al. , B-cell maturation antigen expression across hematologic cancers: a systematic literature review. Blood cancer journal, 2020. 10(6): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. , Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. British journal of haematology, 2012. 158(6): p. 727–738. [DOI] [PubMed] [Google Scholar]
- 29.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. , Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry, 2005. 44(6): p. 1919–1931. [DOI] [PubMed] [Google Scholar]
- 30.Bolkun L, Lemancewicz D, Jablonska E, Kulczynska A, Bolkun-Skornicka U, Kloczko J, et al. , BAFF and APRIL as TNF superfamily molecules and angiogenesis parallel progression of human multiple myeloma. Annals of hematology, 2014. 93(4): p. 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J, Sun Y, Zhang N, Li J, Ta F, Wei W, et al. , Characteristics of BAFF and APRIL factor expression in multiple myeloma and clinical significance. Oncology letters, 2017. 14(3): p. 2657–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreaux J, Legouffe E, Jourdan E, Quittet P, Rème T, Lugagne C, et al. , BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood, 2004. 103(8): p. 3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fragioudaki M, Boula A, Tsirakis G, Psarakis F, Spanoudakis M, Papadakis I, et al. , B cell-activating factor: its clinical significance in multiple myeloma patients. Annals of hematology, 2012. 91(9): p. 1413–1418. [DOI] [PubMed] [Google Scholar]
- 34.Hatzoglou A, Roussel J, Bourgeade M-F, Rogier E, Madry C, Inoue J, et al. , TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-κB, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. The Journal of Immunology, 2000. 165(3): p. 1322–1330. [DOI] [PubMed] [Google Scholar]
- 35.Shen X, Guo Y, Qi J, Shi W, Wu X, and Ju S, Binding of B-cell maturation antigen to B-cell activating factor induces survival of multiple myeloma cells by activating Akt and JNK signaling pathways. Cell biochemistry and function, 2016. 34(2): p. 104–110. [DOI] [PubMed] [Google Scholar]
- 36.Miao YR, Cenik C, Jiang D, Mizuno K, Li GC, Zhao H, et al. , Aberrant BCMA Signaling Promotes Tumor Growth by Altering Protein Translation Machinery, a Therapeutic Target for the Treatment of Relapse/Refractory Multiple Myeloma. bioRxiv, 2021. [Google Scholar]
- 37.Laurent SA, Hoffmann FS, Kuhn P-H, Cheng Q, Chu Y, Schmidt-Supprian M, et al. , γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nature communications, 2015. 6(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meinl E, Thaler FS, and Lichtenthaler SF, Shedding of BAFF/APRIL receptors controls B cells. Trends in immunology, 2018. 39(9): p. 673–676. [DOI] [PubMed] [Google Scholar]
- 39.Ghermezi M, Li M, Vardanyan S, Harutyunyan NM, Gottlieb J, Berenson A, et al. , Serum B-cell maturation antigen: a novel biomarker to predict outcomes for multiple myeloma patients. Haematologica, 2017. 102(4): p. 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. , T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood, The Journal of the American Society of Hematology, 2016. 128(13): p. 1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujarski S, Udd K, Soof C, Spektor TM, Safaie T, Chen H, et al. , Baseline and increases in serum B-cell maturation antigen levels rapidly indicate changes in clinical status among relapsed/refractory multiple myeloma patients undergoing new treatments. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 42.Chen H, Li M, Xu N, Ng N, Sanchez E, Soof CM, et al. , Serum B-cell maturation antigen (BCMA) reduces binding of anti-BCMA antibody to multiple myeloma cells. Leukemia research, 2019. 81: p. 62–66. [DOI] [PubMed] [Google Scholar]
- 43.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. , B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. The Journal of clinical investigation, 2019. 129(6): p. 2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinneer K, Flynn M, Thomas SB, Meekin J, Varkey R, Xiao X, et al. , Preclinical assessment of an antibody–PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia, 2019. 33(3): p. 766–771. [DOI] [PubMed] [Google Scholar]
- 45.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. , Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. The Lancet Oncology, 2018. 19(12): p. 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. , γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood, The Journal of the American Society of Hematology, 2019. 134(19): p. 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cowan AJ, Pont M, Sather BD, Turtle CJ, Till BG, Nagengast AM, et al. , Efficacy and safety of fully human Bcma CAR T cells in combination with a gamma secretase inhibitor to increase Bcma surface expression in patients with relapsed or refractory multiple myeloma. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 48.Kumar S, Migkou M, Bhutani M, Spencer A, Ailawadhi S, Kalff A, et al. Phase 1, first-in-human study of MEDI2228, a BCMA-targeted ADC in patients with relapsed/refractory multiple myeloma. in Proceedings of the 62nd ASH annual Meeting and Exposition, San Diego, CA, USA. 2020. [Google Scholar]
- 49.Sherbenou DW, Behrens CR, Su Y, Wolf JL, Martin TG 3rd, and Liu B, The development of potential antibody-based therapies for myeloma. Blood Rev, 2015. 29(2): p. 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah N, Chari A, Scott E, Mezzi K, and Usmani SZ, B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia, 2020. 34(4): p. 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruins WSC, Zweegman S, Mutis T, and van de Donk N, Targeted Therapy With Immunoconjugates for Multiple Myeloma. Front Immunol, 2020. 11: p. 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pahl A, Lutz C, and Hechler T, Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov Today Technol, 2018. 30: p. 85–89. [DOI] [PubMed] [Google Scholar]
- 53.Bera TK, Anti-BCMA Immunotoxins: Design, Production, and Preclinical Evaluation. Biomolecules, 2020. 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCombs JR and Owen SC, Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. Aaps j, 2015. 17(2): p. 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai Y-T, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. , Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood, The Journal of the American Society of Hematology, 2014. 123(20): p. 3128–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markham A, Belantamab Mafodotin: First Approval. Drugs, 2020. 80(15): p. 1607–1613. [DOI] [PubMed] [Google Scholar]
- 57.Eastman S, Shelton C, Gupta I, Krueger J, Blackwell C, and Bojczuk PM, Synergistic activity of belantamab mafodotin (anti-BCMA immuno-conjugate) with PF-03084014 (gamma-secretase inhibitor) in Bcma-expressing cancer cell lines. Blood, 2019. 134: p. 4401. [Google Scholar]
- 58.de Oca RM, Alavi AS, Vitali N, Bhattacharya S, Blackwell C, Patel K, et al. , Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo. Molecular Cancer Therapeutics, 2021. 20(10): p. 1941–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. , Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer Journal, 2019. 9(4): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. , Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer, 2021. 127(22): p. 4198–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. , Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol, 2020. 21(2): p. 207–221. [DOI] [PubMed] [Google Scholar]
- 62.Richardson PG, Lee HC, Abdallah AO, Cohen AD, Kapoor P, Voorhees PM, et al. , Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J, 2020. 10(10): p. 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahab A, Rafae A, Mushtaq K, Masood A, Ehsan H, Khakwani M, et al. , Ocular Toxicity of Belantamab Mafodotin, an Oncological Perspective of Management in Relapsed and Refractory Multiple Myeloma. Frontiers in Oncology, 2021. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nooka AK, Weisel K, van de Donk NW, Routledge D, Otero PR, Song K, et al. , Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Future Oncol, 2021. 17(16): p. 1987–2003. [DOI] [PubMed] [Google Scholar]
- 65.De Oca RM, Gupta I, and Shelton C, Combinations of belantamab mafodotin with lenalidomide, pomalidomide, bortezomib and/or dexamethasone synergize in vitro and potentiate in vivo anti-tumor activity in multiple myeloma. 2020, AACR. [Google Scholar]
- 66.Popat R, Nooka A, Stockerl-Goldstein K, Abonour R, Ramaekers R, Khot A, et al. , DREAMM-6: Safety, tolerability and clinical activity of belantamab mafodotin (belamaf) in combination with bortezomib/dexamethasone (BorDex) in relapsed/refractory multiple myeloma (RRMM). 2020, American Society of Hematology; Washington, DC. [Google Scholar]
- 67.Nooka AK, Stockerl-Goldstein K, Quach H, Forbes A, Mateos M-V, Khot A, et al. , DREAMM-6: Safety and tolerability of belantamab mafodotin in combination with bortezomib/dexamethasone in relapsed/refractory multiple myeloma (RRMM). Journal of Clinical Oncology, 2020. 38(15_suppl): p. 8502–8502. [Google Scholar]
- 68.Trudel S, Davis R, Lewis NM, Bakshi KK, Chopra B, de Oca RM, et al. , DREAMM-8: a phase III study of the efficacy and safety of belantamab mafodotin with pomalidomide and dexamethasone (B-Pd) vs pomalidomide plus bortezomib and dexamethasone (PVd) in patients with relapsed/refractory multiple myeloma (RRMM). Blood, 2020. 136: p. 4.32614961 [Google Scholar]
- 69.Trudel S, McCurdy A, Sutherland HJ, Louzada ML, Venner CP, Mian HS, et al. , Part 1 Results of a Dose-Finding Study of Belantamab Mafodotin in Combination with Pomalidomide and Dexamethasone for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2021. 138: p. 1653.34734999 [Google Scholar]
- 70.Trudel S MA, Sutherland HJ, et al. Part 1 results of a dose-finding study of belantamab mafodotin (GSK2857916) in combination with pomalidomide and dexamethasone for the treatment of relapsed/refractory multiple myeloma. in 2020 ASH Annual Meeting and Exposition. 2020. [Google Scholar]
- 71.Weisel K, Hopkins TG, Fecteau D, Bao W, Quigley C, Jewell RC, et al. , Dreamm-3: a phase 3, open-label, randomized study to evaluate the efficacy and safety of belantamab mafodotin (GSK2857916) monotherapy compared with pomalidomide plus low-dose dexamethasone (Pom/Dex) in participants with relapsed/refractory multiple myeloma (RRMM). 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 72.Usmani SZ, Terpos E, Janowski W, Quach H, West S, Williams D, et al. , DREAMM-9: Phase III study of belantamab mafodotin plus VRd versus VRd alone in transplant-ineligible newly diagnosed multiple myeloma (TI NDMM). 2020, American Society of Clinical Oncology. [Google Scholar]
- 73.Kinneer K, Meekin J, Varkey R, Xiao X, Zhong H, Breen S, et al. , Preclinical Evaluation of MEDI2228, a BCMA-Targeting Pyrrolobenzodiazepine-Linked Antibody Drug Conjugate for the Treatment of Multiple Myeloma. Blood, 2017. 130(Supplement 1): p. 3153–3153. [Google Scholar]
- 74.Tai Y-T, Xing L, Lin L, Yu T, Cho S-F, Wen K, et al. , MEDI2228, a novel BCMA pyrrolobenzodiazepine antibody drug conjugate, overcomes drug resistance and synergizes with bortezomib and DNA damage response inhibitors in multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia, 2019. 19(10): p. e154–e155. [Google Scholar]
- 75.Taylor NP, AstraZeneca drops BCMA drug after seeing early clinical data. 2021, Questex LLC: Fierce Biotech. [Google Scholar]
- 76.Lutz RJ and Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. in MAbs. 2009. Taylor & Francis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thiant S, Labalette M, Trauet J, Coiteux V, De Berranger E, Dessaint J, et al. , Plasma levels of IL-7 and IL-15 after reduced intensity conditioned allo-SCT and relationship to acute GVHD. Bone marrow transplantation, 2011. 46(10): p. 1374–1381. [DOI] [PubMed] [Google Scholar]
- 78.Figueroa-Vazquez V, Ko J, Breunig C, Baumann A, Giesen N, Pálfi A, et al. , HDP-101, an Anti-BCMA Antibody–Drug Conjugate, Safely Delivers Amanitin to Induce Cell Death in Proliferating and Resting Multiple Myeloma Cells. Molecular Cancer Therapeutics, 2021. 20(2): p. 367–378. [DOI] [PubMed] [Google Scholar]
- 79.Strassz A, Raab MS, Orlowski RZ, Kulke M, Schiedner G, and Pahl A, A First in Human Study Planned to Evaluate Hdp-101, an Anti-BCMA Amanitin Antibody-Drug Conjugate with a New Payload and a New Mode of Action, in Multiple Myeloma. Blood, 2020. 136(Supplement 1): p. 34–34. [Google Scholar]
- 80.Singh RK, Jones RJ, Hong S, Shirazi F, Wang H, Kuiatse I, et al. , HDP101, a Novel B-Cell Maturation Antigen (BCMA)-Targeted Antibody Conjugated to α-Amanitin, Is Active Against Myeloma with Preferential Efficacy Against Pre-Clinical Models of Deletion 17p. Blood, 2018. 132(Supplement 1): p. 593–593. [Google Scholar]
- 81.Fan G, Wang Z, Hao M, and Li J, Bispecific antibodies and their applications. J Hematol Oncol, 2015. 8: p. 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. , Cytokine release syndrome. Journal for immunotherapy of cancer, 2018. 6(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. , Current concepts in the diagnosis and management of cytokine release syndrome. Blood, The Journal of the American Society of Hematology, 2014. 124(2): p. 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riegler LL, Jones GP, and Lee DW, Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Therapeutics and clinical risk management, 2019. 15: p. 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santomasso B, Bachier C, Westin J, Rezvani K, and Shpall EJ, The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. American Society of Clinical Oncology Educational Book, 2019. 39: p. 433–444. [DOI] [PubMed] [Google Scholar]
- 86.Acharya UH, Dhawale T, Yun S, Jacobson CA, Chavez JC, Ramos JD, et al. , Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Review of Hematology, 2019. 12(3): p. 195–205. [DOI] [PubMed] [Google Scholar]
- 87.Morris EC, Neelapu SS, Giavridis T, and Sadelain M, Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nature Reviews Immunology, 2021: p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. , Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer discovery, 2017. 7(12): p. 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borrega JG, Gödel P, Rüger MA, Onur ÖA, Shimabukuro-Vornhagen A, Kochanek M, et al. , In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. Hemasphere, 2019. 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castaneda-Puglianini O. and Chavez JC, Assessing and Management of Neurotoxicity After CAR-T Therapy in Diffuse Large B-Cell Lymphoma. Journal of Blood Medicine, 2021. 12: p. 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. , Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J Clin Oncol, 2020. 38(8): p. 775–783. [DOI] [PubMed] [Google Scholar]
- 92.Lejeune M, Köse MC, Duray E, Einsele H, Beguin Y, and Caers J, Bispecific, T-Cell-Recruiting Antibodies in B-Cell Malignancies. Front Immunol, 2020. 11: p. 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho S-F, Lin L, Xing L, Liu J, Yu T, Wen K, et al. , Anti-BCMA BiTE® AMG 701 potently induces specific T cell lysis of human multiple myeloma (MM) cells and immunomodulation in the bone marrow microenvironment. Blood, 2018. 132: p. 592. [Google Scholar]
- 94.Harrison SJ, Minnema MC, Lee HC, Spencer A, Kapoor P, Madduri D, et al. A Phase 1 First in Human (FIH) Study of AMG 701, an Anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE (R)(bispecific T-cell engager) Molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM). in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 95.Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. , Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer cell, 2017. 31(3): p. 396–410. [DOI] [PubMed] [Google Scholar]
- 96.Costa LJ, Wong SW, Bermúdez A, de la Rubia J, Mateos M-V, Ocio EM, et al. , First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood, 2019. 134(Supplement_1): p. 143–143. [Google Scholar]
- 97.Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. , Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Advances, 2020. 4(18): p. 4538–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Usmani SZ, Mateos M-V, Nahi H, Krishnan AY, Donk N.W.C.J.v.d., Miguel JS, et al. , Phase I study of teclistamab, a humanized B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed/refractory multiple myeloma (R/R MM). Journal of Clinical Oncology, 2020. 38(15_suppl): p. 100–100.31664879 [Google Scholar]
- 99.Krishnan AY, Garfall AL, Mateos M-V, Donk N.W.C.J.v.d., Nahi H, San-Miguel JF, et al. , Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM). Journal of Clinical Oncology, 2021. 39(15_suppl): p. 8007–8007. [Google Scholar]
- 100.Usmani SZ, Garfall AL, van de Donk NW, Nahi H, San-Miguel JF, Oriol A, et al. , Teclistamab, a B-cell maturation antigen× CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. The Lancet, 2021. 398(10301): p. 665–674. [DOI] [PubMed] [Google Scholar]
- 101.Moreau P, Usmani SZ, Garfall AL, van de Donk NW, Nahi H, San-Miguel J, et al. , Updated Results from MajesTEC-1: Phase 1/2 Study of Teclistamab, a B-Cell Maturation Antigen x CD3 Bispecific Antibody, in Relapsed/Refractory Multiple Myeloma. Blood, 2021. 138: p. 896. [Google Scholar]
- 102.Raje NS, Jakubowiak A, Gasparetto C, Cornell RF, Krupka HI, Navarro D, et al. , Safety, Clinical Activity, Pharmacokinetics, and Pharmacodynamics from a Phase I Study of PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2019. 134(Supplement_1): p. 1869–1869. [Google Scholar]
- 103.Lesokhin AM, Raje N, Gasparetto CJ, Walker J, Krupka HI, Joh T, et al. , A Phase I, Open-Label Study to Evaluate the Safety, Pharmacokinetic, Pharmacodynamic, and Clinical Activity of PF-06863135, a B-Cell Maturation Antigen/CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Advanced Multiple Myeloma. Blood, 2018. 132: p. 3229. [Google Scholar]
- 104.Bahlis NJ, Raje NS, Costello C, Dholaria BR, Solh MM, Levy MY, et al. , Efficacy and safety of elranatamab (PF-06863135), a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MM). Journal of Clinical Oncology, 2021. 39(15_suppl): p. 8006–8006. [Google Scholar]
- 105.Sebag M, Raje NS, Bahlis NJ, Costello C, Dholaria B, Solh M, et al. , Elranatamab (PF-06863135), a B-Cell Maturation Antigen (BCMA) Targeted CD3-Engaging Bispecific Molecule, for Patients with Relapsed or Refractory Multiple Myeloma: Results from Magnetismm-1. Blood, 2021. 138: p. 895. [Google Scholar]
- 106.Caraccio C, Krishna S, Phillips DJ, and Schürch CM, Bispecific antibodies for multiple myeloma: a review of targets, drugs, clinical trials, and future directions. Frontiers in immunology, 2020. 11: p. 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiLillo DJ, Olson K, Mohrs K, Meagher TC, Bray K, Sineshchekova O, et al. , A BCMAxCD3 bispecific T cell–engaging antibody demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR T cells. Blood advances, 2021. 5(5): p. 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dilillo DJ, Olson K, Mohrs K, Meagher TC, Bray K, Sineshchekova O, et al. , REGN5458, a Bispecific BCMAxCD3 T Cell Engaging Antibody, Demonstrates Robust In Vitro and In Vivo Anti-Tumor Efficacy in Multiple Myeloma Models, Comparable to That of BCMA CAR T Cells. Blood, 2018. 132: p. 1944. [Google Scholar]
- 109.Zonder JA, Richter J, Bumma N, Brayer J, Hoffman JE, Bensinger WI, et al. , Early, Deep, and Durable Responses, and Low Rates of Cytokine Release Syndrome with REGN5458, a BCMAxCD3 Bispecific Monoclonal Antibody, in a Phase 1/2 First-in-Human Study in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood, 2021. 138: p. 160.33831168 [Google Scholar]
- 110.Buelow B, Choudry P, Clarke S, Dang K, Davison L, Aldred SF, et al. , Pre-clinical development of TNB-383B, a fully human T-cell engaging bispecific antibody targeting BCMA for the treatment of multiple myeloma. Journal of Clinical Oncology, 2018. 36(15_suppl): p. 8034–8034. [Google Scholar]
- 111.Rodriguez C, D’Souza A, Shah N, Voorhees PM, Buelow B, Vij R, et al. , Initial results of a phase I study of TNB-383B, a BCMA x CD3 bispecific T-cell redirecting antibody, in relapsed/refractory multiple myeloma. Blood, 2020. 136(Supplement 1): p. 43–4. [Google Scholar]
- 112.Kumar S, D’Souza A, Shah N, Rodriguez C, Voorhees PM, Bueno OF, et al. , A Phase 1 First-in-Human Study of Tnb-383B, a BCMA x CD3 Bispecific T-Cell Redirecting Antibody, in Patients with Relapsed/Refractory Multiple Myeloma. Blood, 2021. 138: p. 900. [Google Scholar]
- 113.Jayaraman J, Mellody MP, Hou AJ, Desai RP, Fung AW, Pham AHT, et al. , CAR-T design: Elements and their synergistic function. EBioMedicine, 2020. 58: p. 102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sadelain M, Brentjens R, and Rivière I, The basic principles of chimeric antigen receptor design. Cancer discovery, 2013. 3(4): p. 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van de Donk NW, Usmani SZ, and Yong K, CAR T-cell therapy for multiple myeloma: state of the art and prospects. The Lancet Haematology, 2021. 8(6): p. e446–e461. [DOI] [PubMed] [Google Scholar]
- 116.Song D-G, Ye Q, Poussin M, Harms GM, Figini M, and Powell DJ Jr, CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood, The Journal of the American Society of Hematology, 2012. 119(3): p. 696–706. [DOI] [PubMed] [Google Scholar]
- 117.Rafiq S, Hackett CS, and Brentjens RJ, Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nature reviews Clinical oncology, 2020. 17(3): p. 147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eshhar Z, Waks T, Gross G, and Schindler DG, Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences, 1993. 90(2): p. 720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mikkilineni L. and Kochenderfer JN, Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood, 2017. 130(24): p. 2594–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bonifant CL, Jackson HJ, Brentjens RJ, and Curran KJ, Toxicity and management in CAR T-cell therapy. Molecular Therapy-Oncolytics, 2016. 3: p. 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benmebarek M-R, Karches CH, Cadilha BL, Lesch S, Endres S, and Kobold S, Killing mechanisms of chimeric antigen receptor (CAR) T cells. International journal of molecular sciences, 2019. 20(6): p. 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suryadevara CM, Desai R, Farber SH, Choi BD, Swartz AM, Shen SH, et al. , Preventing lck activation in CAR T cells confers Treg resistance but requires 4–1BB signaling for them to persist and treat solid tumors in nonlymphodepleted hosts. Clinical Cancer Research, 2019. 25(1): p. 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. , Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of experimental medicine, 2005. 202(7): p. 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halim L. and Maher J, CAR T-cell immunotherapy of B-cell malignancy: the story so far. Therapeutic advances in vaccines and immunotherapy, 2020. 8: p. 2515135520927164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teoh PJ and Chng WJ, CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer Journal, 2021. 11(4): p. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. , Effective targeting of multiple B-cell maturation antigen–expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Human gene therapy, 2018. 29(5): p. 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bu D-X, Singh R, Choi EE, Ruella M, Nunez-Cruz S, Mansfield KG, et al. , Pre-clinical validation of B cell maturation antigen (BCMA) as a target for T cell immunotherapy of multiple myeloma. Oncotarget, 2018. 9(40): p. 25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Larrea CF, Staehr M, Lopez AV, Ng KY, Chen Y, Godfrey WD, et al. , Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape–driven relapse in multiple myeloma. Blood cancer discovery, 2020. 1(2): p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, et al. , Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. Journal of hematology & oncology, 2020. 13(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wudhikarn K, Mailankody S, and Smith EL, Future of CAR T cells in multiple myeloma. Hematology 2014, the American Society of Hematology Education Program Book, 2020. 2020(1): p. 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garfall AL, Cohen AD, Lacey SF, Tian L, Hwang W-T, Vogl DT, et al. , Combination anti-Bcma and anti-CD19 CAR T cells as consolidation of response to prior therapy in multiple myeloma. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 132.Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. , A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. The Lancet Haematology, 2019. 6(10): p. e521–e529. [DOI] [PubMed] [Google Scholar]
- 133.Simon S. and Riddell SR, Dual Targeting with CAR T Cells to Limit Antigen Escape in Multiple Myeloma. Blood Cancer Discovery, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. , Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. New England Journal of Medicine, 2019. 380(18): p. 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. , Idecabtagene vicleucel in relapsed and refractory multiple myeloma. New England Journal of Medicine, 2021. 384(8): p. 705–716. [DOI] [PubMed] [Google Scholar]
- 136.Anderson Larry D. J., Munshi NC, Shah N, Jagannath S, Berdeja JG, Lonial S, et al. , Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. Journal of Clinical Oncology, 2021. 39(15_suppl): p. 8016–8016. [Google Scholar]
- 137.Usmani SZ, Berdeja JG, Truppel-Hartmann A, Fei Y, Wortman-Vayn H, Shelat S, et al. , KarMMa-4: Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T-cell therapy in high-risk newly diagnosed multiple myeloma. 2021, Wolters Kluwer Health. [Google Scholar]
- 138.Oriol A, Abril L, Torrent A, Ibarra G, and Ribera J-M, The role of idecabtagene vicleucel in patients with heavily pretreated refractory multiple myeloma. Therapeutic Advances in Hematology, 2021. 12: p. 20406207211019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Delforge M, Baz RC, Cavo M, Callander NS, Ghobadi A, Rodriguez-Otero P, et al. , KarMMa-3: a phase 3 study of idecabtagene vicleucel (ide-cel, bb2121), a BCMA-Directed CAR T cell therapy vs standard regimens in relapsed and refractory multiple myeloma. Blood, 2020. 136: p. 24–25.32430494 [Google Scholar]
- 140.Alsina M, Shah N, Raje NS, Jagannath S, Madduri D, Kaufman JL, et al. Updated results from the phase I CRB-402 study of anti-BCMA CAR-T cell therapy bb21217 in patients with relapsed and refractory multiple myeloma: correlation of expansion and duration of response with T cell phenotypes. in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 141.Berdeja JG, Alsina M, Shah ND, Siegel DS, Jagannath S, Madduri D, et al. , Updated results from an ongoing phase 1 clinical study of bb21217 anti-Bcma CAR T cell therapy. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 142.Finney OC, Yeri A, Mao P, Pandya C, Alonzo E, Hopkins G, et al. Molecular and phenotypic profiling of drug product and post-infusion samples from CRB-402, an ongoing: Phase I Clinical Study of bb21217 a BCMA-directed CAR T cell therapy. in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 143.Xu J, Chen L-J, Yang S-S, Sun Y, Wu W, Liu Y-F, et al. , Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proceedings of the National Academy of Sciences, 2019. 116(19): p. 9543–9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. , A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol, 2018. 11(1): p. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martin T, Usmani SZ, Berdeja JG, Jakubowiak A, Agha M, Cohen AD, et al. , Updated Results from CARTITUDE-1: Phase 1b/2Study of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy, in Patients With Relapsed/Refractory Multiple Myeloma. Blood, 2021. 138: p. 549. [Google Scholar]
- 146.Mateos M-V, Weisel K, Martin T, Berdeja JG, Jakubowiak A, Stewart AK, et al. , Ciltacabtagene Autoleucel for Triple-Class Exposed Multiple Myeloma: Adjusted Comparisons of CARTITUDE-1 Patient Outcomes Versus Therapies from Real-World Clinical Practice from the LocoMMotion Prospective Study. Blood, 2021. 138: p. 550. [Google Scholar]
- 147.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. , Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet, 2021. 398(10297): p. 314–324. [DOI] [PubMed] [Google Scholar]
- 148.Cohen YC, Cohen AD, Delforge M, Hillengass J, Goldschmidt H, Weisel K, et al. , Efficacy and Safety of Ciltacabtagene Autoleucel (Cilta-cel), a B-Cell Maturation Antigen (BCMA)-Directed Chimeric Antigen Receptor (CAR) T-Cell Therapy, in Lenalidomide-Refractory Patients with Progressive Multiple Myeloma after 1–3 Prior Lines of Therapy: Updated Results from CARTITUDE-2. Blood, 2021. 138: p. 3866. [Google Scholar]
- 149.Mailankody S, Htut M, Lee KP, Bensinger W, Devries T, Piasecki J, et al. , JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). Blood, 2018. 132(Supplement 1): p. 957–957. [Google Scholar]
- 150.Mailankody S, Jakubowiak AJ, Htut M, Costa LJ, Lee K, Ganguly S, et al. , Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011). 2020, American Society of Clinical Oncology. [Google Scholar]
- 151.Offidani M, Corvatta L, Morè S, and Olivieri A, Novel Experimental Drugs for Treatment of Multiple Myeloma. Journal of experimental pharmacology, 2021. 13: p. 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hao S, Jin J, Jiang S, Li Z, Zhang W, Yang M, et al. Two-year follow-up of investigator-initiated phase 1 trials of the safety and efficacy of fully human anti-Bcma CAR T Cells (CT053) in relapsed/refractory multiple myeloma. in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 153.Jie J, Hao S, Jiang S, Li Z, Yang M, Zhang W, et al. , Phase 1 trial of the safety and efficacy of fully human anti-Bcma CAR T cells in relapsed/refractory multiple myeloma. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 154.Mailankody S, Ghosh A, Staehr M, Purdon TJ, Roshal M, Halton E, et al. , Clinical responses and pharmacokinetics of MCARH171, a human-derived Bcma targeted CAR T cell therapy in relapsed/refractory multiple myeloma: final results of a phase I clinical trial. 2018, Am Soc Hematology. [Google Scholar]
- 155.Paiva B, Puig N, Cedena M-T, de Jong BG, Ruiz Y, Rapado I, et al. , Differentiation stage of myeloma plasma cells: biological and clinical significance. Leukemia, 2017. 31(2): p. 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. , Characterization of clonogenic multiple myeloma cells. Blood, 2004. 103(6): p. 2332–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kang L, Zhang J, Li M, Xu N, Qi W, Tan J, et al. , Characterization of novel dual tandem CD19/BCMA chimeric antigen receptor T cells to potentially treat multiple myeloma. Biomarker Research, 2020. 8(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H, Gao L, Liu L, Wang J, Wang S, Gao L, et al. , A Bcma and CD19 bispecific CAR-T for relapsed and refractory multiple myeloma. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 159.Li C, Mei H, Hu Y, Guo T, Liu L, Jiang H, et al. , A bispecific CAR-T cell therapy targeting Bcma and CD38 for relapsed/refractory multiple myeloma: updated results from a phase 1 dose-climbing trial. 2019, American Society of Hematology; Washington, DC. [Google Scholar]
- 160.Depil S, Duchateau P, Grupp S, Mufti G, and Poirot L, ‘Off-the-shelf’allogeneic CAR T cells: development and challenges. Nature Reviews Drug Discovery, 2020. 19(3): p. 185–199. [DOI] [PubMed] [Google Scholar]
- 161.Graham C, Jozwik A, Pepper A, and Benjamin R, Allogeneic CAR-T cells: more than ease of access? Cells, 2018. 7(10): p. 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sommer C, Boldajipour B, Kuo TC, Bentley T, Sutton J, Chen A, et al. , Preclinical evaluation of allogeneic CAR T cells targeting BCMA for the treatment of multiple myeloma. Molecular therapy, 2019. 27(6): p. 1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mailankody S, Matous JV, Liedtke M, Sidana S, Malik S, Nath R, et al. Universal: an allogeneic first-in-human study of the anti-BCMA ALLO-715 and the anti-CD52 ALLO-647 in relapsed/refractory multiple myeloma. in Blood. 2020. AMER SOC HEMATOLOGY 2021 L ST NW, SUITE 900, WASHINGTON, DC 20036 USA. [Google Scholar]
- 164.Gong Y, Klein Wolterink RG, Wang J, Bos GM, and Germeraad WT, Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. Journal of hematology & oncology, 2021. 14(1): p. 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yilmaz A, Cui H, Caligiuri MA, and Yu J, Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. Journal of hematology & oncology, 2020. 13(1): p. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Williams RL, Cooley S, Bachanova V, Blazar BR, Weisdorf DJ, Miller JS, et al. , Recipient T Cell exhaustion and successful adoptive transfer of haploidentical natural killer cells. Biology of Blood and Marrow Transplantation, 2018. 24(3): p. 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martín EM, Encinas J, García-Ortiz A, Ugalde L, Fernández RA, Leivas A, et al. , Exploring NKG2D and BCMA-CAR NK-92 for adoptive cellular therapy to multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia, 2019. 19(10): p. e24–e25. [Google Scholar]
- 168.Shah NN and Fry TJ, Mechanisms of resistance to CAR T cell therapy. Nature reviews Clinical oncology, 2019. 16(6): p. 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zarour HM, Reversing T-cell dysfunction and exhaustion in cancer. Clinical Cancer Research, 2016. 22(8): p. 1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. , T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer immunology research, 2016. 4(1): p. 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, et al. , T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. Journal of hematology & oncology, 2016. 9(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. , 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine, 2015. 21(6): p. 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, et al. , Tonic 4–1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell reports, 2017. 21(1): p. 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]