Abstract
Despite the fact that we have reported on the dangers of the explosion of hydrogen gas inhalers, hydrogen gas inhalers with explosive hazards are, as a matter of fact, still being sold today. In this study, we investigated past reports of hydrogen gas inhaler explosion accidents to clarify the causes of these explosion incidents. As a result of this investigation, we found that the central cause was the leakage of hydrogen gas inside the hydrogen gas inhaler. Although it is said that the explosive concentration of hydrogen is between 10% and 75%, and that the gas does not explode above 75% due to the lack of oxygen, we confirmed through a series of ignition experiments that explosions can occur even in hydrogen gas inhalers that produce 100% hydrogen gas. Some manufacturers of such highly concentrated hydrogen gas inhalers claim that the high concentration and purity of hydrogen is safe and that there is no risk of explosion. We believe that manufacturing or selling such products that pose a risk of explosion or detonation is a violation of social justice. This paper presents ideas for selecting safe hydrogen gas inhalers based on a survey of past accident cases.
Keywords: detonation, explosion accident, explosive concentration range of hydrogen, flame arresters, hydrogen concentration, hydrogen inhaler, hydrogen, safety
INTRODUCTION
Hydrogen (H2) is a colorless, odorless gas at ambient temperature and pressure.1,2 H2 causes a violent explosion when it reacts with oxygen (O2) to form water (H2O).3 We conducted a joint research study with Keio University and reported that hydrogen gas explodes at concentrations above 10%.4 Especially in the concentration range of 18.3% to 58.9%, hydrogen may cause a detonation, which is an extremely dangerous explosion accompanied by shockwaves at a supersonic speed of Mach 5 (1700 m/s) or higher, and a subsequent flame propagation called a deflagration, which may lead to serious accidents2 In particular, a mixed gas consisting of a 2 to 1 ratio of hydrogen and oxygen is known as an oxyhydrogen detonating gas, which produces a loud explosive sound (detonating sound) and a powerful explosion. The detonating sound that accompanies hydrogen gas explosions may destroy human auditory neurons, causing incurable loss of hearing. When it comes to the use of hydrogen as an energy source, research on the safety of hydrogen and hydrogen explosions has been conducted to prevent such explosions from occurring in hydrogen facilities.5,6 On the other hand, many commercial home-use hydrogen gas inhalers give little consideration to safety with respect to such explosions. While there are ongoing plans to use hydrogen in the medical field,7 explosion incidents involving hydrogen gas inhalers, which itself poses the risk of explosion, may hinder the development of hydrogen medicine.
When using an explosive gas, it is absolutely necessary to ensure safety. Hydrogen explosions sometimes cause tremendous damage, as in the 1937 explosion of the Hindenburg, Poland, in which an airship used hydrogen for buoyancy in the 1983 hydrogen explosion in an industrial park in Stockholm, Sweden, and in hydrogen explosion at the Fukushima nuclear power plant in 2001.8,9,10 Some unscrupulous manufacturers sell hydrogen gas inhalers that generate an explosive concentration of hydrogen while presenting favorable hydrogen gas concentrations that indicate a low risk of explosion so as to lull purchasers into a false sense of safety, and some doctors and medical institutions are affiliated with these manufacturers. Hydrogen gas inhalers are mainly used in homes or hospitals.11,12,13,14,15,16 Therefore, any risk of explosion or detonation may lead to damages to household effects, loss of hearing by the production of a detonating sound, fires, or in some cases, a serious accident that threatens human life.5,6,17 Development of hydrogen gas inhalers that ensure safety requires that the developers have a strong sense of ethics and high level of thought towards technology.
In this study, we investigated past reports of hydrogen gas inhaler explosion accidents and discussed the causes of these explosions. In addition, an ignition experiment was conducted at hydrogen gas concentrations of 75% and higher, which was previously reported to be non-explosive, and the explosive potential of commercially available hydrogen gas inhalers was discussed.4,5,6,8,9,10]
CASES OF HYDROGEN EXPLOSION ACCIDENTS INVOLVING HYDROGEN GENERATORS
The following introduces cases of incidents involving hydrogen explosions. Accident cases 1–3 were reported by the Consumer Affairs Agency of Japan.18]
Accident case 1: Accident involving a hydrogen-oxygen gas inhaler
An accident report can be found in which upon lifting a hydrogen-oxygen gas inhaler, the lid flew off and an explosive sound was produced that caused ringing in the user’s ears (February 19, 2016).
A hydrogen-oxygen gas inhaler allows the user to inhale hydrogen and oxygen at the same time. It can be presumed that inhalers of this kind generate an explosive concentration of hydrogen (66%) and oxygen (34%) by electrolysis of water.
Accident case 2: Explosion accident involving a home-use hydrogen generator
Although this case did not involve a hydrogen gas inhaler, there was an accident report in which hydrogen leakage occurred in a home-use hydrogen bubble generator. The leaked hydrogen accumulated in the body of the unit before causing an explosion, which damaged parts of the house (the bathroom door) and injured the resident (report dated October 25, 2017).
Hydrogen gas inhalers of the midway dilution type, which dilutes hydrogen gas in a location away from the source of generation instead of diluting it to below the lower explosive limit immediately at the source of generation, may allow a high concentration of hydrogen to leak due to deterioration caused by aging or due to the tube and other components falling out of place, which may in turn lead to an explosion caused by the action of electrical energy from the electrical system inside the inhaler.
Accident case 3: Explosion accident involving a hydrogen server
There is an accident report dated November 11, 2015, in which a hydrogen explosion caused a bottle to fly out of a server that generates hydrogen and damaged the ceiling.
This accident was also caused by the leakage of a high concentration of hydrogen inside the product. The same product caused another accident in which the hydrogen-generating server burst and the broken pieces scattered and lacerated a child (report dated November 22, 2015 by the same agency).
Accident case 4: Explosion accident involving an electronic water heater
In the epidemiological database of accidents made public by the U.S. Consumer Product Safety Commission (https://www. cpsc.gov/Data), there is an incident in which an electronic water heater that generates hydrogen ignited when a flame was brought close to its faucet. Though this particular case did not lead to a serious accident, hydrogen leaking inside equipment that generates hydrogen may lead to a serious accident.
IGNITION EXPERIMENTS ON HYDROGEN AND HYDROGEN GAS INHALERS
To investigate the causes of accidents in equipment that generate hydrogen, ignition experiments were conducted on different concentrations of hydrogen trapped inside plastic bags and on commercially available hydrogen gas inhalers that generate different concentrations of hydrogen. In these experiments, the explosive limits of hydrogen gas were verified (ignition experiment 1), and the question of whether hydrogen gas inhalers cause hydrogen gas explosions was tested by directly igniting the inhalers themselves.4]
Ignition experiment 1
Hydrogen gas at concentrations of 4%, 10%, 15%, 20%, and 100% was trapped inside plastic bags and ignited.
A hydrogen cylinder (100%) from Taiyo Nippon Sanso was diluted to prepare these different concentrations of hydrogen, and the hydrogen concentrations were measured with a hydrogen concentration meter (XP-3140) from New Cosmos Electric Co., Ltd. (Osaka, Japan). The experiment results are shown in Table 1.
Table 1.
Ignition experiment results of different concentrations of hydrogen
| Hydrogen concentration (%) | Explosion | Explosive sound |
|---|---|---|
| 4 | No explosion | No explosive sound |
| 10 | No explosion | No explosive sound |
| 15 | Explosion | Explosive sound produced |
| 20 | Violent explosion | Ferocious explosive sound (detonation) produced |
| 100 | Explosion | Explosive sound produced |
Ignition experiment 2: Inhaler with a hydrogen concentration of 66% (electrolysis type)
When a hydrogen gas inhaler (electrolysis type with a hydrogen concentration of 66%) from Company J was ignited, the hydrogen detonated with a ferocious explosive sound, and the inhaler’s lid and wiring parts were scattered by the explosion’s impact (Figure 1). This hydrogen gas inhaler with a hydrogen concentration of 66% allows users to inhale a mixed gas of hydrogen (66%) and oxygen (34%) generated by the electrolysis of water. A mixed gas consisting of a 2 to 1 ratio of hydrogen and oxygen is known as an oxyhydrogen detonating gas, and it causes an explosion accompanied by an especially ferocious explosive sound.
Figure 1.

Ignition experiment result of hydrogen concentration of 66%, electrolysis type.
Ignition experiment 3: Inhaler with a hydrogen concentration of 100% (electrolysis type)
When a hydrogen gas inhaler (electrolysis type with a hydrogen concentration of 100%) from Company I was ignited immediately after it was turned on, transparent smoke was emitted continuously from the hydrogen gas discharge port. When the same hydrogen gas inhaler was ignited several minutes after it was turned on, it exploded with a ferocious explosive sound and the container burst (Figure 2). This experiment revealed that hydrogen gas inhalers with a hydrogen concentration of 100%, which is higher than the upper explosive limit (75%), also pose a risk of explosion.
Figure 2.

Ignition experiment result of hydrogen concentration of 100%, electrolysis type.
Ignition experiment 4: Inhaler with a hydrogen concentration of 100% (hydrogen-generating-agent type)
When a hydrogen gas inhaler (hydrogen-generating-agent type with a hydrogen concentration of 100%) from Company P was ignited, it exploded with a ferocious sound, and the top lid burst and scattered (Figure 3). As in ignition experiment 3, it was found that hydrogen gas inhalers of the hydrogen-generating-agent type with a hydrogen concentration of 100%, which is higher than the upper explosive limit (75%), also pose a risk of explosion.
Figure 3.

Ignition experiment result of hydrogen concentration of 100%, hydrogen-generating-agent type.
SAFE HYDROGEN GAS INHALERS AND DANGEROUS HYDROGEN GAS INHALERS
Most commercially available hydrogen gas inhalers emit hydrogen that is generated by the electrolysis of water directly through the inhalation port, which poses a risk of explosion (Figure 4).
Figure 4.
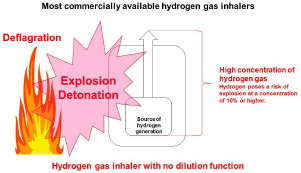
Most commercially available hydrogen gas inhalers pose risks of explosion, detonation, and deflagration.
Generated hydrogen should be diluted with air or another dilution gas in order to ensure that the concentration is below the lower explosive limit. There are two methods for diluting hydrogen: thefirst is direct dilution, in which hydrogen is diluted to concentrations below the lower explosive limit at the source of generation or immediately after generation, and the second is midway dilution, in which generated hydrogen is diluted prior to emission (Figure 5). Hydrogen gas inhalers of the direct dilution type do not allow an explosive concentration of hydrogen to accumulate or flow inside them, and thus ensure safety. On the other hand, hydrogen gas inhalers of the midway dilution type allow the generated hydrogen to be maintained at an explosive concentration from the source of generation until the point where it is mixed with a dilution gas, so they continue to pose a risk of explosion. Hydrogen gas inhalers of the midway dilution type have the risk of interior hydrogen leaks due to deterioration caused by aging or the hydrogen-sending tube falling out of place. In fact, major hydrogen explosion accidents have been caused by hydrogen leakage inside inhalers.2,3]
Figure 5.
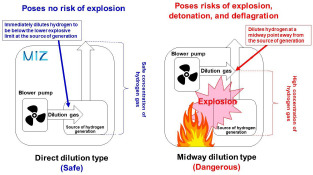
Direct dilution type inhaler (safe) and Midway dilution type inhaler (dangerous).
FLAME ARRESTERS ARE INSUFFICIENT TO PREVENT EXPLOSIONS
Some hydrogen gas inhalers are equipped with a flame arrester to prevent flame backflow in the piping used to carry hydrogen gas.19,20 However, even if flames can be extinguished by the placement of a flame arrester, this does not prevent the detonating sound that accompanies hydrogen explosions. Flame arresters are not intended to prevent hydrogen explosions when hydrogen between the source of hydrogen generation and the flame arrester leaks inside the inhaler, and there is every reason to believe that installing a flame arrester is insufficient to prevent explosions nor to ensure explosion safety (Figure 6).
Figure 6.
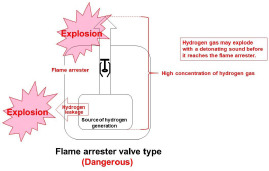
Flame arrester valve type inhaler (dangerous).
FIVE POINTS TO CHECK WHEN SELECTING HYDROGEN GAS INHALERS
Hydrogen has the properties described above. Thus, when purchasing a hydrogen gas inhaler, it is important to select a product that gives due consideration to safety. The requirements that hydrogen gas inhalers should meet are listed below (Figure 7).
Figure 7.
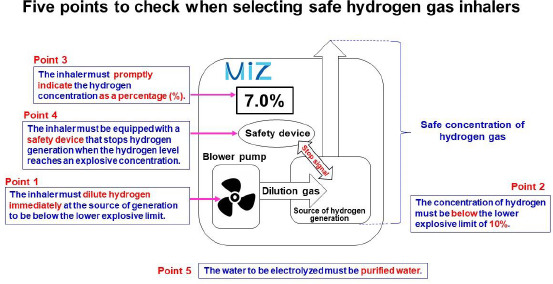
Five points to check when selecting safe hydrogen gas inhalers.
LIST OF COMMERCIALLY AVAILABLE HYDROGEN GAS INHALERS THAT GENERATE A HIGH CONCENTRATION OF HYDROGEN
Table 2 lists the hydrogen gas inhalers on the market that produce high concentrations of hydrogen.
Table 2.
List of commercially available hydrogen gas inhalers that generate a high concentration of hydrogen
| Product name | Supplier | H2 gas concentration (%) | Method |
|---|---|---|---|
| H2JI1 | Doctors Man Co., Ltd. (Kanagawa, Japan) | 99.999 | Electrolysis |
| Cielserein | Inter Christine K.K. (Kanagawa, Japan) | 99.995 | Electrolysis |
| Mintech MT-A100 | Mintech K.K. (Tokyo, Japan) | 99.995 | Electrolysis |
| H2MULTI POD | I•TEC INTERNATIONAL (Tokyo, Japan) | 99.999 | Electrolysis |
| Pure+Cube | Dr’s Choice K.K. (Tokyo, Japan) | 99.99 | Electrolysis |
| LOURDESHYDROFIX | H2FACTORY Inc. (Aichi, Japan) | 99.5 | Electrolysis |
| Hydrogen Inhaler | Kenko Shien Left Co., Ltd. (Aichi, Japan) | 99 | Electrolysis |
| Suiso Choju CH-100 | Eco Higashinippon Co., Ltd. (Fukushima, Japan) | 99 | Electrolysis |
| PHG-150TA | Eco Higashinippon Co., Ltd. (Fukushima, Japan) | 99 | Electrolysis |
| Suiso Care | Kenko Co., Ltd. (Gifu, Japan) | 99 | Electrolysis |
| La Briller Luxe | ISMZ Co., Ltd. (Osaka, Japan) | 99 | Electrolysis |
| La Briller Elan | ISMZ Co., Ltd. (Osaka, Japan) | 99 | Electrolysis |
| SS-100 | SUISO JAPAN (Aichi, Japan) | 99 | Electrolysis |
| HydroRich | Pal Corporation (Kanagawa, Japan) | 99 | Chemical reaction |
| Hydrogen Generator | Kanon Co., Ltd. (Osaka, Japan) | 99 | Chemical reaction |
| WL-959 | Guangzhou Wei Lai Health Management Technology Co., Ltd. (Guangzhou, China) | 99 | Electrolysis |
| AMS-H-03 | Shanghai Asclepius Meditec Co., Ltd. (Shanghai, China) | 66 | Electrolysis |
| BYT-JP-H03 | Shanghai Asclepius Meditec Co., Ltd. (Shanghai, China) | 66 | Electrolysis |
| QM-1500 | Guangzhou Zhongquing Energy Technology Co., Ltd. (Guangzhou, China) | 66 | Electrolysis |
| KENCOS | Aqua Bank Co. Ltd. (Osaka, Japan) | 66 | Electrolysis |
| BYT-JP series | Asklepios Medical Co., Ltd. (Tokyo, Japan) | 66 | Electrolysis |
| Hycellvator | Helix Japan Co., Ltd. (Tokyo, Japan) | 66 | Electrolysis |
| HydroPower | HPJ K.K. (Hokkaido, Japan) | 66 | Electrolysis |
| Beautyfly | Three-E SCIENCE Co., Ltd. (Kyoto, Japan) | 64 | Electrolysis |
| Select air HOME | Brand: select air HOME (Japan) | 66 | Electrolysis |
| Lita Air | WCJ Co., Ltd. (Osaka, Japan) | 3 (midway dilution) | Electrolysis |
| H2 Aroma Generator | Bio Logic Health Inc. (Tokyo, Japan) | 3 (midway dilution) | Electrolysis |
SUMMARY
It has been reported that the explosive concentration range of hydrogen is from 10% to 75%.21,22 Considering this range, it is often assumed that hydrogen gas with a concentration of 100% does not explode. However, in our ignition experiments, hydrogen gas inhalers that generate hydrogen with a concentration of 100% either exploded or caused a deflagration (refer to ignition experiments 1 and 4).
The results of these ignition experiments show that hydrogen with a concentration of 100% may be diluted by the surrounding air to reach a concentration within the explosive range of 10% to 75%. This hydrogen, diluted to an explosive concentration, can then explode in the presence of static electricity or another ignition source. Consequently, even hydrogen gas inhalers that generate hydrogen with a concentration of 100% can still pose a risk of explosion (Figure 8).
Figure 8.
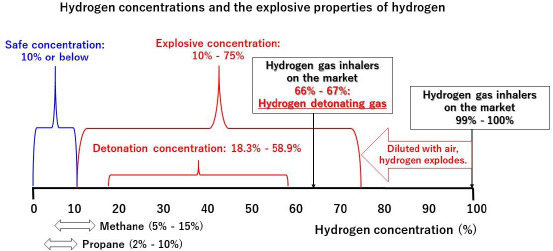
Hydrogen concentrations and the explosive properties of hydrogen.
Some commercially available hydrogen gas inhalers generate hydrogen with a concentration in the range of 66% to 67%. This is an oxyhydrogen detonating gas, which is a mixed gas consisting of hydrogen and oxygen at a ratio of 2 to 1 generated by the electrolysis of water, and it may explode with an extremely ferocious detonating sound. This detonating sound has the risk of damaging the auditory nerve (Figure 8).
The explosive concentrations of flammable gases such as methane and propane are 5% to 15% and 2% to 10%, respectively, which are much narrower than that of hydrogen (10% to 75%). In addition, the minimum ignition energy required for hydrogen is much lower than that of methane and propane.23,24,25,26 The broader explosive concentration and lower ignition energy suggest that hydrogen gas poses a much greater risk of explosion than these other flammable gases.
When choosing a hydrogen inhaler, it is important to search for one with minimal risk of explosion. Given that most commercially available hydrogen inhalers still pose a risk of explosion, the following summary provides guidelines that minimize this risk of explosion. Consulting such guidelines will maximize the safety of the user upon selection of a hydrogen gas inhaler.
Acknowledgements
We are grateful to Ms. Yoko Satoh, Dr. Guannan Shi, Mr. Susumu Mine and Mr. Yoshihiro Mitekura (MiZ Company Limited) for their excellent advice on writing this manuscript.
Footnotes
Conflicts of interest
YI, SH, BS and FS are employees of MiZ Inc.
REFERENCES
- 1.Najjar YSH. Hydrogen safety: The road toward green technology. Int J Hydrogen Energy. 2013;38:10716–10728. [Google Scholar]
- 2.Zhang Y, Tan S, Xu J, Wang T. Hydrogen therapy in cardiovascular and metabolic diseases: from bench to bedside. Cell Physiol Biochem. 2018;47:1–10. doi: 10.1159/000489737. [DOI] [PubMed] [Google Scholar]
- 3.Maas U, Warnatz J. Ignition processes in hydrogen oxygen mixtures. Combust Flame. 1988;74:53–69. [Google Scholar]
- 4.Kurokawa R, Hirano SI, Ichikawa Y, Matsuo G, Takefuji Y. Preventing explosions of hydrogen gas inhalers. Med Gas Res. 2019;9:160–162. doi: 10.4103/2045-9912.266996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorofeev SB, Kochurko AS, Efimenko AA, Chaivanov BB. Evaluation of the hydrogen explosion hazard. Nucl Eng Des. 1994;148:305–316. [Google Scholar]
- 6.Ng HD, Lee JHS. Comments on explosion problems for hydrogen safety. J Loss Prev Process Indust. 2008;21:136–146. [Google Scholar]
- 7.Hirano S-i, Ichikawa Y, Sato B, Satoh F, Takefuji Y. Hydrogen is promising for medical applications. Clean Technol. 2020;2:529–541. [Google Scholar]
- 8.DiLisi GA. The hindenburg disaster: combining physics and history in the laboratory. Phys Teach. 2017;55:268–273. [Google Scholar]
- 9.Venetsanos AG, Huld T, Adams P, Bartzis JG. Source, dispersion and combustion modelling of an accidental release of hydrogen in an urban environment. J Hazard Mater. 2003;105:1–25. doi: 10.1016/j.jhazmat.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Yanez J, Kuznetsov M, Souto-Iglesias A. An analysis of the hydrogen explosion in the Fukushima-Daiichi accident. Int J Hydrogen Energy. 2015;40:8261–8280. [Google Scholar]
- 11.Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. 2019;41:301–311. doi: 10.3892/or.2018.6841. [DOI] [PubMed] [Google Scholar]
- 12.Akagi J, Baba H. Hydrogen gas activates coenzyme Q10 to restore exhausted CD8(+) T cells, especially PD-1(+)Tim3(+)terminal CD8(+) T cells, leading to better nivolumab outcomes in patients with lung cancer. Oncol Lett. 2020;20:258. doi: 10.3892/ol.2020.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sano M, Shirakawa K, Katsumata Y, Ichihara G, Kobayashi E. Low-flow nasal cannula hydrogen therapy. J Clin Med Res. 2020;12:674–680. doi: 10.14740/jocmr4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou ZQ, Zhong CH, Su ZQ, et al. Breathing hydrogen-oxygen mixture decreases inspiratory effort in patients with tracheal stenosis. Respiration. 2019;97:42–51. doi: 10.1159/000492031. [DOI] [PubMed] [Google Scholar]
- 15.Guan WJ, Wei CH, Chen AL, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis. 2020;12:3448–3452. doi: 10.21037/jtd-2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura T, Hayashida K, Sano M, et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome-first-inhuman pilot study. Circ J. 2016;80:1870–1873. doi: 10.1253/circj.CJ-16-0127. [DOI] [PubMed] [Google Scholar]
- 17.Chuai Y, Zhao L, Ni J, et al. A possible prevention strategy of radiation pneumonitis: combine radiotherapy with aerosol inhalation of hydrogen-rich solution. Med Sci Monit. 2011;17:Hy1–4. doi: 10.12659/MSM.881698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consumer Affairs Agency of Japan. Incident Information Database System. [Accessed by February 8, 2021]. https://wwwjikojohocaagojp/ai-national/
- 19.Lin HY, Lai PC, Chen WL. A narrative review of hydrogen-oxygen mixture for medical purpose and the inhaler thereof. Med Gas Res. 2020;10:193–200. doi: 10.4103/2045-9912.295226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hord J. Is hydrogen a safe fuel? Int J Hydrogen Energy. 1978;3:157–176. [Google Scholar]
- 21.The Engineering ToolBox. Gases - Explosion and flammability concentration Limits. [Accessed by September 21, 2021]. https://wwwenginerringtoolboxcom/explosive-concentration-limitits-d_423html.
- 22.Yaws CL, editor. Matheson Gas Data Book. 7th ed. McGraw-Hill Professional; 2001. [Google Scholar]
- 23.Shen X, Zhang B, Zhang X, Wu S. Explosion behaviors of mixtures of methane and air with saturated water vapor. Fuel. 2016;177:15–18. [Google Scholar]
- 24.He S, Su L, Fan H, Ren R. Methane explosion accidents of tunnels in SW China. Geomat Nat Hazards Risk. 2019;10:667–677. [Google Scholar]
- 25.Hjertager BH, Fuhre K, BjØRkhaug M. Concentration effects on flame acceleration by obstacles in large-scale methane-air and propane-air vented explosions. Combust Sci Technol. 1988;62:239–256. [Google Scholar]
- 26.Zhang Q, Li D. Comparison of the explosion characteristics of hydrogen, propane, and methane clouds at the stoichiometric concentrations. Int J Hydrogen Energy. 2017;42:14794–14808. [Google Scholar]


