Abstract

Herein we disclose the synthesis of sterically encumbered dialkylnickel(II) complexes bearing 2,9-dimethyl-1,10-phenanthroline ligands. A comparison with their unsubstituted analogues by both X-ray crystallography and theoretical calculations revealed significant distortions in their molecular structures. Eyring plots along with stoichiometric and photoexcitation studies revealed that sterically encumbered dialkylnickel(II) complexes enable facile C(sp3)–C(sp3) reductive elimination, thus offering an improved understanding of Ni catalysis.
Nickel-catalyzed reactions have gained considerable momentum as enabling techniques for forging new synthetic architectures.1−4 Particularly attractive is the virtue of Ni catalysts for forging C(sp3)–C(sp3) bonds, as these bonds are key motifs in medicinal chemistry programs that modulate solubility, molecular shape, or substrate recognition of drug candidates.5−8 The successful implementation of nickel catalysis in both academic and industrial laboratories is intimately associated with the ease of enabling single-electron-transfer reactivity, the propensity to populate unconventional Ni(I) or Ni(III) manifolds, and the high barrier for β-hydride elimination that allows for forging of sp3 architectures.9 Reviewing the literature data reveals that sterically encumbered polypyridine ligands have proved to be a key contributory factor for success in a myriad of Ni-catalyzed C(sp3)–C(sp3) bond formations.10 Although there exists a reasonable consensus on how Ni–polypyridine complexes enable oxidative addition or transmetalation, the means to trigger C(sp3)–C(sp3) reductive elimination remains the subject of considerable debate due to the inherent difficulty of accessing short-lived yet exceptionally sensitive dialkylnickel(II)–polypyridine species (Scheme 1, top).11−18 Seminal studies by Yamamoto et al. determined that the barrier to reductive elimination from (bpy)NiEt2 was 68 kcal·mol–1.19−24 The extensive literature on the steric implications of phosphine ligands for reductive elimination and the rapid adoption of sterically encumbered polypyridine ligands for the construction of C(sp3)–C(sp3) bonds prompted our interest in studying these systems further (Scheme 1, middle).25,26 We anticipated that a study aimed at unraveling the steric effects of dialkylnickel–polypyridine complexes on C(sp3)–C(sp3) reductive elimination might represent a new lead for future Ni-catalyzed cross-coupling reactions (Scheme 1, bottom).
Scheme 1. Reductive Elimination of Polypyridine-Ligated Ni Complexes.
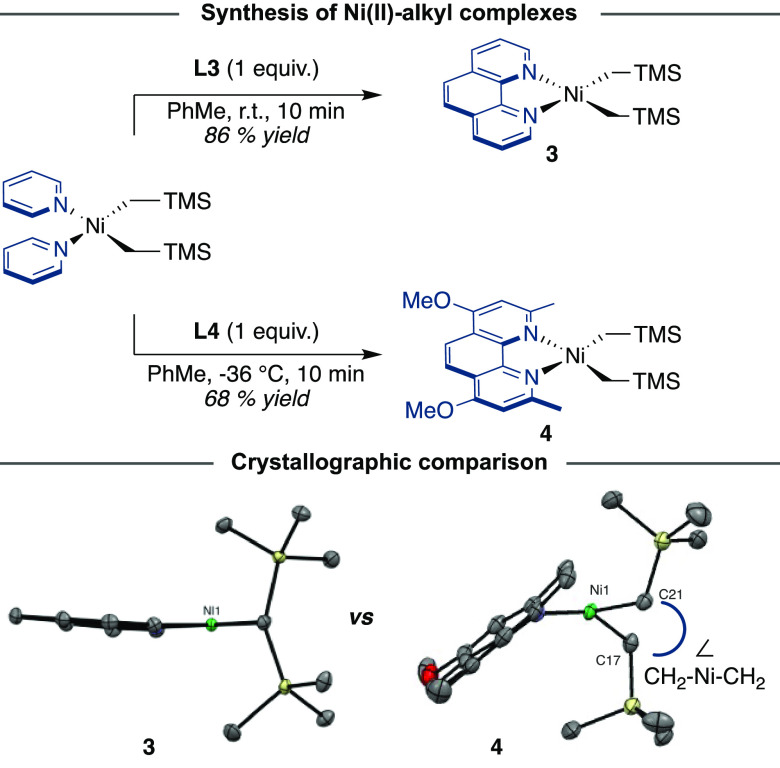
We began our investigations by synthesizing well-defined dialkylnickel(II) complexes bearing variously substituted polypyridine ligands.21 Specifically, we allowed Ni(acac)2 to react with Et2AlOEt in the presence of either 2,2′-bipyridine (L1) or neocuproine (L2) in Et2O at −20 °C (Figure 1). While the synthesis of (L1)NiEt2 (1) posed no problems, the preparation of (L2)NiEt2 was found to be particularly problematic, as (L2)Ni(ethylene) crystallized from the reaction mixture in 60% yield. This product, corroborated by X-ray diffraction, presumably arises from β-hydride elimination. The exceptional ease with which (L2)NiEt2 undergoes β-hydride elimination is in sharp contrast with the high barrier observed in the analogous reaction of (L1)NiEt2. The importance of this observation can hardly be overestimated, as it offers indirect yet long-awaited evidence for the propensity of 2,9-disubstituted phenanthroline to enable Ni-catalyzed chain walking via iterative β-hydride elimination and migratory insertion events.27
Figure 1.
Initial efforts en route to dialkylnickel(II) species.
Aiming to understand the factors influencing reductive elimination of Ni(dialkyl) complexes supported by sterically encumbered polypyridine ligands and the difficulties of synthesizing L2NiR2 complexes by the use of alkylaluminum reagents, we sought out a pathway involving neutral ligand displacement. To this end, we turned our attention to (py)2Ni(CH2TMS)2 (TMS = trimethylsilyl), which is easily prepared by reaction of (py)4NiCl2 with TMSCH2MgCl in Et2O at −60 °C.28 This nickel precursor was chosen because of the ease with which monodentate pyridine ligands could be displaced by 1,10-phenanthroline ligands. Furthermore, we anticipated that the CH2TMS groups would add to the stability of the complexes by hyperconjugation and by preventing β-hydride elimination.29−32 As expected from early studies reported by Carmona and Atwood, (L3)Ni(CH2TMS)2 (3) was prepared in 90% yield by the reaction between (py)2Ni(CH2TMS)2 and 1,10-phenanthroline (L3) at room temperature.28 Notably, the reaction between (py)2Ni(CH2TMS)2 and 4,7-dimethoxy-2,9-dimethyl-1,10-phenanthroline (L4)—a ligand featuring electron-donating methoxy groups via resonance donation to the ligand π system—could be conducted at −36 °C, giving rise to (L4)Ni(CH2TMS)2 (4) in 68% yield as a purple solid (Figure 2). The stability of this complex may arise from the electron-richness of the metal center, which prevents reductive elimination. In keeping with this hypothesis, not even traces of (L5)Ni(CH2TMS)2 (L5 = 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline) were observed upon the reaction of (py)2Ni(CH2TMS)2 with L5, a ligand with inductively withdrawing phenyl groups in place of the methoxy groups of L4. The preparation of 4 is particularly important, as it offers for the first time the opportunity to assess the influence of sterically encumbered polypyridine complexes in the context of C(sp3)–C(sp3) reductive elimination from a well-defined species. This information may therefore allow the parametrization of features that may have an impact in future Ni-catalyzed endeavors.
Figure 2.
Synthesis of (L)Ni(CH2TMS)2 (L = L3, L4) and ORTEP drawings (50%) of 3 and 4. Crystals of 3 and 4 were grown at −36 °C in Et2O/pentane or Et2O. Hydrogen atoms and disordered sections have been omitted in the sake of clarity.
Low-temperature crystallization (−36 °C, Et2O/pentane or Et2O) furnished crystals suitable for X-ray diffraction, thus allowing the structures of 3 and 4 to be determined unambiguously. A simple comparison of their structures is particularly illustrative. While the Ni atom in 3 is in a canonical square planar geometry, the bonding in 4 is fairly distorted from a square planar geometry, although the complex is still diamagnetic. DFT calculations of the gas-phase structure of 4 confirmed that this nonplanarity is a molecular phenomenon, not a result of the crystal packing. Strikingly, the coordination of L4 is ligated at a ca. 35° angle, which results in poor overlap of the σ-sp2 orbital of nitrogen with the central Ni atom. This is reflected in lower values of the delocalization index δ(A, B)—a measure of the orbital overlap and covalent bond order between a pair of atoms A and B—computed within the context of the quantum theory of atoms in molecules (QTAIM):33 in 4, δ(Ni, N) = 0.459 and 0.394, whereas in 3, δ(Ni, N) = 0.499. Natural bond orbital analysis further confirmed a weaker overlap between the lone pairs of the nitrogen atoms and the p orbital of Ni in 4. While second-order perturbation theory for the symmetrical structure of 3 predicted two equally strong interactions from the nitrogen atoms of L3 (E(2) = 35.2 kcal·mol–1), a significant deviation is observed in nonsymmetrical L4 (E(2) = 33.6 and 33.9 kcal·mol–1). We speculate that this indirect binding mode might limit steric pressure surrounding the Ni center, as a direct binding interaction might locate the 2,9-dimethyl substituents of L4 in close proximity to the methylene carbons on the CH2TMS moiety.
Further inspection of the X-ray structure of 4 identified a heavily distorted square planar geometry, with the methylene carbon C17 significantly out of the N1–Ni–N2 plane. A seemingly simple comparison of the CH2–Ni–CH2 angle from the side as shown in Figure 2 reveals that the methylene carbons are distorted by 38.9°, which is in contrast to the distortion of 2.8° observed for 3. Further comparison of the two structures revealed that 4 has longer N–Ni bonds (2.055(3) and 1.982(3) Å in 4 vs 1.9833(16) and 1.9886(15) in 3) and contracted Ni–C linkages (1.930(3) and 1.933(3) Å in 4 vs 1.9441(18) and 1.9442(18) Å in 3). Comparing the QTAIM atomic charges (q(Ni)) and localization index (λ(Ni)) of 3 and 4 suggests that L4 in 4 increases the electron population of Ni more than does coordination of L3 in 3 (q(Ni)4 = +0.612 and λ(Ni)4 = 25.755 vs q(Ni)3 = +0.635 and λ(Ni)3 = 25.699). Analysis of the frontier molecular orbitals of 3 and 4 reveals that the complexation with L4 increases the HOMO energies of 4 more than those of 3 (Figure 3), which is consistent with the highly strained geometry of 4. Consistent with 4 containing a higher-energy HOMO were cyclic voltammetry experiments which found that Ni(II/III) oxidation is easier in 4 (Eox = −0.40 V vs SCE) than in 3 (Eox = 0.22 V vs SCE).
Figure 3.
Frontier molecular orbitals of (left) 3 and (right) 4 by DFT.
In order to study the ligand effects of L3 and L4 on C–C reductive elimination from 3 and 4, respectively, we monitored these compounds at elevated temperature in C6D6. Demonstrating the stability of 3, even after 24 h at 100 °C, 3 showed no reaction. The striking stability of 3 is in sharp contrast to its sterically encumbered analogue 4, which underwent reductive elimination in C6D6 at 60 °C with a first-order decay (k = 3.72 × 10–4 s–1) over 100 min to form insoluble (L4)2Ni and 1,2-bis(trimethylsilyl)ethane (Figure 4).
Figure 4.
Reductive elimination of TMSCH2CH2TMS from 4 under various conditions: thermally, with additives, under ambient light and with an oxidant. ox-1 = 1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate.
Further information on the reductive elimination was gathered by performing an Eyring analysis in C6D6, which determined a particularly low activation barrier (ΔG⧧(50 °C) = 26.3 kcal·mol–1). Qualitative data on the transition state could be obtained by performing a preliminary three-point Eyring plot analysis in THF-d8 (50–70 °C) with single kinetic runs, from which a negative entropy of activation is apparent (Figure S11).34 To gain additional information on the effect of coordinating ligands, we examined whether the inclusion of methyl acrylate (MA) might influence reductive elimination in 4. In line with studies performed by Yamamoto with (bpy)NiEt2, the presence of the π-accepting olefin MA induced rapid reductive elimination (<5 min), which we speculate is due to the intermediacy of five-coordinate species via interaction with the dx2–y2 orbital (Figure 4).35 Taking into consideration that metallaphotoredox scenarios have gained considerable momentum as innovative vehicles for forging sp3 architectures, we next focused our attention on studying whether C(sp3)–C(sp3) reductive elimination of 4 might be facilitated by either photoexcitation or single-electron-transfer oxidation. Notably, a rate enhancement similar to that observed for MA was observed when the reaction of 4 was conducted with 1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate, which acts as a one-electron oxidant,36 thus illustrating the exceptional ease with which Ni(III) intermediates promote reductive elimination. Comparing the rate of reductive elimination of 4 in the dark or under ambient light (4 absorbs (λmax = 399 nm)) revealed a similar rate of reaction (k = 3.66 × 10–6s–1).37 These observations were further corroborated by the lack of changes in the crystal structure of 4 when crystals of 4 were irradiated at 390 nm.
In summary, we have reported the first dialkylnickel(II) complex supported by sterically encumbered 2,9-disubstituted phenanthroline ligands. A comparison of the solid-state geometry with that of its unsubstituted analogue reveals that steric effects destabilize these complexes, setting the basis for promoting C(sp3)−C(sp3) reductive elimination with exceptional ease. Stoichiometric experiments were carried out in different solvents, in the presence of additives, and in the presence of light. We believe that this report might lead to new knowledge in synthetic design while offering a new gateway for studying the intricacies of Ni-catalyzed reactions.
Acknowledgments
We thank thank ICIQ and MICIU (PID2021-133801NB-I00) for financial support. C.S.D. thanks the European Union’s Horizon 2020 Programme under Marie Curie PREBIST Grant Agreement 754558. C.F.N. thanks the National Science Centre, Poland (2020/39/B/ST4/02022) for partially funding this work. This research was supported in part by PLGrid Infrastructure. For Open Access, the authors have applied a CC-BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission. We sincerely thank E. Escudero and J. Benet for X-ray crystallographic data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.organomet.2c00362.
Author Contributions
⊥ S.J.T. and R.T.M. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Hazari N.; Melvin P. R.; Beromi M. M. Well-defined nickel and palladium precatalysts for cross-coupling. Nat. Rev. Chem. 2017, 1 (3), 0025. 10.1038/s41570-017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker S. Z.; Standley E. A.; Jamison T. F. Recent advances in homogeneous nickel catalysis. Nature 2014, 509 (7500), 299–309. 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni J.; Lin Q.; Diao T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53 (4), 906–919. 10.1021/acs.accounts.0c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diccianni J. B.; Diao T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1 (9), 830–844. 10.1016/j.trechm.2019.08.004. [DOI] [Google Scholar]
- Choi J.; Fu G. C. Transition metal-catalyzed alkyl–alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356 (6334), eaaf7230 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M. D.; Schreiber S. L. A Planning Strategy for Diversity-Oriented Synthesis. Angew. Chem., Int. Ed. 2004, 43 (1), 46–58. 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]
- Lipinski C.; Hopkins A. Navigating chemical space for biology and medicine. Nature 2004, 432 (7019), 855–861. 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- Dombrowski A. W.; Gesmundo N. J.; Aguirre A. L.; Sarris K. A.; Young J. M.; Bogdan A. R.; Martin M. C.; Gedeon S.; Wang Y. Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)–C(sp3) Cross-Coupling Methods by Library Synthesis. ACS Med. Chem. Lett. 2020, 11 (4), 597–604. 10.1021/acsmedchemlett.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; Mao E.; Millet A.; Oakley J. V.; Reed N. L.; Sakai H. A.; Seath C. P.; MacMillan D. W. C. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122 (2), 1485–1542. 10.1021/acs.chemrev.1c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected references, see:; a Sun S.-Z.; Börjesson M.; Martin-Montero R.; Martin R. Site-Selective Ni-Catalyzed Reductive Coupling of α-Haloboranes with Unactivated Olefins. J. Am. Chem. Soc. 2018, 140 (40), 12765–12769. 10.1021/jacs.8b09425. [DOI] [PubMed] [Google Scholar]; b Sun S.-Z.; Romano C.; Martin R. Site-Selective Catalytic Deaminative Alkylation of Unactivated Olefins. J. Am. Chem. Soc. 2019, 141 (41), 16197–16201. 10.1021/jacs.9b07489. [DOI] [PubMed] [Google Scholar]; c Sun S.-Z.; Talavera L.; Spieß P.; Day C. S.; Martin R. sp3 Bis-Organometallic Reagents via Catalytic 1,1-Difunctionalization of Unactivated Olefins. Angew. Chem., Int. Ed. 2021, 60 (21), 11740–11744. 10.1002/anie.202100810. [DOI] [PubMed] [Google Scholar]; d Janssen-Müller D.; Sahoo B.; Sun S.-Z.; Martin R. Tackling Remote sp3 C–H Functionalization via Ni-Catalyzed “chain-walking” Reactions. Isr. J. Chem. 2020, 60 (3–4), 195–206. 10.1002/ijch.201900072. [DOI] [Google Scholar]; e Zhou F.; Zhu J.; Zhang Y.; Zhu S. NiH-Catalyzed Reductive Relay Hydroalkylation: A Strategy for the Remote C(sp3)–H Alkylation of Alkenes. Angew. Chem., Int. Ed. 2018, 57 (15), 4058–4062. 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]; f Wang Z.; Yin H.; Fu G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 2018, 563 (7731), 379–383. 10.1038/s41586-018-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhou F.; Zhang Y.; Xu X.; Zhu S. NiH-Catalyzed Remote Asymmetric Hydroalkylation of Alkenes with Racemic α-Bromo Amides. Angew. Chem., Int. Ed. 2019, 58 (6), 1754–1758. 10.1002/anie.201813222. [DOI] [PubMed] [Google Scholar]
- Ju L.; Hu C. T.; Diao T. Strategies for Promoting Reductive Elimination of Bi- and Bis-Oxazoline Ligated Organonickel Complexes. Organometallics 2022, 41 (14), 1748–1753. 10.1021/acs.organomet.2c00091. [DOI] [Google Scholar]
- Dong Z.; MacMillan D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 2021, 598 (7881), 451–456. 10.1038/s41586-021-03920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L.; Till N. A.; Kudisch B.; MacMillan D. W. C.; Scholes G. D. Transient Absorption Spectroscopy Offers Mechanistic Insights for an Iridium/Nickel-Catalyzed C–O Coupling. J. Am. Chem. Soc. 2020, 142 (10), 4555–4559. 10.1021/jacs.9b12835. [DOI] [PubMed] [Google Scholar]
- Kim T.; McCarver S. J.; Lee C.; MacMillan D. W. C. Sulfonamidation of Aryl and Heteroaryl Halides through Photosensitized Nickel Catalysis. Angew. Chem., Int. Ed. 2018, 57 (13), 3488–3492. 10.1002/anie.201800699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1 (7), 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Welin E. R.; Le C.; Arias-Rotondo D. M.; McCusker J. K.; MacMillan D. W. C. Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(II). Science 2017, 355 (6323), 380–385. 10.1126/science.aal2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo K.; Hillhouse G. L. Carbon-Nitrogen Bond Formation by Reductive Elimination from Nickel(II) Amido Alkyl Complexes. Organometallics 1995, 14 (9), 4421–4423. 10.1021/om00009a054. [DOI] [Google Scholar]
- Matsunaga P. T.; Hess C. R.; Hillhouse G. L. Reactions of Organoazides with Nickel Alkyls. Syntheses and Reactions of Nickel(II) Amido Complexes. J. Am. Chem. Soc. 1994, 116 (8), 3665–3666. 10.1021/ja00087a089. [DOI] [Google Scholar]
- Choi B.-K.; Yamamoto T. Electrochemical properties of new n-type π-conjugated polybipyridines and polybiphenylenes with various bridging units. Electrochem. Commun. 2003, 5 (7), 566–570. 10.1016/S1388-2481(03)00119-X. [DOI] [Google Scholar]
- Yamamoto T. Synthesis of π-Conjugated Polymers Bearing Electronic and Optical Functionalities by Organometallic Polycondensations. Chemical Properties and Applications of the π-Conjugated Polymers. Synlett 2003, 2003, 425–450. 10.1055/s-2003-37504. [DOI] [Google Scholar]
- Saito T.; Uchida Y.; Misono A.; Yamamoto A.; Morifuji K.; Ikeda S. Diethyldipyridylnickel. Preparation, Characterization, and Reactions. J. Am. Chem. Soc. 1966, 88 (22), 5198–5201. 10.1021/ja00974a030. [DOI] [Google Scholar]
- Yamamoto T.; Yamamoto A.; Ikeda S. Organo (dipyridyl) nickel complexes. I. Stability and activation of the alkyl-nickel bonds of dialkyl (dipyridyl) nickel by coordination with various substituted olefins. J. Am. Chem. Soc. 1971, 93 (14), 3350–3359. 10.1021/ja00743a009. [DOI] [Google Scholar]
- Yamamoto T.; Abla M. Reductive elimination of Et—Et from NiEt2(bpy) promoted by electron-accepting aromatic compounds. J. Organomet. Chem. 1997, 535 (1), 209–211. 10.1016/S0022-328X(96)06940-9. [DOI] [Google Scholar]
- Yamamoto T.; Yamamoto A.; Ikeda S. Organo (dipyridyl) nickel complexes. II. Stabilities of olefin-nickel bonds in olefin-cooridinated dipyridylnickel and dialkyl (dipyridyl) nickel complexes. J. Am. Chem. Soc. 1971, 93 (14), 3360–3364. 10.1021/ja00743a010. [DOI] [Google Scholar]
- van Leeuwen P. W. N. M.; Kamer P. C. J.; Reek J. N. H.; Dierkes P. Ligand Bite Angle Effects in Metal-catalyzed C–C Bond Formation. Chem. Rev. 2000, 100 (8), 2741–2770. 10.1021/cr9902704. [DOI] [PubMed] [Google Scholar]
- Kohara T.; Yamamoto T.; Yamamoto A. Ligand exchange reaction between NiMe2L2 (L = bpy, PEt3) and ditertiary phosphines Ph2P(CH2)nPPh2 (n = 1–4) and effect of ligand on ease of reductive elimination of C2H6 from NiMe2(Ph2P(CH2)nPPh2). J. Organomet. Chem. 1980, 192 (2), 265–274. 10.1016/S0022-328X(00)94431-0. [DOI] [Google Scholar]
- For selected examples in which 2,9-disubstituted polypyridine ligands promote chain walking, see ref (10d) and the following references:; a Juliá-Hernández F.; Moragas T.; Cornella J.; Martin R. Remote Carboxylation of Halogenated Aliphatic Hydrocarbons with Carbon Dioxide. Nature 2017, 545 (7652), 84–88. 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]; b He Y.; Cai Y.; Zhu S. Mild and Regioselective Benzylic C–H Functionalization: Ni-Catalyzed Reductive Arylation of Remote and Proximal Olefins. J. Am. Chem. Soc. 2017, 139 (3), 1061–1064. 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]; c Wang X.; Nakajima M.; Serrano E.; Martin R. Alkyl Bromides as Mild Hydride Sources in Ni-Catalyzed Hydroamidation of Alkynes with Isocyanates. J. Am. Chem. Soc. 2016, 138 (48), 15531–15534. 10.1021/jacs.6b10351. [DOI] [PubMed] [Google Scholar]; d Chen F.; Chen K.; Zhang Y.; He Y.; Wang Y.-M.; Zhu S. Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of NiH. J. Am. Chem. Soc. 2017, 139 (39), 13929–13935. 10.1021/jacs.7b08064. [DOI] [PubMed] [Google Scholar]; e Tortajada A.; Juliá-Hernández F.; Börjesson M.; Moragas T.; Martin R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem., Int. Ed. 2018, 57 (49), 15948–15982. 10.1002/anie.201803186. [DOI] [PubMed] [Google Scholar]; f Tortajada A.; Menezes Correia J. T.; Serrano E.; Monleón A.; Tampieri A.; Day C. S.; Juliá-Hernández F.; Martin R. Ligand-Controlled Regiodivergent Catalytic Amidation of Unactivated Secondary Alkyl Bromides. ACS Catal. 2021, 11 (16), 10223–10227. 10.1021/acscatal.1c02913. [DOI] [Google Scholar]; g Li Y.; Luo Y.; Peng L.; Li Y.; Zhao B.; Wang W.; Pang H.; Deng Y.; Bai R.; Lan Y.; Yin G. Reaction scope and mechanistic insights of nickel-catalyzed migratory Suzuki–Miyaura cross-coupling. Nat. Commun. 2020, 11 (1), 417. 10.1038/s41467-019-14016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Peng L.; Li Z.; Yin G. Photochemical Nickel-Catalyzed Reductive Migratory Cross-Coupling of Alkyl Bromides with Aryl Bromides. Org. Lett. 2018, 20 (7), 1880–1883. 10.1021/acs.orglett.8b00413. [DOI] [PubMed] [Google Scholar]; i Peng L.; Li Y.; Li Y.; Wang W.; Pang H.; Yin G. Ligand-Controlled Nickel-Catalyzed Reductive Relay Cross-Coupling of Alkyl Bromides and Aryl Bromides. ACS Catal. 2018, 8 (1), 310–313. 10.1021/acscatal.7b03388. [DOI] [Google Scholar]
- Carmona E.; González F.; Poveda M. L.; Atwood J. L.; Rogers R. D. Synthesis and properties of dialkyl complexes of nickel(II). The crystal structure of bis(pyridine)bis(trimethylsilylmethyl)nickel(II). J. Chem. Soc., Dalton Trans. 1981, (3), 777–782. 10.1039/DT9810000777. [DOI] [Google Scholar]
- Kapadia R.; Brian Pedley J.; Brent Young G. Relative metal-carbon bond enthalpies and trans-influences for neopoentylplatinum(II) and trimethysilylmethylplatinum(II) complexes: an unusual case of weaker M-C binding by a β-silylmethyl ligand than its carbon analogue. Inorg. Chim. Acta 1997, 265 (1), 235–239. 10.1016/S0020-1693(97)05744-7. [DOI] [Google Scholar]
- Lappert M. F.; Patil D. S.; Pedley J. B. Standard heats of formation and M–C bond energy terms for some homoleptic transition metal alkyls MR. J. Chem. Soc., Chem. Commun. 1975, (20), 830–831. 10.1039/C39750000830. [DOI] [Google Scholar]
- Fendrick C. M.; Marks T. J. Thermochemically based strategies for carbon-hydrogen activation on saturated hydrocarbon molecules. Ring-opening reactions of a thoracyclobutane with tetramethylsilane and methane. J. Am. Chem. Soc. 1984, 106 (7), 2214–2216. 10.1021/ja00319a054. [DOI] [Google Scholar]
- Sjoegren M.; Hansson S.; Norrby P. O.; Aakermark B.; Cucciolito M. E.; Vitagliano A. Selective stabilization of the anti isomer of (η3-allyl) palladium and-platinum complexes. Organometallics 1992, 11 (12), 3954–3964. 10.1021/om00060a009. [DOI] [Google Scholar]
- Bader R. F. W.Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, U.K., 1990. [Google Scholar]
- Quantification of 1,2-bis(trimethylsilyl)ethane from the crude reaction mixture in C6D6 at 60 °C showed that this product was formed in 51% yield. While we were unable to determine the side products formed, possibly paramagnetic species with ligand C–H activation are generated. For a recent example, see:Davies J.; Janssen-Müller D.; Zimin D. P.; Day C. S.; Yanagi T.; Elfert J.; Martin R. Ni-Catalyzed Carboxylation of Aziridines en Route to β-Amino Acids. J. Am. Chem. Soc. 2021, 143 (13), 4949–4954. 10.1021/jacs.1c01916. [DOI] [PubMed] [Google Scholar]
- Monitoring the reductive elimination of 5 at 60 °C in C6D6 with 1 equiv of DMF revealed a slight reduction in rate (k = 1.16 × 10–4 s–1). We tentatively attribute this to the increase in the polarity of the solution, as an increase in solvent polarity may destabilize a nonpolar transition state.
- For the use of 1-fluoro-2,4,6-trimethylpyridinium tetrafluoroborate as a single-electron oxidant, see:; a Shevick S. L.; Obradors C.; Shenvi R. A. Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation. J. Am. Chem. Soc. 2018, 140 (38), 12056–12068. 10.1021/jacs.8b06458. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kiselyov A. S. Chemistry of N-fluoropyridinium salts. Chem. Soc. Rev. 2005, 34 (12), 1031–1037. 10.1039/b509217p. [DOI] [PubMed] [Google Scholar]
- Experiments with Kessil irradition resulted in a significant rate increase, which we attribute to heating from the lamp. For examples in which ambient light promoted the rate of reaction see:; a Boudjelel M.; Riedel R.; Schrock R. R.; Conley M. P.; Berges A. J.; Carta V. Tungstacyclopentane Ring Contraction Yields Olefin Metathesis Catalysts. J. Am. Chem. Soc. 2022, 144 (24), 10929–10942. 10.1021/jacs.2c03732. [DOI] [PubMed] [Google Scholar]; b Day C. S.; Fogg D. E. High-Yield Synthesis of a Long-Sought, Labile Ru-NHC Complex and Its Application to the Concise Synthesis of Second-Generation Olefin Metathesis Catalysts. Organometallics 2018, 37 (24), 4551–4555. 10.1021/acs.organomet.8b00745. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.