Abstract
Diabetic peripheral neuropathy (DPN) is a complex disorder caused by long-standing diabetes. Oxidative stress was considered the critical creed in this DPN pathophysiology. Hydrogen has antioxidative effects on diabetes mellitus and related complications. However, there is still no concern on the beneficial effects of hydrogen in DPN. This paper aimed to evaluate the effects of exogenous hydrogen to reduce the severity of DPN in streptozotocin-induced diabetic rats. Compared with hydrogen-rich saline treatment, hydrogen inhalation significantly reduced blood glucose levels in diabetic rats in the 4th and 8th weeks. With regard to nerve function, hydrogen administration significantly attenuated the decrease in the velocity of motor nerve conduction in diabetic animals. In addition, hydrogen significantly attenuated oxidative stress by reducing the level of malondialdehyde, reactive oxygen species, and 8-hydroxy-2-deoxyguanosine and meaningfully enhanced the antioxidant capability by partially restoring the activities of superoxide dismutase. Further studies showed that hydrogen significantly upregulated the expression of nuclear factor erythroid-2-related factor 2 and downstream proteins such as catalase and hemeoxygenase-1 in the nerves of diabetic animals. Our paper showed that hydrogen exerts significant protective effects in DPN by downregulating oxidative stress via the pathway of nuclear factor erythroid-2-related factor 2, which suggests its potential value in clinical applications.
Keywords: antioxidant capability, antioxidant therapeutics, diabetic nephropathies, hydrogen, neuroprotective agents, nuclear factorerythroid-2-related factor 2, oxidative stress, reactive oxygen species
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is a serious complication of long-term diabetes mellitus (DM). DPN is categorized by paresthesia, pain and numb. It upsets about half of DM patients due to high prevalence, high rate of mortality and poor quality of daily life.1 Oxidative stress caused by chronic hyperglycemia was assumed to be the main culprit in the introduction of DPN.2 The unbalance between free radicals production, such as reactive oxygen species (ROS) and antioxidant species which is defined as oxidative stress, causes potential tissue damage. It has been proved in vitro and in humans that excessive ROS leads to injury to DNA, proteins and lipids,3 causing their instability and consequent function loss. Overproduction of ROS increases DNA damage and lipid peroxidation and results in axonal loss and disorder of the peripheral nervous system by destroying lipids in the neural myelinated structures. ROS could cause oxidative damage to dorsal root ganglion neurons and is suggested as a major mechanism in the DPN pathogenesis.4 Furthermore, the immune system in DM patients is disturbed by free radicals.5 High glucose has been postulated to generate oxidative stress, which ultimately causes nerve dysfunction essentially via several established pathways, such as increased advanced glycation end-product formation, increased hexosamine pathway flux, protein kinase C activation, glucose autoxidation, and increased polyol pathway flux.2,6 Oxidative stress is greatly important in the progress of DPN. And antioxidant strategies have been the research focus in search of an efficient, efficacious and safe treatment for nerve dysfunction in DM within the past decades. Considerable evidence has indicated that antioxidants, such as α-lipoic acid,7 flavonoids,8,9 rutin,10 Tang-Luo-Ning (an Chinese recipe for the treatment of DPN),11 and N-acetyl-L-cysteine,12 have definite therapeutic effects against oxidative stress in DM-induced DPN. In clinical trials, some antioxidants that are designed to slow the progression of diabetic complications have been withdrawn for efficacy and safety reasons.13 Only three drugs got permission from the Food and Drug Administration of the USA for DPN: pregabalin, gabapentin and duloxetine.14 Due to the development of potential toxicity drug tolerance, and inadequate relief, it is urgent to search for an helpful and harmless drug for DPN.
Hydrogen, the formula of which is H2, is a tasteless, colorless, odorless, and highly combustible gas. It has been reported to act as an inactive gas in mammalian cells at body temperature. In 2007, a publication shifted our view.15 Hydrogen was suggested to extinguish selectively strong oxidants such as hydroxyl radicals (•OH) and peroxynitrite (ONOO-). This mechanism was the explanation for beneficial effects of hydrogen.15 Afterwards, numerous studies have assured the assumption that hydrogen was protective in almost all organs.16,17,18,19 Hydrogen showed better prospects as an alternative antioxidant.First, it distributes everywhere, which allows it to penetrate bio-membranes and diffuse into the cytosol. Second, it has nothing to interfere with biological and beneficial ROS. Hydrogen has no cytotoxicity, even at high concentrations, which is its most important advantage.19 Hydrogen has also shown protective and therapeutic effects in DM20,21,22,23 and related complications.24,25 Nevertheless, the role of hydrogen in DPN is less reported. Our study aimed to reveal the neuroprotective effects of hydrogen against DPN. And the mechanism of it was also well explored.
MATERIALS AND METHODS
Animals
The study protocol was approved by the Experimentation Ethics Committee of the Sixth Medical Center of PLA General Hospital in Beijing, China (approval No. 2016).
According to the previously published protocol,42 male rats were selected because estrogen has neuroprotective effects. Twenty-four male specific pathogen free (SPF) Sprague-Dawley rats were obtained from Vital River Laboratories in Beijing, China (license No. SCXK (Jing) 2012-0001), weighing 200–220 g, 4 weeks old. The animals were offered free access to water and rodent diet under a 12-hour light/dark cycle at 21 ± 2°C and 30–70% of humidity.
Study groups and induction of diabetes
The sample size was estimated according to the preliminary experiment, which indicated that the blood glucose level would be approximately 17 mM for DM rats and 16 mM for hydrogen rats. It was calculated that 8 rats per group would provide 80% power showing a significant difference in the incidence based on a two-tailed significance level of 0.05.
Using a previously published protocol,7,8,10 24 rats were randomly divided into 3 groups, including a control group (n = 8), a DM group (n = 8), and DM + hydrogen group (DM + H2 group, n = 8). Diabetic rats in the DM and DM + H2 groups were induced by a single intraperitoneal injection of streptozotocin (Sigma, St. Louis, MO, USA) at a dose of 65 mg/kg body mass, which was freshly dissolved in 0.1 M citrate buffer, pH 4.5. Control animals received an equal volume (1 mL) of citrate buffer. Seventy-two hours after induction of diabetes, blood glucose level was measured in samples taken from the tail vein. The diabetic state was confirmed as the glucose concentration exceeded 16.67 mM.
Three days after the induction of diabetes, rats in the DM + H2 group received an intraperitoneal injection of H2 saline (10 mL/kg) every day, and rats in the other two groups received the same volume (10 mL/kg) of physiological saline. During the 8-week experimental duration, no animals were given insulin or antidiabetic drugs.
Preparation of hydrogen saline
Hydrogen was dissolved in normal saline for 3 hours, which was to form a supersaturated state at 0.4 MPa pressure. The saturated hydrogen saline was stored at 4°C under atmospheric pressure for 24 hours. To ensure a stable concentration of 0.6 mM, hydrogen saline was prepared 1 day before the animal experimentation. The content of hydrogen in saline was confirmed through a gas chromatography analysis described by Ohsawa et al.15]
Measurement of body mass and blood glucose
Body mass was measured on the 1st day, 4th and 8th weeks of the experiment using an electronic balance (Dingguo Changsheng, Beijing, China). Blood glucose levels of rats were measured using glucose test reagent strips and a glucometer (Roche, Mannheim, Germany). Blood glucose measurements were performed 3 days after streptozotocin injection and the day before sacrifice, on the same days as the body mass measurements.
Measurement of motor nerve conduction velocity
The motor nerve conduction velocity (MNCV) of the rats in the three groups was measured in the 8th week of the experiment. The animals were anesthetized. MNCV was measured with the Functional Experiment System (BL-420s, Taimeng, Chengdu, China) as previously described26 in the nerve branch to the tibialis anterior muscle, keeping the limbic temperature at 37°C. MNCV is representative of the whole sciatic nerve in terms of susceptibility to DM and treatment effects.26]
Specimen and homogenate preparation
After measurement of MNCV, animals were euthanized. With fixation of the rat in the supine position, the sciatic nerve was exposed and cut with sharp scissors, and the blood was rinsed off with ice-cold saline. The left sciatic nerve was cut into two segments. One segment was fixed in 10% formalin and embedded in paraffin for myelin staining, and the other segment was stored at –80°C for Luxol fast blue staining. The right sciatic nerve was made into a 10% homogenate immediately (tissue grams/saline volume in milliliters = 1:9) for oxidative stress measurement.
Luxol fast blue staining
Paraffin-embedded sciatic nerve tissue was cut into 4-μm sections and analyzed by Luxol fast blue (LFB) staining to determine changes in myelin morphology. The LFB staining result for the tissue was observed under an optical microscope (Olympus, Tokyo, Japan), and five random sections were evaluated under 400× magnification for the density and average cross-sectional area of myelinated nerve.
Measurement of malondialdehyde, ROS, 8-hydroxy-2-deoxyguanosine and superoxide dismutase
The malondialdehyde (MDA) content, ROS, superoxide dismutase (SOD) activity in sciatic nerve tissue homogenates was measured by an assay kit (Jiancheng Institute of Biotechnology, Nanjing, China) as described previously.27 8-Hydroxy-2-deoxyguanosine (8-OHdG) protein levels in sciatic nerve tissue homogenate were measured using a commercial enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., Ltd., Wuhan, China) according to the instructions.
Western blot detection of nuclear factor erythroid-2-related factor 2, heme oxygenase-1 and catalase
Antibodies against nuclear factor erythroid-2-related factor 2 (Nrf-2; rabbit, 1:1000, Cat# ab71890, RRID: AB_1603893), heme oxygenase-1 (HO-1; rabbit, 1:1000, Cat# 1922-1, RRID: AB_764541) and catalase (CAT; rabbit, 1:1000, Cat# ab48613, RRID: AB_868693) were obtained from Abcam (Cambridge, UK). The sciatic nerve tissue homogenates were homogenized and centrifuged at 12,000 × g for 10 minutes at 4°C. Supernatants were collected, and protein concentrations were determined using a bicinchoninic acid kit (Jiancheng Institute of Biotechnology, Nanjing, China). The protein samples were separated using 10–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked using 5% nonfat dry milk in Tris-buffered saline for 2 hours, followed by incubation with primary antibodies for Nrf-2, HO-1 and CAT as appropriate overnight at 4°C. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:10,000; ZSGB-BIO, Beijing, China, Cat# PV-9001, RRID: AB_2868452) at room temperature for 2 hours. Antigen-antibody complexes were detected by an ECL Plus Chemiluminescence Reagent kit (Millipore, Billerica, MA, USA). The membranes were exposed to X-ray film (Eastman Kodak Company, Rochester, NY, USA) for detection. The band densities were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). Tubulin (Dingguo Changsheng, Beijing, China) represents an internal control.
Statistical analysis
All values are presented as the mean ± standard error of the mean (SEM) and were analyzed with SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). Means of different groups were analyzed using one-way analysis of variance followed by the Newman-Keuls test for multiple pairwise comparisons between the various treatment groups. A P-value < 0.05 was considered to indicate statistical significance.
RESULTS
Hydrogen improves body mass and blood glucose in DM rats
Throughout the experimental period, the weight of rats was measured to research the hydrogen effect on diabetic rats. Body mass gains in the DM group in the 4th and 8th weeks were significantly lower than control group (P < 0.05). Mass gains in the DM + H2 group in the 4th and 8th weeks were higher than those in the DM group (P > 0.05) (Figure 1).
Figure 1.
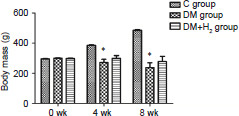
The effects of hydrogen on body mass in diabetic rats.
Note: Data are showed as mean ± SEM (n = 8). *P < 0.05, vs. control group (Newman-Keuls test). C: Control; DM: diabetes mellitus; H2: hydrogen.
Regarding the antidiabetic effects of hydrogen, subcutaneous injection of hydrogen could reduce the blood glucose levels of DM rats. As shown in Figure 2, blood glucose levels were significantly increased in the DM rats compared with control animals (P < 0.05). And blood glucose levels were significantly reduced by hydrogen injection in the DM + H2 group compared with the DM group in the 4th (P < 0.05) and 8th (P < 0.05) weeks of the experiment.
Figure 2.
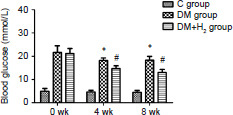
The effects of hydrogen on blood glucose in diabetic rats.
Note: Data were showed as mean ± SEM (n = 8). *P < 0.05, vs. control group; #P < 0.05, vs. DM group (Newman-Keuls test). C: Control; DM: diabetes mellitus; H2: hydrogen.
Hydrogen attenuates the decrease in MNCV in nerve branch to the tibialis anterior muscle of DM rats
Decreased MNCV suggests the nerve dysfunction in DPN.26 In the 8th week of the experiment, a significant decline in the DM group, compared with the control group, was presented (P < 0.05). MNCV of diabetic rats receiving hydrogen administration was significantly improved compared with that in the DM group (P < 0.05) (Figure 3).
Figure 3.
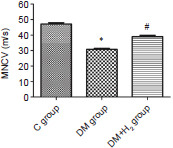
The effects of hydrogen on MNCV in nerve branch to the tibialis anterior muscle of diabetic rats.
Note: Data are presented as the mean ± SEM (n = 8). *P < 0.05, vs. control group; #P < 0.05, vs. DM group (one-way analysis of variance followed by the Newman-Keuls test). C: Control; DM: diabetes mellitus; H2: hydrogen; MNCV: motor nerve conduction velocity.
Hydrogen reduces the injury to diabetic nerves due to hyperglycemia
The LFB staining results shown in Figure 4A disclosed that fibers of the sciatic nerve in the DM group were broken, damaged and missing. The morphology of the fibers of the sciatic nerve was kept relatively intact in the DM + H2 group after hydrogen administration. In addition, the density and average sectional area of myelinated nerve were evaluated. As shown in Figure 4B and C, both the density and average sectional area of myelinated nerve were significantly increased due to hydrogen administration (P < 0.05).
Figure 4.
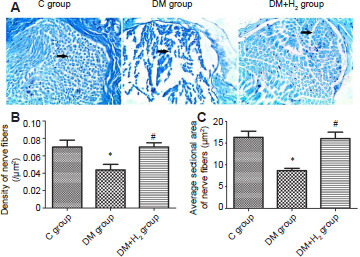
The effects of hydrogen on myelin morphology of sciatic nerve in diabetic rats via Luxol fast blue staining.
Note: (A) Myelin morphology of the sciatic nerve (original magnification 400×). With hydrogen administration, the morphology of the nerve fibers of the sciatic nerve was more complete. There were few cases of looseness and disorder, fiber rupture and breakage (arrows). The Schwann cells were clearly increased, and the axons were more clearly visible. (B, C) Density (B) and average sectional area (C) of myelinated nerve. Data are presented as the mean ± SEM (n = 8). *P < 0.05, vs. control group, #P < 0.05, vs. DM group. C: Control; DM: diabetes mellitus; H2: hydrogen.
Hydrogen reduces the levels of oxidative stress due to hyperglycemia in DM nerves
In the 8th week of the experiment, the increased levels of MDA, ROS, and 8-OHdG in the sciatic nerves of diabetic rats in DM group were significantly attenuated by hydrogen administration in the DM + H2 group (P < 0.05; Figure 5A-C), suggesting that hydrogen has the capacity of inhibiting lipid peroxidation, ROS generation and DNA damage caused by oxidation in diabetic rats.
Figure 5.
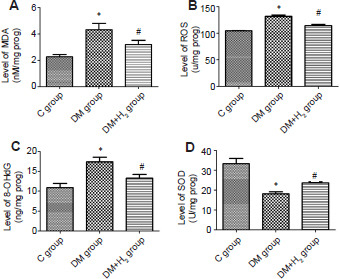
The effects of hydrogen on oxidation stress in sciatic nerve of diabetic rats.
Note: (A–D) Levels of MDA (A), ROS (B), 8-OHdG (C) and SOD (D). Data are presented as the mean ± SEM (n = 8). *P < 0.05, vs. control group; #P < 0.05, vs. DM group. 8-OHdG: 8-Hydroxy-2-deoxyguanosine; C: control; DM: diabetes mellitus; H2: hydrogen; MDA: malondialdehyde; ROS: reactive oxygen species; SOD: superoxide dismutase.
Afterwards, the effects of hydrogen on an antioxidant enzyme in DM rats were explored. The activities of SOD were meaningfully reduced in DM nerves (P < 0.05). Meanwhile, the activities of SOD was partially restored by hydrogen administration (P < 0.05; Figure 5D).
Hydrogen increased the levels of Nrf-2 and downstream proteins in diabetic nerves
In diabetic rats, the level of Nrf-2 was significantly decreased. Same trend went with the downstream proteins of Nrf-2 (P < 0.05). This indicated persistent hyperglycemia injures the body’s antioxidant system. The levels of these three molecular were significantly improved by hydrogen (P < 0.05; (Figure 6).
Figure 6.
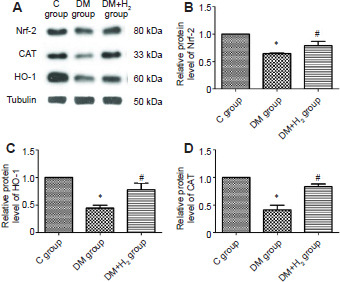
The effects of hydrogen on Nrf-2, HO-1 and CAT in sciatic nerves in diabetic rats using western blot.
Note: (A) Bands of Nrf-2, HO-1 and CAT. (B–D) Quantitative results of Nrf-2 (B), HO-1 (C) and CAT (D). Relative protein level was normalized to tubulin. Data are presented as the mean ± SEM (n = 8). *P < 0.05, vs. control group, #P < 0.05, vs. DM group. 8-OHdG: 8-Hydroxy-2-deoxyguanosine; C: control; CAT : catalase; DM: diabetes mellitus; H2: hydrogen; HO-1: heme oxygenase-1; Nrf-2: 8-hydroxy-2-deoxyguanosine.
DISCUSSION
In our work, we found that hydrogen administration partially attenuated the progression of DPN and improved the neurological function of peripheral nerves in diabetic rats. Hydrogen administration significantly decreased oxidative stress levels and significantly increased the antioxidant capacity, perhaps by increasing Nrf-2 levels.
Hyperglycemia is the basic onset of damage in peripheral neuropathy. Blood glucose control is the key to preventing diabetic complications. The effect of hydrogen on levels of blood glucose is not stable. Zhang et al.20 found that subcutaneous injection of hydrogen gas ameliorated hyperglycemia and improved glucose homeostasis and insulin sensitivity in diabetic mice. Our study found that hydrogen administration meaningfully decreased the blood glucose level, which fits the previous studies about other antioxidants10 and the results of Zhang et al.20 However, the exact mechanisms are unknown. Hydrogen might upregulate the expression of Nrf-2 to maintain the homeostasis of glucose metabolism (discussed in the following), which seems to play a vital role in the maintenance of glucose metabolism. The exact mechanism has not yet been confirmed, which might be through utilization of glucose in insulin-sensitive tissues, insulin secretion, and regulation of metabolism-related gene expression.28
DPN is characterized by symptoms ranging from sensory deficits to allodynia and hyperalgesia. In our study, we found that, the nerve dysfunction indicator, MNCV, was significantly decreased in DM rats compared with the control group. Hydrogen administration reversed the MNCV decline in diabetic rats partially. Similarly, hydrogen supplementation could also partially protect peripheral nerves against the injuries caused by hyperglycemia according to the results of LFB staining. These findings suggested that hydrogen supplementation partially attenuated the progression of DPN and protected the structure and function of peripheral nerves in diabetic rats.
Hyperglycemia-induced oxidation injury has been considered as part of the major mechanisms promoting DPN under circumstances of diabetes.6 Overproduction of ROS, DNA damage, lipid peroxidation, and reduction in cellular antioxidants are engaged in the initiation of oxidative stress-induced nerve dysfunction.2 Upregulation of MDA, 8-OHdG and ROS, indication of oxidative stress, and downregulation of antioxidants have been found in several studies related to diabetes.28 Increased ROS is capable of causing microvasculature break and axons loss of the peripheral nervous system by damaging lipids as part of the neural myelin sheath.2 Apoptosis of neurons could be triggered by cumulative oxidative stress and Schwann cell nerve could be injured as a result.29 Based on the pathogenesis of DPN, antioxidant strategies may exhibit their antidiabetic effects in DPN. Antioxidants work in two different ways: reducing the harmful effects of free radicals through inactivating oxidative damage or reinforcement the natural defense system by increasing the activities of antioxidant enzymes. Studies have revealed that antioxidants such alpha-lipoic acid,7 vitamins,30 and flavonoids,9,10 all have the capability to protect against the damage of hyperglycemia-induced oxidative damage and reduce the progression of DPN. Hydrogen has the clear function on the scavenging of ROS and has significant protective effects on diverse diseases.15,16,17 In previous studies, hydrogen has been shown to improved DM patients23 and metabolic syndrome31 by reducing oxidation injury. Additionally, hydrogen gas alleviated type II DM and diabetic nephropathy-related symptoms via subcutaneous injection in a mouse model.20 There is a reaction between hydrogen and strong oxidants, such as hydroxyl and/or nitrosyl radicals, which indicates hydrogen is a candidate drug for preventive and therapeutic purposes.32 Hydrogen could permeate into cells and tissues with no reactions with biological procedures. Therefore, hydrogen is not expected to have adverse effects, which is the most considerable difference between it and conventional pharmaceutical drugs and its greatest advantage over them. Our study results supported the view that oxidant levels dramatically increase in nerve tissue injury due to persistent hyperglycemia.6,33 Moreover, hydrogen could increase the antioxidants activities, such as SOD.20,31 After administration, hydrogen reduced oxidant capacities by inhibiting levels of MDA, ROS, and 8-OHdG and significantly increased the antioxidant states by increasing the levels of SOD, which is in accord with previous results revealing the antioxidant effects of hydrogen.15,16,18 These findings show that for protection against hyperglycemia, hydrogen might affect oxidant amounts.
Nrf-2 is a member of Cap’n‘Collar family exerting function as a basic leucine zipper transcription factor,14 which is one of the key molecules in the maintenance of cellular homeostasis activated by various stimuli. Nrf-2 activity insufficiency, such as decreased clearance of ROS or misfolded and aggregated proteins, is engaged in the etiology of DM and its complications, which is proved by lower levels of Nrf-2 in diabetes. In the case of acute hyperglycemia, increasing levels of Nrf-2 protects against the onset of diabetes and its complications.34 However, Nrf-2 is downregulated in chronic or persistent hyperglycemia and diabetic complications.34 Nrf-2 has emerged as a hopeful target to the prevention of DPN.4,14 Studies have shown that Nrf-2 and its target gene were activated with the protective effects of melatonin,36 rutin,10 and fisetin37 against streptozotocin-induced DM and DPN. Epalrestat,38,39 which is used in the treatment of DPN, leads to upregulated HO-1, CAT, and SOD levels in a Nrf-2-dependent manner. Activated Nrf-2 could reduce cellular distress, increase insulin sensitivity, and preserve β-cell function, to play a protective role.41 In the present study, inhibition of Nrf-2 was observed due to persistent hyperglycemia, which is consistent with previous findings.35,40 Hydrogen application could activate the Nrf-2 pathway in neurons in DPN. Furthermore, HO-1 and CAT expression was also increased after hydrogen administration, which is thought to be one of the causes of the antioxidant capacity of Nrf-2 activation.41]
Hydrogen can alleviate hyperglycemia-induced DPN, inhibit oxidative stress, improve antioxidant capacity, and prevent peripheral nerve injury through the Nrf-2 signaling pathway. Here are the limitations of this study.Firstly, there is a lack of experiments on the exact molecular mechanism of hydrogen in DPN. Second, the role of Nrf-2 signaling pathway in the beneficial effects of hydrogen needs further investigation.
Footnotes
Funding: This work was supported by grants from the Innovation Cultivation Foundation of Navy General Hospital (which was renamed to the Sixth Medical Center, Chinese PLA General Hospital), China, No. CXPY201617.
Conflicts of interest
The authors have no conflicts of interests to declare.
REFERENCES
- 1.Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938–948. doi: 10.1016/S2213-8587(19)30081-6. [DOI] [PubMed] [Google Scholar]
- 2.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015:515042. doi: 10.1155/2015/515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014:674987. doi: 10.1155/2014/674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasznicki J, Kosmalski M, Sliwinska A, et al. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012 Sep 39;((9)):8669–8678. doi: 10.1007/s11033-012-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang L, Lian X, Liu H, et al. Understanding diabetic neuropathy: focus on oxidative stress. Oxid Med Cell Longev. 2020;2020:9524635. doi: 10.1155/2020/9524635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baydas G, Donder E, Kiliboz M, et al. Neuroprotection by alpha-lipoic acid in streptozotocin-induced diabetes. Biochemistry (Mosc) 2004;69:1001–1005. doi: 10.1023/b:biry.0000043542.39691.95. [DOI] [PubMed] [Google Scholar]
- 8.Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med. 2011;2011:323171. doi: 10.1155/2011/323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher P, Dargusch R, Ehren JL, Okada S, Sharma K, Schubert D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS One. 2011;6((6)):e21226. doi: 10.1371/journal.pone.0021226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol. 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Yao W, Li Q, et al. Mechanism of Tang Luo Ning effect on attenuating of oxidative stress in sciatic nerve of STZ-induced diabetic rats. J Ethnopharmacol. 2015 Nov 4;174:1–10. doi: 10.1016/j.jep.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 12.Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia-induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem. 2010;112:77–91. doi: 10.1111/j.1471-4159.2009.06435.x. [DOI] [PubMed] [Google Scholar]
- 13.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology. 2017;25:393–402. doi: 10.1007/s10787-017-0339-y. [DOI] [PubMed] [Google Scholar]
- 15.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 16.Iketani M, Ohsawa I. Molecular hydrogen as a neuroprotective agent. Curr Neuropharmacol. 2017;15:324–331. doi: 10.2174/1570159X14666160607205417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slezák J, Kura B, Frimmel K, et al. Preventive and therapeutic application of molecular hydrogen in situations with excessive production of free radicals. Physiol Res. 2016;65(Suppl 1):S11–28. doi: 10.33549/physiolres.933414. [DOI] [PubMed] [Google Scholar]
- 18.Huang L. Molecular hydrogen: a therapeutic antioxidant and beyond. Med Gas Res. 2016;6:219–222. doi: 10.4103/2045-9912.196904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu P, Liu Y, Zhang J. Recent advances in studies of molecular hydrogen against sepsis. Int J Biol Sci. 2019;15:1261–1275. doi: 10.7150/ijbs.30741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Liu J, Jin K, et al. Subcutaneous injection of hydrogen gas is a novel effective treatment for type 2 diabetes. J Diabetes Investig. 2018;9:83–90. doi: 10.1111/jdi.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amitani H, Asakawa A, Cheng K, et al. Hydrogen improves glycemic control in type1 diabetic animal model by promoting glucose uptake into skeletal muscle. PLoS One. 2013;8:e53913. doi: 10.1371/journal.pone.0053913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirahata S, Hamasaki T, Haramaki K, et al. Anti-diabetes effect of water containing hydrogen molecule and Pt nanoparticles. BMC Proc. 2011;5(Suppl 8):P18. doi: 10.1186/1753-6561-5-S8-P18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kajiyama S, Hasegawa G, Asano M, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137–143. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura N, Nishimaki K, Ohsawa I, Ohta S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring) 2011;19:1396–1403. doi: 10.1038/oby.2011.6. [DOI] [PubMed] [Google Scholar]
- 25.Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478:1021–1027. doi: 10.1016/j.bbrc.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YZ, Zhou ZC, Song CY, Chen X. The protective effect and mechanism of dexmedetomidine on diabetic peripheral neuropathy in rats. Front Pharmacol. 2020;11:1139. doi: 10.3389/fphar.2020.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padiya R, Chowdhury D, Borkar R, Srinivas R, Pal Bhadra M, Banerjee SK. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One. 2014;9:e94228. doi: 10.1371/journal.pone.0094228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uruno A, Yagishita Y, Yamamoto M. The Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys. 2015;566:76–84. doi: 10.1016/j.abb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Sekido H, Suzuki T, Jomori T, Takeuchi M, Yabe-Nishimura C, Yagihashi S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem Biophys Res Commun. 2004;320:241–248. doi: 10.1016/j.bbrc.2004.05.159. [DOI] [PubMed] [Google Scholar]
- 31.Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Luna R, Talanki Manjunatha R, et al. A comprehensive review of neuronal changes in diabetics. Cureus. 2021;13:e19142. doi: 10.7759/cureus.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–114. doi: 10.1016/j.phrs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J Pineal Res. 2011;50:124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 37.Sandireddy R, Yerra VG, Komirishetti P, Areti A, Kumar A. Fisetin imparts neuroprotection in experimental diabetic neuropathy by modulating Nrf2 and NF-κB pathways. Cell Mol Neurobiol. 2016;36((6)):883–892. doi: 10.1007/s10571-015-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Yama K, Murao Y, Tatsunami R, Tampo Y. Epalrestat increases intracellular glutathione levels in Schwann cells through transcription regulation. Redox Biol. 2013 Nov 19;2:15–21. doi: 10.1016/j.redox.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yama K, Sato K, Murao Y, Tatsunami R, Tampo Y. Epalrestat up-regulates heme oxygenase-1, superoxide dismutase, and catalase in cells of the nervous system. Biol Pharm Bull. 2016;39:1523–1530. doi: 10.1248/bpb.b16-00332. [DOI] [PubMed] [Google Scholar]
- 40.de Haan JB. Nrf2 activators as attractive therapeutics for diabetic nephropathy. Diabetes. 2011;60:2683–2684. doi: 10.2337/db11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matzinger M, Fischhuber K, Heiss EH. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol Adv. 2018;36:1738–1767. doi: 10.1016/j.biotechadv.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peri A. Neuroprotective effects of estrogens: the role of cholesterol. J Endocrinol Invest. 2016;39:11–18. doi: 10.1007/s40618-015-0332-5. [DOI] [PubMed] [Google Scholar]


