Abstract
Previous studies have revealed the usefulness of neoadjuvant chemotherapy (NAC) followed by surgery for clinical stage III gastric cancer (GC). The tumor volume at the primary tumor site (PT) is sometimes difficult to measure because of the luminal structure; therefore, evaluation of the lymph node (LN) response to NAC may help to more accurately predict survival outcomes. The present study therefore evaluated the LN response to NAC for prediction of survival outcomes in patients with GC. The study involved 160 patients with clinical stage III GC who underwent NAC (n=14) and upfront surgery (n=146). PT and LN responses to NAC were evaluated, various clinicopathological factors were evaluated and Cox proportional hazard regression analyses were performed to determine survival outcomes. Overall survival (OS) and disease-free survival (DFS) were not significantly different between patients who underwent NAC and those who underwent upfront surgery (OS, P=0.71; DFS, P=0.50). However, although there were no significant differences in PT responses, patients classified as LN non-responders to NAC had a significantly worse prognosis compared with patients classified as LN responders in terms of DFS (PT, P=0.93; LN, P<0.01) and OS (PT, P=0.84; LN, P<0.01). Moreover, a higher neutrophil-lymphocyte ratio tended to be associated with poor DFS [univariate: hazard ratio (HR)=4.23, P=0.06; multivariate: HR=6.45, P=0.04]. Finally, an LN response to NAC was significantly better for prediction of recurrence (univariate, HR=7.79, 95% confidence interval=1.16-63.51, P=0.02; multivariate, HR=7.44, P=0.01). Overall, the current study revealed the clinical importance of the LN response to NAC for predicting survival outcomes in patients with GC. These findings highlight the potential clinical impact of optimizing treatment strategies to improve the selection and management of patients.
Keywords: neoadjuvant chemotherapy, lymph node, response prediction marker, gastric cancer, neutrophil-lymphocyte ratio
Introduction
Gastric cancer (GC) is the fourth leading cause of cancer death worldwide (1). Although surgical resection with D2 lymphadenectomy is currently a standard strategy for advanced GC, the survival outcome of patients with stage III GC remains poor, with a 5-year overall survival (OS) rate of 40 to 70% (2). Neoadjuvant chemotherapy (NAC) for advanced GC has been used in clinical practice since the 1990s (3). NAC aims to control microscopic metastasis and decrease the tumor volume. For patients with clinically resectable disease at high risk of recurrence, such as patients with extensive lymph node (LN) metastasis or large type 3 or 4 tumors, NAC is more commonly used than adjuvant chemotherapy. Although no randomized trials have been performed to compare these approaches, NAC is more likely to provide maximum response to systemic therapy. NAC is a promising strategy that is associated with significantly higher curative resection and downstaging rates than provided by surgery alone, highlighting its potential to improve the OS of patients with GC (4). The MAGIC trial was a landmark study that evaluated the survival benefit of perioperative chemotherapy plus surgery vs. surgery alone in patients with curative GC (4). The study was concluded by a phase III trial comparing surgery with vs. without perioperative chemotherapy, and the results showed a similar 5-year OS rate in favor of perioperative chemotherapy (5). In the randomized phase II PETRARCA trial, the addition of trastuzumab and pertuzumab in patients with human epidermal growth factor 2-positive resectable esophagogastric adenocarcinoma resulted in a high pathologic complete response rate and significantly improved LN-negative rates (6). Because accumulating reports including the above-mentioned clinical trials have shown that the response to NAC is considered an important factor related to survival outcomes in patients with GC, NAC followed by surgery is more likely to improve disease-free survival (DFS) and OS (7–9). However, optimal methods to predict the therapeutic effect in patients with GC have not been developed, and a novel approach to evaluate the response to NAC for prediction of survival outcomes is required.
Tumor markers and imaging modalities, such as endoscopy and computed tomography (CT), have been used to evaluate the response to NAC. However, these evaluation methods involve measurement of the tumor volume at the primary tumor site (PT), which is sometimes difficult because of the luminal structure (10). In contrast, LNs have a clearer border and are included in the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Thus, NAC response evaluations that target LNs instead of the PT might provide a useful alternative for predicting survival outcomes in patients with GC. The presence of LN metastasis (LNM) is considered one of the most important prognostic factors after curative resection. More than half of patients with GC have LNM at the time of diagnosis, resulting in a 5-year OS rate of <30% (11). Because LNM is considered to be more closely associated with micrometastases than is the PT, evaluating the LN response to NAC might be more accurate than assessing the PT for predicting treatment benefits and survival outcomes and managing treatment strategies.
In the present study, by analyzing PT and LN responses to NAC using CT images, we evaluated the clinical use of NAC responses for predicting survival outcomes. Our simple approach may be used in clinical decision-making for patients with GC before and after NAC.
Materials and methods
Patient cohorts
This study involved 1220 patients with GC who underwent standard surgical procedures (gastrectomy and regional lymphadenectomy) at Tokushima University from 1994 to 2020. All patients were diagnosed with GC by pathologists according to the 8th edition of the American Joint Committee on Cancer/Union for International Cancer TNM staging system. Of these 1220 patients, we retrospectively reviewed 160 patients with clinical stage III GC who underwent NAC followed by surgery (n=14) and upfront surgery (n=146). The exclusion criteria were the inability to detect the size of the PT or LNs by CT images, the presence of non-adenocarcinoma, and lack of available clinical information. The pathological findings were evaluated by pathologists of Tokushima University, and the histological grade was determined according to the Japanese classification of GC; the evaluation criteria are provided in Fig. 1 (12). The patients' immunonutritional status was assessed using the neutrophil-lymphocyte ratio (NLR), lymphocyte-C-reactive protein ratio (LCR), and prognostic nutritional index (PNI) (13–15). C-reactive protein levels were assessed by using LABOSPECT 008 α (HITACHI, Tokyo, Japan). Neutrophils were calculated by using SIEMENS ADVIA 2120i Hematology System (Siemens Health care Co., Tokyo, Japan). Present study was approved in advance by the Institutional Review Board of the University of Tokushima Graduate School of Medical Science (TOCMS: 3215-1).
Figure 1.
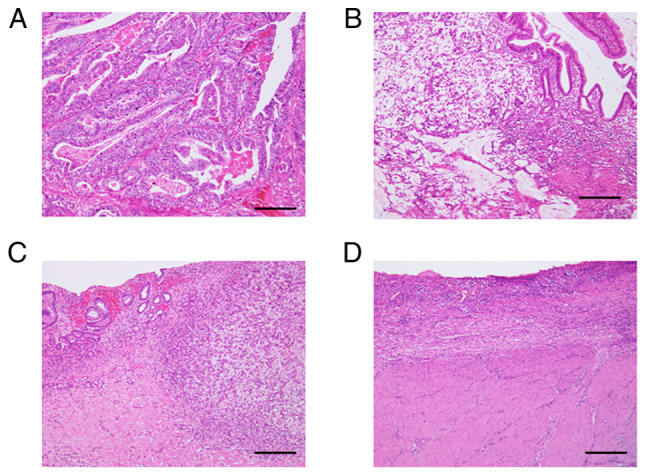
Histological grade of tumor response in patients treated by neoadjuvant chemotherapy. Pathological appearances of histological grades are shown (HE staining). (A) Grade 0 (no effect): No evidence of treatment effect. (B) Grade 1 (slight effect): Viable tumor cells occupy >1/3 of the tumor area. (C) Grade 2 (considerable effect): Viable tumor cells remain in <1/3 of the tumor area. (D) Grade 3 (complete response): No viable tumor cells remain. HE staining (magnification, ×20). Scale bar: 50 µm. HE, hematoxylin and eosin.
NAC
Routine examinations before treatment included a complete physical examination; assessment of medical and surgical history; chest, abdominal, and pelvic CT scans; and endoscopy. NAC consisted of a docetaxel, cisplatin, and S-1 (DCS) regimen or S-1 and oxaliplatin (SOX) regimen. DCS therapy involved two to six cycles of docetaxel (60 mg/m2), cisplatin (60 mg/m2), and S-1 (40 mg/m2 twice daily on days 1–14) every 3 weeks. SOX therapy involved two to six cycles of S-1 (40 mg/m2 twice daily on days 1–14) and oxaliplatin (130 mg/m2) via rapid intravenous infusion every 3 weeks. Radical resection with D2 lymphadenectomy was performed 2 to 4 weeks after NAC.
Evaluation of response to NAC
The response to NAC was evaluated by the volume of the PT and the short axis of the LN lesion with the greatest diameter on enhanced thin-slice (1-mm) CT scans before NAC and 2 to 4 weeks after NAC. The response to NAC in the PT and LNs was evaluated as the percent reduction in the PT area and LN length, respectively. We classified positive LNs as those of >8 mm in size because a threshold of 8 mm is often arbitrarily used for perigastric LNs (16). We evaluated the diameter of each LN lesion and the median short axis of the total LN lesions in patients with multiple LNM. Based on the RECIST classification, the patients were classified into a responder group (complete response and partial response) and a non-responder group (stable disease and progressive disease) (10). All patients underwent an abdominal CT scan using a 320-slice Aquilion One scanner (Toshiba, Tokyo, Japan). The CT device had auto exposure control and was linked to a networked medical imaging system through which images were electronically transferred to a centralized data system and then retrieved at a workstation.
Statistical analysis
The cutoff thresholds of continuous variables were divided by the median value among the total participants. The nutrition indices (NLR, LCR, and PNI) were also divided by the median value among the total participants. The patients' clinicopathological characteristics were compared between responders and non-responders using the chi-square test for categorical data. DFS and OS were analyzed by the Kaplan-Meier method using a weighted test. Univariate and multivariate Cox proportional hazard regression models were used to identify independent prognostic markers. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using MedCalc 16.2.0 statistical software (MedCalc Software bvba, Ostend, Belgium) and JMP Pro 13 statistical software (SAS Institute Japan, Tokyo, Japan).
Results
Patient characteristics and CT measurements
We retrospectively reviewed 160 patients with clinical stage III GC who had been diagnosed with one or more positive LNs before treatment. Among these 160 patients, 14 underwent NAC followed by surgery and 146 underwent standard radical surgery (gastrectomy and D2 lymphadenectomy). The patients' detailed clinicopathological characteristics are provided in Table I. There were no significant differences in the clinicopathological characteristics between the two groups. The median PT area before and after NAC was 924 mm2 (range: 600–2649 mm2) and 665 mm2 (range: 332–1956 mm2), respectively. The median short axis of LN lesions before and after NAC was 11 mm (range: 8–20 mm) and 7 mm (range: 5–12 mm), respectively. We divided the patients into responder and non-responder groups based on the RECIST criteria; six patients were PT responders and eight were PT non-responders, and eight patients were LN responders and six were LN non-responders. We then examined the difference in the response to NAC between PTs and LNs (Table II).
Table I.
Clinicopathological characteristics of patients who underwent upfront surgery vs. NAC followed by surgery.
| Factors | Upfront surgery (n=146) | NAC (n=14) | P-value |
|---|---|---|---|
| Age | 0.19 | ||
| ≥70 years | 79 | 5 | |
| <70 years | 67 | 9 | |
| Sex | 0.75 | ||
| Male | 109 | 11 | |
| Female | 37 | 3 | |
| Tumor location | 0.96 | ||
| Upper/Middle | 84 | 8 | |
| Lower | 62 | 6 | |
| Tumor size | 0.62 | ||
| ≥50 mm | 83 | 7 | |
| <50 mm | 63 | 7 | |
| Differentiation | 0.08 | ||
| Well/Moderate | 74 | 3 | |
| Poor | 58 | 7 | |
| Others | 14 | 4 | |
| cT stage | 0.75 | ||
| T1-3 | 76 | 6 | |
| T4 | 70 | 8 | |
| cN stage | 0.73 | ||
| N0-1 | 80 | 7 | |
| N2-3 | 66 | 7 | |
| Surgical procedure | 0.62 | ||
| DG | 62 | 5 | |
| TG | 84 | 9 | |
| NAC regimen | - | ||
| DCS | - | 9 | |
| SOX | - | 5 | |
| Histological grade | - | ||
| 0, 1 | - | 6 | |
| 2, 3 | - | 8 |
DCS, docetaxel + cisplatin + S-1; DG, distal gastrectomy; NAC, neoadjuvant chemotherapy; SOX, S-1 + oxaliplatin; TG, total gastrectomy.
Table II.
Clinicopathological characteristics according to PT and LN responses to NAC.
| Primary tumor | Lymph node | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factors | Res (n=6) | Non-res (n=8) | P-value | Res (n=8) | Non-res (n=6) | P-value |
| Age | 0.33 | |||||
| ≥70 years | 3 | 2 | 2 | 3 | ||
| <70 years | 3 | 6 | 0.33 | 6 | 3 | |
| Sex | 0.35 | |||||
| Male | 6 | 5 | 7 | 4 | ||
| Female | 0 | 3 | 0.10 | 1 | 2 | |
| Tumor location | 0.12 | |||||
| Upper/Middle | 5 | 3 | 6 | 2 | ||
| Lower | 1 | 5 | 0.09 | 2 | 4 | |
| Tumor size | 0.12 | |||||
| ≥50 mm | 2 | 5 | 2 | 4 | ||
| <50 mm | 4 | 3 | 0.28 | 6 | 2 | |
| Differentiation | 0.08 | |||||
| Well/Moderate | 1 | 2 | 2 | 1 | ||
| Poor | 4 | 3 | 5 | 2 | ||
| Others | 1 | 3 | 0.54 | 1 | 3 | |
| cT stage | 0.53 | |||||
| T1-3 | 3 | 3 | 4 | 2 | ||
| T4 | 3 | 5 | 0.64 | 4 | 4 | |
| cN stage | 0.28 | |||||
| N1 | 2 | 5 | 3 | 4 | ||
| T2-3 | 4 | 3 | 0.28 | 5 | 2 | |
| Surgical procedure | 0.33 | |||||
| DG | 2 | 3 | 2 | 3 | ||
| TG | 4 | 5 | 0.87 | 6 | 3 | |
| NAC regimen | 0.87 | |||||
| DCS | 4 | 5 | 5 | 4 | ||
| SOX | 2 | 3 | 0.87 | 3 | 2 | |
| Histological grade | 0.12 | |||||
| 0, 1 | 2 | 4 | 2 | 4 | ||
| 2, 3 | 4 | 4 | 0.53 | 6 | 2 | |
| LCR | 0.53 | |||||
| High | 4 | 2 | 4 | 4 | ||
| Low | 2 | 6 | 0.12 | 4 | 2 | |
| NLR | 0.35 | |||||
| High | 5 | 2 | 7 | 2 | ||
| Low | 1 | 6 | 0.71 | 1 | 4 | |
| PNI | 0.20 | |||||
| High | 5 | 4 | 4 | 1 | ||
| Low | 1 | 4 | 0.20 | 4 | 5 | |
DCS, docetaxel + cisplatin + S-1; DG, distal gastrectomy; LCR, lymphocyte-C-reactive protein ratio; LN, lymph node; NAC, neoadjuvant chemotherapy; NLR, neutrophil-lymphocyte ratio; Non-res, nonresponse; PNI, prognostic nutritional index; PT, primary tumor site; Res, response; SOX, S-1 + oxaliplatin; TG, total gastrectomy.
Prognostic potential of NAC response for predicting survival outcomes in patients with GC
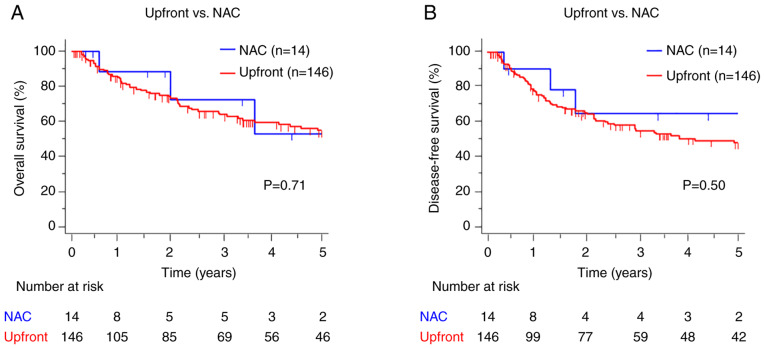
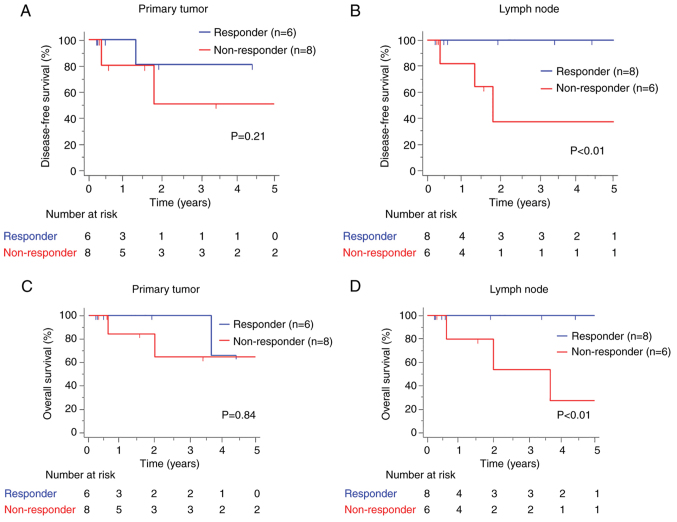
To evaluate the prognostic potential of NAC responses, we performed a survival analysis to compare OS and DFS between patients who underwent NAC and those who underwent upfront surgery. There were no significant differences in survival outcomes between the two groups (OS: P=0.71, DFS: P=0.50) (Fig. 2A and B). We then investigated the prognostic potential of NAC responses between patients categorized as responders and non-responders for PTs and LNs. The results showed no significant differences in the 5-year DFS rate between the PT non-responder and responder groups (64.3% vs. 66.7%, P=0.93) (Fig. 3A). More importantly, we observed that LN non-responders had significantly worse DFS than LN responders (30.0% vs. 100%, P<0.01) (Fig. 3B). Moreover, LN non-responders had significantly worse OS, whereas no significant difference in OS was observed between PT responders and non-responders (PT: 62.5% vs. 50.0%, P=0.84; LN: 26.7% vs. 100%, P<0.01) (Fig. 3C and D). These findings highlight the clinical importance of LN responses to NAC.
Figure 2.
Prognosis of patients after upfront surgery and NAC. Comparison of (A) OS and (B) DFS in patients with clinical stage III gastric cancer who underwent upfront surgery (n=146) and NAC followed by surgery (n=14). The OS and DFS times were calculated from the date of surgery to the date of death of any cause, recurrence, or the last follow-up. OS and DFS were estimated using the Kaplan-Meier method. NAC, neoadjuvant chemotherapy; OS, overall survival; DFS, disease free survival.
Figure 3.
Prognosis of patients in responder and non-responder groups based on PT and LN responses to NAC. Comparison of DFS between patients in the responder and non-responder groups according to (A) PT and (B) LN responses to NAC. Comparison of OS between patients in the responder and non-responder groups according to (C) PT and (D) LN responses to NAC. OS and DFS were estimated using the Kaplan-Meier method using a weighted test. PT, primary tumor site; LN, lymph node; NAC, neoadjuvant chemotherapy; DFS, disease-free survival; OS, overall survival.
Furthermore, we conducted univariate and multivariate Cox proportional hazard regression analyses to identify prognostic factors, and patients categorized as LN non-responders to NAC had significantly worse DFS than patients categorized as LN responders [univariate: hazard ratio (HR)=7.79, 95% confidence interval (CI)=1.16-63.51, P=0.02; multivariate: HR=7.44, 95% CI=1.45-56.78, P=0.01] (Table III). In contrast, PT responders and non-responders to NAC showed no significant differences in DFS (univariate: HR=2.35, 95% CI=0.62-8.85, P=0.21). Among the clinicopathological factors, an elevated NLR tended to be associated with poor DFS (univariate: HR=4.23, 95% CI=0.57-31.42, P=0.06; multivariate: HR=6.45, 95% CI=1.23-45.34, P=0.04). These results indicate that the LN response to NAC and the NLR have prognostic potential in predicting the survival outcomes of patients with GC.
Table III.
Univariate and multivariate Cox proportional hazard regression analyses for disease-free survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | ||||||
| (≥70 vs. <70 years) | 2.10 | 0.59-7.43 | 0.25 | |||
| Gender | ||||||
| (Male vs. Female) | 0.31 | 0.04-2.53 | 0.28 | |||
| Tumor location | ||||||
| (Upper/Middle vs. Lower) | 1.45 | 0.37-5.77 | 0.59 | |||
| Tumor size | ||||||
| (≥50 mm vs. <50 mm) | 0.64 | 0.16-2.56 | 0.53 | |||
| Differentiation | ||||||
| (Well/Moderate vs. Poor/Others) | 1.13 | 0.28-4.41 | 0.88 | |||
| cT | ||||||
| (T4 vs. <T3) | 2.61 | 0.73-9.40 | 0.14 | |||
| cN | ||||||
| (≥N2 vs. <N1) | 1.18 | 0.31-4.48 | 0.81 | |||
| Surgical procedure | ||||||
| (TG vs. DG) | 1.29 | 0.33-5.08 | 0.71 | |||
| NAC regimen | ||||||
| (DCS vs. SOX) | 3.27 | 0.78-13.73 | 0.09 | 2.37 | 0.67-8.98 | 0.45 |
| Histological grade | ||||||
| (0, 1 vs. 2, 3) | 1.71 | 0.32-9.00 | 0.53 | |||
| LCR | ||||||
| (Low vs. High) | 2.53 | 0.67-9.60 | 0.17 | |||
| NLR | ||||||
| (High vs. Low) | 4.23 | 0.57-31.42 | 0.06 | 6.45 | 1.23-45.34 | 0.04a |
| PNI | ||||||
| (Low vs. High) | 1.90 | 0.45-8.07 | 0.39 | |||
| PT response | ||||||
| (Non-res vs. Res) | 2.35 | 0.62-8.85 | 0.21 | |||
| LN response | ||||||
| (Non-res vs. Res) | 7.79 | 1.16-63.51 | 0.02a | 7.44 | 1.45-56.78 | 0.01a |
P<0.05. CI, confidence interval; DCS, docetaxel + cisplatin + S-1; DG, distal gastrectomy; HR, hazard ratio; LCR, lymphocyte-C-reactive protein ratio; LN, lymph node; NAC, neoadjuvant chemotherapy; NLR, neutrophil-lymphocyte ratio; Non-res, nonresponse; PNI, prognostic nutritional index; PT, primary tumor site; Res, response; SOX, S-1 + oxaliplatin; TG, total gastrectomy.
Discussion
Radical resection with D2 lymphadenectomy is currently the gold standard for advanced GC worldwide (12,17–19). However, GC is one of the most aggressive malignancies; it has high metastatic potential, and the 5-year OS rates of patients after D2 lymphadenectomy remain poor (18). Therefore, a multi-treatment strategy consisting of NAC has been suggested for GC because NAC reportedly reduces the tumor volume and micrometastases and improves the R0 resection rate (4). Since the 1990s, NAC has been used in clinical practice for advanced GC (3). Furthermore, several clinical studies on the efficacy of NAC or different chemotherapeutic regimens have recently demonstrated survival benefits compared with surgery alone in patients with advanced GC (4,5,20), emphasizing the clinical significance of predicting the response to NAC.
Imaging modalities, including fluoroscopy, CT, and endoscopy, are standard tools for confirming the efficacy of NAC (21–23). Although the most accurate approach for evaluating the response to NAC is pathological classification, some studies have shown that the pathological grading scores of PTs have no predictive value for survival outcomes, and the predictive effect of the pathological complete response rate is not associated with that of the LNM rate (24,25). In the present study, we investigated the clinicopathological factors and PT/LN responses to NAC by CT imaging. The results showed that the PT response to NAC had no predictive significance, whereas patients classified as LN responders to NAC had a significantly higher 5-year DFS rate (30.0% vs. 100%, P<0.01) and 5-year OS rate (26.7% vs. 100%, P<0.01) than non-responders. Accumulating reports have shown that the rate of LNM predicts survival outcomes and local recurrence in patients with GC (26–28), emphasizing that the LN response to NAC has prognostic potential.
Additionally, we evaluated the LCR, PNI, and NLR, which have been suggested to reflect the balance between the pro-tumor inflammatory status and anti-tumor immune status. In previous studies, patients with several cancers (including GC) who had an increased NLR showed neutrophilic leukocytosis and lymphocytopenia (29–33). In the present study, an elevated NLR was associated with recurrence in patients with GC before treatment and with the LN response to NAC, emphasizing the clinical importance of this nutritional index. Several reports have also shown that cancer-related systemic inflammatory responses are associated with increased numbers of circulating neutrophils. Neutrophils secrete cytokines and chemokines, which play an important role in cancer progression (34), and the NLR is one of the most robust biomarkers for predicting the prognosis of various types of cancer (35,36). In the present study, an elevated NLR tended to be associated with poor DFS. However, other nutritional indices (lower PNI and LCR) were not associated with poor survival. A lower PNI and LCR have also been reported to be independently associated with poor survival (33,37–40). Therefore, additional studies involving more patients are needed to address the potential prognostic value of nutritional indices in GC.
This study has several limitations. First, its retrospective and single-institute design may have resulted in selection bias. Thus, a prospective clinical trial is required to confirm our findings. However, the patient population was uniform, and all patients received the same treatment strategy [i.e., NAC (DCS or SOX regimen) followed by distal or total gastrectomy with D2 lymphadenectomy]. Second, we analyzed a small number of patients who underwent gastrectomy with NAC. As a result, the findings of the present study are only applicable to patients with positive LNM who undergo NAC followed by surgery. We must confirm the accuracy of our findings in larger numbers of patients, and future studies should evaluate the feasibility of targeting distant metastatic lesions in addition to LNs to predict the response to chemotherapy. Third, we validated the predictive potential of LN responses to NAC by analyzing CT images and clinicopathological factors. Future experiments investigating other previously reported prognostic factors, such as the microsatellite instability status and GC subtypes (41–44), are needed to increase the convenience and prognostic accuracy of our approach. Finally, diagnosing clinical stage III GC by imaging modalities is difficult. However, our study provides important evidence for predicting survival outcomes in patients with GC, and these findings may be a significant step toward the management of lethal malignancies.
In conclusion, we have revealed the clinical importance of the LN response to NAC with CT imaging for prediction of survival outcomes in patients with GC. Our findings highlight the potential clinical impact of optimizing treatment strategies and improving the selection and management of patients with this malignancy.
Acknowledgements
The authors would like to thank Dr Melissa Crawford and Dr Angela Morben for editing a draft of this manuscript.
Glossary
Abbreviations
- CI
confidence interval
- CT
computed tomography
- DCS
docetaxel, cisplatin, and S-1
- DFS
disease-free survival
- GC
gastric cancer
- HR
hazard ratio
- LCR
lymphocyte-C-reactive protein ratio
- LN
lymph node
- LNM
lymph node metastasis
- NAC
neoadjuvant chemotherapy
- NLR
neutrophil-lymphocyte ratio
- OS
overall survival
- PNI
prognostic nutritional index
- PT
primary tumor site
- RECIST
Response Evaluation Criteria in Solid Tumors
- SOX
S-1 and oxaliplatin
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YW, MN and MS designed the study. YW, MN, KY, CT, TT and TN performed the data analyses. YW, MN, MS, HK and TY acquired the clinical data and drafted the manuscript. MS revised the manuscript. All authors read and approved the final manuscript. YW and MN confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Tokushima Graduate School (approval no. 3215). Consent to participate was not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 3.Songun I, Keizer HJ, Hermans J, Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van Krieken JH, van de Velde CJ. Chemotherapy for operable gastric cancer: Results of the Dutch randomised FAMTX trial. The Dutch gastric cancer group (DGCG) Eur J Cancer. 1999;35:558–562. doi: 10.1016/S0959-8049(98)00429-8. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 6.Hofheinz RD, Merx K, Haag GM, Springfeld C, Ettrich T, Borchert K, Kretzschmar A, Teschendorf C, Siegler G, Ebert MP, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2-positive resectable esophagogastric adenocarcinoma: A randomized phase II Trial of the AIO EGA study group. J Clin Oncol. 2022 doi: 10.1200/JCO.22.00380. JCO2200380. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Peng L, Yang W, Zhang Z, Liu H, Hua Y. Clinical features and prognosis analysis of 21 gastric cancer patients with pathological complete response after neoadjuvant chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:1168–1173. (In Chinese) [PubMed] [Google Scholar]
- 8.Cho H, Nakamura J, Asaumi Y, Yabusaki H, Sakon M, Takasu N, Kobayashi T, Aoki T, Shiraishi O, Kishimoto H, et al. Long-term survival outcomes of advanced gastric cancer patients who achieved a pathological complete response with neoadjuvant chemotherapy: A systematic review of the literature. Ann Surg Oncol. 2015;22:787–792. doi: 10.1245/s10434-014-4084-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Peng Z, Chen L. Survival analysis of gastric cancer cases with pathological complete response received neoadjuvant chemotherapy. Zhonghua Yi Xue Za Zhi. 2016;96:1582–1584. doi: 10.3760/cma.j.issn.0376-2491.2016.20.008. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepatogastroenterology. 2002;49:874–877. [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association, corp-author. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, Bridgewater J. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: Exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27:687–692. doi: 10.1093/annonc/mdw012. [DOI] [PubMed] [Google Scholar]
- 14.Bruixola G, Caballero J, Papaccio F, Petrillo A, Iranzo A, Civera M, Moriana M, Bosch N, Maroñas M, González I, et al. Prognostic nutritional index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open. 2018;3:e000425. doi: 10.1136/esmoopen-2018-000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, Fujikawa H, Yasuda H, Hiro J, Yoshiyama S, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272:342–351. doi: 10.1097/SLA.0000000000003239. [DOI] [PubMed] [Google Scholar]
- 16.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 17.Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 18.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A, ESMO Guidelines Working Group Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21((Suppl 5)):v50–v54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 19.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO) Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24((Suppl 6)):vi57–vi63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 20.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SR, Lee JS, Kim CG, Kim HK, Kook MC, Kim YW, Ryu KW, Lee JH, Bae JM, Choi IJ. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer. 2008;112:2368–2376. doi: 10.1002/cncr.23483. [DOI] [PubMed] [Google Scholar]
- 22.Redondo-Cerezo E, Martínez-Cara JG, Jiménez-Rosales R, Valverde-López F, Caballero-Mateos A, Jérvez-Puente P, Ariza-Fernández JL, Úbeda-Muñoz M, López-de-Hierro M, de Teresa J. Endoscopic ultrasound in gastric cancer staging before and after neoadjuvant chemotherapy. A comparison with PET-CT in a clinical series. United European Gastroenterol J. 2017;5:641–647. doi: 10.1177/2050640616684697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial. J Clin Oncol. 2016;34:2721–2727. doi: 10.1200/JCO.2015.65.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasello G, Petrelli F, Ghidini M, Pezzica E, Passalacqua R, Steccanella F, Turati L, Sgroi G, Barni S. Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: A meta-analysis of 17 published studies. Eur J Surg Oncol. 2017;43:1607–1616. doi: 10.1016/j.ejso.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Takagane A, Terashima M, Abe K, Araya M, Irinoda T, Yonezawa H, Nakaya T, Inaba T, Oyama K, Fujiwara H, Saito K. Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer. 1999;2:122–128. doi: 10.1007/s101200050034. [DOI] [PubMed] [Google Scholar]
- 27.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Metastatic gastric lymph node rate is a significant prognostic factor for resectable stage IV stomach cancer. J Am Coll Surg. 1997;185:65–69. doi: 10.1016/S1072-7515(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 28.Komatsu S, Ichikawa D, Miyamae M, Kosuga T, Okamoto K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki A, et al. Positive lymph node ratio as an indicator of prognosis and local tumor clearance in N3 gastric cancer. J Gastrointest Surg. 2016;20:1565–1571. doi: 10.1007/s11605-016-3197-9. [DOI] [PubMed] [Google Scholar]
- 29.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: An analysis of Japan multinational trial organisation LC00-03. Eur J Cancer. 2009;45:1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, Kim HJ. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng CB, Zhang QX, Zhuang LP, Sun JW. Prognostic value of lymphocyte-to-C-reactive protein ratio in patients with gastric cancer after surgery: A multicentre study. Jpn J Clin Oncol. 2020;50:1141–1149. doi: 10.1093/jjco/hyaa099. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. 2017;7:17166. doi: 10.1038/s41598-017-17130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, Cai S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139:220–231. doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 36.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, Suzuki A, Fujikawa H, Yasuda H, Hiro J, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2020;39:1209–1217. doi: 10.1016/j.clnu.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Hirahara N, Tajima Y, Fujii Y, Kaji S, Yamamoto T, Hyakudomi R, Taniura T, Kawabata Y. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: A propensity score matching analysis. BMC Cancer. 2018;18:285. doi: 10.1186/s12885-018-4201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N, Nakajima Y. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20:2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 40.Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10:17373. doi: 10.1038/s41598-020-74525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017;23:4441–4449. doi: 10.1158/1078-0432.CCR-16-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer Genome Atlas Research Network, corp-author. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Wang X. Identification of gastric cancer subtypes based on pathway clustering. NPJ Precis Oncol. 2021;5:46. doi: 10.1038/s41698-021-00186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriquenz MG, Roviello G, D'Angelo A, Lavacchi D, Roviello F, Polom K. MSI and EBV positive gastric cancer's subgroups and their link with novel immunotherapy. J Clin Med. 2020;9:1427. doi: 10.3390/jcm9051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.