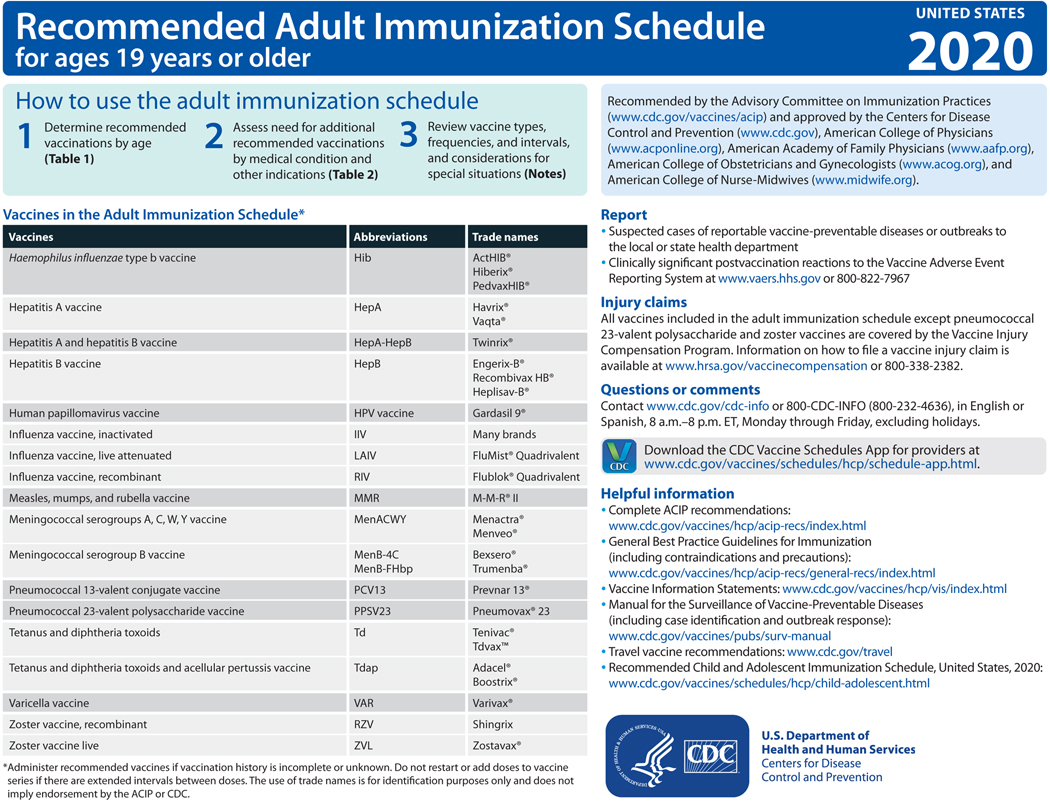
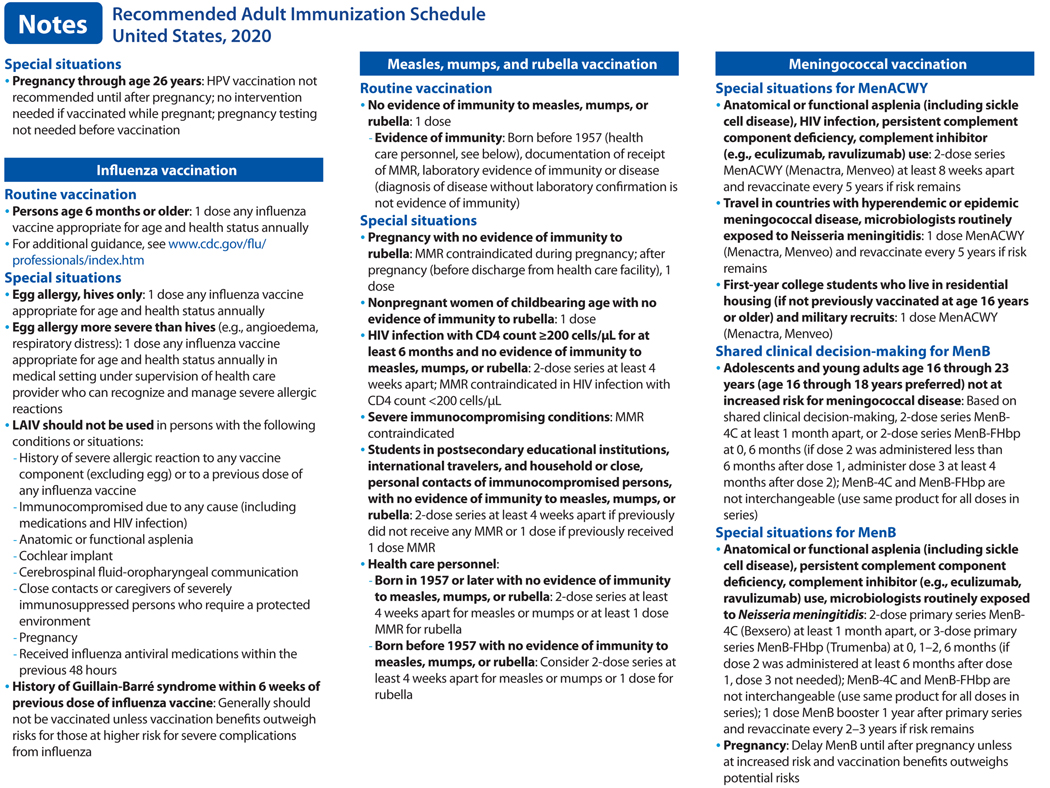
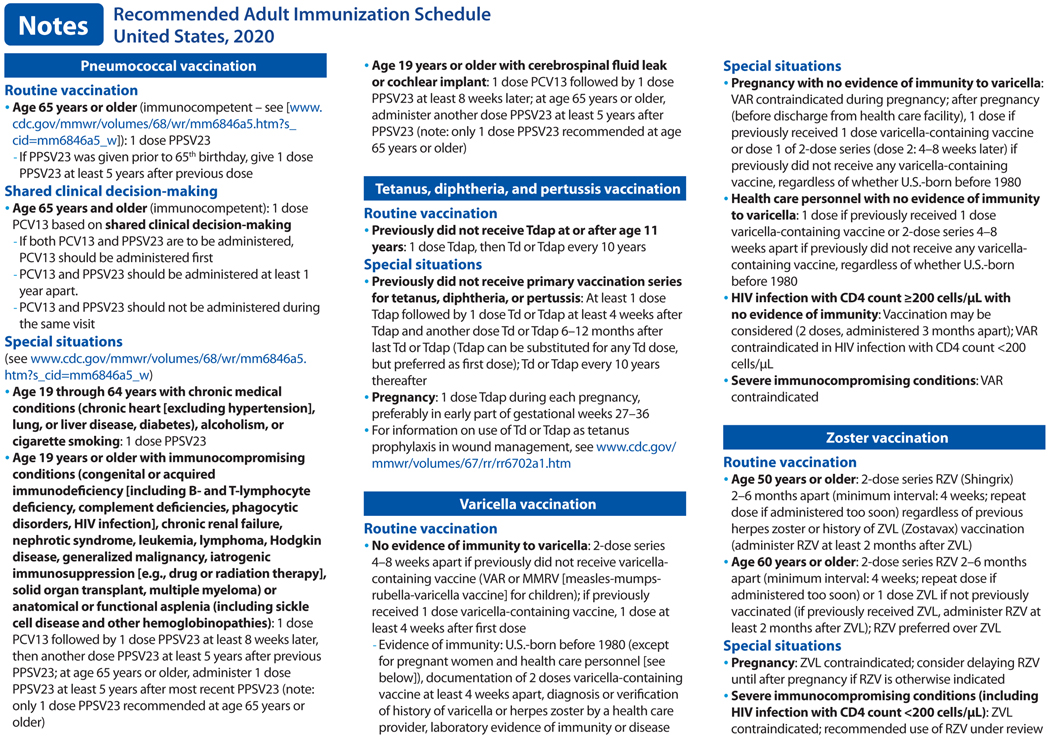
In October 2019, the Advisory Committee on Immunization Practices (ACIP) voted to approve the Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2020. The 2020 adult immunization schedule, available at https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html, summarizes ACIP recommendations in two tables and accompanying notes (Figure). The full ACIP recommendations for each vaccine are available at https://www.cdc.gov/vaccines/hcp/acip-recs/index.html. The 2020 schedule has also been approved by the director of the Centers for Disease Control and Prevention (CDC) and by the American College of Physicians (https://www.acponline.org), the American Academy of Family Physicians (https://www.aafp.org), the American College of Obstetricians and Gynecologists (http://www.acog.org), and the American College of Nurse-Midwives (https://www.midwife.org).
Figure.
Recommended Adult Immunization Schedule, United States, 2020
The ACIP develops recommendations on the use of each vaccine after in-depth review of vaccine-related data, such as the epidemiology and burden of the vaccine-preventable disease, vaccine efficacy and effectiveness, vaccine safety, the quality of evidence, feasibility of program implementation, and economic analyses of immunization policy (1). ACIP recommendations can be complex and challenging to implement. The purpose of the schedule, published annually, is to consolidate and summarize updates to ACIP recommendations on vaccination of adults and assist providers in implementing current ACIP recommendations. The use of vaccine trade names in this article and in the schedule is for identification purposes only and does not imply endorsement by the ACIP or CDC.
Changes to the 2020 Adult Immunization Schedule
Influenza vaccination (2).
Updates to the recommendations reflect discussions during public meetings of ACIP held on October 25, 2018, February 27, 2019, and June 27, 2019. For the 2019–2020 flu season, routine annual influenza vaccination is recommended for all persons age 6 months and older who do not have contraindications. No preferential recommendation is made for one influenza vaccine product over another for persons for whom more than one licensed, recommended, and appropriate product is available. LAIV is an option for adults through age 49 years, except for those who have immunocompromising conditions, including HIV infection; have anatomical or functional asplenia; are pregnant; have close contact with or are caregivers of severely immunocompromised persons in a protected environment; have received influenza antiviral medications in the previous 48 hours; or have cerebrospinal fluid leak or a cochlear implant. Those with a history of Guillain-Barré syndrome within 6 weeks of a previous dose of influenza vaccine generally should not be vaccinated, unless vaccination benefits outweigh risks for those at higher risk for severe complications from influenza.
Hepatitis A vaccination (3).
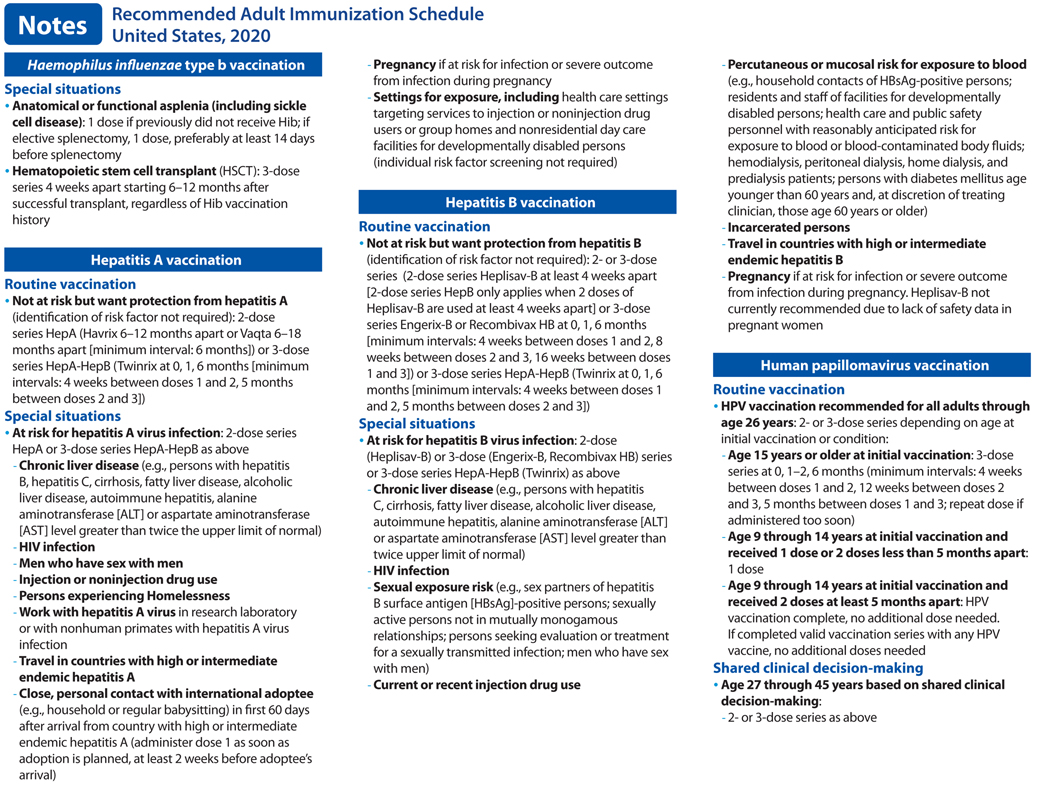
In June 2019, ACIP recommended all persons with HIV age 1 year or older be routinely vaccinated with hepatitis A vaccine. The list of other populations at risk for hepatitis A infection or severe hepatitis A disease has not changed substantially and includes persons with chronic liver disease, travelers in countries with high or intermediate endemic hepatitis A, persons with close, personal contact with an international adoptee in the first 60 days after arrival from a country with high or intermediate endemic hepatitis A, men who have sex with men, persons who use injection or noninjection drugs, persons experiencing homelessness, and persons who work with hepatitis A virus in a laboratory or nonhuman primates infected with the virus (3–6). Clotting factor disorders has been removed from the list. The definition of chronic liver disease has been expanded and includes, but is not limited to, persons with hepatitis B, hepatitis C, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase [ALT] or aspartate aminotransferase [AST] level greater than twice the upper limit of normal. A 2-dose series HepA (or 3-dose series HepA-HepB) is recommended for pregnant women if they are at risk for infection or severe outcome from infection during pregnancy. Lastly, hepatitis A vaccination is recommended for persons working in settings of exposure (e.g., those working in health care settings for injection or noninjection drug users or group homes and nonresidential day care facilities for developmentally disabled persons). In addition, any person who is not at risk for hepatitis A virus infection but wants protection against it may be vaccinated.
Hepatitis B vaccination (7).
The list of populations at risk for hepatitis B infection or severe hepatitis B disease has not changed and includes persons with hepatitis C, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, persons with HIV infection, sexual exposure risk (e.g., sex partners of hepatitis B surface antigen (HBsAg)-positive persons, sexually active persons not in mutually monogamous relationships, persons seeking evaluation or treatment for a sexually transmitted infection, men who have sex with men), current or recent injection drug use, percutaneous or mucosal risk for exposure to blood (e.g., household contacts of HBsAg-positive persons, residents and staff of facilities for developmentally disabled persons, health care and public safety personnel with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids, hemodialysis, peritoneal dialysis, home dialysis, and predialysis patients, persons with diabetes mellitus younger than 60 years of age and, at the discretion of the treating clinician, persons with diabetes age 60 years or older), incarcerated persons, and persons traveling in countries with high or intermediate endemic hepatitis B. Pregnancy, if at risk for infection or severe outcome from infection during pregnancy, has been added as a population at risk. In addition, HepB-Cpg (Heplisav B) administration is not recommended during pregnancy due to a lack of safety data.
Human papillomavirus (HPV) vaccination (8).
In June 2019, ACIP recommended catch-up HPV vaccination for all adults through age 26 years who are not adequately vaccinated. Previously, catch-up vaccination was recommended through age 26 years for females and 21 years for males; the updated recommendation is harmonized across genders. In addition, ACIP recommended shared clinical decision-making regarding HPV vaccination for adults aged 27 through 45 years who are not adequately vaccinated. Public health benefit of HPV vaccinations for adults in this age range is minimal, yet some persons who are not adequately vaccinated might benefit. HPV vaccination does not need to be discussed with most adults older than 26 years of age, but clinicians can consider discussing HPV vaccination with persons who are most likely to benefit. HPV vaccines are not licensed for use in adults older than age 45 years.
Measles, mumps, and rubella (MMR) vaccination (9).
Language has been added in the notes section to clarify indications for MMR vaccine in health care workers (HCP). For HCP born in 1957 or later without evidence of immunity to measles, mumps, or rubella, a 2-dose series at least 4 weeks apart should be administered for measles or mumps immunity and at least 1 dose of MMR should be administered for rubella immunity. For HCP born before 1957 without evidence of immunity to measles, mumps, or rubella, consider a 2-dose series at least 4 weeks apart for measles or mumps immunity and at least 1 dose MMR for rubella immunity.
Meningococcal B vaccination.
In June 2019, ACIP recommended persons age 10 years or older with complement deficiency, complement inhibitor use, or asplenia or who are microbiologists should receive a MenB booster dose 1 year following completion of a MenB primary series, followed by MenB booster doses every 2–3 years thereafter, for as long as the increased risk remains. For persons age 10 years or older determined by public health officials to be at increased risk during an outbreak, ACIP recommends a one-time booster dose if it has been 1 year or more since completion of a MenB primary series. Additionally, a booster dose interval of 6 months or more may be considered by public health officials depending on the specific outbreak, vaccination strategy, and projected duration of elevated risk.
Pneumococcal vaccination (10).
In June 2019, ACIP recommended PCV13 based on shared clinical decision-making for adults 65 years or older who do not have an immunocompromising condition, cerebrospinal fluid leak, or cochlear implant and who have not previously received PCV13. All adults 65 years or older should receive a dose of PPSV23.
Tetanus, diphtheria, and pertussis vaccination (11).
In October 2019, ACIP recommended either Td or Tdap vaccine be used in situations where only Td vaccine is currently recommended for the decennial booster, tetanus prophylaxis for wound management, and in the catch-up immunization schedule, including in pregnant women.
Varicella vaccination (12).
Vaccination may be considered for persons with HIV without evidence of varicella immunity who have CD4 counts ≥200 cells/μL.
Revised Content, Format, and Graphics
The cover page of the 2020 schedule provides basic instructions on how to use the schedule to systematically identify vaccination needs of adults and lists routinely recommended vaccines and their standardized abbreviations and trade names. Web links are provided, where providers can download the CDC Vaccine Schedules app and access reference materials for the surveillance of vaccine-preventable diseases, including case identification and disease outbreak response. Instructions on reporting suspected cases of reportable vaccine-preventable diseases to local or state health departments and significant postvaccination adverse events to the Vaccine Adverse Event Reporting System are listed. Information on the Vaccine Injury Compensation Program is provided, as well as web links to other resources, such as vaccine information statements and recommended vaccines for travelers.
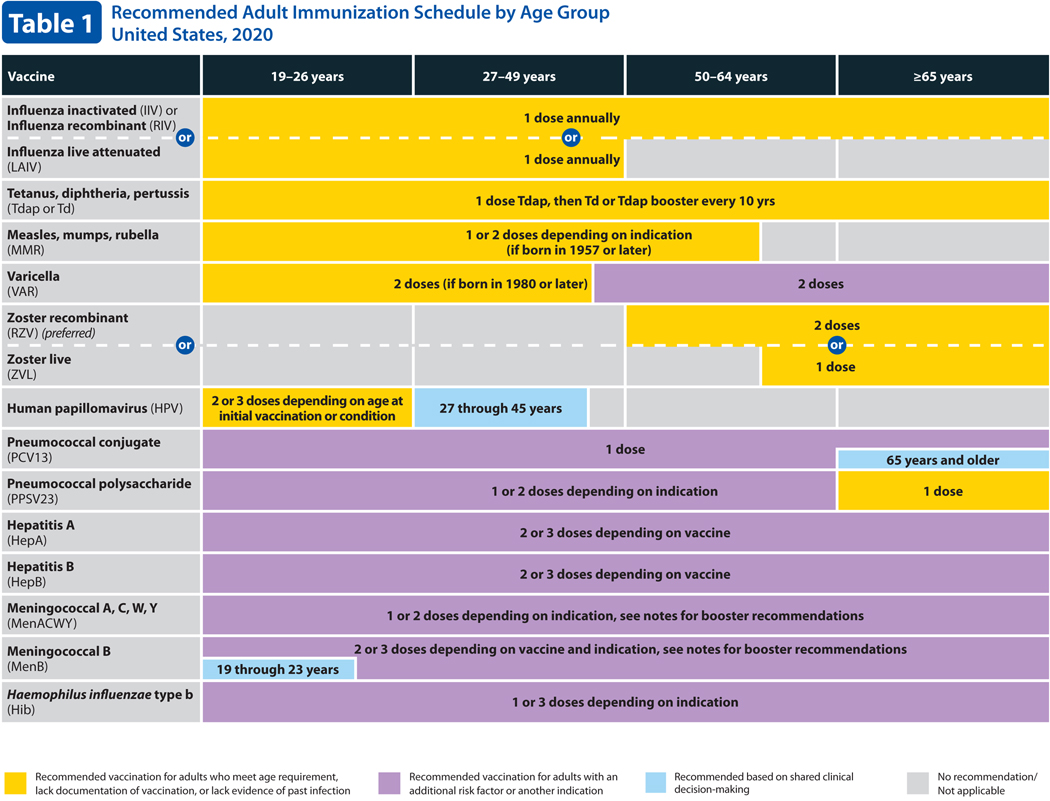
Table 1. Recommended Adult Immunization Schedule by Age Group.
Table 1 describes routine and catch-up vaccination recommendations for adults by age. For 2020, the columns for ages 19–21 and 22–26 years have been combined into a single column for 19–26 years and there is a single row for HPV vaccine (previously there were separate rows for females and males). Additionally, a new color (blue) has been added to indicate shared clinical decision-making for HPV vaccination in persons 27–45 years of age, for meningococcal B vaccination for adolescents and young adults age 16 through 23 years (age 16 through 18 years preferred) not at increased risk for meningococcal disease, and for pneumococcal conjugate (PCV13) vaccination in immunocompetent persons 65 years of age and older.
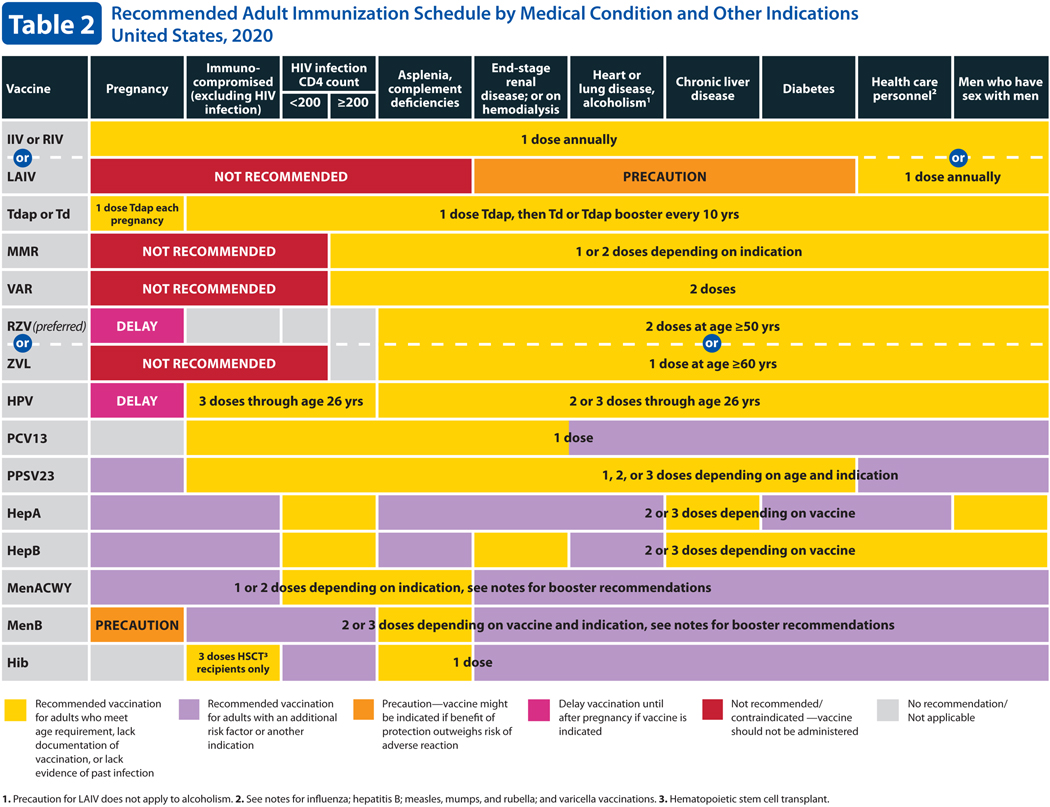
Table 2. Recommended Adult Immunization Schedule by Medical Condition and Other Indications.
Table 2 describes the recommended adult immunization schedule by medical condition and other indications. For 2020, the HPV row has been combined into a single row for all genders, including females and males. In the hepatitis A row, the box for all persons living with HIV, regardless of CD4 count, has been changed to yellow. Lastly, the red color now indicates the vaccine is not recommended/contraindicated.
Notes. Recommended Adult Immunization Schedule.
Each recommended vaccine for adults in Tables 1 and 2 is accompanied by a note (previously known as a footnote), which is designed to provide additional information on routine vaccination and recommendations in special situations. Each section contains concise information on vaccine indications, dosing frequencies and intervals, and other published ACIP recommendations. New or revised recommendations for influenza, hepatitis A, HPV, pneumococcal, and meningococcal B vaccines have been added to their respective sections in the notes. CDC has provided interim guidance and implementation considerations for shared clinical decision-making recommendations, available at URL. All vaccines identified in Tables 1 and 2 (except zoster vaccines) also appear in the Recommended Child and Adolescent Immunization Schedule for Ages 18 Years or Younger, United States, 2020 (ref). The notes for vaccines that appear in both the adult immunization schedule and the child and adolescent immunization schedule have been harmonized to the extent possible.
Human Papillomavirus Vaccination
HPV is a preventable, potentially cancer-causing infection commonly acquired soon after first sexual activity (13). Most HPV infections are transient, asymptomatic and do not progress to cancer. However, persistent infections with high-risk (oncogenic) HPV types can lead to development of cervical, anal, penile, vaginal, vulvar, and oropharyngeal cancers, usually after several decades (13). Most new HPV infections occur in adolescents and young adults. Although most sexually active adults have been exposed to HPV (14), new infections can occur with a new sex partner at any age (15).
In October 2018, the Food and Drug Administration expanded the approved age range for 9vHPV use from 9 through 26 years to 9 through 45 years in women and men (https://www.fda.gov/media/90064/download). After reviewing evidence related to HPV vaccination of adults, in June 2019, ACIP updated recommendations for catch-up vaccination and for vaccination of adults older than the recommended catch-up age.
ACIP does not recommend catch-up HPV vaccination for all adults older than age 26 years. Instead, shared clinical decision-making regarding HPV vaccination is recommended for some adults age 27 through 45 years who are not adequately vaccinated. For these adults, clinicians can consider discussing HPV vaccination with persons who are most likely to benefit, using the following considerations (8):
HPV is a very common sexually transmitted infection. Most HPV infections are transient and asymptomatic and cause no clinical problems.
Although new HPV infections are most commonly acquired in adolescence and young adulthood, some adults are at risk for acquiring new HPV infections. At any age, having a new sex partner is a risk factor for acquiring a new HPV infection.
Persons who are in a long-term, mutually monogamous sexual partnership are not likely to acquire a new HPV infection.
Most sexually active adults have been exposed to some HPV types, although not necessarily all of the HPV types targeted by vaccination.
No clinical antibody test can determine whether a person is already immune or still susceptible to any given HPV type.
HPV vaccine efficacy is high among persons who have not been exposed to vaccine-type HPV before vaccination.
Vaccine effectiveness might be low among persons with risk factors for HPV infection or disease (e.g., adults with multiple lifetime sex partners and likely previous infection with vaccine-type HPV), as well as among persons with certain immunocompromising conditions.
HPV vaccines are prophylactic (i.e., they prevent new HPV infections). They do not prevent progression of HPV infection to disease, decrease time to clearance of HPV infection, or treat HPV-related disease.
Pneumococcal Conjugate Vaccination
Streptococcus pneumoniae (pneumococcus) has the potential to cause serious illness, including sepsis, meningitis, and pneumonia, both with bacteremia (invasive) or without bacteremia (noninvasive). Herd immunity due to routine vaccination of children with pneumococcal conjugate vaccine (PCV) protects adults from pneumococcal disease. There have been sharp declines in pneumococcal disease in unvaccinated children and adults due to the widespread use of PCV7 and PCV13 in children. This has resulted in the prevention of carriage and, thereby, transmission, of vaccine-type strains. In 2014, ACIP recognized that, while in the short term, routine PCV13 use among adults age 65 years and older was warranted, in the long term, continued indirect effects from PCV13 use in children might limit the utility of this recommendation. In addition, an economic model estimated that vaccinating adults would have limited public health benefits in the long term, given the relatively low remaining PCV13-type disease burden (16). Therefore, in 2014, ACIP recommended that routine PCV13 use among adults age 65 years and older be re-evaluated in or after 2018 (10).
Indirect effects of the pediatric use of PCV13 has reduced the incidence of PCV13-type disease to historically low levels among adults age 65 years and older. Implementation of a PCV13 recommendation for all adults age 65 years and older in 2014 has had minimal impact on PCV13-type disease at the population level in this age group. However, PCV13 has been shown to be a safe and effective vaccine that can reduce the risk for PCV13-type invasive pneumococcal disease and noninvasive pneumonia among persons age 65 years and older. Balancing this evidence and considering acceptability and feasibility concerns, in June 2019, ACIP voted to no longer routinely recommend PCV13 for all adults age 65 years and older and, instead, to recommend PCV13 based on shared clinical decision-making for adults age 65 years and older who do not have an immunocompromising condition, CSF leak, or cochlear implant (10).
When patients and vaccine providers engage in a discussion to determine whether PCV13 is right for an immunocompetent individual age 65 years or older, the following considerations may be used (10):
PCV13 is a safe and potentially effective vaccine for older adults.
- The following adults age 65 years and older are potentially at increased risk for exposure to PCV13 serotypes and might attain higher than average benefit from PCV13 vaccination. Clinicians and practices caring for many patients in these groups may consider regularly offering PCV13 to their patients age 65 years and older who have not previously received PCV13:
- Persons residing in nursing homes or other long-term care facilities.
- Persons residing in settings with low pediatric PCV13 uptake.
- Persons traveling to settings with no pediatric PCV13 program.
Incidence of PCV13-type invasive pneumococcal disease and pneumonia increases with increasing age and is higher among persons with chronic heart, lung, or liver disease, diabetes, or alcoholism, and those who smoke cigarettes or who have more than one chronic medical condition. Although indirect effects from pediatric PCV13 use were documented for these groups of adults and were comparable to those observed among healthy adults, the residual PCV13-type disease burden remains higher in these groups. Clinicians and practices caring for patients with these medical conditions may consider offering PCV13 to such patients who are age 65 years and older and who have not previously received PCV13.
ACIP continues to recommend that all adults age 65 years and older receive 1 dose of PPSV23. A single dose of PPSV23 is recommended for routine use among all adults age 65 years and older (16).
Supplementary Material
Footnotes
Disclosures: To ensure the integrity of the ACIP, the U.S. Department of Health and Human Services has taken steps to ensure there is technical adherence to ethics statutes and regulations regarding financial conflicts of interest. Concerns regarding the potential for the appearance of a conflict are addressed or avoided altogether through preappointment and post-appointment considerations. Individuals with particular vaccine-related interests will not be considered for appointment to the committee. Potential nominees are screened for conflicts of interest and, if any are found, are asked to divest or forgo certain vaccine-related activities. In addition, at the beginning of each ACIP meeting, each member is asked to declare his or her conflicts. Members with conflicts are not permitted to vote if the conflict involves the vaccine or biologic being voted on. Details can be found at www.cdc.gov/vaccines/acip/committee/structure-role.html. Dr. Freedman and Dr. Kroger have nothing to disclose. Dr. Hunter reports travel expenses to ACIP meetings paid by the Centers for Disease Control and Prevention, grants from the Wisconsin Department of Health Services for speaking to clinicians in Milwaukee about adult vaccinations, and board membership in Immunize Milwaukee! (https://www.immunizationcoalitions.org/network-members/?coal=immunize-milwaukee_oid457), an ad hoc, nonincorporated, unfunded community coalition seeking to increase vaccination rates in metro Milwaukee. Dr. Ault reports travel expenses to the ACIP meetings paid by the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists (ACOG). Dr. Ault is also on committees for ACOG and the National Cancer Institute and has travel expenses paid by those organizations. Dr. Ault was a member of a data safety and monitoring committee for an immunization trial and received consultant fees and travel expenses from ACI Clinical for this activity. Dr. Ault is also a volunteer medical advisor for ‘Families Fighting Flu’ (www.familiesfightingflu.org) and receives no compensation from that nonprofit organization. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-3600.
The 2020 adult immunization schedule appeared in Annals of Internal Medicine and on the Centers for Disease Control and Prevention website at www.cdc.gov/vaccines/schedules. An announcement summarizing changes to the 2020 adult immunization schedule is published concurrently in the Morbidity and Mortality Weekly Report. Readers can cite the 2020 adult immunization schedule as follows: Freedman MS, Hunter P, Ault K, Kroger A; Advisory Committee on Immunization Practices. Recommended adult immunization schedule, United States, 2020. Ann Intern Med. 2020 (add volume and issue info here).
Contributor Information
Mark Freedman, Centers for Disease Control and Prevention, Atlanta, Georgia.
Andrew Kroger, Centers for Disease Control and Prevention, Atlanta, Georgia.
Paul Hunter, City of Milwaukee Health Department, Milwaukee, WI.
Kevin Ault, University of Kansas Medical Center, Kansas City, Kansas.
References:
- 1.CDC. Charter of the Advisory Committee on Immunization Practices. [updated April 1, 2018; cited 2019 September 6]; Available from: https://www.cdc.gov/vaccines/acip/committee/acip-charter.pdf. [Google Scholar]
- 2.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2019–20 Influenza Season. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2019. Aug 23;68(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doshani M, Weng M, Moore KL, Romero JR, Nelson NP. Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Persons Experiencing Homelessness. MMWR Morbidity and mortality weekly report. 2019. Feb 15;68(6):153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morbidity and mortality weekly report. 2007. Oct 19;56(41):1080–4. [PubMed] [Google Scholar]
- 5.Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morbidity and mortality weekly report. 2009. Sep 18;58(36):1006–7. [PubMed] [Google Scholar]
- 6.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2006. May 19;55(Rr-7):1–23. [PubMed] [Google Scholar]
- 7.Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2018. Jan 12;67(1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report. 2019. Aug 16;68(32):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2013. Jun 14;62(Rr-04):1–34. [PubMed] [Google Scholar]
- 10.Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged >/=65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report. 2019. Nov 22;68(46):1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havers FP, Moro PL, Hunter P, et al.Use of tetanus toxoid, re-duced diphtheria toxoid, and acellular pertussis vaccines: updatedrecommendations of the Advisory Committee on Immunization Practices–United States, 2019. MMWR Morb Mortal Wkly Rep.2020;69:77–83. doi: 10.15585/mmwr.mm6903a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin M, Güris D, Chaves SS, et al. ; Advisory Committee on Im-munization Practices, Centers for Disease Control and Prevention(CDC).Prevention of varicella: recommendations of the advisorycommittee on immunization practices (ACIP). MMWR Recomm Rep.2007;56:1–40. [PubMed] [Google Scholar]
- 13.Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for DiseaseControl and Prevention (CDC).Human papillomavirus vaccination:recommendations of the advisory committee on immunization prac-tices (ACIP). MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 14.Chesson HW, Dunne EF, Hariri S, et al.The estimated lifetimeprobability of acquiring human papillomavirus in the United States.Sex Transm Dis. 2014;41:660–4. doi: 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer RL, Hughes JP, Feng Q, et al.Incident detection of high-risk human papillomavirus infections in a cohort of high-risk womenaged 25–65 years. J Infect Dis. 2016;214:665–75. doi: 10.1093/infdis/jiw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomczyk S, Bennett NM, Stoecker C, et al. ; Centers for DiseaseControl and Prevention (CDC).Use of 13-valent pneumococcal con-jugate vaccine and 23-valent pneumococcal polysaccharide vaccineamong adults aged ≥65 years: recommendations of the AdvisoryCommittee on Immunization Practices (ACIP). MMWR Morb MortalWkly Rep. 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.