Abstract
Li-Fraumeni syndrome (LFS) is an inherited autosomal-dominant tumor-predisposition disorder caused by germline mutations in the TP53 tumor suppressor gene. Since patients with LFS are likely to develop therapy-related cancers, radiation therapy should be avoided if breast cancer is found in these individuals. Herein, we present a case of secondary breast cancer in an LFS patient after radiation and chemotherapy for the first diagnosed breast sarcoma.
Keywords: Breast Neoplasms, Li-Fraumeni Syndrome, Sarcoma, Second Primary
Abstract
Li-Fraumeni 증후군(이하 LFS)은 TP53 종양 억제 유전자의 생식세포 돌연변이로 인한 상염색체 우성 종양 유발 유전 질환이다. LFS 환자에서는 치료 후 치료와 관련된 암이 발생하기 때문에 LFS 환자에서 발생한 유방암의 치료에 있어 방사선 요법을 피해야 한다. 저자들은 LFS 환자에서 발생한 유방 육종의 방사선 및 화학 요법 치료 후 발생한 유방암 증례를 보고한다.
INTRODUCTION
Li-Fraumeni syndrome (LFS) is an inherited autosomal-dominant tumor-predisposition disorder caused by germline mutations in the TP53 tumor suppressor gene. It is associated with the early onset of various cancers and induces a high cumulative cancer risk (1). Individuals with LFS have a high risk of developing multiple neoplasms during their lifetime, especially breast cancer (50%), soft tissue sarcomas (15%), brain tumors (6%), and osteosarcomas (5%) (1). Around 50% of breast cancer patients with LFS develop secondary cancer, of which breast cancer is the most common followed by sarcomas, then brain and lung cancers (2). For female with LFS, the cumulative breast cancer incidence by age 60 is 85%, so multimodality screening protocols should be considered for these individuals (2,3). It is important to diagnose LFS for the first time, but it is also important to reduce the risk of subsequent cancer, which can be prevented (2). Second primary malignancy means a new cancer that occurs in an individual and certain type of it may be a result of previous treatment, such as radiation and chemotherapy (4). Here in, we present a case of the second breast cancer in a 29-year-old female with LFS after radiation and chemotherapy for previously diagnosed breast sarcoma and include a review of the literature.
CASE REPORT
A 29-year-old female was referred to our institution due to suffering from a huge fast-growing mass and abnormality on outside breast ultrasonography. The results of histologic examination on outside biopsy revealed a possibility of malignant phyllodes tumor. She had a family history of breast cancer occurring in her mother and grandmother in their 40s and her sister in her 20s, but no genetic mutation in the BRCA1 and BRCA2 genes. Contrast-enhanced chest CT showed a 100 mm × 70 mm size and heterogeneous low density mass with peripheral enhancement in the right upper breast (Fig. 1A).
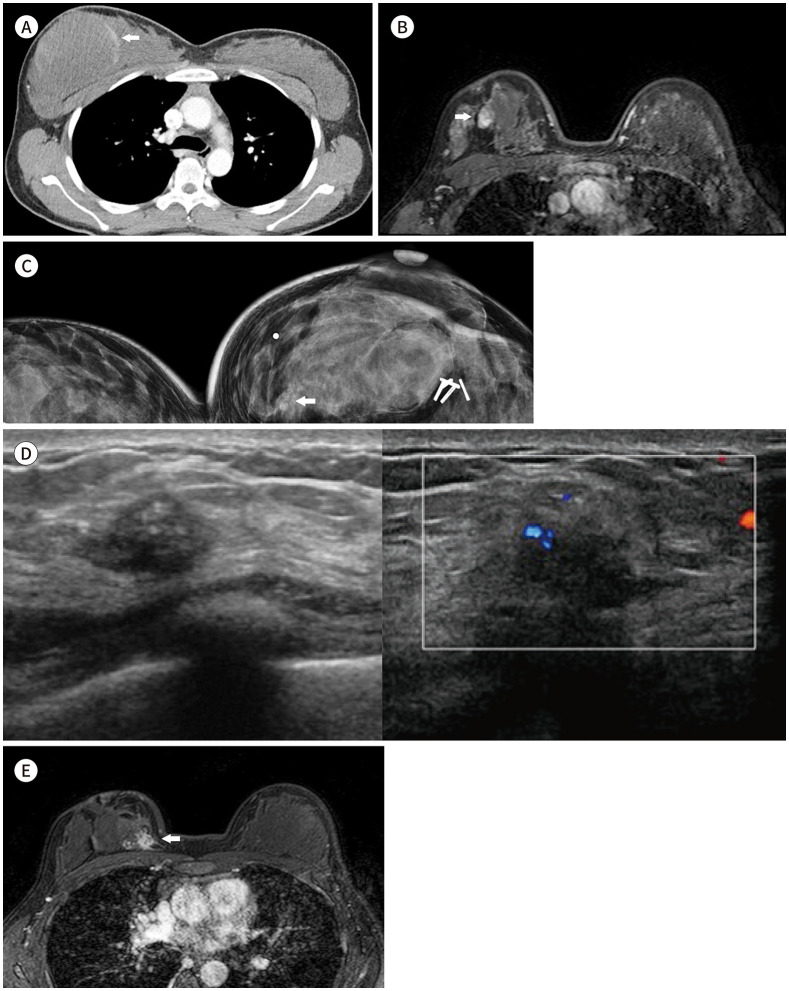
Fig. 1. A 29-year-old female with Li-Fraumeni syndrome and combined breast cancer.
A. Contrast-enhanced chest CT shows a 100 mm × 70 mm-sized heterogeneous low-density soft mass with a peripheral enhancing solid component (arrow) diagnosed as myxoid malignant fibrous histiocytoma (recently changed to pleomorphic undifferentiated sarcoma).
B. Dynamic contrast-enhanced MR with fat-suppressed T1-weighed gradient echo after wide excision of the mass shows a nearly 15-mm-sized irregularly shaped mass with heterogeneous enhancement in the right upper central breast (arrow), suggesting remnant mass.
C. 6 years after breast surgery shows focal asymmetry with grouped amorphous and pleomorphic calcifications (arrow) in the far inner portion of the right breast.
D. US image shows an irregular hypoechoic mass with internal echogenic dots corresponding to calcifications in the upper inner region of the right breast. Combined increased vascularity within the mass was demonstrated (transverse view and Doppler ultrasonography from the left side).
E. Dynamic contrast-enhanced T1-weighted fat-suppressed gradient-echo axial MR image shows a 26 mm × 18 mm-sized irregularly shaped mass with a spiculated margin and heterogeneous enhancement in the right upper inner breast (arrow).
She was treated via a wide excision. It was diagnosed with myxoid malignant fibrous histiocytoma of the right breast that has recently been classified as pleomorphic undifferentiated sarcoma. Adjuvant chemotherapy with an alkylating agent and an anthracycline was performed. However, remnants of the tumor were detected on dynamic contrast-enhanced (DCE)-MRI after surgery followed by adjuvant chemotherapy (Fig. 1B).
She then underwent re-excision, residual adjuvant chemotherapy, and radiation therapy performed using 5000 cGy in 25 fractions on the right breast followed by additional boost irradiation using 1000 cGy in 5 fractions on the right breast tumor bed for 2 months in 2013.
However, in 2018, five years later, she was diagnosed with LFS at the age of 35 in a genetic testing conducted after she learned that her sister in United States had been diagnosed with LFS. The following year, she presented with a palpable mass in the upper inner quadrant of the right breast. On cleavage mammogram, focal asymmetry with grouped amorphous and pleomorphic calcifications in the far inner portion of the right breast was noted (Fig. 1C). An irregular-shaped hypoechoic mass with internal echogenic dots in the far upper inner of the right breast was demonstrated (Fig. 1D). Invasive ductal carcinoma was confirmed via US guided core needle biopsy. The results of immunohistochemical staining showed negative for estrogen and progesterone receptors but were positive for human epidermal growth factor receptor 2 receptor and positive for Ki-67 with 27%. After biopsy, on axial DCE-MRI, a 26 mm × 18 mm-sized irregular shaped mass with spiculated margin and heterogeneous enhancement (Fig. 1E). Wash-out kinetics was also noted on DCE-MRI.
She underwent a mastectomy followed by adjuvant chemotherapy with paclitaxel plus trastuzumab.
This study was approved by the Institutional Review Board of our institution and the requirement for informed consent was waived (IRB No. EUMC 2020-03-033).
DISCUSSION
Classic LFS is defined by the presence of all of the following criteria: 1) a proband with a sarcoma diagnosed before 45 years old, 2) a first-degree relative with any form of cancer before 45 years old, and 3) a first- or second-degree relative with any form of cancer before 45 years old or a sarcoma at any age (1). Breast cancer is the most common cancer in LFS patients with early onset diagnosed before age 34 years (1). In LFS, 50% of patients develop subsequent cancers, but for female breast cancer in LFS patients, the cancer-free survival rate by age 70 was 15% (1). Most LFS-associated breast cancers are invasive ductal carcinomas with human epidermal growth factor receptor 2/neu amplification or estrogen and progesterone receptor positivity (1). It is well-known that TP53 mutations in human cancer give rise to neoplastic properties in cells (5). A germline mutation in TP53 is present in 56%–70% of families with classical LFS (5). The central role of TP53 is to block the mechanisms involved in cell cycle arrest, apoptosis, and DNA repair, particularly in response to DNA damage and other cellular stressors (1). The cumulative cancer incidence in patients with germline TP53 mutations is 50% by 31 years old among females and nearly 100% by 70 years old for females and males (2).
Only 5%–10% of breast cancer patients are associated with hereditary syndromes, and the rates of the germline BRCA1, BRCA2, and TP53 mutations in early-onset breast cancer patients are 11%, 6%, and 5%, respectively (6). However, the incidence in Asia has been poorly reported due to a lack of resources (6). LFS is such a rare genetic disorder, but the importance of its diagnosis is also closely related to the occurrence of radiation-induced secondary malignancies. Radiation-induced second primary cancer is a very rare event with an incidence of less than 2% that can develop 10 years after irradiation (7). However, breast cancer patients with LFS have a higher risk of developing radiation-induced second primary cancer and have a shorter onset time (8). Hendrickson et al. (8) reported that four of fourteen (29%) cancer patients with LFS developed radiation-induced cancer after treatment. Sarcoma is one of the common secondary malignancy occurring within radiation filed after radiation therapy. In breast, chest wall angiosarcoma and malignant histiocytofibroma have been reported, and in addition, various cancers such as papillary thyroid carcinoma have been also reported (7). While data showing the factors contributing to therapy-associated cancer incidence in patients with LFS are limited, the second primary cancer is likely to occur due to the carcinogenic effects of the radiation and/or chemotherapy agents previously used to treat malignancies (1). This is because damaged TP53 function not only affects the tumor response to radiation therapy and chemotherapy, but also increases the risk of therapy-associated malignancy (4). In a study by Boyle et al. (9), cells with or without germline TP53 mutation have been found to have a decreased capacity for eliminating or repairing radiation-induced DNA damage that occurs in LFS patients who have undergone radiation therapy; for that reason the authors concluded that LFS may be a predisposing factor for radiation-induced second primary cancer.
The recommendation for clinical monitoring of LFS in National Comprehensive Cancer Network (NCCN) was specified to detect tumors early and reduce treatment-related morbidity and mortality. According to the NCCN v.1 2021 guidelines on LFS management for breast cancer, it is recommended that ages 20–29 take MRI every year and those aged 30–75 take MRI and mammography with consideration of tomosynthesis for breast screening (10). In addition, it also states that it is recommended to consult about options for risk-reducing mastectomy and avoid therapeutic radiation therapy in breast cancer as well as other cancers (10). However, since the management of LFS is complex, it should be considered on a case-by-case in consideration of the balance between benefits and risks.
In our case, the risk of radiation was not assessed because the diagnosis of LFS was delayed. We showed imaging features of breast sarcoma and a second breast cancer arising from the breast undergone radiation treatment. Imaging findings of invasive ductal carcinoma after radiation therapy were not significantly different from those of usual breast cancer, which are commonly seen with irregularly shaped masses and suspicious calcification. However, we believe that this is the case of second primary breast cancer occurred in the radiation field six years after completion of radiation therapy. Through this case, we would like to emphasize the importance of early diagnosis of LFS, prevention and prediction of radiation-induced malignancies, and to help in establishing an appropriate screening and treatment plan.
Footnotes
- Conceptualization, Y.I.N., C.E.S.
- data curation, all authors.
- investigation, Y.I.N., C.E.S.
- formal analysis, all authors.
- project administration, Y.I.N., C.E.S.
- resources, Y.I.N., C.E.S.
- supervision, Y.I.N., C.E.S.
- visualization, all authors.
- writing—original draft, Y.I.N., C.E.S.
- writing—review & editing, all authors.
Conflicts of Interest: Eun Suk Cha has been an Editorial Board Member of Journal of the Korean Society of Radiology since 2008; however, she was not involved in the peer reviewer selection, evaluation, or decision process of this article. Otherwise, no other potential conflicts of interest relevant to this article were reported.
Funding: None
References
- 1.Valdez JM, Nichols KE, Kesserwan C. Li-Fraumeni syndrome: a paradigm for the understanding of hereditary cancer predisposition. Br J Haematol. 2017;176:539–552. doi: 10.1111/bjh.14461. [DOI] [PubMed] [Google Scholar]
- 2.Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122:3673–3681. doi: 10.1002/cncr.30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyder Z, Harkness EF, Woodward ER, Bowers NL, Pereira M, Wallace AJ, et al. Risk of contralateral breast cancer in women with and without pathogenic variants in BRCA1, BRCA2, and tp53 genes in women with very early-onset (< 36 Years) breast cancer. Cancers (Basel) 2020;12:378. doi: 10.3390/cancers12020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimatani A, Aono M, Hoshi M, Oebisu N, Iwai T, Takada N, et al. Secondary osteosarcoma in patients previously treated for childhood cancer: three case reports. Mol Clin Oncol. 2019;10:153–158. doi: 10.3892/mco.2018.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji M, Wang L, Shao Y, Cao W, Xu T, Chen S, et al. A novel dysfunctional germline P53 mutation identified in a family with Li-Fraumeni syndrome. Am J Cancer Res. 2018;8:165–169. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DS, Yoon SY, Looi LM, Kang P, Kang IN, Sivanandan K, et al. Comparable frequency of BRCA1, BRCA2 and TP53 germline mutations in a multi-ethnic Asian cohort suggests TP53 screening should be offered together with BRCA1/2 screening to early-onset breast cancer patients. Breast Cancer Res. 2012;14:R66. doi: 10.1186/bcr3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann S, Delaloge S, Rahal A, Caron O, Frebourg T, Barreau L, et al. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol. 2010;5:104. doi: 10.1186/1748-717X-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrickson PG, Luo Y, Kohlmann W, Schiffman J, Maese L, Bishop AJ, et al. Radiation therapy and secondary malignancy in Li-Fraumeni syndrome: a hereditary cancer registry study. Cancer Med. 2020;9:7954–7963. doi: 10.1002/cam4.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle JM, Spreadborough A, Greaves MJ, Birch JM, Varley JM, Scott D. The relationship between radiation-induced G1arrest and chromosome aberrations in Li-Fraumeni fibroblasts with or without germline TP53 mutations. Br J Cancer. 2001;85:293–296. doi: 10.1054/bjoc.2001.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]