SUMMARY
We implemented a quality management system (QMS) and documented our improvements in a tuberculosis (TB) laboratory in Kisumu, Kenya. After implementation of the QMS, a sustained reduction in culture contamination rates for solid (from 15.4% to 5.3%) and liquid media (from 15.2% to 9.3%) was observed, and waste from product expiry was reduced significantly. External quality assurance (EQA) results were satisfactory before and after QMS implementation, and a client survey after implementation revealed 98% satisfaction. The laboratory attained ISO 15189 accreditation in October 2013. The implementation of QMS facilitated the attainment of target quality indicators, reduced waste due to expiry and led to high client satisfaction.
Keywords: accreditation, ISO 15189, tuberculosis, laboratory, quality
RESUME
Nous avons mis en œuvre un système de contrôle de qualité (QMS) et nous avons documenté nos améliorations dans un laboratoire de tuberculose à Kisumu, Kenya. Après la mise en œuvre du QMS, nous avons observé une diminution durable des taux de contamination des cultures en milieu solide (de 15,4% à 5,3%) et liquide (de 15,2% à 9,3%), et le taux de gaspillage de produits expirés a été significativement réduit. Les résultats de l’assurance de qualité externe ont été satisfaisants avant et après la mise en œuvre du QMS, et une enquête auprès des clients après la mise en œuvre a révélé un taux de satisfaction de 98%. Le laboratoire a atteint l’accréditation ISO 15189 en octobre 2013. En conclusion, la mise en œuvre du QMS a facilité l’atteinte d’indicateurs de qualité ciblés, a réduit le taux de produits expirés et obtenu une satisfaction élevée des clients.
RESUMEN
En un laboratorio de tuberculosis de Kisimu, en Kenia, se introdujo un sistema de gestión de la calidad (QMS) y se documentaron los progresos alcanzados. Tras la puestaenmarchadelsistema,seobservó una disminución continua de la tasa de contaminación de los cultivos en medio sólido (de 15,4% a 5,3%) y en medio líquido (de 15,2% a 9,3%), además de una reducción considerable del desperdicio de productos por cumplimiento de su fecha de caducidad. Los resultados del control externo de la calidad fueron satisfactorios antes y después de la aplicación del QMS y una encuesta realizada después de la introducción reveló 98% de satisfacción de los usuarios. El laboratorio logró la homologación ISO 15189 en octubre del 2013. En conclusión, la ejecución de un QMS facilita el cumplimiento de las metas de los indicadores de calidad, disminuye las pérdidas por caducidad de los productos y procura gran satisfacción de los usuarios.
LABORATORIES ARE A FUNDAMENTAL component of tuberculosis (TB) control, providing testing at every level of the health care system.1 A key component of strengthening laboratory capacity is the implementation of a quality management system (QMS), leading to the laboratory’s accreditation according to international standards such as the International Organization for Standardization (ISO) 15189.2,3 However, in sub-Saharan Africa, which has a high TB burden, laboratories are among the most ill-equipped and poorly resourced facilities, with few TB laboratories currently accredited.4
In collaboration with the US Centers for Disease Control and Prevention (CDC), the Kenya Medical Research Institute (KEMRI) established a TB biosafety level (BSL) 2 laboratory in 2005 to support smear microscopy, epidemiology studies and specimen referral. In 2009, the laboratory was upgraded to a BSL3 facility, and it currently performs fluorescence and bright-field microscopy, mycobacterial culture, molecular testing, first-line drug susceptibility testing (DST), immunology assays and genetic sequencing. In 2011, the laboratory began implementing a QMS to improve its services. In the present article, we share our experiences in implementing a QMS based on quality indicators, including culture contamination rates, cost savings associated with improved inventory management, external quality assurance (EQA) results and a client satisfaction survey.
METHODS
Implementation of the quality management system
Initially, we assessed pre-analytical, analytical and post-analytical phases of testing and developed a quality manual, laboratory policies, and standard operating procedures (SOPs) for all assays. A health and safety review was also conducted of all laboratory processes from specimen reception up to management of biohazard waste, in an effort to minimize the distance between sequential process steps and improve operations management. The overall workflow was streamlined through the establishment of organized work stations.
Quality management system: evaluating progress
QMS evaluation involved conducting internal audits, monitoring of all processes by quality assurance personnel and training additional staff as internal auditors. A gap analysis was conducted by an external auditor, and two audits were conducted by A Global Healthcare Public Foundation, Nairobi, Kenya. The laboratory applied for accreditation with the South African National Accreditation System (SANAS), Pretoria, South Africa, by submitting the required documentation and preparing the laboratory for final assessment.
Measurement of impact of quality management system implementation
Culture contamination rates
The proportion of cultures that were contaminated with organisms other than mycobacteria (typically fungi and other bacteria) was calculated for liquid and solid culture before (2011) and after QMS implementation (2012–2013) and compared to established acceptable ranges for both.5,6 Once baseline performance was established, we assessed the proficiency of staff performing culture, and optimised specimen processing procedures where indicated.
Waste due to product expiry
We calculated the cost of expired items as a precentage of the total cost of operation before (2010–2011) and after (2012–2013) QMS implementation. The laboratory implemented an electronic inventory system in 2012 which captured the stock levels, the amounts ordered, the re-order triggers, the amounts issued and the expiration dates of each item. The system provided alerts on expired items and those items below re-order level to improve projections and tracking to avoid expiries and stock-outs.
Participation in external quality assessment programs
To determine staff proficiency and the overall quality of services provided, we enrolled in the National Health Laboratory Service (College of American Pathologists, Northfield, IL, USA), and Introl proficiency testing programmes for microscopy, Xpert® MTB/RIF, QuantiFERON® assays, culture and DST.7
Client satisfaction
A client satisfaction survey was conducted in 2012 and included questions such as the scope of services offered, quality, reliability and turnaround times for test results, responses to technical queries, quality of communication with staff, clarity and usefulness of the laboratory report and service costs.
Ethical considerations
No ethics review was required for this study, as no data on human subjects were used.
RESULTS
Contamination rates
The contamination rates for solid media declined from 15.4% to 5.3% (odds ratio [OR] 0.31, 95% confidence interval [CI] 0.21–0.45, P < 0.001), and for liquid media they declined from 15.2% to 9.3% (OR 0.58, 95%CI 0.49–0.68, P < 0.001) (Table, Figure).
Table.
Summary of quality indicators before and after QMS implementation
| Quality indicator | Before QMS implementation % | After QMS implementation % |
|---|---|---|
| Contamination rates | ||
| MGIT | 15.2 | 9.3 |
| LJ | 15.4 | 5.3 |
| Waste from product expiry | 6.1 | 1.3 |
| EQA performance for microscopy, culture, DST and Xpert | 90–100 | 90–100 |
| Client satisfaction survey | Not done | 98 |
QMS = quality management system; MGIT = Mycobacteria Growth Indicator Tube™; LJ = Löwenstein-Jensen; EQA = external quality assurance, DST = drug susceptibility testing, Xpert = Xpert® MTB/RIF.
Figure.
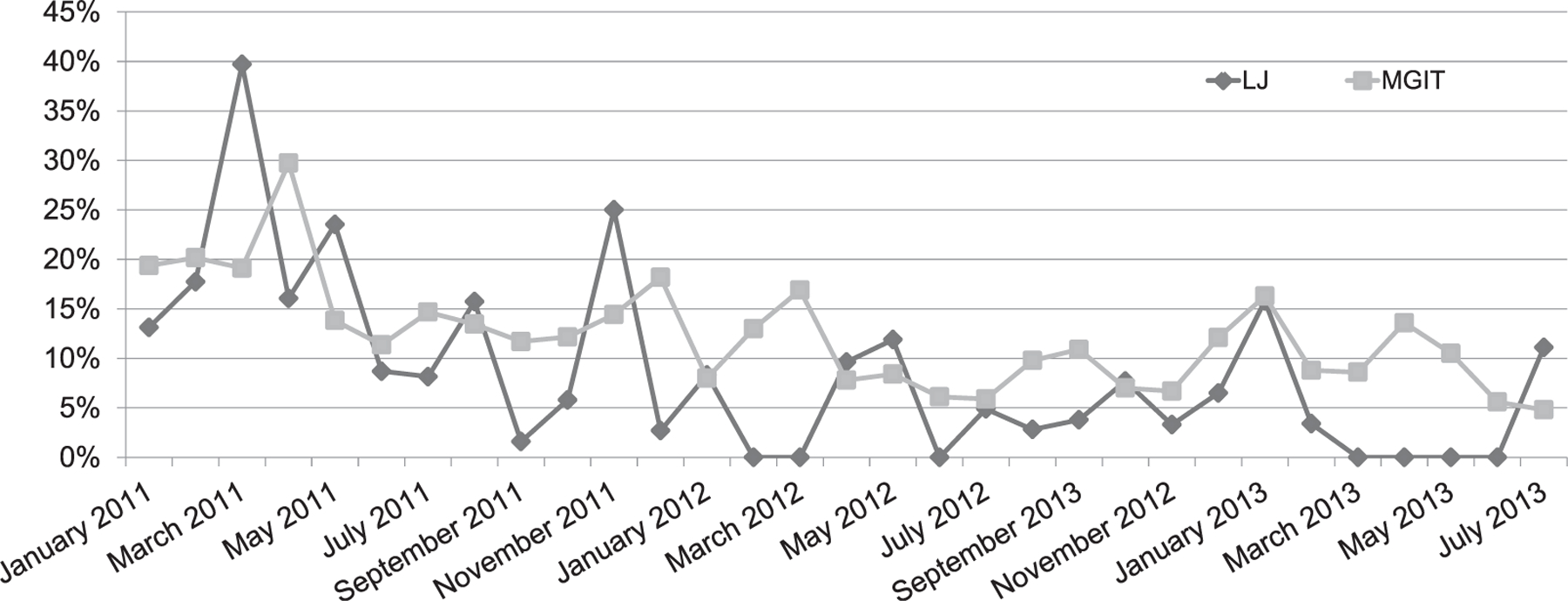
Contamination rates of LJ and MGIT media before and after implementation of a quality management system in a tuberculosis laboratory, Kisumu, Kenya. LJ = Löwenstein-Jensen; MGIT = Mycobacterial Growth Indicator Tube.
Assessment of waste from product expiry
Before QMS implementation, (2010–2011), expired supplies accounted for US$13 324 (6.1%) of the total cost of US$217 137, while items ordered after QMS implementation (2012–2013) accounted for US$2320 (1.3%) of the total cost of US$181 281(Table).
External quality assessment results
EQA results for all enrolled assays were satisfactory before and after QMS implementation with scores of 90–100% (Table).
Client satisfaction survey
A 2012 client survey revealed 98% satisfaction based on eight key indicators (Table).
Accreditation
The laboratory was enrolled in the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA)8 in February 2012. We attained a 3-star baseline rating and a 5-star rating for the second and final assessment. The laboratory was assessed by SANAS in July 2013 with three major and six minor non-conformances, and was recommended for accreditation. The non-conformances were cleared in 10 working days, and the laboratory was awarded full accreditation effective from 15 October 2013.
DISCUSSION
Efforts to accredit TB laboratories have an important impact on public health, as accreditation helps ensure accurate diagnostic testing. Implementing QMS improved the efficiency of our laboratory services, including identification of the most probable causes of elevated contamination rates, such as suboptimal specimen collection and processing procedures, and introduction of targeted interventions (including development of an SOP on specimen collection, retraining of field staff on the correct approach and modifying the decontamination regimen in the laboratory). This resulted in a reduced need for retesting due to lower culture contamination rates, interruption of the loss of reagents due to expiration (e.g., cost savings of more than US$10 000), supervision and EQA programs that assure accuracy of testing with few false-positive and false-negative results, and high client satisfaction. Specifically, this process enabled us to strengthen our workforce through additional technical training courses and an introduction to quality assurance concepts, and was an essential initial step in preparing our laboratory for ISO accreditation.
While variation in laboratory infrastructure, personnel and resources in other settings may influence the implementation of QMS, resulting in differences in experiences, implementing a QMS and working towards accreditation can benefit any laboratory. A baseline assessment enables laboratories to identify opportunities for improvement. Tools such as the Global Laboratory Initiative Stepwise Process Towards Tuberculosis Laboratory Accreditation9 and Strengthening Laboratory Management towards Accreditation (SLMTA) programmes10 are available to help laboratories implement and improve their QMS and performance. These phased processes include tools, performance indicators and quality measures that should be useful in producing meaningful improvements in quality, reliability and timeliness of diagnostic testing.
Acknowledgements
The authors thank the Director of the Kenya Medical Research Institute Centre for Global Health Research, the tuberculosis laboratory team for their efforts and input in the implementation of the quality management system, and TB branch management and the United States Agency for International Development and the Centers for Disease Control and Prevention for providing the funding needed to help the laboratory gain accreditation.
Footnotes
Conflicts of interest: none declared.
References
- 1.Shinnick TM, Iademarco MF, Ridderhof JC. National plan for reliable tuberculosis laboratory services using a systems approach. MMWR Recomm Rep 2005; 54 (RR-6): 1–12. [PubMed] [Google Scholar]
- 2.Ridderhof JC, van Deun A, Kam KM, et al. Roles of laboratories and laboratory systems in effective tuberculosis programmes. Bull World Health Organ 2007; 85: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeh CE, Inzaule SC, Magero VO, et al. Field experience in implementing ISO 15189 in Kisumu, Kenya. Am J Clin Pathol 2010; 134: 410–418. [DOI] [PubMed] [Google Scholar]
- 4.Mboowa G Genetics of sub-Saharan African human population: implications for HIV/AIDS, tuberculosis, and malaria. Int J Evol Biol 2014; 2014: 108–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqi SH, Rüsch-Gerdes S. MGIT™ procedure manual Geneva, Switzerland: Foundation for Innovative New Diagnostics, 2006. [Google Scholar]
- 6.McCarthy KD, Metchock B, Kanphukiew A, et al. Monitoring the performance of mycobacteriology laboratories: a proposal for standardized indicators. Int J Tuberc Lung Dis 2008; 12: 1015–1020. [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services. Mycobacteriology. 42 CFR 493.825 - Standard Independence, MI, USA: United States Department of Health and Human Services, 2010. [Google Scholar]
- 8.World Health Organization. WHO guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region (with checklist) Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 9.Datema TAM, Oskam L, Engelberts MFM, et al. Global laboratory initiative stepwise implementation guide towards TB laboratory accreditation. Intl J Tuberc Lung Dis 2012; 16: 704–705. [DOI] [PubMed] [Google Scholar]
- 10.Yao K, McKinney B, Murphy A, et al. Improving quality management systems of laboratories in developing countries: an innovative training approach to accelerate laboratory accreditation. Am J Clin Pathol 2010; 134: 401–409. [DOI] [PubMed] [Google Scholar]


