Abstract
Hepatocellular carcinoma (HCC) is one of the most common types of cancer diagnosed worldwide. After a decade of stagnation, several novel compounds have recently been shown to be effective in the treatment of HCC. Since immunotherapy is associated with important clinical benefits in some, but not all patients, it is essential to identify reliable predictive biomarkers. As the complex interplay between hepatocytes and immune cells is highly dependent on the tumor microenvironment, the tumor microenviroment has been suggested to be an important factor associated with the response to therapy and is currently being extensively investigated. Within this network, several important factors should be highlighted. Most of the cells are hepatocytes, but fibroblasts, endothelial cells, and immune cells are also present. Tumor-infiltrating leukocytes include several populations of cells and each of them plays a role in forming the tumor environment. Some of these cells may have antitumor effects, whereas others may be associated with the progression of the disease. The most important subsets include tumor-associated macrophages, tumor-associated neutrophils, and lymphocytes. These groups are described in the present review. The immune response is controlled by immune checkpoint molecules. One of the most important molecules involved in this checkpoint process seems to be the programmed death-1 (PD-1) receptor, which typically is induced on activated T cells, natural killer (NK) cells, B cells, and antigen-presenting cells. On the other hand, programmed death ligand 1 (PD-L1) is expressed by tumor cells, hepatocytes and hepatic stellate cells, and Kupffer cells or liver sinusoidal cells. Complex interactions between ligands and receptors are dependent on the signals from the microenvironment leading to either cancer development or apoptosis. Evidence from several studies indicates that patients with higher expression levels of PD-L1 on tumor cells or immune cells are more likely to achieve beneficial results from treatment with checkpoint blockers. This review focuses on the basic information regarding the microenvironment and its components, particularly on immune system involvement.
Keywords: hepatocellular carcinoma, immunotherapy, tumour microenvironment, tumour-associated macrophages, tumour-infiltrating lymphocytes
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of cancer diagnosed worldwide (1). For several years, the only treatment option for patients with advanced disease was sorafenib (2,3). However, more recently, promising results have been obtained from trials with immunotherapy. For example, the use of atezolizumab with bevacizumab provided better outcomes than sorafenib (4). Since immunotherapy may be associated with clinical benefits in certain patients, it is essential to identify reliable predictive biomarkers of treatment response. Therefore it is crucial to better understand the molecular and microenvironmental characteristics of the tumor.
Carcinogenesis is associated with cirrhosis, chronic inflammation, injuries, and regeneration of hepatocytes (5). The interplay between hepatocytes and immune cells depends on the tumor microenvironment which forms the complex network involved in hepatocarcinogenesis. Within this network most of the cells are hepatocytes, but fibroblasts, endothelial cells and immune cells [such as tumor-associated macrophages, T lymphocytes] also play a critical role. The microenvironment also includes growth factors, enzymes, and extracellular matrix proteins, and cytokines may also contribute to carcinogenesis (6). Due to the association between the gut and liver, the microbiome may also play a role in forming the tumor microenvironment (7).
In this review, we have summarized the basic data regarding immune cells and their role in hepatocarcinogenesis. Tumor-infiltrating leukocytes (TILs) include several populations of cells with different roles. Some of these may have antitumor effects, while others may be associated with the progression of the disease. The most important subsets include tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), and T lymphocytes, and are briefly described in Table I. Furthermore, we briefly focused on the prognostic and predictive factors associated with outcomes of immunotherapy.
Table I.
Summary of immune cells and their potential impact on the treatment and prognosis of HCC.
| Cell type | Impact | Potential prognostic/predictive factor | (Refs.) |
|---|---|---|---|
| MDSCs | Negative impact on immunoregulation | Yes | (10–14) |
| Increased neoangiogenesis | |||
| M2 polarization | |||
| Targeting MDSCs are associated with increased response to sorafenib | |||
| Higher MDSC levels are inversely correlated with overall and relapse-free survival | |||
| TAMs | |||
| M1 macrophages (pro-inflammatory) | Antitumor response, but CD68+ M1 | Yes | (16,17,26) |
| TAMs may be associated with the induction of PD-L1 in HCC cells (pro-tumor role) | |||
| M2 macrophages (anti-inflammatory) | Promotion of tumor progression | Yes | (18,19,23,24,30) |
| Enhanced angiogenesis and metastasis | |||
| Poorer prognosis | |||
| Resistance to sorafenib | |||
| TILs | |||
| NK cells | Targets tumor cells to prevent tumor | Yes | (38) |
| Cytotoxic T lymphocytes (CD8+) | progression | Yes | (38,43) |
| Regulatory T lymphocytes (CD4+) | May promote immune tolerance and inhibit the anti-tumor-promoting roles of other cells | Yes | (36,39) |
| Promotes disease progression by promoting angiogenesis | |||
| TANs | Able to promote or inhibit the progression of the disease | Yes | (51,52) |
| Resistance to sorafenib |
HCC, hepatocellular carcinoma; MDSCs, myeloid-derived suppressor cells; TAMs, tumor-associated macrophages; PD-L1, programmed death ligand 1; TILs, tumor-infiltrating lymphocytes; NK, natural killer; TANs, tumor-associated neutrophils.
2. Myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous subset of myeloid cells that play an essential role in the immunosuppressive network. MDSCs may differentiate into granulocytes, dendritic cells, and macrophages. Due to the hypoxic microenvironment, the process of differentiation can be prevented in HCC (8,9). It was observed that in patients with HCC, MDSCs inhibit T-cell proliferation and induce T-regulatory cells (10). The proliferation of T cells is inhibited through the depletion of arginine due to increased arginase activity. Furthermore, the production of matrix metallopeptidase 9 (MMP-9) by MDSCs contributes to increased neoangiogenesis through increasing vascular endothelial growth factor (VEGF) bioavailability (11). MDSCs can secrete IL-10, which results in M2 polarization as well as inhibition of natural killer (NK) cells, CD4+ and CD8+ lymphocytes through the expression of transforming growth factor β (TGF-β) (12,13). In a mouse model, targeting MDSCs was associated with a better response to sorafenib (14). A recent meta-analysis highlighted several observations. Firstly, the percentage of MDSCs in HCC patients was higher than in healthy individuals or patients with other chronic liver diseases. Moreover, higher quantities of MDSCs were inversely correlated with overall survival (OS) and relapse-free survival (RFS).
3. Tumor-associated macrophages (TAMs)
The population of liver macrophages includes Kupffer cells that reside within the liver and originate from the yolk sack and infiltrating hematopoietic stem cells/bone marrow-derived monocytes. TAMs are derived from monocytes recruited at the tumor site by molecules produced by neoplastic or stromal cells (15). However, they may be associated with a poorer prognosis, as some TAMs exhibit antitumor properties. There are two subsets of TAMs.
M1 macrophages are activated by cytokines such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF), or in response to microbial infection. This mechanism is vital for pro-inflammatory and antitumor responses. Production of interleukin (IL)-12 and other pro-inflammatory cytokines leads to the initiation of a Th-1 dependent immune response. They are also capable of cytotoxic activity against cancer cells (16,17).
Conversely, M2 macrophages play an anti-inflammatory role and are associated with the promotion of tumor progression. They may be alternatively activated by IL-4, IL-10, IL-13, or macrophage colony-stimulating factor (M-CSF), and glucocorticoid hormones. M2-like macrophages are further sub-divided into four subtypes, with different roles (18,19). M2a macrophages are involved in tissue repair as well as cell growth and endocytic processes. M2b cells regulate the immune response. M2c cells are responsible for apoptosis. Finally, M2d macrophages enhance tumor growth and angiogenesis (20). TAMs may secrete pro-angiogenic cytokines such as VEGF, TGF-β, or platelet-derived growth factor (PDGF) which contributes to the progression of cancer (21). Promoting tumor progression by M2 macrophages is associated with increased recruitment of regulatory T cells leading to the suppression of T effector cells. This mechanism results from the secretion of cytokines such as IL-10, TGF-β, or chemokines such as C-C motif chemokine ligand (CCL)17, CCL18, CCL22, and CCL24 (22).
A high count of M2 cells is associated with enhanced angiogenesis and metastasis, which leads to a poorer prognosis. Moreover, the production of IL-17 by M2 TAMs has been shown to suppress oxaliplatin-induced apoptosis in HCC (23,24).
It was suggested that CD206+ M2, but not CD68+ TAMs may be used as a prognostic biomarker in HCC (25). A recently published study indicated that CD68+ M1 TAMs are associated with the induction of programmed death-ligand 1 (PD-L1) in HCC cells, which suggest they possessed a pro-tumor role (26). Another analysis showed that CD68+ TAMs were associated with a poor prognosis (27). These results highlight the need for further investigations.
There are several mechanisms involved in immune suppression associated with TAMs. One of these is the interaction with PD-L1, which was confirmed in vivo in mouse models where TAM-derived PD-L1 contributed more significantly to suppressing antitumor immunity than the host-derived PD-L1 (28). PD-L2 expressed by TAMs is also involved in suppressing the host antitumor response in the murine microenvironment (29).
It is suggested that TAMs are involved in resistance to sorafenib through sustaining tumor growth and metastasis by secreting hepatocyte growth factor (HGF). This in turn may activate the HGF/c-Met, ERK1/2/MAPK, and PI3K/AKT pathways in tumor cells. An interesting concept would be combining sorafenib with HGF inhibitors such as cabozantinib to improve treatment outcomes (30).
Most of the TAMs within the tumor microenvironment are M2 macrophages. Since M2 cells promote tumor development, downregulate M1 functions and adaptive immunity, and favor angiogenesis and regeneration of tissues, they could be a potential target for novel therapies. In a rat model, zoledronic acid treatment enhanced the effects of transarterial chemoembolization by inhibiting TAM infiltration and tumor angiogenesis (31).
As TAMs may influence tumor progression, several studies have attempted to neutralize their role. One of the approaches is to re-educate TAMs using the M-CSF receptor with pexidartinib (PLX3397), which inhibited tumor growth and increased the CD8+ lymphocyte count (32). In a preclinical setting, another agent that proved efficacious was baicalin (8-bromo-7-methoxychrysin) (33). Tocilizumab inhibits IL-6 produced by TAMs, which may contribute to the suppression of tumorigenesis (34). Inhibition of TAM recruitment was also suggested as a therapeutic option; this has been tested in studies targeting chemokine receptor type 2 (CCR2) antagonists and showed promising results (35,36).
4. Tumor-infiltrating leukocytes (TILs)
TILs may be involved in the antitumor responses through the direct mechanisms of the adaptive immune system and modulation of the innate response or angiogenesis (37). Various cell subsets play different roles. First, NK cells and cytotoxic T lymphocytes are able to target tumor cells and thus prevent their progression, especially in the early stages. Later, the antitumor response is less pronounced, which correlates with patient prognosis (38).
Another subset of cells, regulatory T lymphocytes (Tregs, CD4+), may promote immune tolerance and inhibit the antitumor roles of other cells. One of these mechanisms is the secretion of TGF-β and IL-10, which inhibits CD8+ T-cells (39). One subset, Th-17 cells, secrete IL-17 and this promotes disease progression by inducing angiogenesis, which is associated with poorer outcomes (36).
One of the roles of cytotoxic CD8+ lymphocytes is to prevent tumor progression by killing cancer cells. However, in the case of prolonged exposure to antigens, CD8+ lymphocytes differentiate into so-called exhausted CD8+ cells with impaired cytotoxic functions (40). These exhausted CD8+ cells may be associated with decreased expression of cytokines, but also with increased expression of inhibitory receptors, including programmed cell death 1 (PD-1), lymphocyte-activation gene 3, cytotoxic T lymphocyte-associated antigen (CTLA-4), and T cell immunoglobulin domain (41,42). TILs with high levels of PD-1 expression exhibit higher expression levels of genes regulating T-cell exhaustion (43).
The low presence of intratumoral Tregs with high intratumoral activated CD8+ cytotoxic cells (CTLs) was found to be associated with improved disease-free survival (DFS) and OS (44). This is supported by the observation, that increased levels of regulatory T cells were correlated with CD8+ T-cell impairment and poor survival in HCC patients (45). Low CD8+ cell counts were found to be associated with poorer survival in another study (46). It was also observed, that CD8+ infiltration was associated with a so-called ‘immune cell stroma’ HCC type, which in comparison with ‘conventional stroma’, is characterized by the lack of catenin β1 (CTNNB1) mutations, global hypermethylation, expression of PD-1 and PD-L1 in TILs, and expression of PD-L1 in tumors. This type was also associated with an improved prognosis (47). The results of several studies indicate that the inhibition of Treg-induced suppression may be effective in restoring antitumor immune responses in several types of cancer (48–50).
5. Tumor-associated neutrophils (TANs)
The role of TANs in carcinogenesis is complex. TANs can promote as well as inhibit the progression of the disease, dependent on the cytokines released. The secretion of CCL-2 or CCL17 is associated with poorer outcomes. Those cytokines may also promote the infiltration of Tregs and macrophages. This infiltration negatively correlates with survival. It is suggested that TANs are involved in sorafenib resistance (49). In vitro, when HCC cell lines were cocultured with TANs, colony formation, cell migration, invasion, and sphere formation were enhanced, while apoptosis was inhibited.
There is growing evidence, that miRNAs may regulate tumor progression in HCC. For example, miR-301b-3p was correlated with the tumor size and advanced stages of the disease. Knockdown of miR-301b-3p reduced proliferation, induced cell cycle arrest in the G2/M phase, and induced apoptosis. Furthermore, TANs secrete bone morphogenetic protein 2 and TGF-β2 and increased miR-301-3p expression in HCC cells, which subsequently suppressed gene expression of limbic system-associated membrane protein (LSAMP) and CYLD lysine 63 deubiquitinase (CYLD), and increased the acquisition of stem cell characteristics in HCC cells. TAN-induced HCC stem-like cells were hyperactive and further increased TAN infiltration, suggesting a positive feedback loop. In clinical HCC samples, increased quantities of TANs were correlated with elevated miR-301b-3p levels, decreased LSAMP and CYLD expression, and increased nuclear p65 accumulation and C-X-C motif chemokine ligand 5 (CXCL5) expression, all of which were associated with patient outcomes (50).
6. Tumor microenvironment and the response to systemic treatment
Sorafenib has been the standard of care for advanced HCC for several years. However, there are several challenges associated with sorafenib treatment, including low response rates, toxicity, and acquisition of drug resistance. The tumor microenvironment plays an essential role in mechanisms associated with resistance. Several studies have demonstrated that the infiltration of TANs and TAMs may be correlated with the sensitivity to sorafenib (51). Furthermore, treatment with sorafenib results in hypoxia related to the depletion of pericytes and a decreased number of vessels. It is suggested that sorafenib-induced hypoxia may be associated with subsequent exosome-mediated resistance to sorafenib (52). Characterization of the tumor microenvironment has led to the division of HCC into four immune subclasses. About 20% of patients present with an immunogenic subclass characterized by massive T cell infiltration and activation of the immune checkpoint pathway. These patients exhibit the best response not only to immunotherapy, but also to sorafenib (53).
In 2018, the results of a phase III trial with lenvatinib showed it to be effective in the first-line treatment of HCC. It was also evaluated in combination with immunotherapy. Lenvatinib reduced the number of TAMs and increased the percentage of activated CD8+ T cells secreting IFN-γ+ and granzyme B. It is worth noting that the exhaustion of CD8+ lymphocytes is one of the mechanisms involved in immune escape and cancer development. On the other hand, it is regulated by the PD-1 signaling (54). These findings provide a scientific rationale for the combination therapy of lenvatinib with PD-1 blockade (55). In a mouse model of HCC, the immunomodulatory activity of lenvatinib led to an enhanced response to anti-PD-1 treatment. In 2021, it was also suggested in a small retrospective study in which patients with HCC received lenvatinib with pembrolizumab or nivolumab (56,57).
7. Immune checkpoint inhibition
Immune checkpoint molecules control the immune response. One of the most important seems to be the PD-1 receptor, which typically is induced on activated T cells, NK cells, B cells, and antigen-presenting cells. Conversely, PD-L1 is expressed by tumor cells, hepatocytes, hepatic stellate cells, and Kupffer cells or liver sinusoidal cells (58). PD-L2 is another known ligand for PD-1, and it is present on dendritic cells. Complex interactions between ligands and receptors dependent on the signals from the microenvironment lead to either cancer development or apoptosis.
Evidence from several studies indicates that patients with higher expression of PD-L1 on tumor cells or immune cells are more likely to achieve benefits from treatment with checkpoint blockade (59,60).
The impact of PD-1/PD-L1 on the prognosis and treatment outcomes in HCC has been studied in several studies. However, the results have proven to be conflicting, and the underlying mechanism is not fully understood. It has been shown that patients with high expression of PD-L1 and a high TIL presence tend to have better prognoses, whereas low expression of PD-L1 and galectin-9 and low CD8+ TIL counts are associated with poor HCC-specific survival (46). On the other hand, the results of another study, where the PD-L1 expression was correlated with clinical and pathological features suggested that PD-L1 expression by either neoplastic or intratumoral inflammatory cells was associated with tumor aggressiveness (61). These conflicting results clearly show the need for further investigation of the immune landscape of the HCC microenvironment.
Of note, it has been suggested that stratifying tumors according to the expression of PD-L1 and TILs could be helpful for predicting the response to treatment. Type 1 cancers are characterized by PD-L1+TILs+, and benefit from the single-agent anti-PD-1/L1 blockade. Type 2 cancers (PD-L1−TIL−) have been predicted to have poor responses to single-agent immunotherapy and poorer prognoses. In type 3 cancers (PD-L1+TIL−), PD-L1 positivity is not a single predictive factor for determining the response to anti-PD-1/PD-L1 treatment, as without TILs, a reaction to blocking PD1/L1 is unlikely. Finally, other suppressive mechanisms may be dominant for type 4 cancers (PD-L1−TIL+) (62). Thus, a simple distinction between the presence or absence of TILs or PD-L1 may not be sufficient. It is well established that other immune checkpoint molecules may be co-expressed on T cells. For example, lymphocyte activation gene-3 (LAG-3), a negative regulator of T cells is also expressed on T cells and its inhibition increases the antitumor response (63). It has been shown that HCCs may contain CD8+ T cells that express different levels of PD-1. Those cells with a discrete population of PD-1 high CD8+ T cells express T cell immunoglobulin and mucin-domain-containing protein-3 (TIM-3) or LAG-3 and can produce low levels of IFN-γ or TNF in response to anti-CD3. Incubation of these cells with antibodies against PD1 and TIM-3 or LAG-3 further restored proliferation and production of cytokines. The results of this study indicate that HCCs with a discrete population of PD1-high CD8+ T cells may be more susceptible to combined immune checkpoint blockade-based therapies (43).
Kurebayashi et al suggested that the tumor microenvironment can be classified into three subtypes based on immunohistochemical analyses of the immune regulatory molecules; namely, Immune-high, Immune-mid, and Immune-low. The Immune-high subtype is characterized by increased B-/plasma-cell and T-cell infiltration. The Immune-high subtype and B-cell infiltration were identified as independent positive prognostic factors. Furthermore, the Immune-high subtype was found to be associated with poorly differentiated HCC, cytokeratin 19 (CK19)+, or Sal-like protein 4 (SALL4)+ high-grade HCC, and Hoshida's S1/Boyault's G2 subclasses. Finally, patients with high-grade HCC of the predominant Immune-high subtype had significantly better prognoses. The Immune-mid subtype is characterized by moderately increased T-cell and other immune cell infiltration with lesser B- and plasma-cell infiltration, while the Immune-low subtype is associated with even lower levels of infiltration. Depending on the Treg/CD4+ ratio, the Immune-low type was subdivided into 2 classes (1-lower Treg/CD4+ ratio; 2-higher) (64).
As mentioned above, TIM-3 is another regulatory molecule that plays a role in tumor progression. Its levels may be increased in CD4+ and CD8+ cells or TAMs, and this is correlated with a poorer prognosis. On the other hand, its suppression resulted in an increased antitumor response (13).
8. Immune checkpoint inhibitors assessed for treatment of HCC
Recently several immune checkpoint inhibitors have been evaluated as treatments for HCC. Most of the studies have focused on PD-1/PD-L1 inhibitors and CTLA-4 inhibitors. Anti-PD-1 treatment with monoclonal antibodies was found to result in the blockade of PD-1 interaction with PD-L1 or PD-L2 expressed in antigen-presenting cells, which is involved in the T-cell antitumoral response. Checkpoint inhibition enhances T-cell response and normalizes the immune response within the microenvironment (65).
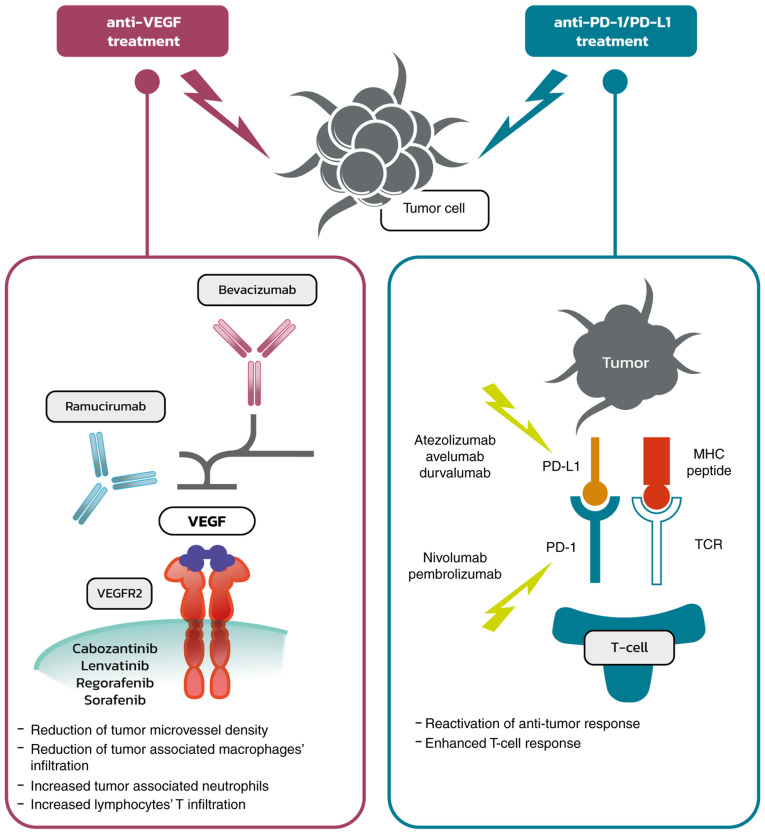
From a clinical point of view, the most important inhibitors assessed were atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) in the ImBrave150 trial, which showed a significant response to the combined treatment, and has since become the recommended standard of care (66,67). Bevacizumab was shown to reduce tumor microvessel density and modulate the immune microenvironment through the reduction of infiltration of tumor-associated macrophages and the increase in tumor-associated neutrophils (68). VEGF-A produced in the tumor microenvironment enhances the expression of PD-1 and other inhibitory checkpoints involved in CD8+ T-cell exhaustion, and this could be inhibited using anti-angiogenic agents targeting VEGF-A and/or VEGFR (69). Thus, anti-VEGF therapy leads to the reduction of immunosuppressive factors within the tumor and its microenvironment and is associated with increased T-cell infiltration, which together enhances the response to immunotherapy. Atezolizumab is a monoclonal antibody that directly binds to PD-L1 and provides a dual blockade of the PD-1 and B7.1 receptors, releasing PD-L1/PD-1 mediated inhibition of the immune response, including the reactivation of the antitumor immune response without inducing antibody-dependent cellular cytotoxicity. The effect of anti-VEGF and anti-PD-1/PD-L1 treatment is illustrated in Fig. 1.
Figure 1.
Anti-VEGF and anti-PD-1/PD-L1 treatment: Mechanism of action. PD, programmed death; PD-L1, programmed death-ligand 1; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
Of note, combined treatment with bevacizumab and atezolizumab was associated not only with improved survival [median progression-free survival (PFS) was 6.8 months vs. 4.3 months in the sorafenib group; hazard ratio (HR) 0.59; 95% confidence interval (CI), 0.47-0.76; P<0.001], but also with the maintenance of the quality of life (11.2 months vs. 3.6 months in patients treated with sorafenib; HR 0.63; 95% CI, 0.46-0.85) (70).
The Check-Mate 459 trial compared nivolumab (anti-PD-1) with sorafenib as a first-line treatment for HCC. Although the objective response rate was higher in the investigational arm, the increase in OS was not significant (71). Nivolumab was also evaluated in combination with ipilimumab (anti-CTLA-4) in the phase 1/2 trial CheckMate 040, where manageable safety, promising objective response rates, and durable responses were shown (72).
Pembrolizumab is a humanized monoclonal antibody that blocks the interaction of the PD-1 receptor with PD-L1 and PD-L2, which results in the enhancement of the antitumor effect of the immune system associated with T-cell responses (73). Based on the promising results of the phase II trial Keynote 224 (74), pembrolizumab was evaluated as a second-line treatment for HCC in the Keynote 240 trial. However, the results did not show a notable clinical benefit (75).
Combined treatment with tremelimumab, an anti-CTLA4 antibody, and durvalumab showed promising results in patients with advanced HCC in a phase I/II trial (76). It is also being investigated in the phase III HIMALAYA trial (77).
Several other immunotherapeutic agents are under evaluation in various combinations with tyrosine kinase inhibitors or other immunotherapeutic drugs. Select phase III trials are summarized in Table II (78–91). The results of the already published trials suggest that there are groups of patients that may benefit more from immunotherapy compared with others. Currently, it seems to be crucial to identify predictive factors for the response to targeted therapy. It is suggested that the microenvironment may play an important role in the response to the treatment.
Table II.
Selected phase III trials with various immunotherapy approach for HCC.
| Clinicaltrials.gov number | Design | Status | (Refs.) |
|---|---|---|---|
| NCT02851784 | Combination therapy of microwave ablation and expanding activated autologous lymphocytes | Completed | (78) |
| NCT02562755 | Vaccinia virus-based immunotherapy (Pexa-Vec) followed by sorafenib vs. sorafenib | Completed | (79) |
| NCT05033522 | Living allogeneic Th1-like cells with anti-CD3/CD28 microbeads attached derived from precursors purified from healthy blood donors that are differentiated and expanded ex-vivo vs. FOLFOX regimen | Not yet recruiting | (80) |
| NCT02576509 | Nivolumab vs. sorafenib as first-line treatment | Active, not recruiting | (81) |
| NCT02678013 | RFA+highly-purified CTL vs. RFA alone for recurrent HCC | Active, not recruiting | (82) |
| NCT04167293 | Combination of sintilimab and stereotactic body radiotherapy | Recruiting | (83) |
| NCT03949231 | Infusion of PD-1/PDL-1 inhibitor via hepatic arterial vs. vein | Recruiting | (84) |
| NCT04229355 | DEB-TACE plus lenvatinib or sorafenib or PD-1 inhibitor for unresectable HCC | Recruiting | (85) |
| NCT00699816 | Adjuvant adoptive immune therapy using a CIK cell agent (Immuncell-LC) vs. non-treatment group in HCC | Completed | (86) |
| NCT02709070 | Resection+highly purified cytotoxic T lymphocytes vs. resection alone | Active | (87) |
| NCT04268888 | Nivolumab in combination with TACE/TAE | Recruiting | (88) |
| NCT03867084 | Adjuvant pembrolizumab vs. placebo in HCC with complete radiological response after surgical resection or local ablation | Recruiting | (89) |
| NCT04682210 | Adjuvant sintilimab plus bevacizumab in HCC after resection | Not yet recruiting | (90) |
| NCT01749865 | Adjuvant cytokine-induced killer cell treatment in HCC after resection | Completed | (91) |
HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; CTL, cytotoxic T lymphocytes; HCC, hepatocellular carcinoma; PD, programmed death; L, ligand; CIK, cytokine-induced killer cell; DEB-TACE, drug-eluting bead transarterial chemoembolization; TAE, trans-arterial embolization.
A very interesting topic is the use of immunotherapy in patients that qualify for liver transplants. There are limited data regarding the use of immunotherapy for downstaging or bridging therapy from clinical case reports (92,93). The use of adoptive immunotherapy with liver allograft-derived NK cells was evaluated in a study presented during the 2015 American Transplant Congress, and it was suggested that this approach may improve RFS (94). Immunotherapy was also used for recurrent HCC treatment after liver transplantation. Interestingly, a case report where ipilimumab was used in a patient with recurrent HCC after liver transplantation described a durable response and no rejection of the organ (95). However, it should be noted that immunotherapy may increase the risk of liver rejection and therefore should be considered rather as a salvage therapy rather than a first-line approach (96). Clearly, there is a need for additional data.
9. Immune microenvironment factors associated with the response to immunotherapy
The response to treatments depends on several mechanisms and the specific characteristics of the tumor, the local microenvironment, as well as the host itself. Firstly, since most of the immunotherapeutics that showed clinical efficacy are checkpoint inhibitors, it has been suggested that the expression of PD-1 may be a reliable predictive factor. It has been shown that in patients with head and neck cancers (97), melanoma (98), or lung cancer (99), increased PD-1 expression was associated with better treatment outcomes.
Interestingly, in the Check-Mate 040 trial, expression of PD-L1 in >1% of the tumor cells was detected in 20% of patients. However, the objective response between patients with higher expression of PD-L1 in comparison to those with low expression did not differ significantly: 26 vs. 19% (95% CI 13–26). This suggests that other mechanisms may be involved in the response to immunotherapy (66). Potentially, this could be related to the expression of PD-1 and PD-L1 on tumor-infiltrating lymphocytes.
Furthermore, in a small phase II trial where patients with advanced HCC were treated with pembrolizumab after sorafenib failure, the response was not correlated with either PD-L1 tumor staining, prior sorafenib therapy or a history of hepatitis. Correlative studies revealed high baseline plasma TGF-β levels (≥200 pg/ml) were significantly correlated with poor treatment outcomes after pembrolizumab. Tumor PD-L1 and plasma PD-L1/PD-1 levels were associated with plasma IFN-γ or IL-10. However, those results need caution due to the low number of patients (n=28) (100).
Recently it was demonstrated that androgen receptors may inhibit the expression of PD-L1 in HCC. It was found that the androgen receptor is overexpressed in the nucleus of ~37% of HCC tumors, which is significantly associated with an advanced disease stage and poorer survival rates. It has also been suggested that the overexpression of androgen receptors may be related to a worse response to PD-L1 inhibitors (101).
Studies conducted in non-small cell lung cancer highlighted the role of EGFR signaling in the regulation of the host immunity and tumor microenvironment. Cell lines with mutant EGFR exhibit increased expression of PD-L1 compared to wild-type EGFR cells (102).
There are several questions regarding the potential use of PD1/PD-L1 expression as a predictive factor. Firstly, the expression of PD-L1 could be present on tumor cells or on immune cells, which may play an essential role in the response to immunotherapy (103). In patients with HCC, a low baseline intratumoral CD4+/CD8+ T-cell ratio was associated with a better OS. Response to PD-1/PD-L1 therapy showed a trend of high baseline frequency of intratumoral PD-1 CD8+ T cells. However, LAG-3 and TIM-3 upregulation was also observed in circulating T cells in patients who did not respond to PD-1/PD-L1 pathway blockade (103).
Furthermore, currently, there is no standard for assessing the cut-off value for PD-L1 positivity and there are various kits used, which makes the comparison between studies more difficult. It was also shown that the expression of PD-L1 may change during the disease (104,105). On the other hand, it has been suggested that other factors associated with the tumor microenvironment may be necessary for predicting the response to immunotherapy. Recently, a classification of tumor microenvironments based on TILs and PD-L1 expression was described. Furthermore, it is suggested, that there is an immune class of HCC tumors which may be further divided into two subtypes, characterized by markers of an adaptive T-cell response or exhausted immune response. The exhausted immune response subclass expresses several genes regulated by TGF-β1 that mediate immunosuppression. According to the authors, these findings indicate that some HCCs may be susceptible to therapeutic agents designed to block the regulatory pathways in T cells, such as PD-L1, PD-1, or TGF-β1 inhibitors (106). In another study, a high number of PD-1+ TILs was shown to be associated with prolonged OS and DFS in patients with HCC who received surgery and adjuvant cytokine-induced killer cells treatment (107).
Of note, it was suggested that the response to immunotherapy may depend on the specific liver disease background, such as a viral infection. However, a recently published meta-analysis of 8 studies suggested that there was no significant difference in objective response rate between virally infected HCC and non-viral HCC patients [OR=1.03 (95% CI, 0.77-1.37; I2=30.9%, pH=0.152)]. Similarly, there was no difference between HBV-HCC and HCV-HCC patients in terms of objective response rate [OR=0.74 (95% CI, 0.52-1.06; I2=7.4%, pH=0.374)]. The infiltration of immune cells in the tumor microenvironment did not differ by etiology except for M0 macrophages, M2 macrophages, regulatory T cells, naive B cells, follicular helper T cells, activated dendritic cells, activated mast cells, and plasma cells. Despite differences in infiltration observed in specific cell types, the immune score and stromal score were generally comparable among the different etiological groups (108).
Interestingly, it was suggested that immune checkpoint inhibitors may be ineffective in non-alcoholic steatohepatitis (NASH)-related HCC. This was observed in a preclinical model as well as in a meta-analysis of three randomized phase III clinical trials that tested inhibitors of PD-L1 or PD-1 in >1,600 patients with advanced HCC. According to the results, immunotherapy did not improve survival in patients with non-viral HCC. In two additional cohorts, patients with NASH-driven HCC who received anti-PD-1 or anti-PD-L1 treatment showed reduced OS compared with patients with other aetiologias (109).
10. Additional potential biomarkers and predictive factors for the response to immunotherapy
Since radical treatment options are limited, and the prognosis for patients with advanced HCC remains poor, there is a need to identify reliable biomarkers of HCC to better tailor patient therapy. Several potential biomarkers for the response to immunotherapy have been investigated in HCC.
Several other mechanisms may be involved in the response to immunotherapy. The gut-liver axis and the colon microbiome are some of the suggested factors associated with the response to immunotherapy. In a small study, where 8 patients with progressive disease after sorafenib failure were treated with nivolumab, fecal samples were collected and analyzed at baseline, after 1 week, and every 3 weeks after that. Variations in the gut bacteria were analyzed by metagenomic sequencing. It was observed that fecal samples from patients responding to immunotherapy showed higher taxa richness and increased gene counts than non-responders (110). Another mechanism potentially involved in the response to immunotherapy includes exosomes due to their role in the communication between host and tumor cells. Exosomes are involved in the transfer of proteins, DNA, and RNA. Exosomes could serve as biomarkers for early-stage HCC and as a possible target for therapy (111). There are several exosomal biomarkers for the prediction of survival in HCC, i.e. proteins involved in angiogenesis (S100A11, S100 calcium-binding protein A11) and numerous microRNAs such as miR-29b-3p, miR-30d-5p which are involved in cell migration, or miR-210 which is associated with angiogenesis (112,113). Another example may be exosomal circulating RNA (circPTGR1) which was suggested to promote HCC metastasis. Finally, exosome-based strategies to deliver drugs into tumors and the microenvironment showed promising results in preclinical and clinical trials (114–116). The complexity of the interplay between host and tumor requires further studies to identify reliable predictive factors. Other factors include tumor mutational burden (TMB), defined as the total number of mutations in the tumor exome. However, it seems to be relatively rare in HCC; in a recently published report median TMB was 4 mutations/Mb and only 6 tumors (0.8%) were classed as TMB-high. Out of 542 cases assessed for microsatellite instability (MSI), one (0.2%) was MSI-high and TMB-high. TMB may be associated with MSI or DNA mismatch repair gene deficiency. In a recently published analysis, the most commonly altered genes were TERT (44%), TP53 (35%), CTNNB1 (31%), ARID1A (12%), and MYC (12%) (117).
Currently, there are a vast group of biomarkers being investigated for HCC. They include post-translational modifications such as phosphorylation, glycosylation, ubiquitination, or acetylation. These changes are involved in various physiological processes, but also in disease progression (118). Next, generation sequencing techniques (NGS) are proving to be very powerful and valuable methods. Potentially, thanks to NGS profiling, patient responses to treatments may be predictable. An analysis showed that in patients with HCC, the WNT/β-catenin pathway (45%) and TP53 (33%) alterations were frequent and represented mutually exclusive molecular subsets. In sorafenib-treated patients (n=81), oncogenic PI3K-mTOR pathway alterations were associated with lower disease control rates (DCR, 8.3 vs. 40.2%), shorter median PFS (1.9 vs. 5.3 months), and shorter median OS (10.4 vs. 17.9 months). Conversely, patients treated with immune checkpoint inhibitors (n=31) activating altered WNT/β-catenin signaling had lower DCR (0% vs. 53%), shorter median PFS (2.0 vs. 7.4 months), and shorter median OS (9.1 vs. 15.2 months) (119,120). Potential factors involved in response to immunotherapy are summarized in Fig. 2.
Figure 2.
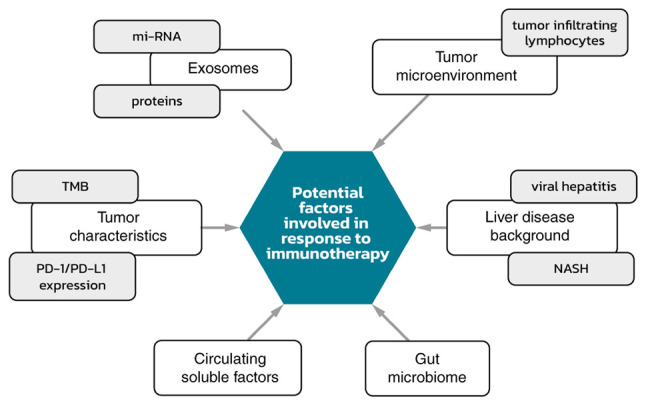
Potential factors involved in response to immunotherapy. mi-RNA, microRNA; NASH, non-alcoholic steatohepatitis; PD-1/PD-L1, programmed death 1/programmed death ligand 1; TMB, tumor mutational burden.
Since histological specimens may not always be available in HCC, factors that could be assessed from blood examinations would be of value. A number of soluble factors such as cytokines are of interest. In a phase II study, where several circulating biomarkers were evaluated, low baseline levels of TGF-β were significantly associated with improved OS and PFS after treatment with pembrolizumab in advanced, unresectable HCC (100,120). However, a detailed analysis is beyond the scope of this review. A recently published analysis showed that in patients with unresectable HCC, treated with nivolumab or pembrolizumab, an early decrease in α-fetoprotein levels was associated with an increased objective response and increased survival. Moreover, albumin/bilirubin grade and Child-Pugh classification determined survival based on immunotherapy treatment (121).
11. Conclusions
This review briefly summarizes the current body of knowledge regarding immune cells within the tumor microenvironment in HCC. However, other factors may play important roles, such as tumor mutational burden or microsatellite instability (122). The changing landscape of the treatment possibilities in advanced HCC makes the role of predictive factors rise. Thus, there is a need to identify reliable factors that may help tailor treatments to the specific disease characteristics presented.
Acknowledgements
The authors would like to thank Mr. Jakub Jaworski for their assistance with the images used in this review.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
MG was responsible for conception and the design, data analysis and interpretation of the data as well as draft preparation of this review. KW conducted the draft preparation, critical revisions and approved the final manuscript version to be published; and agrees to be accountable for all aspects of the work. LK was responsible for draft preparing, critical revisions; approved of the final manuscript version to be published; and agrees to be accountable for all aspects of the work. LR conducted the draft preparation, critical revisions; approved of the final manuscript version to be published; and agrees to be accountable for all aspects of the work. RS conducted the draft preparing, critical revisions; approved of the final manuscript version to be published; and agrees to be accountable for all aspects of the work. All authors have read and approved the final manuscript. Data authentication is not applicable..
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Authors' information
MG (ORCID: 0000-0003-1344-5001) is a medical oncologist, who graduated at the Medical University of Warsaw, and has worked for several years at the Maria Curie-Sklodowska Cancer Center-Institute in Warsaw. He is currently preparing a PhD programme at the University Clinical Center, Warsaw. His focus is on gastrointestinal cancers, particularly hepatobiliary cancer and he is involved in several scientific projects, such as MentorEye Project (development of Polish complementary system of molecular surgical navigation for the purposes of cancer treatment); attendee of several international trainings led by ESMO and ESDO; member of the Polish Society of Clinical Oncology, ESMO (European Society for Medical Oncology) and ESDO (European Society of Digestive Oncology). KW is a medical oncologist and lecturer who graduated at the Medical University of Warsaw, and is an assistant at the Central Clinical Hospital, Oncology. His focus is on hepatobiliary and pancreatic cancer. He was born in 1984 in Czestochowa, Poland. He completed specialization in medical oncology in October 2016. In clinical practice he takes care mostly of patients suffering from gastrointestinal neoplasms. He is an author of more than 10 publications in medical journals and conferences reports. LK (ORCID: 0000-0001-6509-5993) is a medical oncologist, researcher and lecturer who graduated at the Medical University of Warsaw (PhD) and is mainly focused on pancreatic and hepatobiliary cancers. LK is the winner of several domestic grants and awards for young researchers. LR is a medical oncology resident, involved in several scientific projects, and an academic teacher at the Medical University of Warsaw. Professor Rafal Stec (ORCID: 0000-0002-3291-6422) is a graduate of the Medical University of Lublin and conducted post-graduate study ‘Innovation Management in the Health Sector’ of the Academy of Leon Koźmiński in Warsaw. He has been associated with oncology since 2000, initially at the Maria Curie-Sklodowska Cancer Center-Institute in Warsaw, and then at the Department of Oncology at the Military Medical Institute in Warsaw. From September 2018, he was head of the Oncology Clinic of the Medical University of Warsaw. He is also editor-in-chief of the journal ‘Personalized Oncology’. He is co-author of over 100 publications in Polish and foreign journals as well as books and textbooks, and is a member of the Polish Society of Clinical Oncology and ESMO (European Society for Medical Oncology). He was a winner of the awards of the Polish Society of Clinical Oncology and is the Director of the Military Medical Institute in Warsaw. His area of particular interest remains molecular and precise oncology as well as personalized (individual) and interdisciplinary oncology treatment. He is head of the Oncology Department at the Medical University of Warsaw.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A, Qin S, Ikeda M, Galle PR, Ducreux MP, Zhu AX, Kim T, Kudo M, Breder V, Merle P, et al. Imbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC)'. Ann Oncol. 2019;30((Suppl 9)):ix183–ix202. doi: 10.1093/annonc/mdz446.002. [DOI] [Google Scholar]
- 5.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novikova MV, Khromova NV, Kopnin PB. Components of the hepatocellular carcinoma microenvironment and their role in tumor progression. Biochemistry (Mosc) 2017;82:861–873. doi: 10.1134/S0006297917080016. [DOI] [PubMed] [Google Scholar]
- 7.Jia W, Rajani C, Xu H, Zheng X. Gut microbiota alterations are distinct for primary colorectal cancer and hepatocellular carcinoma. Protein Cell. 2021;12:374–393. doi: 10.1007/s13238-020-00748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8:517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 13.Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, Liang X, Ma C. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut. 2015;64:1593–1604. doi: 10.1136/gutjnl-2014-307671. [DOI] [PubMed] [Google Scholar]
- 14.Chang CJ, Yang YH, Chiu CJ, Lu LC, Liao CC, Liang CW, Hsu CH, Cheng AL. Targeting tumor-infiltrating Ly6G+ myeloid cells improves sorafenib efficacy in mouse orthotopic hepatocellular carcinoma. Int J Cancer. 2018;142:1878–1889. doi: 10.1002/ijc.31216. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan S, Kuo N, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304–1312. doi: 10.1002/hep.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo B, Li L, Guo J, Liu A, Wu J, Wang H, Shi J, Pang D, Cao Q. M2 tumor-associated macrophages produce interleukin-17 to suppress oxaliplatin-induced apoptosis in hepatocellular carcinoma. Oncotarget. 2017;8:44465–44476. doi: 10.18632/oncotarget.17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu XT, Song K, Zhou J, Shi YH, Liu WR, Shi GM, Gao Q, Wang XY, Ding ZB, Fan J. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019;19:71. doi: 10.1186/s12935-019-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Chang L, Zhang X, Zhou Z, Gao Y. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in hepatocellular carcinoma. J Invest Surg. 2021;34:297–306. doi: 10.1080/08941939.2019.1631411. [DOI] [PubMed] [Google Scholar]
- 26.Zong Z, Zou J, Mao R, Ma C, Li N, Wang J, Wang X, Zhou H, Zhang L, Shi Y. M1 macrophages induce PD-L1 expression in hepatocellular carcinoma cells through IL-1β signaling. Front Immunol. 2019;10:1643. doi: 10.3389/fimmu.2019.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding W, Tan Y, Qian Y, Xue W, Wang Y, Jiang P, Xu X. Clinicopathologic and prognostic significance of tumor-associated macrophages in patients with hepatocellular carcinoma: A meta-analysis. PLoS One. 2019;14:e0223971. doi: 10.1371/journal.pone.0223971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8:14572. doi: 10.1038/ncomms14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umezu D, Okada N, Sakoda Y, Adachi K, Ojima T, Yamaue H, Eto M, Tamada K. Inhibitory functions of PD-L1 and PD-L2 in the regulation of anti-tumor immunity in murine tumor microenvironment. Cancer Immunol Immunother. 2019;68:201–211. doi: 10.1007/s00262-018-2263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong N, Shi X, Wang S, Gao Y, Kuang Z, Xie Q, Li Y, Deng H, Wu Y, Li M, Li JL. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br J Cancer. 2019;121:22–33. doi: 10.1038/s41416-019-0482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou DY, Qin J, Huang J, Wang F, Xu GP, Lv YT, Zhang JB, Shen LM. Zoledronic acid inhibits infiltration of tumor-associated macrophages and angiogenesis following transcatheter arterial chemoembolization in rat hepatocellular carcinoma models. Oncol Lett. 2017;14:4078–4084. doi: 10.3892/ol.2017.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY, Zhang KZ, Kong LQ, Zhang N, Ye BG, Ma DN, Sun HC. Colony-stimulating factor 1 receptor blockade inhibits tumour growth by altering the polarization of tumour-associated macrophages in hepatocellular carcinoma. Mol Cancer Ther. 2017;16:1544–1554. doi: 10.1158/1535-7163.MCT-16-0866. [DOI] [PubMed] [Google Scholar]
- 33.Sun S, Cui Y, Ren K, Quan M, Song Z, Zou H, Li D, Zheng Y, Cao J. 8-bromo-7-methoxychrysin reversed M2 polarization of tumour-associated macrophages induced by liver cancer stem-like cells. Anticancer Agents Med Chem. 2017;17:286–293. doi: 10.2174/1871520616666160204112556. [DOI] [PubMed] [Google Scholar]
- 34.Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 36.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H. A natural CCR93 antagonist relieves tumour-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- 40.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, Yoo C, Yi K, Kim KH, Eo S, et al. Association between expression level of PD1 by tumor-infiltrating CD8(+) T cells and features of hepatocellular carcinoma. Gastroenterology. 2018;155:1936–1950.e17. doi: 10.1053/j.gastro.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 44.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 45.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 46.Sideras K, Biermann K, Verheij J, Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA, Sprengers D, Buschow SI, et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. OncoImmunology. 2017;6:e1273309. doi: 10.1080/2162402X.2016.1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang HJ, Oh JH, Chun SM, Kim D, Ryu YM, Hwang HS, Kim SY, An J, Cho EJ, Lee H, et al. Immunogenomic landscape of hepatocellular carcinoma with immune cell stroma and EBV-positive tumor-infiltrating lymphocytes. J Hepatol. 2019;71:91–103. doi: 10.1016/j.jhep.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: Implications for prognosis and therapeutic applications. Liver Int. 2019;39:1608–1621. doi: 10.1111/liv.14192. [DOI] [PubMed] [Google Scholar]
- 49.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646–1658.e17. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 50.Zhou SL, Yin D, Hu ZQ, Luo CB, Zhou ZJ, Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, et al. A positive feedback loop between cancer stem-like cells and tumor-associated neutrophils controls hepatocellular carcinoma progression. Hepatology. 2019;70:1214–1230. doi: 10.1002/hep.30630. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 52.Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X, Huang H, Wang Y, Pan C, Yin S, Zhou L, Zheng S. Tumor immune microenvironment characterization in hepatocellular carcinoma identifies four prognostic and immunotherapeutically relevant subclasses. Front Oncol. 2021;10:610513. doi: 10.3389/fonc.2020.610513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budimir N, Thomas GD, Dolina JS, Salek-Ardakani S. Reversing T-cell exhaustion in cancer: Lessons learned from PD-1/PD-L1 immune checkpoint blockade. Cancer Immunol Res. 2022;10:146–153. doi: 10.1158/2326-6066.CIR-21-0515. [DOI] [PubMed] [Google Scholar]
- 55.Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu M, Zhang X, Gao X, Sun S, Wei X, Hu X, Huang C, Xu H, Wang B, Zhang W, et al. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer. Pharmacol Res. 2021;174:105829. doi: 10.1016/j.phrs.2021.105829. [DOI] [PubMed] [Google Scholar]
- 57.Huang X, Xu L, Ma T, Yin X, Huang Z, Ran Y, Ni Y, Bi X, Che X. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: A Retrospective Study. Front Oncol. 2021;11:751159. doi: 10.3389/fonc.2021.751159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–3329. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 60.Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128:805–815. doi: 10.1172/JCI120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, Luciani A, Zafrani ES, Laurent A, Azoulay D, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship With clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 62.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurebayashi Y, Ojima H, Tsujikawa H, Kubota N, Maehara J, Abe Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025–1041. doi: 10.1002/hep.29904. [DOI] [PubMed] [Google Scholar]
- 65.Abdou Y, Pandey M, Sarma M, Shah S, Baron J, Ernstoff MS. Mechanism-based treatment of cancer with immune checkpoint inhibitor therapies. Br J Clin Pharmacol. 2020;86:1690–1702. doi: 10.1111/bcp.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Medicine Agency, corp-author. Bevacizumab, Avastin Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf. [ June 12; 2022 ]; [Google Scholar]
- 67.European Medicine Agency, corp-author. Atezolizumab Summary of Product Characteristics. [ June 12; 2022 ]; [Google Scholar]
- 68.Roland CL, Dineen SP, Lynn KD, Sullivan LA, Dellinger MT, Sadegh L, Sullivan JP, Shames DS, Brekken RA. Inhibition of vascular endothelial growth factor reduces angiogenesis and modulates immune cell infiltration of orthotopic breast cancer xenografts. Mol Cancer Ther. 2009;8:1761–1771. doi: 10.1158/1535-7163.MCT-09-0280. [DOI] [PubMed] [Google Scholar]
- 69.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 71.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 72.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.European Medicine Agency, corp-author. Pembrolizumab Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. [ June 12; 2022 ]; [Google Scholar]
- 74.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 75.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 76.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT03298451. NIH; Bethesda, MD: 2017. [ April 19; 2021 ]. Study of Durvalumab and Tremelimumab as First-line Treatment in Patients With Advanced Hepatocellular Carcinoma (HIMALAYA) [Google Scholar]
- 78.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT02851784. NIH; Bethesda, MD: 2016. [ November 20; 2021 ]. Combination Therapy of Microwave Ablation and Cellular Immunotherapy for Hepatocellular Carcinoma. [Google Scholar]
- 79.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT02562755. NIH; Bethesda, MD: 2015. [ November 20; 2021 ]. Hepatocellular Carcinoma Study Comparing Vaccinia Virus Based Immunotherapy Plus Sorafenib vs Sorafenib Alone (PHOCUS) [Google Scholar]
- 80.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT05033522. NIH; Bethesda, MD: 2021. [ November 20; 2021 ]. FImmunotherapy for Advanced Liver Cancer (ALIVE) [Google Scholar]
- 81.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT02576509. NIH; Bethesda, MD: 2015. [ November 20; 2021 ]. An Investigational Immuno-therapy Study of Nivolumab Compared to Sorafenib as a First Treatment in Patients With Advanced Hepatocellular Carcinoma. [Google Scholar]
- 82.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT02678013. NIH; Bethesda, MD: 2016. [ November 20; 2021 ]. RRFA+Highly-purified CTL vs. RFA Alone for Recurrent HCC. [Google Scholar]
- 83.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT04167293. NIH; Bethesda, MD: 2019. [ November 20; 2021 ]. Combination of Sintilimab and Stereotactic Body Radiotherapy in Hepatocellular Carcinoma (ISBRT01) (ISBRT01) [Google Scholar]
- 84.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT03949231. NIH; Bethesda, MD: 2019. [ November 20; 2021 ]. Infusion of PD1/PDL1 Inhibitor Via Hepatic Arterial Versus Vein for Immunotherapy of Advanced Hepatocellular Carcinoma. [Google Scholar]
- 85.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT04229355. NIH; Bethesda, MD: 2020. [ November 20; 2021 ]. DEB-TACE Plus Lenvatinib or Sorafenib or PD-1 Inhibitor for Unresectable Hepatocellular Carcinoma. [Google Scholar]
- 86.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT00699816. NIH; Bethesda, MD: 2008. [ November 20; 2021 ]. Efficacy and Safety of Immuncell-LC Group and Non-treatment Group in Hepatocelluar Carcinoma Patients. [Google Scholar]
- 87.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT02709070. NIH; Bethesda, MD: 2016. [ November 20; 2021 ]. Resection+Highly Purified CTL Versus Resection Alone for HCC. [Google Scholar]
- 88.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT04268888. NIH; Bethesda, MD: 2020. [ November 20; 2021 ]. Nivolumab in Combination With TACE/TAE for Patients With Intermediate Stage HCC (TACE-3) [Google Scholar]
- 89.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT03867084. NIH; Bethesda, MD: 2019. [ November 20; 2021 ]. Safety and Efficacy of Pembrolizumab (MK-3475) Versus Placebo as Adjuvant Therapy in Participants With Hepatocellular Carcinoma (HCC) and Complete Radiological Response After Surgical Resection or Local Ablation (MK-3475-937 / KEYNOTE-937) [Google Scholar]
- 90.U.S. National Library of Medicine (NIH), corp-author ClinicalTrials.gov Identifier: NCT04682210. NIH; Bethesda, MD: 2020. [ November 20; 2021 ]. Sintilimab Plus Bevacizumab as Adjuvant Therapy in HCC Patients at High Risk of Recurrence After Curative Resection (DaDaLi) [Google Scholar]
- 91.U.S. National Library of Medicine (NIH):CIK Treatment for HCC Patient Underwent Radical Resection, corp-author. ClinicalTrials.gov Identifier NCT01749865. NIH; Bethesda, MD: 2012. [ November 20; 2021 ]. [Google Scholar]
- 92.Schwacha-Eipper B, Minciuna I, Banz V, Dufour JF. Immunotherapy as a downstaging therapy for liver transplantation. Hepatology. 2020;72:1488–1490. doi: 10.1002/hep.31234. [DOI] [PubMed] [Google Scholar]
- 93.Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021;21:1979–1980. doi: 10.1111/ajt.16448. [DOI] [PubMed] [Google Scholar]
- 94.Tanimine N, Tanaka Y, Ishiyama K, Ohira M, Shimizu S, Yano T, Ohdan H. Adoptive Immunotherapy with Liver allograft-derived NK Cells Improves Recurrence-free Survival after Living-donor Liver Transplantation in Patients with Hepatocellular Carcinoma. Am J Transplant. 2015;15((Suppl 3)):317. [Google Scholar]
- 95.Pandey A, Cohen DJ. Ipilumumab for hepatocellular cancer in a liver transplant recipient, with durable response, tolerance and without allograft rejection. Immunotherapy. 2020;12:287–292. doi: 10.2217/imt-2020-0014. [DOI] [PubMed] [Google Scholar]
- 96.Luo Y, Teng F, Fu H, Ding GS. Immunotherapy in liver transplantation for hepatocellular carcinoma: Pros and cons. World J Gastrointest Oncol. 2022;14:163–180. doi: 10.4251/wjgo.v14.i1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Jr, Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 98.Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed Death-Ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian P, He B, Mu W, Liu K, Liu L, Zeng H, Liu Y, Jiang L, Zhou P, Huang Z, et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics. 2021;11:2098–2107. doi: 10.7150/thno.48027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, Madrazo B, Kwon D, Garcia-Buitrago M, Martin P, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125:3603–3614. doi: 10.1002/cncr.32339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Li XX, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross-talk in hepatocellular carcinoma. Hepatology. 2018;67:2271–2286. doi: 10.1002/hep.29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 103.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumour immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macek Jilkova Z, Aspord C, Kurma K, Granon A, Sengel C, Sturm N, Marche PN, Decaens T. Immunologic features of patients with advanced hepatocellular carcinoma before and during sorafenib or anti-programmed death-1/programmed death-L1 treatment. Clin Transl Gastroenterol. 2019;10:e00058. doi: 10.14309/ctg.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kleinovink JW, van Hall T, Ossendorp F, Fransen MF. PD-L1 immune suppression in cancer: Tumor cells or host cells? OncoImmunology. 2017;6:e1325982. doi: 10.1080/2162402X.2017.1325982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vilain RE, Menzies AM, Wilmott JS, Kakavand H, Madore J, Guminski A, Liniker E, Kong BY, Cooper AJ, Howle JR, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res. 2017;23:5024–5033. doi: 10.1158/1078-0432.CCR-16-0698. [DOI] [PubMed] [Google Scholar]
- 107.Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153:812–826. doi: 10.1053/j.gastro.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang B, Shen L, Wang K, Jin J, Huang T, Chen Q, Li W, Wu P. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int. 2018;38:1449–1458. doi: 10.1111/liv.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding Z, Dong Z, Chen Z, Hong J, Yan L, Li H, Yao S, Yan Y, Yang Y, Yang C, Li T. Viral status and efficacy of immunotherapy in hepatocellular carcinoma: A systematic review with meta-analysis. Front Immunol. 2021;12:733530. doi: 10.3389/fimmu.2021.733530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, Jiang W, Cai S, Zhao P, Song R, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S, Moriyama M. Exosomes and hepatocellular carcinoma: From bench to bedside. Int J Mol Sci. 2019;20:1406. doi: 10.3390/ijms20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hao J, Liang C, Jiao B. Eukaryotic translation initiation factor 3, subunit C is overexpressed and promotes cell proliferation in human glioma U-87 MG cells. Oncol Lett. 2015;9:2525–2533. doi: 10.3892/ol.2015.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, Zhuang SM. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi: 10.1016/j.omtn.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci. 2018;109:2364–2374. doi: 10.1111/cas.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo J, Zhang Y, Li H, Zhang Q, Yang Y, Chen G. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine. 2019;40:432–445. doi: 10.1016/j.ebiom.2018.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ang C, Klempner SJ, Ali SM, Madison R, Ross JS, Severson EA, Fabrizio D, Goodman A, Kurzrock R, Suh J, Millis SZ. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10:4018–4025. doi: 10.18632/oncotarget.26998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen F, Wang J, Wu Y, Gao Q, Zhang S. Potential biomarkers for liver cancer diagnosis based on multi-omics strategy. Front Oncol. 2022;12:822449. doi: 10.3389/fonc.2022.822449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dominguez DA, Wang XW. Impact of next-generation sequencing on outcomes in hepatocellular carcinoma: How precise are we really? J Hepatocell Carcinoma. 2020;7:33–37. doi: 10.2147/JHC.S217948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika I, et al. Prospective genotyping of hepatocellular carcinoma: Clinical Implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JY, Kronbichler A, Eisenhut M, Hong SH, van der Vliet HJ, Kang J, Shin JI, Gamerith G. Tumour mutational burden and efficacy of immune checkpoint inhibitors: A systematic review and meta-analysis. Cancers (Basel) 2019;11:1798. doi: 10.3390/cancers11111798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.