Abstract
OBJECTIVE
The World Health Organization has recently declared a monkeypox outbreak as a public health emergency of global concern. The main aim of this systematic review was to ascertain the maternal and perinatal outcomes of pregnancies complicated by monkeypox infection.
DATA SOURCES
The Medline, Embase, and Cochrane databases were searched on June 25, 2022 utilizing combinations of the relevant medical subject heading terms, key words, and word variants for “monkeypox” and “pregnancy.”
STUDY ELIGIBILITY CRITERIA
The search and selection criteria were restricted to the English language.
METHODS
The outcomes observed were miscarriage; intrauterine, neonatal, and perinatal death; preterm birth, vertical transmission, and maternal symptoms. A metaanalysis of proportions was used to analyze the data.
RESULTS
Four studies were included. All the cases in the present systematic review presented with symptoms and signs of monkeypox infection. There was no case of maternal death. Miscarriage occurred in 39% of cases (95% confidence interval, 0–89.0), whereas intrauterine fetal death occurred in 23.0% (95% confidence interval, 0–74.0) of cases. The overall incidence of late fetal and perinatal loss was 77.0% (95% confidence interval, 26.0–100), whereas only 23% (95% confidence interval, 0–74.0) of the included fetuses survived to birth. The incidence of preterm birth before 37 weeks of gestation was 8.0% (95% confidence interval, 0–62.0). Vertical transmission occurred in 62.0% (95% confidence interval, 3.0–100) of cases. When stratifying the analysis according to gestational age at infection, fetal loss was found to occur in 67.0% (95% confidence interval, 9.0–99.0) of cases with first-trimester infection and in 82.0% (95% confidence interval, 17.0–100) of those with second-trimester infection.
CONCLUSION
Monkeypox infection in pregnancy is associated with a high risk of perinatal loss and vertical transmission. The preliminary results from this systematic review affected by a very small number of included cases highlight the need for thorough maternal and fetal surveillance in pregnancies complicated by monkeypox infection.
Key words: monkey pox, outcome, pregnancy
AJOG MFM at a Glance.
Why was this study conducted?
Most studies on monkeypox infection in humans do not include pregnant people. The sporadic case reports published mainly in developing countries do not allow to extrapolate robust evidence on the actual risk of adverse maternal and perinatal outcomes in people with monkeypox infection in pregnancy.
This systematic review and metaanalysis aimed to report the maternal and perinatal outcomes of pregnancies complicated by monkeypox infection.
Key findings
The findings from this systematic review showed that monkeypox acquired in pregnancy is associated with a high risk of miscarriage, intrauterine fetal death, and vertical transmission. The risk of fetal loss was similar in infections acquired in the first or second trimester.
What does this add to what is known?
This is the first systematic review on monkeypox infection in pregnancy. A thorough literature search and multitude of outcomes explored represent the main strengths of the present study. A large worldwide registry of monkeypox infection in pregnant people is urgently needed to elucidate the actual burden of this disease in pregnancy.
Objectives
Monkeypox is a viral zoonosis induced by an enveloped, double-stranded DNA virus belonging to the Orthopoxvirus genus of the Poxviridae family. Several outbreaks of monkeypox infection have been reported in the last decades, including those in the Democratic Republic of the Congo, Nigeria, and Gambia.1 More recently, multiple cases of monkeypox were identified in several nonendemic countries in May 2022, mainly the United Kingdom, thus highlighting the role of monkeypox as a disease of public health importance. In view of the rapid spread of human monkeypox worldwide, on July 23, 2022, the World Health Organization (WHO) declared monkeypox a global health emergency.
The course of monkeypox infection in humans not been completely explored yet, but it is commonly characterized by a prodroma with fever, intense headache, lymphadenopathy, myalgia, and intense asthenia followed by a skin rash that tends to be more concentrated on the face and extremities. The course of the infection is commonly self-limiting, but immunocompromised individuals are vulnerable to more serious disease and have a higher case fatality rate.2
Most studies on monkeypox infection in humans do not include pregnant people. However, previous studies in pregnancy on the outcome of infection by smallpox—a virus belonging to the Poxviridae family—have reported a high fatality rate for the infection once acquired in pregnancy, especially when it was acquired in the third trimester of pregnancy or in patients with hemorrhagic disease. Despite the potential similarity between the 2 viral strains, there are currently no robust data on the outcome of monkeypox infection in pregnancy. The sporadic case reports published mainly in developing countries do not allow to extrapolate robust evidence on the actual risk of adverse maternal and perinatal outcome in people with monkeypox infection in pregnancy.3
This systematic review and metaanalysis aimed to report the maternal and perinatal outcomes of pregnancies complicated by monkeypox infection.
Methods
Protocol, information sources, and literature search
This review was performed according to a protocol designed a-priori and recommended for systematic reviews and metaanalysis.4 , 5 The International Prospective Register of Systematic Reviews registration number is CRD42022351339. The Medline, Embase, and Cochrane databases were searched electronically on June 25, 2022, utilizing combinations of the relevant medical subject heading (MeSH) terms, key words, and word variants for “monkeypox” and “pregnancy” (Supplementary Table 1). The search and selection criteria were restricted to the English language. Reference lists of relevant articles and reviews were hand searched for additional reports. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) were followed.6
Outcome measures, study selection, and data collection
The outcomes observed were as follows:
-
•
Fetal loss, including miscarriage and intrauterine fetal death (IUFD)
-
•
Neonatal death (NND), defined as death occurring at up to 28 days of life
-
•
Perinatal loss, including both fetal loss and neonatal death
-
•
Fetal anomalies detected either at ultrasound or birth
-
•
Preterm birth, defined as birth before 37 weeks’ gestation
-
•
Maternal symptoms and severe disease requiring hospitalization
-
•
Maternal death
-
•
Vertical transmission, defined as the presence of laboratory-confirmed analysis on fetal or neonatal sample (amniotic fluid or cord blood) or the presence of clinical manifestations suggestive of monkeypox infection
Both prospective and retrospective studies were included. Two authors reviewed all abstracts independently. Agreement regarding potential relevance was reached by consensus. Full-text copies of those articles were obtained, and the same 2 reviewers independently extracted relevant data regarding study characteristics and pregnancy outcome. Inconsistencies were discussed by the reviewers and were resolved by consensus or by discussion with a third author. If >1 study was published for the same cohort with identical endpoints, the report containing the most comprehensive information on the population was included to avoid overlapping populations.
Quality assessment, risk of bias, and statistical analysis
Although case series and case reports are generally biased, specific tools to assess their quality in the context of a systematic review have been specifically developed. The quality assessment of the included studies was performed using a standardized tool adapted from Murad et al7. Studies were rated as having low, moderate, or high risk of bias.
We used random effect metaanalyses of proportions to analyze the data. Between-study heterogeneity was explored using the I 2 statistic, which represents the percentage of between-study variation that is owing to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, whereas I 2 values of ≥50% indicate a substantial level of heterogeneity.
All analyses were carried out using Stata, version 13.1 (StataCorp, College Station, TX).
Results
Study selection and characteristics
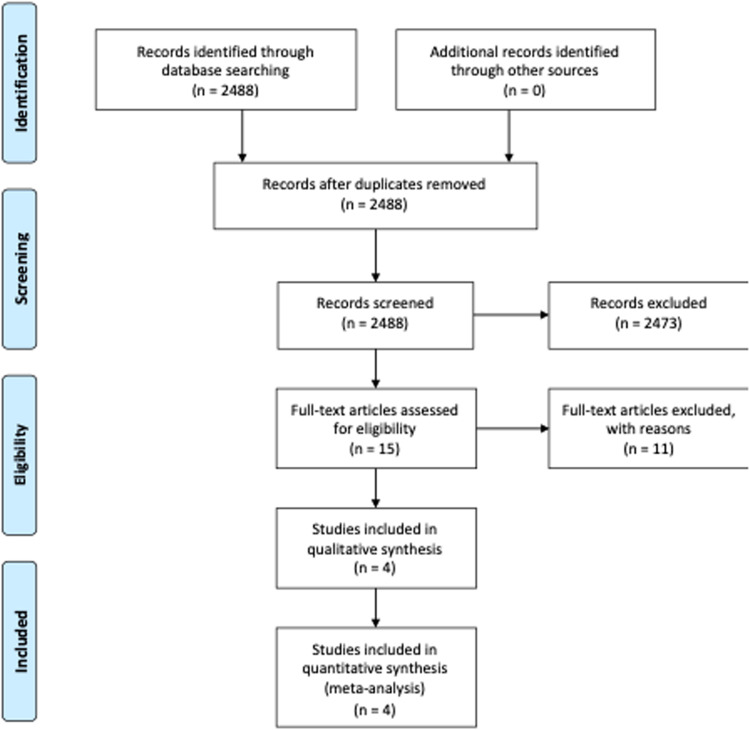
A total of 2488 articles were identified; 15 were assessed with respect to their eligibility for inclusion, and 4 studies were included in the systematic review (Table 1 , Figure , Supplementary Table 2).8, 9, 10, 11 These 4 studies included 7 cases with monkeypox infection acquired during pregnancy. All the studies were from countries in which monkeypox infection is endemic (2 from Nigeria, 1 from Democratic Republic of the Congo, and 1 from Zaire). All the included cases acquired the infection during the first and second trimesters of pregnancy and were hospitalized. The results of the quality assessment of the included studies are presented in Supplementary Table 2. Three of the included studies showed an overall low risk, whereas 1 showed a high risk of bias. For each outcome, the total number of publications included in the metaanalyses was <10. We were thus unable to assess publication bias, either graphically, through funnel plots, or formally through Egger's regression asymmetry test (in such cases, the power is too low to distinguish chance from real asymmetry).12, 13, 14
Table 2.
Pooled proportions for the outcomes observed in the present systematic review in pregnant people with monkeypox infection
| Outcome | Studies | Fetuses (n/N) | Pooled proportions (95% confidence interval) | I2 (%) |
|---|---|---|---|---|
| Maternal death | 4 | 0/7 | 0.00 (0.00–0.27) | 0.00 |
| Miscarriage | 4 | 3/7 | 0.39 (0.00–0.89) | 0.00 |
| Intrauterine demise | 4 | 2/7 | 0.23 (0.00–0.74) | 0.00 |
| Neonatal death | 4 | 0/7 | 0.00 (0.00–0.27) | 0.00 |
| Overall fetal or neonatal losses | 4 | 5/7 | 0.77 (0.26–1.00) | 0.00 |
| Alive | 4 | 2/7 | 0.23 (0.00–0.74) | 0.00 |
| Fetal anomalies | 4 | 2/7 | 0.23 (0.00–0.74) | 0.00 |
| Preterm birth <37 wk | 2 | 1/5 | 0.08 (0.00–0.62) | no value (nv) |
| Vertical transmission | 3 | 3/6 | 0.62 (0.03–1.00) | 23.84 |
| Clinical manifestations | 4 | 7/7 | 1.00 (0.73–1.00) | 0.00 |
| Maternal symptoms | 4 | 7/7 | 1.00 (0.73–1.00) | 0.00 |
| Severe maternal symptoms requiring hospitalization | 4 | 7/7 | 1.00 (0.73–1.00) | 0.00 |
D'Antonio. Monkeypox in pregnancy. Am J Obstet Gynecol MFM 2022.
Table 1.
General characteristics of the studies included in the systematic review
| Author | Year | Country | Study design | Period considered | Gestational age at diagnosis | Stratification according to the disease severity | Cases (n) |
|---|---|---|---|---|---|---|---|
| Ogoina et al8 | 2020 | Nigeria | Case series | 2017–2018 | II trimester | No | 1 |
| Yinka-Ogunleye et al9 | 2019 | Nigeria | Case report | 2017–2018 | II trimester | No | 1 |
| Mbala et al10 | 2017 | Democratic Republic of the Congo | Case series | 2007–2011 | I–II trimester | Yes | 4 |
| Jezek et al11 | 1983 | Zaire | Case report | Not applicable | I trimester | No | 1 |
D'Antonio. Monkeypox in pregnancy. Am J Obstet Gynecol MFM 2022.
Figure.
Systematic review flowchart
D'Antonio. Monkeypox in pregnancy. Am J Obstet Gynecol MFM 2022.
Synthesis of the results
All the cases included in the present systematic review presented with symptoms and signs of monkeypox infection and tested positive after polymerase chain reaction (PCR). There was no case of maternal death. Miscarriage occurred in 39% (95% confidence interval [CI], 0–89.0), whereas IUFD occurred in 23.0% (95% CI, 0–74.0) of the cases. There was no case of NND within 30 days from birth, but 1 case reported by Jezek and Fenner11 had NND at 6.5 weeks of age, apparently owing to malnutrition; however, the newborn showed a neonatal rash, clinically consistent with congenital monkeypox virus infection.14 The overall incidence of fetal and perinatal loss was 77.0% (95% CI, 26.0–100), whereas only 23% (95% CI, 0–74.0) of the included fetuses survived to birth. The incidence of preterm birth before 37 weeks of gestation was 8.0% (95% CI, 0–62.0). Assessment of vertical transmission was hampered by the facts that some of the fetal losses occurred in the early second trimester and no formal PCR analysis was performed on the aborted tissue. The overall incidence of vertical transmission was 62.0% (95% CI, 3.0–100). A meaningful subgroup analysis according to severity of maternal infection could not be performed, whereas that according to the trimester of infection was affected by the very small sample size. The overall incidence of fetal loss was 67.0% (95% CI, 9.0–99.0) following first-trimester infection and 82.0% (95% CI, 17.0–100) in those with second-trimester infection.
Comment
Main findings
The findings from this systematic review showed that monkeypox acquired in pregnancy is associated with a high risk of miscarriage, IUFD, and vertical transmission. The risk of fetal loss was similar if the infection was acquired in the first or second trimester. Unfortunately, the very small number of included cases do not allow to extrapolate robust evidence or conclusions.
Strengths and limitations
This is one of the earliest systematic reviews on monkeypox infection in pregnancy. A thorough literature search and multitude of outcomes explored represent the main strengths of the study. The very small number of cases included in the present review, retrospective design of the studies, the fact that most cases were case reports, and lack of knowledge on HIV status and other comorbidities represent its main limitations. Furthermore, assessment of vertical transmission of the virus was not based exclusively on the virological analysis of fetal tissues but rather on the presence of macroscopic signs of the disease, thus making it difficult to extrapolate the actual risk of congenital infection. Finally, all the included cases were admitted to the hospital owing to maternal symptoms. In this scenario, the very high rates of adverse perinatal outcomes reported in the present review may represent an overestimation of the actual burden of the disease in pregnancy.
Clinical and research implications
The recent outbreaks of monkeypox infection in several nonendemic countries have highlighted this infection as a major issue of public health importance. In nonpregnant individuals, the course of the infection is usually uneventful; immunocompromised individuals are more likely to develop the severe form of the disease and have a higher case fatality rate. Monkeypox usually causes a systemic illness that includes fevers, chills, myalgias, and a characteristic rash. However, during the 2022 outbreak, some patients presented with genital, anal, and/or oral lesions without systemic illness.
Most cases of monkeypox have occurred in Central and West Africa, though recent outbreaks have also been reported in nonendemic countries. In endemic countries, the strain isolated from West Africa appears to be less virulent than the one from Central Africa.
Conversely, data on outcomes in pregnant persons with monkeypox infection are clearly limited and are subject to reporting bias. Previous studies on smallpox infection—a virus belonging to the Poxviridae family—have reported a high fatality rate once it is acquired in pregnancy, especially when it is acquired in the third trimester of pregnancy or in patients with hemorrhagic disease.3 15 However, it is not entirely certain whether these findings are applicable to persons with monkeypox infection.
The findings from this systematic review, which is limited by the very small number of included cases, suggest a high rate of miscarriage and perinatal loss in people with monkeypox infection in pregnancy. However, these results should be interpreted with caution. The systematic review included only 7 cases of pregnant people with monkeypox infection. Furthermore, all the included cases were hospitalized owing to moderate or severe disease, thus potentially overestimating the perinatal risks associated with the infection. Finally, there was no formal assessment of pregnancy status in all the included studies.
Although it has been suggested that transplacental transmission from the mother to fetus may be responsible for congenital monkeypox, this risk is far from established. There were 2 cases of fetal demise in the third trimester; however, there was no information on other potential causes of fetal death, including chromosomal anomalies or malformation. Of the 2 cases that were reported to have vertical transmission and delivered in the third trimester, both showed clinical signs of monkeypox infection, including diffuse cutaneous maculopapillary lesions on the skin of the head, trunk, and extremities; 1 fetus also showed hydrops. More importantly, worse pregnancy outcomes were mainly observed in cases with severe disease. Based on this, accurate fetal surveillance is warranted when maternal monkeypox infection is confirmed, especially when the infection is severe and requires hospitalization.
Several guidelines for the management of pregnancy with suspected monkeypox infection have been recently published.16 , 17 Once a diagnosis of the infection is made by PCR analysis, patients should be carefully monitored to identify the early signs of progression of the severity of the disease. Tecovirimat and vaccinia immune globulin can be considered for those with severe disease; cidofovir and brincidofovir have shown evidence of teratogenicity in animal studies. Modified Vaccinia Ankara-Bavarian Nordic is a third-generation smallpox vaccine recently approved in the United States, Canada, and the European Union; it is considered relatively safe because it contains nonreplicating virus and should be administered to pregnant people who have close contacts with individuals with confirmed monkeypox infection. The decision whether to treat and monitor a pregnant person as an outpatient or in the inpatient setting should be individualized depending on the specific impact of any treatment on pregnancy and lactation.
However, more evidence is needed from larger studies to guide more appropriate management.
Conclusions
Monkeypox infection in pregnancy seems to be associated with a high risk of perinatal loss; this is probably owing to vertical transmission of the virus, at least in hospitalized patients. Despite being limited by the very small number of included cases, preliminary results from this systematic review highlight the need for thorough maternal and fetal surveillance in pregnancies complicated by monkeypox infection. A large worldwide registry of monkeypox infection in pregnant people is urgently needed to elucidate the actual burden of this disease in pregnancy.
Acknowledgments
We thank Dr Adesola Ogunleye, and Dr Dimie Ogoina, for the additional information provided.
Footnotes
The authors report no conflict of interest.
No funding was obtained for this systematic review.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ajogmf.2022.100747.
Appendix. Supplementary materials
References
- 1.Gong Q, Wang C, Chuai X, Chiu S. Monkeypox virus: a re-emergent threat to humans. Virol Sin. 2022;37:477–482. doi: 10.1016/j.virs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg Infect Dis. 2006;12:1119–1121. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson LK, Craig JC, Willis NS, Tovey D, Webster AC. How to write a Cochrane systematic review. Nephrology (Carlton) 2010;15:617–624. doi: 10.1111/j.1440-1797.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.NHS Centre for Reviews and Dissemination . 2009. Systematic reviews: CRD's guidance for undertaking reviews in health care.https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf Available at: Accessed December 3, 2016. [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 9.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 11.Jezek Z, Gromyko AI, Szczeniowski MV. Human monkeypox. J Hyg Epidemiol Microbiol Immunol. 1983;27:13–28. [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Manzoli L, De Vito C, Salanti G, D'Addario M, Villari P, Ioannidis JP. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One. 2011;6:e24384. doi: 10.1371/journal.pone.0024384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . 2022. WHO disease outbreak news: monkeypox, all items related to multi-country outbreak.https://www.who.int/emergencies/emergency-events/item/2022-e000121 Available at. Accessed 29 May 2022. [Google Scholar]
- 17.Centers for Disease Control and Prevention . 2022. Clinical considerations for monkeypox in people who are pregnant or breastfeeding.https://www.cdc.gov/poxvirus/monkeypox/clinicians/pregnancy.html Available at: Accessed 01 Aug 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.