Abstract
Rise in the incidences of chronic degenerative diseases with aging makes wound care a socio-economic burden and unceasingly necessitates a novel, economical, and efficient wound healing treatment. Platelets have a crucial role in hemostasis and thrombosis by modulating distinct mechanistic phases of wound healing, such as promoting and stabilizing the clot. Platelet-rich plasma (PRP) contains a high concentration of platelets than naïve plasma and has an autologous origin with no immunogenic adverse reactions. As a consequence, PRP has gained significant attention as a therapeutic to augment the healing process. Since the past few decades, a robust volume of research and clinical trials have been performed to exploit extensive role of PRP in wound healing/tissue regeneration. Despite these rigorous studies and their application in diversified medical fields, efficacy of PRP-based therapies is continuously questioned owing to the paucity of large samplesizes, controlled clinical trials, and standard protocols. This review systematically delineates the process of wound healing and involvement of platelets in tissue repair mechanisms. Additionally, emphasis is laid on PRP, its preparation methods, handling, classification,application in wound healing, and PRP as regenerative therapeutics combined with biomaterials and mesenchymal stem cells (MSCs).
Keywords: Wound healing, Platelet, Platelet-rich plasma, Growth factors, Biomaterials, Mesenchymal stem cells
Introduction
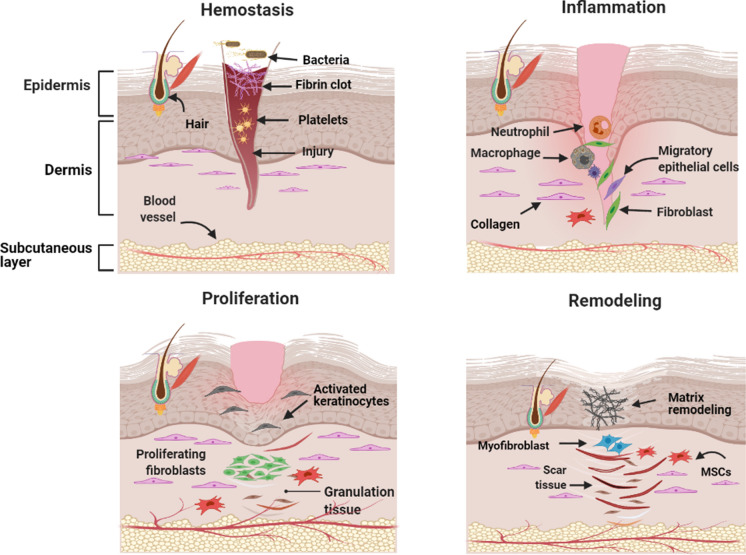
Millennia of evolution have created our skin; it is the largest organ of the human body and contributes10% of the total body mass (Theoret 2009; Hanson et al. 2010; Maxson et al. 2012). Itis a highly adaptive, multifunctional organ that serves as a shield for internal organs and protects body from the onslaught of mechanical damage, microbial infections,andother environmental extremities. Additionally, any imbalance in the skin's structural, anatomical, and functional integrity may result in wound formation. Interestingly, wound healing is a dynamic physiological process that restores typical structure and function of damaged tissue (Shaw and Martin 2009). When tissue gets damaged, wide varieties of cells, growth factors, cytokines, and chemokines underneath the skin layers coordinate to stimulate and complete different steps of wound healing cascade viz. hemostasis, inflammation, angiogenesis, epithelization, and tissue remolding (Cristina De Oliveira Gonzalez et al. 2016) (Fig. 1). According to statistical data of Medicare retrospective analysis 2014, for all wounds, including acute and chronic wounds, it was observed that approximately 8.2 million Medicare beneficiaries had at least one type of wound or related infection. Medicare budget for all wound treatments (infection management) ranged from $28.1 billion to $96.8 billion; a significant portion was contributed by surgical wounds and diabetic ulcers as they were more expensive to treat (Sen et al. 2009; Nussbaum et al. 2018; Sen 2019). In 2014, globally, the annual cost required for global wound care was estimated to be $2.8 billion, which will hike up to $3.5 billion at the end of 2021. According to a market research study, due to advancing technology, expensive wound care procedures, and increasing geriatric population, wound closure and dressing market will expand to $15 billion and $22 billion by 2022 and 2024, respectively. In progressing biomedical research era, several methods such as conventional and advanced dressing, biomaterial-based matrices, growth factors (GFs), cell-based therapies, and nanotechnology procedures are used to overcome wound healing complications (Gimble et al. 2007; Engel et al. 2008; Mason and Dunnill 2008; Negut et al. 2018). However, due to the economic burden of wound healing procedures, there is a great demand for effective, economical, and side-effect-free healing strategies.
Fig. 1.
The four phases of wound healing. It starts with hemostasis, where blood loss is prevented by platelet plug and fibrin matrix formation. The inflammation phase ensures the removal of dead cell debris and prevents further infection after neutrophil influx (stimulated by histamine release from the mast cell). Monocytes also get differentiated into macrophages to clear leftover dead cells and neutrophils around the wounded area. In the proliferative phase, various cascade culminates, such as keratinocytes migrate to seal the wound; angiogenesis starts for new blood vessel formation, and fibroblast triggers granulation tissue formation. Finally, fibroblast, blood vessels, MSCs, and myofibroblasts trigger tissue remolding, resulting in complete wound closure
Interestingly, it has been found that the regenerative potential of stem cells and platelets (especially platelet-rich plasma (PRP)) can serve as an appropriate alternate method for wound healing. Platelets are anucleated blood components, they circulate for 7–10 days in blood, critically modulating hemostasis and thrombo-inflammation (Ni and Freedman 2003; Versteeg et al. 2013). They secrete ample cytoplasmic granules, lysosomal content, microparticles, and exosomes, that play a pivotal role in regulating wound healing signalling mechanisms (Blair and Flaumenhaft 2009; De Pascale et al. 2015a; Golebiewska and Poole 2015). PRP (an autologous biological product isolated from a patient's blood)has a high platelet concentration compared to naïve plasma. According to some previous studies, PRP has a copious number of growth factors and cytokines, and these factors promote proliferation, differentiation, and migration of cells such as fibroblast, epithelial, endothelial, and mesenchymal stem cells (MSCs) and are responsible for wound healing (Blair and Flaumenhaft 2009). Moreover, they are involved inhemostasis, angiogenesis, collagen synthesis, and revascularization of the damaged tissue. Clinical studies on different research models also substantiated the efficiency and efficacy of PRP in improving wound and tissue regeneration. This compilation highlights the process of wound healing, its phases, strategies used to cure wounds and reviews the involvement of platelet, its secretome, and autologous product (PRP) as a cost-effective, easy to handle regenerative method for wound healing/tissue regeneration.
Platelets in wound healing
One microlitre of human blood contains approximately 150,000–450,000 platelets (anucleate biconvex discoid cell fragments of diameter 2–3 µm and 0.5 µm thick) in circulation (Kaushansky 2005, 2008). Due to various cellular receptors on their surface, they are the first responder to a wound/tissue repair and play a critical role in wound healing mechanism (Harrison 2005; Rivera et al. 2009; Kauskot and Hoylaerts 2012). These receptor proteins bind to von Willebrand factor (vWF), thrombin, and fibrinogen, resulting in platelet plug formation and platelet morphological transition at the injury site.
Moreover, platelets also accommodate several secretory cytoplasmic and lysosomal granules, microparticles, and exosomes,which release various factors as platelets secretome (GFs, cytokines, adhesive molecules, chemokines, and other signalling molecules)that significantlyparticipatein wound repair mechanism (Anitua et al. 2004, 2006; Pietrzak and Eppley 2005; Fitch-Tewfik and Flaumenhaft 2013; Golebiewska and Poole 2015; Heijnen and van der Sluijs 2015). These secretomes regulate diverse biochemical, molecular, and cellular aspects of wound niche, such as inflammation, recruitment of neutrophils and macrophages, promoting angiogenesis, ECM formation, and tissue remodelling (Etulain et al. 2014; Burnouf et al. 2016a; Gresele et al. 2017; Etulain 2018; Nurden 2018; Everts et al. 2020). There are three types of platelet secretome: α-granules, dense granules, and lysosomal granules, approximately 50–80 granules are present in each platelet (Lacci and Dardik 2010; Heijnen and van der Sluijs 2015; Sekhon and Sen Gupta 2018)(Pietrzak and Eppley 2005). After activation or programmed cell-death (apoptosis), platelet also shed some small evagination-mediated microparticles {platelet microparticles (PMPs)} of size 0.05–1 µm, also known as platelet dust or platelet-derived microvesicles (Varon and Shai 2015; Wojtukiewicz et al. 2017). They promote transfer of different platelet antigens viz CD41, CD61, CD62P, CXCR4, and PAR-1, to hematopoietic stem cell progenitor, and contain various proteinaceous wound healing factors like RANTES that stimulates multiple responses such as coagulation, inflammation,angiogenesis, neovascularization, and tissue regeneration (Janowska-Wieczorek et al. 2001; Ohtsuka et al. 2013). Apart from PMP, platelets also secrete exosomes by direct exocytosis; they are rich in various microRNAs and tetraspanin family of proteins. Some studies (in vitro and preclinical models) substantiate thatexosomes positively influence wound healing. However, detailed mechanism of platelet exosomes mediated wound recovery is still unclear, and it is one of the objectives of future research (Gawaz and Vogel 2013; Torreggiani et al. 2014; Rani et al. 2015; Guo et al. 2017).
Platelet rich plasma
PRP, also termed as autologous plasma, is rich in growth factors (PRGF), platelet-rich fibrin (PRF) matrix, and platelet concentrate. Haematologists introduced this concept in 1970 to describe elevated platelet level in a small amount of plasma, used initially to treat patients with thrombocytopenia (Pietrzak and Eppley 2005); (Jayadev et al. 2013). PRP has a high concentration of growth factors and cytokines that participate in various cellular, immune, and regenerative processes, such as wound healing and tissue regeneration, with sufficient tissue reparative efficacy (Currie et al. 2001; Kawase et al. 2003; Christgau et al. 2006; Banerjee et al. 2009; Lyras et al. 2010). In recent years, many controversies have arisen regarding the definition and nomenclature of PRP. Anitua and co-workers proposed definition of PRP as a vague and imprecise term because blood preparation differs in their production, resulting in variation in quantitative and qualitative characteristics of isolated PRP (Chicharro-Alcántara et al. 2018). All these complications stress the need for standard processing and preparation methods for PRP, which can compare different aspects of studies.
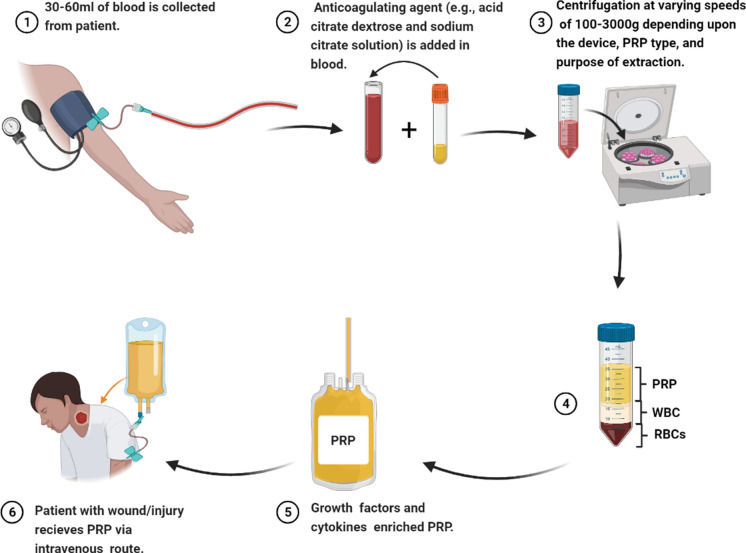
Preparation of PRP
Various systems can synthesize PRP in a reproducible manner, and its preparation procedures mainly rely on the type of device and instruction provided by the manufacturers (Table 1). Most of the devices obtain PRP with platelet concentrations higher than naïve plasma, but their platelet and leucocyte concentrations differ due to variability in isolation methods and centrifugation time. Hence, it is challenging to decide which preparatory kit is best and which is worst. All these systems generally operate on a small volume of blood and centrifugation principle. PRP is prepared through differential centrifugation, in which individual blood components are sedimented based on specific gravity (ratio of density of an object to reference’s density) (Dhurat and Sukesh 2014). The procedure of PRP preparation usually includes three sequential steps. The blood of an individual is collected by venipuncture in a tube with an adequate anticoagulating agent (e.g., acid citrate dextrose and sodium citrate solution). It is followed by centrifugation at varying speeds of 100-3000 g, depending upon the device, PRP type, and purpose of extraction. After centrifugation, blood sample separates into three layers: bottom layer (RBC with leucocytes deposited immediately above), middle layer (contains PRP), and top layer (contains platelet-poor plasma (PPP) (Fig. 2). Usually, two centrifugation spins are applied; the first spin (hard spin) separates PRP from RBCs, while the second spin (soft spin) separates PRP from platelet-poor plasma (PPP) (Mishra et al. 2009; Dohan Ehrenfest et al. 2009; Lyras et al. 2010). Inducers of aggregation like bovine thrombin and 10% of calcium chloride are used for PRP's activation to stimulate degranulation, further releasing GFs. These activators increase platelet concentration up 3–5 times within 15 min as compared to native plasma (Mishra et al. 2009; Everts et al. 2012; Dhurat and Sukesh 2014; De Pascale et al. 2015b; Burnouf et al. 2016b).
Table 1.
| S. No | Device | Company | Blood Collected | Anti-coagulant | Platelet Activator | Description |
|---|---|---|---|---|---|---|
| 1 | Angel® | Arthrex, Inc. Corporate Naples, Florida, USA | Syringe 40–180 mL | Acid citrate dextrose | 10% (v/v) calcium gluconate | It is a closed technique, using software and programmed centrifugation to isolate PRP with a wide range of platelet concentrations from 3 to 18X compared to naïve plasma |
| 2 | Cascade® or Selphyl® | Musculoskeletal Transplant Foundation, Edison, New Jersey, USA | Tube 9 mL | Sodium citrate | CaCl2 | Pellet of activated PRP is prepared after centrifuging 18 ml of blood (9 ml in each tube) at 1100 g for 10 min |
| 4 | C-Punt® | Biomed Device, Modena MO, Italy | Syringe 60 mL | Sodium citrate | CaCl2 | A volume of 9 ml of PRP was harvested after centrifugation at 1200 rpm for 10 min |
| 5 | i-Stem® Preparation System | i-Stem, Biostems, Co., LTD., Seoul, Korea | Tube 20 mL | Sodium citrate | CaCl2 | After first centrifuging at 3000 rpm for 6 min, 1 ml of PPP and 2 ml of RBCs are removed. Suspension is again centrifuged at 3000 rpm for 3 min to obtain 15 ml of A-PRP |
| 6 | MAG-18® | DTS MG Co., Ltd., Seoul, Korea | Tube 19 mL | Sodium citrate | CaCl2 | The sample is collected and centrifuged twice, firstly at 3000 rpm for 6 min and secondly at 3400 rpm for 2 min to harvest 1.5 mL of PRP |
| 8 | Regenlab® | EnBudron b2, 1052 Le Mont-sur-Lausanne, Swiss | 40 mL | Sodium citrate | Thrombin | Blood is collected in five ATS (autologous thrombin serum) Regen tubes (8 mL each). All tubes are centrifuged at 1500 g for 15 min at room temperature using the universal centrifuge (Regen Lab PRP-Centrig) |
| 9 | PRGF Endoret® | Biotechnology Institute (BTI) | Tube 9 mL | sodium citrate | CaCl2 | Sample is centrifuged at 270 g for 7 min |
Fig. 2.
Preparation of PRP for treatment
After successful extraction of PRP from patient's blood, it is either used or stored. For storage of PRP, some scientists claim that in circulating blood PRP could not be preserved beyond 6 h of blood collection, while others observed that additives solution might enhance their stability, viability and enable their storage up to 7 h (Sweeney et al. 2006; Etulain 2018). Moreover, frozen PRP can be stored for longer. In some studies, frozen PRP was stockpiled with a 3-D scaffold (Lee and Blajchman 2001; Li et al. 2017). According to Shinga and co-workers, level of growth factors present in PRP reduce after 2 weeks of storage at room temperature. Whereas, in freeze-dried PRP, a baseline level of growth factors is maintained up to 8 weeks of storage. Therefore, PRP's freeze-dried form can be stored for an extended period with bioactivity and efficacy, a prerequisite for PRP's multiple applications in the same patient (Shiga et al. 2017).
Classification of PRP
Depending upon different parameters and their clinical applications, PRP is categorized into four distinct groups: activated PRP, non-activated PRP, leucocyte rich, and leucocyte poor. Activated PRP is prepared with the aid of CaCl2 and with or without use of thrombin. They stimulate cytokine release from plateletgranules, while non-activated PRP synthesis includes platelet contact with intrinsic collagen and thrombin. The presence of leukocytes in PRP impedes bacterial growth and enhances soft tissue injury repair. In 2016, Magalon and co-workers postulated a DEAP classification of PRP based on dose of injected platelets, production efficiency, PRP activation, and PRP purity (Magalon et al. 2016). Moreover, some studies categorized PRP; based onmethods used in their preparation (centrifugation and anti-coagulation), content, and composition of platelets, leucocytes, growth factors, and medical applications.
Ehrenfest and his colleagueproposed another way to classify PRP based on the presence and absence of leucocytes and fibrin.
The purest form of PRP: After activation, theyhave a low concentration of fibrin.
Leucocytes and PRP: This composition contains leucocytes with a low density of fibrin.
Pure-platelet-rich fibrin: They have a high density of fibrin network, but leucocytes are more or less absent.
Leucocyte and platelet-rich fibrin: This preparation has a high leucocyte concentration anda high fibrin network density.
3Growth factors present in PRP
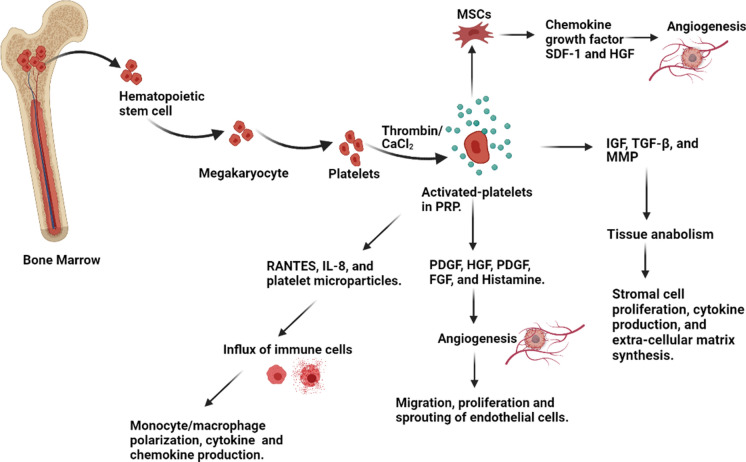
PRP has a significant role in hemostasis, innate immunity, angiogenesis, stem cell migration, proliferation, and wound healing (Andia and Abate 2013; Shin et al. 2014; Anitua et al. 2016; Suthar et al. 2017a; Guszczyn et al. 2017). It contains many growth factors, cytokines, and chemokines, as they stimulate downstream signaling pathways required to synthesize proteins necessary for collagen, osteoid, and extracellular matrix formation(Jee et al. 2016) (Brissett and Hom 2003a) (Table 2). Platelets are a reservoir of more than 800 proteins,interacting with stem cells, fibroblast, endothelial and epithelial cells. PRP is a natural source of many growth factors (PDGF, IGF, VEGF, TGF-β), primarily stored in platelets α-granules (Anitua et al. 2004). The activating agents or stimuli like thrombin, CaCl2, and collagen could trigger release of these growth factors (Fig. 3), which are further involved in crucial stages of wound healing and regenerative processes like chemotaxis, proliferation, differentiation, and angiogenesis (Bennett and Schultz 1993). In addition to these growth factors, PRP also contains some adhesive molecules such as fibronectin, vitronectin, fibrinogen, and sphingosine-1-phosphate. These are also essential for completing wound healing and bone formation process (Fernández-Barbero et al. 2006).
Table 2.
Growth factors regulating wound healing process
| S. No | Growth factor | Origin | Function | Future prospective |
|---|---|---|---|---|
| 1 | Platelet-derived growth factor (PDGF) | Platelets, macrophages, endothelial cells, keratinocytes, and muscle cells | It is the first growth factor that is secreted just after injury and regulates various cellular reactions throughout wound healing | The application of PDGF onsets wound healing. Therefore harnessing the effective use of PDGF in wound care increases its economic uses. The chemotaxis nature of PDGF would also be helpful in treating rare diseases |
| Promotes synthesis of TGF-β and IGF-1 | ||||
| It stimulates collagen synthesis and chemotaxis of macrophages and neutrophils | ||||
| 4. Increases hair growth. (Steed 2006; Graham et al. 2009; Takikawa et al. 2011; Shah et al. 2014) | ||||
| 2 | Epidermal growth factor (EGF) | Mainly secreted by platelets and cells like macrophages, fibroblast,and MSCs | Involved in proliferation, migration, and differentiation of epithelial cells and keratinocytes | The application of EGF in wound enhances proliferation of healthy cells, facilitating wound repair |
| Promotes angiogenesis | ||||
| Stimulates hair cell proliferation and regeneration | ||||
| It also triggers epithelization of burn and granulation of wound. (Kim et al. 2010; Makki et al. 2013; Nanba et al. 2013; Lin et al. 2015; Takabayashi et al. 2016; Chicharro-Alcántara et al. 2018) | ||||
| 3 | Transforming growth factor-β (TGF-β) | Macrophages, T-lymphocytes, and keratinocytes | There are three isoforms of TGF-β; TGF-β1, -β2, -β3, having overlapping but unique function in wound healing | The application of TGF-β stimulates key processes of wound healing such as angiogenesis, fibroblast proliferation, collagen synthesis and thus accelerate wound healing |
| TGF-β1 promotes angiogenesis, | ||||
| TGF-β2 and β3 are linked with scarring and fibrosis. They enhance fibroblast and myofibroblast differentiation, extracellular matrix deposition, wound contraction, and scar formation | ||||
| TGF-β triggers proliferation of undifferentiated MSCs, regulates mitogenesis of endothelial, fibroblast, and osteoblasts | ||||
| Inhibits proliferation (of macrophages and lymphocytes) and metalloproteins activity | ||||
| Regulates synthesis of collagen and secretion of collagenase. (Enoch et al. 2006; Le et al. 2012; Kofler and Simons 2016; Lamora et al. 2016; Etulain 2018) | ||||
| 4 | Vascular endothelial growth factor (VEGF) | Platelets, keratinocytes, macrophages, and fibroblasts | It has a robust paracrine influence on endothelial cells and also promotes angiogenesis of wounds | The VEGF promotes angiogenesis, which ensures a constant supply of oxygen at the site of injury. For instance, a decrease in the supply of oxygen drastically reduces the healing potential of the wound |
| VEGF is a regulator of processes, such as vasculogenesis, lymphangiogenesis, and vascular permeability. (Werner and Grose 2003; Hsu and Chang 2004; Tammela et al. 2005; Hanft et al. 2008; Karayannopoulou et al. 2014; Shibuya 2015; Ferrara and Adamis 2016) | ||||
| 5 | Fibroblast growth factor-2 (FGF-2) | Platelets, macrophages, mesenchymal cells, chondrocytes, and osteoblasts | Involved in re-epithelization, angiogenesis, and granulation tissue formation | Previous reports has suggested that FGF-2 is effective in comorbidity such as diabetic cases in which wound healing processes get slow or decline |
| Indirectly stimulates the release of TGF-α | ||||
| Promotes fibroblast proliferation, collagen accumulation and accelerates granulation tissue formation. (Enoch et al. 2006; Niu et al. 2007; Xie et al. 2008; Shi et al. 2013; Shah et al. 2014; Maddaluno et al. 2017, Koike et al. 2020) | ||||
| 6 | Insulin-like growth factor (IGF-1) | Platelets, plasma, epithelial cells, endothelial cells, fibroblasts, osteoblasts, and bone matrix | Mainly involved in inflammatory and proliferative phase of wound healing | Among all, IGF-1 significantly promotes wound healing as well as self-renewal and differentiation of cells. For a constant supply of new cells in a dysfunctional tissues, the role of IGF-1 becomes necessary |
| In combination with other growth factors like PDGF and EGF, it can exert a strong synergistic effect and promotes keratinocyte migration and tissue repair. (Reckenbeil et al. 2017; Yu et al. 2007) | ||||
| 7 | Hepatocyte growth factor (HGF) | Platelets and mesenchymal cells | Regulates cell growth, mortality, and morphogenesis in epithelial and endothelial cells | There is a direct effect of HGF on liver and kidney. HGF helps in the activation of VEGF and stimulates angiogenesis |
| Directly involved in epithelial repair, granulation tissue formation, and neovascularization | ||||
| In combination with VEGF, it exerts a robust cooperative effect that enhances angiogenesis at the injury site. (Conway et al. 2006; Anitua et al. 2005) | ||||
| 8 | Keratinocyte growth factor-2 (KGF-2), also described as fibroblast growth factor-10 (FGF-10) | Fibroblasts and MSCs | It is involved in remolding phase of wound healing: it induces migration and proliferation of keratinocytes | The fouth phase of wound healing lasts for 61 days to 2 years. In long run, the role of KGF-2 is very significant for proper healing of damage tissues |
| Increases the proliferation of epithelial cells. (Enoch et al. 2006; Everts et al. 2020; Jimenez et al. 1999) | ||||
| 9 | Transforming growth factor (TNF) | Macrophages, mast cells, and T-lymphocytes | Regulates monocyte migration, fibroblast, proliferation, macrophage activation, and angiogenesis. (Giusti et al. 2020) | Transforming growth factor (TNF) activates macrophages which kill the microorganism and drastically reduce further infection |
Fig. 3.
Mechanistic role of PRP in tissue repair
PRP as a regenerative therapeutic agent
Since year 1990, platelets derived products are efficiently been used in sub-fields of regenerative medicine. Over the past few years, PRP-based treatments are continuously in the limelightto curemultiple clinical challenges such as wound healing, skin and bone regeneration, ophthalmology, ulcer, burn, muscle repair, and others (De Pascale et al. 2015b; Burnouf et al. 2016b; Gresele et al. 2017). PRP influencesbone, tendon, and cartilage regeneration by modulating MSCsproliferation, chondrogenic differentiation, bone cell proliferation, and differentiation. It mobilizes circulating cells for tendon healing, matrix biosynthesis, and angiogenesis in acute tissue injury (Kajikawa et al. 2008; Lin et al. 2013; Kreuz et al. 2015). Reportedly, PRP is predominantly utilized in dermatology, especially in tissue regeneration, wound healing, acute and chronic ulcers, due to their impact on mitogenesis, angiogenesis, chemotaxis, type-I collagen synthesis, and proliferation and migration of keratinocytes, dermal fibroblast cells, and endothelial cells (Shin et al. 2014; Anitua et al. 2016; Guszczyn et al. 2017).
Application of PRP in wound healing
Advancing medical field is trying to trailblaze less invasive and cost-effective treatments (Lacci and Dardik 2010; Yung et al. 2017). Over the last few decades, PRP-based treatment had a potent impact in reducing economic cost of standard medical treatment and served as a potential competitor for replacing conventional therapies. PRP-based therapies supplement wound sites with a high concentration of GFs, cytokines, and chemokines, which play a crucial role in tissue repair (Glover 1992; Brissett and Hom 2003b; Jee et al. 2016). These factors also regulate inflammation, angiogenesis, synthesis of extracellular matrix, and newly formed tissue remodeling. An increased concentration of GFs also stimulates regeneration of epithelial and endothelial cells and synthesized collagen. For the first time, the PRP-based treatment method was used to treat chronic leg ulcers, which successfully resulted in vascularized connective tissue formation (Andia and Abate 2013; Suthar et al. 2017a). Besides humans, clinical studies have suggested that PRP-based treatment enhances wound healing in dogs, horses, and other animals (Carter et al. 2003; Kimura et al. 2005; Lee et al. 2008; Sardari et al. 2011; CH et al. 2016; Suthar et al. 2017b). In chronic diseases such as diabetic ulcers, excess reactive oxygen species (ROS) are generated, resulting in an imbalance between pro-inflammatory and anti-inflammatory cytokines. PRP contains a high concentration of GFs and cytokines that maintain ROS levels and reduces wound recovery time (Lacci and Dardik 2010). Several studies have been performed to observe clinical effects of PRP-based therapies, majority of them showed a significant reduction in wound size without side-effects. Subcutaneous PRP administration in patients suffering from nonhealing ulcers demonstrated decreased wound size, pain, and inflammation. According to Babaei et al., after topical PRP application in 150 patients diagnosed with a diabetic foot ulcer, a significant granulation tissue formation, and early wound closure was observed (Babaei et al. 2017). In a study by Man et al., a quantitative improvement in human skin wound healing was also reported after using a cutaneous flap with autologous PRP (Man et al. 2001). Even AIDS patients suffering from crural ulcers showed increased neovascularization and re-epithelization after PRP and platelet application to achieve faster wound healing than other conventional methods (Cieslik-Bielecka et al. 2018).
The second intension wound (SIH) occurs when a significant tissue loss and edges cannot be brought together by granulation, contraction, and epithelization. It can be affected by a wide variety of factors, such as inadequate blood supply, previous infection, and systemic disease that result in imperfect wound healing (Schreml et al. 2010; Zaman Phull et al. 2018). For efficient closure, proper regulation of granulation tissue formation, angiogenesis, collagen synthesis, and epithelization is highly needed. Karayannopoulou et al. evaluated the effect of intra-lesional PRP administrationon SIH involving acute full-thickness skin defect in dogs. They observed improved tissue perfusion that uplifted granulation tissue formation and attracted nutrients and oxygen towards the wound, simultaneously accelerating collagen formation and wound healing process (Karayannopoulou et al. 2015). A full-thickness wound was treated with PRP in a study by Ostvar and co-workers on rabbits and other small research models. The PRP application increased vascular density, angiogenesis, granulation tissue formation, and healing rate compared to thestandard method (Ostvar et al. 2015). Some studies have also mentioned PRP's synergistic effect combined with bone-morrow-derived mesenchymal stem cells (BM-MSCs) (Lian et al. 2014). This combination offers a suitable microenvironment for proliferation and differentiation for facilitated wound healing. In a study by Park et al., when wounded mice were treated with a combination of PRP and hydrogel, a marked improvement in wound healing was observed in comparison to control and PRP/hydrogel (Park et al. 2017).
Platelet inspired biomaterials
As previously discussed, platelet and their secretory molecules are used in wound healing procedures. But during their administration, various constraints have been observed, like prominent risk of contamination, low viability, and portability (Spinella et al. 2012, 2016; Lambert et al. 2013; Shin et al. 2014; Miron et al. 2017). These shortcomings limit the efficiency and efficacy of platelet-based therapies. Several biomaterial and nanotechnology procedures are considered to develop synthetic and non-synthetic platelet mimics to overcome these limitations. For designing platelet mimics, nanotechnology approaches are used in which polymer nanoparticles of polylactic acid (PLA) and poly-N-isopropyl acrylamide-co-acrylic acid (pNIPAm-AAc) are coated with fibrinogen (Fg) or Fg-mimetic Arginine-Glycine-Aspartic Acid (RGD) peptides. Also, surface of liposome was decorated with fibrinogen γ-chain dodecapeptide. These synthetical designs reduce bleeding in various injury models and are used in platelet-inspired drug delivery (Coller 1980; Takeoka et al. 2001, 2003; Bertram et al. 2009; Ravikumar et al. 2012; Modery-Pawlowski et al. 2013a, b; Anselmo et al. 2014; Brown et al. 2014; Shukla et al. 2017).
In some cases, these synthetic platelets are also loaded with some anti-infective agents and GFs (PDGF, VEGF, and others); they are released in spatio-temporally controlled manner during hemostasis to stimulate post-hemostasis wound healing mechanism. Even fibrin-coagulated PGFM, bFGF, and PDGF are incorporated in gelatine hydrogel; they collectively promote tissue and blood regeneration (Matsui and Tabata 2012; Leotot et al. 2013; Santo et al. 2015; Mittermayr et al. 2016; Robinson et al. 2016). Furthermore, synthetic platelets integrated biomaterial matrix system was also used to develop multi-component technology, affecting different aspects of wound healing and assisting direct loading and delivery of platelet-relevant biomolecules. Some studies have demonstrated that recombinant FGF in combination with collagen sponge system reduces recovery and wound closure time. Tuneable hydrogel has been shown to regulate delivery of various cell secretory GFs and cytokines (e.g., interferon-γ and IL-4), resulting in macrophages transition to promotetissue regeneration (Yao et al. 2006; Spiller et al. 2015; Skardal et al. 2017). Even PEG-fibrin gel also secretes some muscle cell markers, for instance, α-smooth muscle actin, PDGF-β, NG2 proteoglycan, and angiopoietin-1 that assist the development of vascular structure in a wound area. For sustained degradation, release, and activation, PRP was encapsulated within enzyme-degradable hydrogel matrices to modulate wound healing mechanism. These combinations of polymer, synthetic platelets, and nanotechnology systems are administered via topical, intracavitary, or intravascular passage to interact with bleeding site and damaged tissue directly. These systems release several GFs and biomolecules to enhance wound healing (Zamora et al. 2013).
Synergism of PRP and MSCs in wound healing
Treatment of chronic and nonhealing wounds is a tedious and challenging task for the health sector as it involves replacing cutaneous lesions with new regenerative skin. Also, pre-existing strategies (bioengineered dressings and cell therapies) were not optimal for chronic wound treatment as these wounds persist as an unmet medical need. Wound care products should have a similar composition to normal skin, which constitutes a proper amalgamation of GFs, extracellular proteins, MSCs, fibroblast, and endothelial cells. Even presence of endogenous MSCs in skin and their involvement in various phases of wound healing substantiate the application of exogenous MSCs combined with other tissue repair therapies (Paquet-Fifield et al. 2009; Sellheyer and Krahl 2010). In the inflammatory phase, these cells prevent deleterious effects of inflammatory cytokines (TNF and IFN-γ) and secrete several antimicrobials factors to facilitate wound clearance via stimulating phagocytosis through immune cell and promote transition from inflammatory to proliferative phase in a chronic wound, otherwise which is hindered due to high level of inflammation (Robson et al. 2001; Ramasastry 2005; Velnar et al. 2009). Like PRP, MSCs also secrete numerous soluble factors (VEGF and SDF-1), growth factors and cytokines, micro-vesicles/exosomes with cytoprotective, proangiogenic, and anti-inflammatory properties. MSCs associated secretomes are adopted well in their niche, and their paracrine effect lasts for a more extended period post-engraftment (Yong et al. 2018)(Yiou et al. 2016). During proliferative and remodeling phase, MSCs release GFs like VEGF, bFGF, and KGF; they promote granulation, neovascularization, tissue epithelization, ECM organization, and mobilization of stem cells at the wound site (Clark 1993, 2001; Tonnesen et al. 2000; Mulder and Vande Berg 2002; Baum and Arpey 2006; Koellensperger et al. 2014; Marfia et al. 2015). A considerable number of completed clinical trials are available, which validate safety and efficacy of MSCs in stimulating regeneration of damaged tissues, including the skin. Based on genetic modification, pharmacological pre-conditioning, and use with biomaterial, robust studies have been performed to ameliorate viability, retention, and functionality of MSCs, but these approaches are pretty expensive and non-feasible to translate in humans (Sheykhhasan et al. 2015; Li et al. 2016; Frese et al. 2016).
Barbara Hersant and her colleague performed a studyto evaluaterole of a combination of MSCs and PRP in wound healing. According to their observations, this amalgamation is more efficient in promoting vascularization, proangiogenic potential, and tissue regeneration in wound as compared to individual treatment of PRP and MSCs (Hersant et al. 2019). After PRP treatment, MSCs secrete VEGF and SDF-1 in higher concentration, resulting in more significant vessel formation and endothelial cell migration. Moreover, PRP also serves as a clinical-grade adjuvant to elevate therapeutic efficacy of engrafted MSCs and increase its adaptability, paracrine effect, retention, and persistence at wound site and shields MSCs from oxidative damage (as cytoprotectant) by increasing oxygen consumption and ATP-linked respiration (Badiavas and Falanga 2003; Falanga et al. 2007; Yoshikawa et al. 2008; Dash et al. 2009; Lu et al. 2011; Martínez et al. 2016; Chen and Liao 2018; Samberg et al. 2019). Still, there is a scope for exploring different mechanisms that affect regenerative properties of MSCs to develop a more efficient protocol for tissue repair and other degenerative diseases.
Merit and demerits of using PRP based therapies
The prime advantage of using platelets as a regenerative agent in wound healing/tissue regeneration is that they can be prepared instantly and do not require any advanced preparation facilities. They are safe and natural due to their direct extraction from a patient's blood, and this method even demolishes probability of any adverse immune response and blood-born contaminations (Lyras et al. 2010) (Bianco et al. 2008).
There are as such no such demerits of platelet-based treatments. However, infection site morbidity, infection, and blood vessel injury were reported in some cases due to formation of tissue scars and calcification at injection site. Platelet and its secretomes and autologous PRPare generally injected intravenously; sometimes, it might damage arteries and veins, resulting in blood coagulation. Patients with ahistory of platelet dysfunctions syndromes, thrombocytopenia, hyper-fibrogenemia, hemodynamic instability, chronic heart disease, and cancerexperienceseveral complications during platelet-based treatment (Bianco et al. 2008).
Cost effectiveness of PRP and comparision with standard treatment
Through Meta-analysis using the Markov Mode, the cost of PRP in skin ulcers was calculated. The comparative result showed that the probability of healing was 56% using PRP and 31% with standard treatment. The associated costs were €5224 and €5133 respectively. The major benefit of PRP treatment is associated with reducing the average length of hospital stay which compensates for the normal cost of treatment. The incremental cost to achieve additional healing was €364, within a 48-week time of treatment. In an another comparative study, the cost of PRP treatment in 81 patients with ulcers demonstrated that the average length of stay with PRP was (11 ± 2.5 days) and cost € 785.25, whereas the standard treatment average length of stay in the hospital was 23.1 ± 1.5 days with cost € 1649.02. The overall study demonstrated that PRP therapy would significantly reduce the length of hospital stay and directly becomes economic. The major reason for the slightly high cost of PRP treatment is the procedure of PRP preparation. Scientists are exploring new approaches to reduce the cost of preparation of PRP so that a significant reduction in cost can be observed (Oliveira et al., 2020).
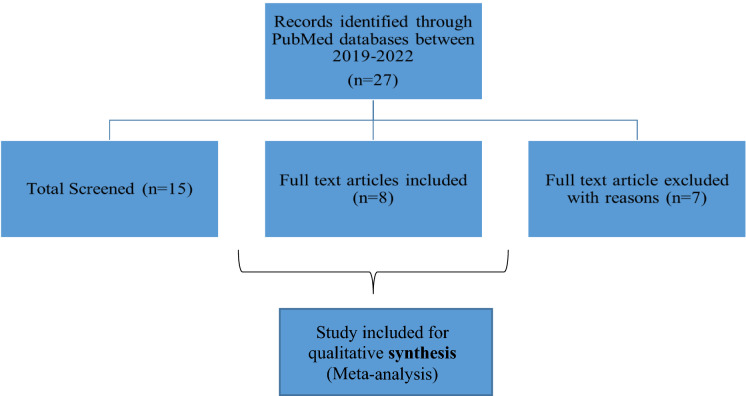
Meta-analysis for cost effectiveness of PRP therapy
The cost-effective comparison was done through meta-analysis (Fig. 4). A total of 27 published papers were found with the search term (Economic cost effectiveness PRP) term from the Pubmed database. Papers were screed, out of 27 papers, 7 paper was considered for analysis because the data and study were more relevant to our study. In one study, Linertová et.al observed that PRP treatment with the manual method was more effective and less costly compared to PRP treatment with the commercial kit and standard methods (Linertová et.al. 2021). The cost of PRP treatment with the manual method was significantly less but not as effective as PRP treatment with a commercial kit. According to Alcerro et al. (2019) when PRP was compared with Stem cells Therapy (SCT), the mean cost of PRP injection was $897, and for SCT injection, it was $3,100. It was also observed that about 36% of people preferred PRP whereas 24.5% accepted SCT. The cost-effectiveness of PRP therapy and hyaluronic acids (HA) was compared by Samuelson et al., it was observed that PRP injection was more effective and less economical as compared to HA (Samuelson et al. 2020). Randomized controlled clinical trials (RCTs) indicate that autologous PRP was associated with an increase in hair density when compared to placebo but the economic cost-effective measurement was not done (Dervishi et al. 2020). Bendich et al. also conducted RCTs for PRP, HA, and Saline groups, the result showed that the lowest total cost for HA and saline were $681.93 and $516.29 respectively. For PRP to be cost-effective, total treatment cost would have to be less than $3,703.03 and $1,192.08 for 6-month and 12-month outcomes respectively (Bendich et al. 2020). When PRP gel and gas dressing was compared by Uçar et al. 2020, the result showed PRP gel had a positive effect on the healing of stage II pressure ulcers with PRP gel dressings. In addition, when evaluated in the long term, it was concluded that PRP gel is easily accessible and less costly than serum physiological dressing (Uçar et al. 2020). The overall analysis from different papers suggested that the PRP is therapeutically effective but more research are required to minimize the cost. Many studies have suggested that PRP treatment was cost-effective but need more clarity.
Fig. 4.
Meta-analysis of cost effectiveness of PRP therapy
Future prospects and market value
The total market valuation of PRP therapy has reached US$ 370.78 million in 2021. The data also demonstrated that the market is projected to expand at a steady 6% compound annual growth rate (CAGR) through 2031. According to Future Market Insights (FMI) analysis on PRP, growth prospects will remain positive because of gaining traction in diverse medical procedures such as orthopaedic and neurological surgeries. In recent years, the application of PRP in regenerative therapies and surgical procedures has created prospects for intensive medical research. There was significant use of PRP therapy observed in COVID-19. The growth of PRP in the year 2021 was 8.8% [https://www.futuremarketinsights.com/reports/platelet-rich-plasma-market].
Conclusion
In wound healing, several intracellular, intercellular, and extracellular signaling mechanisms regulate distinct phases of healing. Some studies substantiate significant involvement of platelets and their related products such as PMPs and exosomes in wound healing phases. Due to these characteristics, PRP is continuously explored for its role in wound healing/tissue regeneration as they have higher platelet concentrations (Lacci and Dardik 2010; De Pascale et al. 2015b). Apart from natural platelets, robust research has been done to develop platelets' bio-mimics. An amalgamation of PRP-based components with synthetic biomaterials was also used to designs several biohydride systems (Oryan et al. 2016) (Salamanna et al. 2015). These approaches enhance wound healing, prompt site-specific delivery, and even regulate loaded drugs' release patterns. However, wide variability in preparation, composition, and concentration of these platelet products makes standardized correlation a tedious task. In recent years, various clinical trials have been performed to evaluate the significance of platelet-based products; they showed several beneficial results in clinical conditions with minimal sideeffects. However, their efficacy as regenerative medicine is still in its infancy owing to a lack of accepted standard preparation protocol. For illustration, PRP-based therapy has shown its salutary role in many health complications, but their significance is continuously undermined. Metanalysis of clinical trials also showed a disparity in the results of these trials, which might be due to variation in preparation, activation, and administration procedure. Therefore, it is imperative to understand the mechanism of PRP in regeneration, step-wise preparation, long-term side effects, and anti-aggregating drug effects on PRP-based treatment. There is dearth of data that could substantiate long-term outcome of cutaneous wound healing with PRP application. Therefore, controlled studies with a significant sample size are highly needed to validate PRP's efficacy as regenerative medicine to treat wound healing. Despite these complications and controversies in PRP-based approaches, recent clinical trials show promising results of PRP application in dermatology, dentistry, ophthalmology, orthopedics, and other fields. Further insights can be made after completion of phase 3 and phase 4 trials. Therefore, PRP can serve as a potentform of therapeutic, solo, or in combination with other regenerative approaches for wound healing and tissue regeneration. Due to economic sufficiency, these therapies also have the potential to replace conventional treatments. Thus, it is essential to collect more consensus data obtained from various clinicaltrials and standardize application of its formulation as a potent regenerative therapy for wound healing/tissue regeneration.
Acknowledgements
We thank Director, INMAS, DRDO, for his continuous support. Images are created with BioRender.com.
Abbreviations
- PRP
Platelet-rich plasma
- MSCs
Mesenchymal stem cells
- GFs
Growth factors
- PDGF
Platelet-derived growth factor
- EGF
Epidermal growth factor
- FGF
Fibroblast growth factor
- IGF
Insulin-like growth factor
- VEGF
Vascular endothelial growth factor
- TGF-β
Transforming growth factor-beta
- KGF
Keratinocyte growth factor
- HGF
Hepatocyte growth factor
- TNF
Transforming growth factor
- PEG
Poly (ethylene glycol)
- CD
Cluster of differentiation
- RANTES
Regulated on activation, normal T expressed, and secreted
- PMPs
Platelet microparticles
- PRF
Platelet-rich fibrin
- RBCs
Red blood cells
- WBC
White blood cells
- PPP
Platelet-poor plasma
- DEAP
Dose of injected platelets, production efficiency, activation
- ROS
Reactive oxygen species
- PLA
Polylactic acid
- RGD
Arginine-glycine-aspartic acid
- SDF
Stromal cell-derived factor-1
- IFN-γ
Interferon-gamma
- MMPs
Matrix metalloproteinases
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alcerro JC, Lavernia CJ. Stem cells and platelet-rich plasma for knee osteoarthritis: prevalence and cost in South Florida. JAAOS-J Am Acad Orthopaed Surg. 2019;27(20):779–783. doi: 10.5435/JAAOS-D-18-00343. [DOI] [PubMed] [Google Scholar]
- Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Ski Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013;8:645–658. doi: 10.2217/rme.13.59. [DOI] [PubMed] [Google Scholar]
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- Anitua E, Andía I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–286. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Anitua E, Sánchez M, Nurden AT, et al. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–234. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Anitua E, Pino A, Orive G. Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care. 2016;25:680–687. doi: 10.12968/jowc.2016.25.11.680. [DOI] [PubMed] [Google Scholar]
- Anselmo AC, Lynn Modery-Pawlowski C, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8:11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei V, Afradi H, Gohardani HZ, et al. Management of chronic diabetic foot ulcers using platelet-rich plasma. J Wound Care. 2017;26:784–787. doi: 10.12968/jowc.2017.26.12.784. [DOI] [PubMed] [Google Scholar]
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Intwala AR, Baudino TA. IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. Am J Physiol - Hear Circ Physiol. 2009 doi: 10.1152/ajpheart.00908.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2006;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- Bendich I, Rubenstein WJ, Cole BJ, Ma CB, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from level I randomized controlled trials. Arthrosc J Arthrosc Relat Surg. 2020;36(7):1983–1991. doi: 10.1016/j.arthro.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/S0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- Bertram JP, Williams CA, Robinson R, et al. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med. 2009 doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair P, Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissett AE, Hom DB. The effects of tissue sealants, platelet gels, and growth factors on wound healing. Curr Opin Otolaryngol Head Neck Surg. 2003;11:245–250. doi: 10.1097/00020840-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Brown AC, Stabenfeldt SE, Ahn B, et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater. 2014;13:1108–1114. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- Carter CA, Jolly DG, Worden CE, et al. Platelet-rich plasma gel promotes differentiation and regeneration during equine wound healing. Exp Mol Pathol. 2003;74:244–255. doi: 10.1016/S0014-4800(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Ch J, Ny E, Hm J, et al. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J Vet Sci. 2016 doi: 10.4142/JVS.2016.17.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Liao HT. Platelet-rich plasma enhances adipose-derived stem cellmediated angiogenesis in a mouse ischemic hindlimb model. World J Stem Cells. 2018;10:212–227. doi: 10.4252/wjsc.v10.i12.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018 doi: 10.3390/jfb9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgau M, Moder D, Hiller KA, et al. Growth factors and cytokines in autologous platelet concentrate and their correlation to periodontal regeneration outcomes. J Clin Periodontol. 2006;33:837–845. doi: 10.1111/j.1600-051X.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- Cieslik-Bielecka A, Skowroński R, Jędrusik-Pawłowska M, Pierchała M. The application of L-PRP in AIDS patients with crural chronic ulcers: a pilot study. Adv Med Sci. 2018;63:140–146. doi: 10.1016/j.advms.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci. 1993;306:42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Clark RAF. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14:2–10. doi: 10.1111/j.1743-6109.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359–366. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- De Oliveira C, Gonzalez A, Fortuna Costa T, De Z, et al. Wound healing-a literature review. An Bras Dermatol. 2016;91:614–634. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascale MR, Sommese L, Casamassimi A, Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015;29:52–61. doi: 10.1016/j.tmrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Dervishi G, Liu H, Peternel S, et al. Autologous platelet-rich plasma therapy for pattern hair loss: a systematic review. J Cosmet Dermatol. 2020;19(4):827–835. doi: 10.1111/jocd.13113. [DOI] [PubMed] [Google Scholar]
- Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author′s perspective. J Cutan Aesthet Surg. 2014;7:189. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Engel E, Michiardi A, Navarro M, et al. Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol. 2008;26:39–47. doi: 10.1016/j.tibtech.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ. 2006;332:962–965. doi: 10.1136/bmj.332.7547.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–568. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- Etulain J, Negrotto S, Schattner M. Role of platelets in angiogenesis in health and disease. Curr Angiogenes. 2014;3:48–57. doi: 10.2174/2211552802666140404002756. [DOI] [Google Scholar]
- Everts PAM, Hoogbergen MM, Weber AT, et al. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol. 2012;13:1163–1172. doi: 10.2174/138920112800624346. [DOI] [PubMed] [Google Scholar]
- Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:1–36. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- Fernández-Barbero JE, Galindo-Moreno P, Ávila-Ortiz G, et al. Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin Oral Implants Res. 2006;17:687–693. doi: 10.1111/j.1600-0501.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- Fitch-Tewfik JL, Flaumenhaft R. Platelet granule exocytosis: a comparison with chromaffin cells. Front Endocrinol (Lausanne) 2013 doi: 10.3389/fendo.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother. 2016;43:268–274. doi: 10.1159/000448180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–2554. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- Gentile P, Bottini DJ, Spallone D, et al. Application of platelet-rich plasma in maxillofacial surgery: clinical evaluation. J Craniofac Surg. 2010;21:900–904. doi: 10.1097/SCS.0b013e3181d878e9. [DOI] [PubMed] [Google Scholar]
- Gentile P, Cole JP, Cole MA, et al. Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017 doi: 10.3390/ijms18020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile P, Calabrese C, De Angelis B, et al. Impact of the different preparation methods to obtain autologous non-activated platelet-rich plasma (A-PRP) and activated platelet-rich plasma (AA-PRP) in plastic surgery: wound healing and hair regrowth evaluation. Int J Mol Sci. 2020 doi: 10.3390/ijms21020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti I, D’Ascenzo S, MacChiarelli G, Dolo V. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020;18:117–129. doi: 10.2450/2019.0164-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JL. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg. 1992;16:124–125. doi: 10.1016/0741-5214(92)90430-G. [DOI] [PubMed] [Google Scholar]
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S, Leonidou A, Lester M, et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18:1633–1654. doi: 10.1517/13543780903241607. [DOI] [PubMed] [Google Scholar]
- Gresele P, Lopez JA, Kleiman NS, Page CP. Platelets in thrombotic and non-thrombotic disorders: pathophysiology, pharmacology and therapeutics: an update. Cham: Springer; 2017. [Google Scholar]
- Guo SC, Tao SC, Yin WJ, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guszczyn T, Surażyński A, Zaręba I, et al. Differential effect of platelet-rich plasma fractions on β1-integrin signaling, collagen biosynthesis, and prolidase activity in human skin fibroblasts. Drug Des Devel Ther. 2017;11:1849–1857. doi: 10.2147/DDDT.S135949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft JR, Pollak RA, Barbul A, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care. 2008;17:1–30. doi: 10.12968/jowc.2008.17.1.27917. [DOI] [PubMed] [Google Scholar]
- Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125:510–516. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. Platelet function analysis. Blood Rev. 2005;19:111–123. doi: 10.1016/j.blre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015;13:2141–2151. doi: 10.1111/jth.13147. [DOI] [PubMed] [Google Scholar]
- Hersant B, Sid-Ahmed M, Braud L, et al. Platelet-rich plasma improves the wound healing potential of mesenchymal stem cells through paracrine and metabolism alterations. Stem Cells Int. 2019 doi: 10.1155/2019/1234263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29:551–563. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Majka M, Kijowski J, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.V98.10.3143. [DOI] [PubMed] [Google Scholar]
- Jayadev M, Vr M, Naik B, Karunakar P. Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent. 2013;16:284. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee CH, Eom NY, Jang HM, et al. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J Vet Sci. 2016;17:79–87. doi: 10.4142/jvs.2016.17.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez PA, Rampy MA. Keratinocyte growth factor-2 accelerates wound healing in incisional wounds. J Surg Res. 1999;81(2):238–242. doi: 10.1006/jsre.1998.5501. [DOI] [PubMed] [Google Scholar]
- Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215:837–845. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- Karayannopoulou M, Papazoglou LG, Loukopoulos P, et al. Locally injected autologous platelet-rich plasma enhanced tissue perfusion and improved survival of long subdermal plexus skin flaps in dogs. Vet Comp Orthop Traumatol. 2014;27:379–386. doi: 10.3415/VCOT-14-02-0030. [DOI] [PubMed] [Google Scholar]
- Karayannopoulou M, Psalla D, Kazakos G, et al. Effect of locally injected autologous platelet-rich plasma on second intention wound healing of acute full-thickness skin defects in dogs. Vet Comp Orthop Traumatol. 2015;28:172–178. doi: 10.3415/VCOT-14-06-0088. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–986. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauskot A, Hoylaerts MF. Platelet receptors. Handb Exp Pharmacol. 2012;210:23–57. doi: 10.1007/978-3-642-29423-5_2. [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74:858–864. doi: 10.1902/jop.2003.74.6.858. [DOI] [PubMed] [Google Scholar]
- Kim YS, Lew DH, Tark KC, et al. Effect of recombinant human epidermal growth factor against cutaneous scar formation in murine full-thickness wound healing. J Korean Med Sci. 2010;25:589–596. doi: 10.3346/jkms.2010.25.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Ogata H, Yazawa M, et al. The effects of platelet-rich plasma on cutaneous incisional wound healing in rats. J Dermatol Sci. 2005;40:205–208. doi: 10.1016/j.jdermsci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Koellensperger E, Lampe K, Beierfuss A, et al. Intracutaneously injected human adipose tissue-derived stem cells in a mouse model stay at the site of injection. J Plast Reconstr Aesthetic Surg. 2014;67:844–850. doi: 10.1016/j.bjps.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Kofler N, Simons M. The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature. Curr Opin Hematol. 2016;23:260–267. doi: 10.1097/MOH.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike Y, Yozaki M, Utani A, Murota H. Fibroblast growth factor 2 accelerates the epithelial–mesenchymal transition in keratinocytes during wound healing process. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-75584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz PC, Krüger JP, Metzlaff S, et al. Platelet-rich plasma preparation types show impact on chondrogenic differentiation, migration, and proliferation of human subchondral mesenchymal progenitor cells. Arthrosc J Arthrosc Relat Surg. 2015;31:1951–1961. doi: 10.1016/j.arthro.2015.03.033. [DOI] [PubMed] [Google Scholar]
- Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83:1–9. [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Sullivan SK, Fuentes R, et al. Challenges and promises for the development of donor-independent platelet transfusions. Blood. 2013;121:3319–3324. doi: 10.1182/blood-2012-09-455428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamora A, Talbot J, Mullard M, et al. TGF-β signaling in bone remodeling and osteosarcoma progression. J Clin Med. 2016;5:96. doi: 10.3390/jcm5110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le M, Naridze R, Morrison J, et al. Transforming growth factor beta 3 is required for excisional wound repair in vivo. PLoS ONE. 2012;7:e48040. doi: 10.1371/journal.pone.0048040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Blajchman MA. Novel treatment modalities: new platelet preparations and subsititutes. Br J Haematol. 2001;114:496–505. doi: 10.1046/j.1365-2141.2001.03004.x. [DOI] [PubMed] [Google Scholar]
- Lee H-W, Reddy MS, Geurs N, et al. Efficacy of platelet-rich plasma on wound healing in rabbits. J Periodontol. 2008;79:691–696. doi: 10.1902/jop.2008.070449. [DOI] [PubMed] [Google Scholar]
- Leotot J, Coquelin L, Bodivit G, et al. Platelet lysate coating on scaffolds directly and indirectly enhances cell migration, improving bone and blood vessel formation. Acta Biomater. 2013;9:6630–6640. doi: 10.1016/j.actbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Li J, Chen M, Wei X, et al. Evaluation of 3D-printed polycaprolactone scaffolds coated with freeze-dried platelet-rich plasma for bone regeneration. Materials (basel) 2017 doi: 10.3390/ma10070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Z, Yin X, Li H, et al. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol. 2014;26:1–10. doi: 10.5021/ad.2014.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BN, Whu SW, Chen CH, et al. Bone marrow mesenchymal stem cells, platelet-rich plasma and nanohydroxyapatite-type I collagen beads were integral parts of biomimetic bone substitutes for bone regeneration. J Tissue Eng Regen Med. 2013;7:841–854. doi: 10.1002/term.1472. [DOI] [PubMed] [Google Scholar]
- Lin WH, Xiang LJ, Shi HX, et al. Fibroblast growth factors stimulate hair growth through β -Catenin and shh expression in C57BL/6 mice. Biomed Res Int. 2015 doi: 10.1155/2015/730139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linertová R, del Pino-Sedeño T, et al. Cost-effectiveness of platelet-rich plasma for diabetic foot ulcer in Spain. Int J Lower Extrem Wounds. 2021;20(2):119–127. doi: 10.1177/1534734620903239. [DOI] [PubMed] [Google Scholar]
- Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Lyras DN, Kazakos K, Agrogiannis G, et al. Experimental study of tendon healing early phase: Is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res. 2010;96:381–387. doi: 10.1016/j.otsr.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Dev. 2017;144:4047–4060. doi: 10.1242/dev.152587. [DOI] [PubMed] [Google Scholar]
- Magalon J, Chateau AL, Bertrand B, et al. DEPA classification: a proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016;2:e000060. doi: 10.1136/bmjsem-2015-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki N, Thiel KW, Miller FJ. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci. 2013;14:20597–20613. doi: 10.3390/ijms141020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–236. doi: 10.1097/00006534-200101000-00037. [DOI] [PubMed] [Google Scholar]
- Marfia G, Navone SE, Di Vito C, et al. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis. 2015;11:183–206. doi: 10.1080/15476278.2015.1126018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez CE, González SA, Palma V, Smith PC. Platelet-poor and platelet-rich plasma stimulate bone lineage differentiation in periodontal ligament stem cells. J Periodontol. 2016;87:e18–e26. doi: 10.1902/jop.2015.150360. [DOI] [PubMed] [Google Scholar]
- Mason C, Dunnill P. A brief definition of regenerative medicine. Regen Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- Matsui M, Tabata Y. Enhanced angiogenesis by multiple release of platelet-rich plasma contents and basic fibroblast growth factor from gelatin hydrogels. Acta Biomater. 2012;8:1792–1801. doi: 10.1016/j.actbio.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron RJ, Fujioka-Kobayashi M, Bishara M, et al. Platelet-rich fibrin and soft tissue wound healing: a systematic review. Tissue Eng - Part B Rev. 2017;23:83–99. doi: 10.1089/ten.teb.2016.0233. [DOI] [PubMed] [Google Scholar]
- Mishra A, Woodall J, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Mittermayr R, Slezak P, Haffner N, et al. Controlled release of fibrin matrix-conjugated platelet derived growth factor improves ischemic tissue regeneration by functional angiogenesis. Acta Biomater. 2016;29:11–20. doi: 10.1016/j.actbio.2015.10.028. [DOI] [PubMed] [Google Scholar]
- Modery-Pawlowski CL, Tian LL, Pan V, et al. Approaches to synthetic platelet analogs. Biomaterials. 2013;34:526–541. doi: 10.1016/j.biomaterials.2012.09.074. [DOI] [PubMed] [Google Scholar]
- Modery-Pawlowski CL, Tian LL, Ravikumar M, et al. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34:3031–3041. doi: 10.1016/j.biomaterials.2012.12.045. [DOI] [PubMed] [Google Scholar]
- Mulder GD, Vande Berg JS. Cellular senescence and matrix metalloproteinase activity in chronic wounds: relevance to debridement and new technologies. J Am Podiatr Med Assoc. 2002;92:34–37. doi: 10.7547/87507315-92-1-34. [DOI] [PubMed] [Google Scholar]
- Nanba D, Toki F, Barrandon Y, Higashiyama S. Recent advances in the epidermal growth factor receptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation. J Dermatol Sci. 2013;72:81–86. doi: 10.1016/j.jdermsci.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23:2392. doi: 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci. 2003;28:257–264. doi: 10.1016/S1473-0502(03)00044-2. [DOI] [PubMed] [Google Scholar]
- Niu J, Chang Z, Peng B, et al. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-κB transcription factors. J Biol Chem. 2007;282:6001–6011. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
- Nurden AT. The biology of the platelet with special reference to inflammation, wound healing and immunity. Front Biosci - Landmark. 2018;23:726–751. doi: 10.2741/4613. [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Heal. 2018;21:27–32. doi: 10.1016/j.jval.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Ohtsuka M, Sasaki K, Ueno T, et al. Platelet-derived microparticles augment the adhesion and neovascularization capacities of circulating angiogenic cells obtained from atherosclerotic patients. Atherosclerosis. 2013;227:275–282. doi: 10.1016/j.atherosclerosis.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016;16:213–232. doi: 10.1517/14712598.2016.1118458. [DOI] [PubMed] [Google Scholar]
- Ostvar O, Shadvar S, Yahaghi E, et al. RETRACTED ARTICLE: effect of platelet-rich plasma on the healing of cutaneous defects exposed to acute to chronic wounds: a clinico-histopathologic study in rabbits. Diagn Pathol. 2015 doi: 10.1186/s13000-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Paquet-Fifield S, Schlüter H, Li A, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YG, Lee IH, Park ES, Kim JY. Hydrogel and platelet-rich plasma combined treatment to accelerate wound healing in a nude mouse model. Arch Plast Surg. 2017;44:194–201. doi: 10.5999/aps.2017.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull QZ, et al. Wound healing effects of bentonite: a rabbit model experimental study. Biomed J Sci Tech Res. 2018 doi: 10.26717/BJSTR.2018.10.001921. [DOI] [Google Scholar]
- Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16:1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- Ramasastry SS. Acute wounds. Clin Plast Surg. 2005;32:195–208. doi: 10.1016/j.cps.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar M, Modery CL, Wong TL, et al. Mimicking adhesive functionalities of blood platelets using ligand-decorated liposomes. Bioconjug Chem. 2012;23:1266–1275. doi: 10.1021/bc300086d. [DOI] [PubMed] [Google Scholar]
- Reckenbeil J, Kraus D, Stark H, et al. Insulin-like growth factor 1 (IGF1) affects proliferation and differentiation and wound healing processes in an inflammatory environment with p38 controlling early osteoblast differentiation in periodontal ligament cells. Arch Oral Biol. 2017;73:142–150. doi: 10.1016/j.archoralbio.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Rivera J, Lozano ML, Navarro-Núñez L, Vicente García V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ST, Douglas AM, Chadid T, et al. A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater. 2016;36:86–98. doi: 10.1016/j.actbio.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:A1. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- Samberg M, Stone R, Natesan S, et al. Platelet rich plasma hydrogels promote in vitro and in vivo angiogenic potential of adipose-derived stem cells. Acta Biomater. 2019;87:76–87. doi: 10.1016/j.actbio.2019.01.039. [DOI] [PubMed] [Google Scholar]
- Samuelson EM, Ebel JA, et al. The cost-effectiveness of platelet-rich plasma compared with hyaluronic acid injections for the treatment of knee osteoarthritis. Arthrosc J Arthrosc Relat Surg. 2020;36(12):3072–3078. doi: 10.1016/j.arthro.2020.07.027. [DOI] [PubMed] [Google Scholar]
- Santo VE, Popa EG, Mano JF, et al. Natural assembly of platelet lysate-loaded nanocarriers into enriched 3D hydrogels for cartilage regeneration. Acta Biomater. 2015;19:56–65. doi: 10.1016/j.actbio.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Sardari K, Emami MR, Kazemi H, et al. Effects of platelet-rich plasma (PRP) on cutaneous regeneration and wound healing in dogs treated with dexamethasone. Comp Clin Path. 2011;20:155–162. doi: 10.1007/s00580-010-0972-y. [DOI] [Google Scholar]
- Schreml S, Szeimies RM, Prantl L, et al. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63:866–881. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Sekhon UDS, Sen Gupta A. Platelets and platelet-inspired biomaterials technologies in wound healing applications. ACS Biomater Sci Eng. 2018;4:1176–1192. doi: 10.1021/acsbiomaterials.7b00013. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Krahl D. Cutaneous mesenchymal stem cells: status of current knowledge, implications for dermatopathology. J Cutan Pathol. 2010;37:624–634. doi: 10.1111/j.1600-0560.2009.01477.x. [DOI] [PubMed] [Google Scholar]
- Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care. 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy: perspective article. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Keppler L, Rutkowski J. A review of platelet derived growth factor playing pivotal role in bone regeneration. J Oral Implantol. 2014;40:330–340. doi: 10.1563/AAID-JOI-D-11-00173. [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheykhhasan M, Qomi RT, Kalhor N, et al. Evaluation of the ability of natural and synthetic scaffolds in providing an appropriate environment for growth and chondrogenic differentiation of adipose-derived mesenchymal stem cel. Indian J Orthop. 2015;49:561–568. doi: 10.4103/0019-5413.164043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shi H-X, Lin C, Lin B-B, et al. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE. 2013;8:e59966. doi: 10.1371/journal.pone.0059966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. VEGF-VEGFR system as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Targets. 2015;15:135–144. doi: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Kubota G, Orita S, et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J. 2017;11:329–336. doi: 10.4184/asj.2017.11.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Lee JW, Il KY, et al. The effects of platelet-rich clot releasate on the expression of MMP-1 and type I collagen in human adult dermal fibroblasts: PRP is a stronger MMP-1 stimulator. Mol Biol Rep. 2014;41:3–8. doi: 10.1007/s11033-013-2718-9. [DOI] [PubMed] [Google Scholar]
- Shukla M, Sekhon UDS, Betapudi V, et al. In vitro characterization of SynthoPlate™ (synthetic platelet) technology and its in vivo evaluation in severely thrombocytopenic mice. J Thromb Haemost. 2017;15:375–387. doi: 10.1111/jth.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal A, Murphy SV, Crowell K, et al. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res - Part B Appl Biomater. 2017;105:1986–2000. doi: 10.1002/jbm.b.33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Nassiri S, Witherel CE, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella PC, Dunne J, Beilman GJ, et al. Constant challenges and evolution of US military transfusion medicine and blood operations in combat. Transfusion. 2012;52:1146–1153. doi: 10.1111/j.1537-2995.2012.03594.x. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Pidcoke HF, Strandenes G, et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion. 2016;56:S190–S202. doi: 10.1111/trf.13491. [DOI] [PubMed] [Google Scholar]
- Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143–149. doi: 10.1097/01.prs.0000222526.21512.4c. [DOI] [PubMed] [Google Scholar]
- Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017 doi: 10.1186/s12929-017-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney J, Kouttab N, Holme S, et al. Storage of platelet-rich plasma-derived platelet concentrate pools in plasma and additive solution. Transfusion. 2006;46:835–840. doi: 10.1111/j.1537-2995.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- Takabayashi Y, Nambu M, Ishihara M, et al. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin Cosmet Investig Dermatol. 2016;9:127–134. doi: 10.2147/CCID.S108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka S, Teramura Y, Okamura Y, et al. Fibrinogen-conjugated albumin polymers and their interaction with platelets under flow conditions. Biomacromol. 2001;2:1192–1197. doi: 10.1021/bm015554o. [DOI] [PubMed] [Google Scholar]
- Takeoka S, Okamura Y, Teramura Y, et al. Function of fibrinogen γ-chain dodecapeptide-conjugated latex beads under flow. Biochem Biophys Res Commun. 2003;312:773–779. doi: 10.1016/j.bbrc.2003.10.184. [DOI] [PubMed] [Google Scholar]
- Takikawa M, Nakamura S, Nakamura S, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721–1729. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Theoret C. Tissue engineering in wound repair: the three “r”s - repair, replace, regenerate. Vet Surg. 2009;38:905–913. doi: 10.1111/j.1532-950X.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Feng X, Clark RAF. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Torreggiani E, Perut F, Roncuzzi L, et al. Exosomes: novel effectors of human platelet lysate activity. Eur Cells Mater. 2014;28:137–151. doi: 10.22203/eCM.v028a11. [DOI] [PubMed] [Google Scholar]
- Uçar Ö, Çelik S. Comparison of platelet-rich plasma gel in the care of the pressure ulcers with the dressing with serum physiology in terms of healing process and dressing costs. Int Wound J. 2020;17(3):831–841. doi: 10.1111/iwj.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon D, Shai E. Platelets and their microparticles as key players in pathophysiological responses. J Thromb Haemost. 2015;13:S40–S46. doi: 10.1111/jth.12976. [DOI] [PubMed] [Google Scholar]
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New Fundamentals in Hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wojtukiewicz MZ, Sierko E, Hempel D, et al. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36:249–262. doi: 10.1007/s10555-017-9673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Bian H, Qi S, et al. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin Exp Dermatol. 2008;33:176–182. doi: 10.1111/j.1365-2230.2007.02573.x. [DOI] [PubMed] [Google Scholar]
- Yao C, Yao P, Wu H, Zha Z. Acceleration of wound healing in traumatic ulcers by absorbable collagen sponge containing recombinant basic fibroblast growth factor. Biomed Mater. 2006;1:33–37. doi: 10.1088/1748-6041/1/1/005. [DOI] [PubMed] [Google Scholar]
- Yiou R, Mahrouf-Yorgov M, Trébeau C, et al. Delivery of human mesenchymal adipose-derived stem cells restores multiple urological dysfunctions in a rat model mimicking radical prostatectomy damages through tissue-specific paracrine mechanisms. Stem Cells. 2016;34:392–404. doi: 10.1002/stem.2226. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- Yu DH, Mace KA, Hansen SL, et al. Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1-α protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen. 2007;15:628–635. doi: 10.1111/j.1524-475X.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Yung YL, Fu SC, Cheuk YC, et al. Optimisation of platelet concentrates therapy: composition, localisation, and duration of action. Asia-Pac J Sport Med Arthrosc Rehabil Technol. 2017;7:27–36. doi: 10.1016/j.asmart.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora DO, Natesan S, Becerra S, et al. Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis. 2013;16:745–757. doi: 10.1007/s10456-013-9352-y. [DOI] [PubMed] [Google Scholar]
- Patents Assigned to MOSAIC BIOSCIENCES, INC. - Justia Patents Search. https://patents.justia.com/assignee/mosaic-biosciences-inc. Accessed 22 Feb 2021d
- Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization - PubMed. https://pubmed.ncbi.nlm.nih.gov/25785127/. Accessed 21 Jan 2021b [PMC free article] [PubMed]
- Coller BS (1980) Interaction of normal, thrombasthenic, and Bernard-Soulier platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia - PubMed. https://pubmed.ncbi.nlm.nih.gov/6766334/.Accessed 22 Feb 2021 [PubMed]
- Li L, Chen X, Wang WE, Zeng C (2016) How to improve the survival of transplanted mesenchymal stem cell in ischemic heart?. Stem Cells Int 2016 [DOI] [PMC free article] [PubMed]
- Oliveira BGRBD, Carvalho MRD and Ribeiro APL (2020) Cost and effectiveness of platelet rich plasma in the healing of varicose ulcer: meta-analysis. Revista Brasileira de Enfermagem, 73 https://www.futuremarketinsights.com/reports/platelet-rich-plasma-market [DOI] [PubMed]
- Salamanna F, Veronesi F, Maglio M, et al (2015) New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int [DOI] [PMC free article] [PubMed]
- TGF-beta-induced interleukin-6 participates in transdifferentiation of human Tenon’s fibroblasts to myofibroblasts - PubMed. https://pubmed.ncbi.nlm.nih.gov/19862334/. Accessed 21 Jan 2021a [PMC free article] [PubMed]
- Transfusion of blood and blood products: indications and complications - PubMed. https://pubmed.ncbi.nlm.nih.gov/21404983/. Accessed 22 Feb 2021c
- US9988433B2 - Covalent modification of biological macromolecules - Google Patents. https://patents.google.com/patent/US9988433B2/en. Accessed 22 Feb 2021e
- Yong KW, Choi JR, Mohammadi M, et al (2018) Mesenchymal stem cell therapy for ischemic tissues. Stem Cells Int [DOI] [PMC free article] [PubMed]