Abstract
The abundance of docosahexaenoic acid (DHA) in phospholipids in the brain and retina has generated interest to search for its role in mediating neurological functions. Besides the source of many oxylipins with pro-resolving properties, DHA also undergoes peroxidation, producing 4-hydroxyhexenal (4-HHE), although its function remains elusive. Despite wide dietary consumption, whether supplementation of DHA may alter the peroxidation products and their relationship to phospholipid species in brain and other body organs have not been explored sufficiently. In this study, adult mice were administered a control or DHA-enriched diet for three weeks, and phospholipid species and peroxidation products were examined in brain, heart and plasma. Results demonstrated that this dietary regimen increased (n-3) and decreased (n-6) species to different extent in all major phospholipid classes (PC, dPE, PE-pl, PI and PS) examined. Besides changes in phospholipid species, DHA-enriched diet also showed substantial increases in 4-HHE in brain, heart and plasma. Among different brain regions, the hippocampus responded to the DHA-enriched diet showing significant increase in 4-HHE. Considering the pro- and anti-inflammatory pathways mediated by the (n-6) and (n-3) polyunsaturated fatty acids, unveiling the ability for DHA-enriched diet to alter phospholipid species and lipid peroxidation products in the brain and in different body organs may be an important step forward towards understanding the mechanism(s) for this (n-3) fatty acid on health and diseases.
Keywords: docosahexaenoic acid, lipidomics, lipid peroxidation, hippocampus, heart, plasma
1. INTRODUCTION
Docosahexaenoic acid (DHA, 22:6n-3) is an (n-3) polyunsaturated fatty acid (PUFA) abundant in the phospholipids in the brain and retina. DHA not only plays a role in the maintenance of cell membrane fluidity, it also is involved in the production of lipid mediators important in regulating intracellular signaling activities (Belkouch et al., 2016; Jump, Depner, & Tripathy, 2012; Maulucci et al., 2016; Mita et al., 2016; G. Y. Sun et al., 2018). Its pleiotropic properties has generated interest in examining its role and mechanism(s) of action in health and diseases (Avallone, Vitale, & Bertolotti, 2019; Nicolas G. Bazan, Molina, & Gordon, 2011; Lauritzen et al., 2016). There is suggestion that abnormalities in DHA during brain development may be an underlying factor for some psychiatric disorders including attention deficit, hyperactivity, and autism spectrum disorders (Agostoni et al., 2017; Clayton, Hanstock, Garg, & Hazell, 2007; Hashimoto, Maekawa, Katakura, Hamazaki, & Matsuoka, 2014; Muller et al., 2015). Furthermore, studies with animal models have demonstrated ability for (n-3) PUFAs to ameliorate age-related neurodegenerative diseases, and to facilitate brain recovery after ischemic stroke and traumatic brain injuries (Nicolas G. Bazan et al., 2011; Zhang et al., 2014; Zhu, Ding, Kong, Li, & Chen, 2018). Aside from its effects on the central nervous system, DHA and other (n-3) PUFAs also offer beneficial effects on other body systems, including the cardiovascular system (Duda, O’Shea, & Stanley, 2009; Jump et al., 2012; Sakamoto, Saotome, Iguchi, & Maekawa, 2019). Subsequently, it is not surprising that DHA is widely used as a dietary supplement and a nutraceutical across different human populations (Calzada et al., 2010).
DHA and arachidonic acid (ARA, 20:4n-6) are major PUFAs linked to the sn-2 position of membrane phospholipids. These PUFAs are metabolically active mediated by phospholipases A2 (PLA2) and energy dependent fatty acid CoA ligase and acyltransferases through the deacylation-reacylation cycle, also known as the “Land’s cycle” (Granger et al., 2018; G. Y. Sun & MacQuarrie, 1989). However, depending on the cell type and conditions, different types of PLA2 are responsible for regulating the metabolism of DHA and ARA (G. Y. Sun et al., 2014; G. Y. Sun et al., 2018; B. Yang, Fritsche, et al., 2019). For example, ARA in phospholipids is released through the action of cytosolic PLA2 (cPLA2), and this pathway is known to play a role in production of prostaglandins, thromboxanes, and leukotrienes, lipid mediators which are largely pro-inflammatory (G. Y. Sun et al., 2014). In contrary, the release of DHA through the Ca+2-independent PLA2 (iPLA2) is linked to production of resolvins, protectins and marisins, which are pro-resolving mediators (N. G. Bazan, 2018; Serhan, Chiang, & Dalli, 2015).
Besides interaction with cyclooxygenases and lipoxygenases, PUFAs are also susceptible to enzymatic and non-enzymatic reactions with oxygen free radicals, leading to the production of reactive aldehydes, namely, 4-hydroxyhexenal (4-HHE) from DHA and 4-hydroxynonenal (4-HNE) from ARA (Long & Picklo, 2010; B. Yang, Fritsche, et al., 2019). There is evidence that these lipid peroxidation products may offer important physiological functions, due mainly to their ability to form adducts with proteins, nucleic acids and phospholipids (Cohen et al., 2013; Long & Picklo, 2010; Nakagawa et al., 2014; Riahi, Cohen, Shamni, & Sasson, 2010). However, in light of the “Yin-Yang” mechanism for the metabolism of DHA and ARA, there is also evidence that these peroxidation products are also regulated differently (B. Yang, Fritsche, et al., 2019). For example, our study with microglial cells demonstrated the increase in 4-HNE but not 4-HHE upon stimulation of the cPLA2/ARA pathway by lipopolysaccharides (B. Yang et al., 2018). Increases in 4-HNE are also observed in brain associated with neuroinflammatory diseases, including stroke and spinal cord injury (Lee et al., 2012; Ungerer et al., 2020; Zhang et al., 2014). On the other hand, increases in 4-HHE have been shown upon consumption of diets enriched in DHA or fish oil (Calzada et al., 2010; Nakagawa et al., 2014; B. Yang, Li, et al., 2019). Since both peroxidation products are readily detected in tissues and body fluids, there is increasing interest to examine regulation of these products in health and diseases.
Our recent study demonstrated the effects of maternal DHA-enriched diet to alleviate stress-induced behavioral deficits in offspring mice (Matsui et al., 2018). Subsequently, a follow-up study using the same dietary regimen showed that administration of the DHA-enriched diet to pregnant mothers resulted in altered fatty acid composition in all brain regions, but increase in 4-HHE levels mainly in the cerebral cortex and hippocampus in the offspring pups (B. Yang, Li, et al., 2019). In addition to the brain tissue, this study also demonstrated substantial changes in fatty acid composition and levels of lipid peroxidation products in the heart and plasma (B. Yang, Li, et al., 2019). Considering that (n-3) and (n-6) PUFAs are linked to different classes of membrane phospholipids, an important goal in this study is to determine whether the DHA-supplemented diet may alter lipid peroxidation products and molecular species in different phospholipids in adult mice administered a control or DHA-enriched diet. In addition to examining the changes in the brain, this study also included determination of lipid peroxidation products and phospholipids species in the heart and plasma.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
4-Hydroxyhexenal (4-HHE, 1mg in 100μL of ethanol), 4-hydroxynonenal (4-HNE, 1mg in 100μL of ethanol), and 4-hydroxy hexenal-d3 (4-HHE-d3, 100μg in 100μL of methyl acetate) were purchased from Cayman Chemical Co. (Ann Arbor, MI). 1,3-Cyclohexanedione (CHD, 97%), ammonium acetate (HPLC grade), acetic acid (ACS grade) and formic acid (mass spectrometry grade), were purchased from Sigma-Aldrich (St. Louis, MO). C18 Sep-Pak cartridges (1 mL, 100 mg) were obtained from Waters Corporation (Milford, MA). Phospholipid removal cartridges (Phree™, 1 mL) were purchased from Phenomenex Inc. (Torrance, CA). All solvents (HPLC grade) used for sample preparation, UHPLC, and MS analysis were obtained from Thermo Fisher Scientific Inc. (Fair Lawn, NJ).
2.2. Animals and diets
Three month-old male mice (C57Bl/6J) were purchased from Jackson Laboratories (Bar Harbor, ME), and were given a nutritionally-complete, control (CTL) diet (modified AIN-93G #103619) from Dyets Inc. (Bethleham, PA). The CTL diet contained no preformed DHA (Supplemental Table S1), but did contain sufficient amounts of alpha-linolenic acid (ALA,18:3n-3) to meet normal brain DHA requirements (Domenichiello, Kitson, & Bazinet, 2015). At the beginning of the study, animals (n = 12) were randomly divided into two groups. The control group (n = 5 mice) remained on the CTL diet, whereas the experimental group (n = 7 mice) was fed a diet containing 1%, by weight, DHA (#103598) for three weeks. The reason for the three-week diet is because this period of time was used in a study in which adult mice fed a fish oil diet with a similar amount of DHA resulted in substantial changes in fatty acids and lipid peroxidation products in different body organs (Nakagawa et al., 2014). The composition of the control and DHA diets have been described previously (Matsui et al., 2018; B. Yang, Li, et al., 2019) as well as the final fatty acid profiles (Supplemental Tables S1 and S2). The diets were stabilized against auto-oxidation with 0.02 g tertiary-butylhydroquinone/100 g fat. All experiments were approved by the University of Missouri Animal Care and Use Committee (#8945) and were performed in compliance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
2.3. Sample preparation
After the feeding regimen, animals were anesthetized with isoflurane and blood was obtained by heart puncture using heparin as an anticoagulant, followed by perfusion with physiological saline. The brain was dissected to obtain left and right cerebral cortices, striatum, hippocampus and cerebellum. In addition, the perfused heart was also obtained. The blood was centrifuged at 16,100 g for 5–8 min to obtain plasma and was transferred to Eppendorf tubes and frozen at −80 °C until use. Samples were placed in sterilized aluminum foil, immediately flash frozen in liquid nitrogen, and stored at −80 °C until use.
For the experiments using LC-MS/MS to determine 4-HHE and 4-HNE, the left cerebral cortex, hippocampus, striatum and cerebellum, along with the heart were weighed and homogenized for in 8-fold volumes of HPLC water using a bullet blender (Next Advance, Inc., Averill Park, NY) as described previously (B. Yang, Li, et al., 2019). The heart tissue was first pulverized in liquid nitrogen prior to homogenization, and the homogenates were centrifuged at 16,100 g for 20 min at 4 °C. The supernatant was collected and stored at −80 °C prior to use. Total protein concentration in the supernatant was determined using the Bicinchoninic Acid Assay (Sigma-Fisher, St. Louis, MO).
2.4. LC-MS/MS analysis of 4-HHE and 4-HNE
The sample preparation stage involved three main steps: phospholipids removal, derivatization, and desalting as previously described (G.Y. Sun et al., 2019; B. Yang, Li, et al., 2019). Briefly, phospholipids were removed via solid phase extraction using a 1 mL Phree™ cartridge. This step removes phospholipids and helps eliminate matrix effects to allow for lower limits of detection of the peroxidation products. The bed was loaded with 30 μL of homogenized sample and an equal volume of internal standard (4-HHE-d3, 1000 ng/mL). Acetonitrile (500 μL) was spiked with 1% formic acid and was used to elute the analyte of interest. The clear elute was dried under a stream of nitrogen and used for derivatization with a freshly prepared acidified CHD reagent. The mixture was incubated at 60 °C for 1 h and then cooled on ice prior to desalting using a C18 SPE cartridge. The C18 cartridge was pre-conditioned with methanol (0.7 mL) and equilibrated with water (0.7 mL). The cooled derivatized mixture was loaded onto the cartridge, washed twice with water (0.7 mL) and followed by 5% acetonitrile in water (0.7 mL). The derivatized analytes of interest (4-HHE, 4-HNE, and 4-HHE-d3 derivatives) were eluted with 100% acetonitrile (0.7 mL) and dried with a steady stream of nitrogen gas. The samples were reconstituted in 40% methanol in water with 0.1% formic acid (300 μL) and were ready for LC-MS/MS analysis. A Waters Acquity UHPLC system equipped with a quaternary solvent manager was used in conjunction with a C18 reverse phase column (Luna Omega, Phenomenex). A previously developed and validated UHPLC-MS/MS method with a 7 min LC gradient including equilibration was used for the separation and detection of the 4-HHE and 4-HNE derivatives (B. Yang, Li, et al., 2019). A Waters Xevo TQ-S triple quadrupole mass spectrometer with an electrospray ionization source operated in the positive-ion mode was used. Multiple reaction monitoring transition m/z 326.3 -> 216.1Da, m/z 284.2 -> 216.1 Da and 287.2 -> 216.1 Da were chosen for simultaneous monitoring of 4-HNE, 4-HHE and 4- HHE-d3 (internal standard) derivatives, respectively.
2.5. Analysis of phospholipid species
2.5.1. Sample preparation
Four randomly selected frozen samples (half of the left cerebral cortex, half of the heart tissue, and plasma) from the control and DHA groups were sent to Han’s laboratory (University of Texas Health Science Center at San Antonio, TX) for analysis of phospholipid species. The reason for selecting only cerebral cortex was because a substantial amount of brain tissue is required for this type of lipid analysis, and in our previous study, similar changes in fatty acid composition (i.e., decrease in ARA and increase in DHA) were observed in all brain regions (B. Yang, Li, et al., 2019). Tissue samples (~10 mg) were pulverize and homogenized in 0.5 mL of ice-cold diluted PBS (0.1x) with a Potter-Elvehjem tissue grinder. Protein assay on individual homogenates was conducted. An aliquot of homogenate was transferred to a disposable glass test tube. A mixture of lipid internal standards for quantification of all reported lipid classes was added to the tube based on the tissue protein content (Wang, Wang, & Han, 2017). Lipid extraction was performed by a modified Bligh and Dyer method as previously described (Wang & Han, 2014). All of the lipid extracts were flushed with nitrogen, capped, and stored at −20ºC.
2.5.2. Mass spectrometric analysis of lipids
Mass spectrometric analysis of lipids was performed with an Altis triple-quadrupole mass spectrometer (Thermo Fisher Scientific, San Jose, CA) or a Q-Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an automated nanospray device (Triversa Nanomate, Advion Biosciences, Ithaca, NY) and operated with the Xcalibur software as previously described (Han, Yang, & Gross, 2008; K. Yang, Cheng, Gross, & Han, 2009). Identification and quantification of all reported lipid molecular species were performed using an in-house automated software program (Wang et al., 2017; K. Yang et al., 2009). All lipid levels were normalized to sample protein content.
2.6. Statistical Analysis
For the determination of 4-HHE and 4-HNE levels via LC-MS/MS, three biological replicates of each tissue sample and three analytical replicates of each biological replicate were completed for each sample. Statistical analyses were performed with GraphPad Prism (version 8.3; GraphPad Prism Software Inc., San Diego, CA). Results are expressed as the mean ± standard error of mean (SEM). Depending on the experiments, samples were analyzed by non-parametric t-tests or one-way ANOVA to compare among control and DHA diet groups. Differences were considered significant at p < 0.05 for all analyses.
3. RESULTS
3.1. Feeding mice with control and DHA-enriched diets
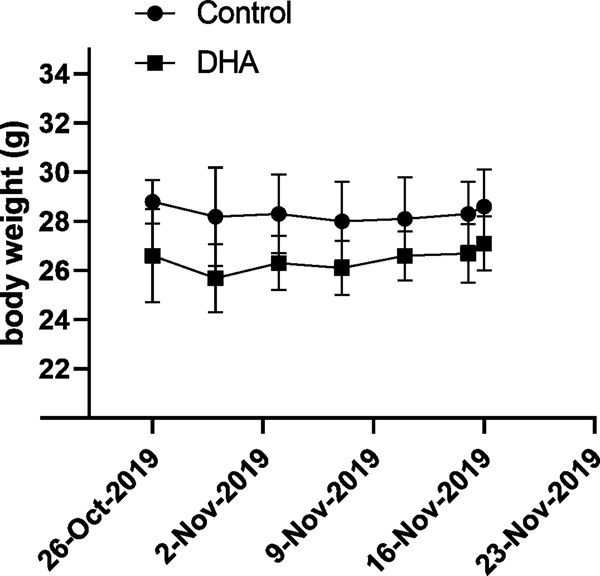
In this study, adult mice were given control and 1% DHA-enriched diets for three weeks. Fresh diets were provided every other day, and body weights of each mouse was obtained every 4 days. As shown in Figure 1, there was no significant change in body weights between the two groups during the three-week period with the control and DHA-enriched diets.
Figure 1.
Body weights of mice in the control and DHA group over the time of the study.
3.2. Determination of 4-HHE and 4-HNE
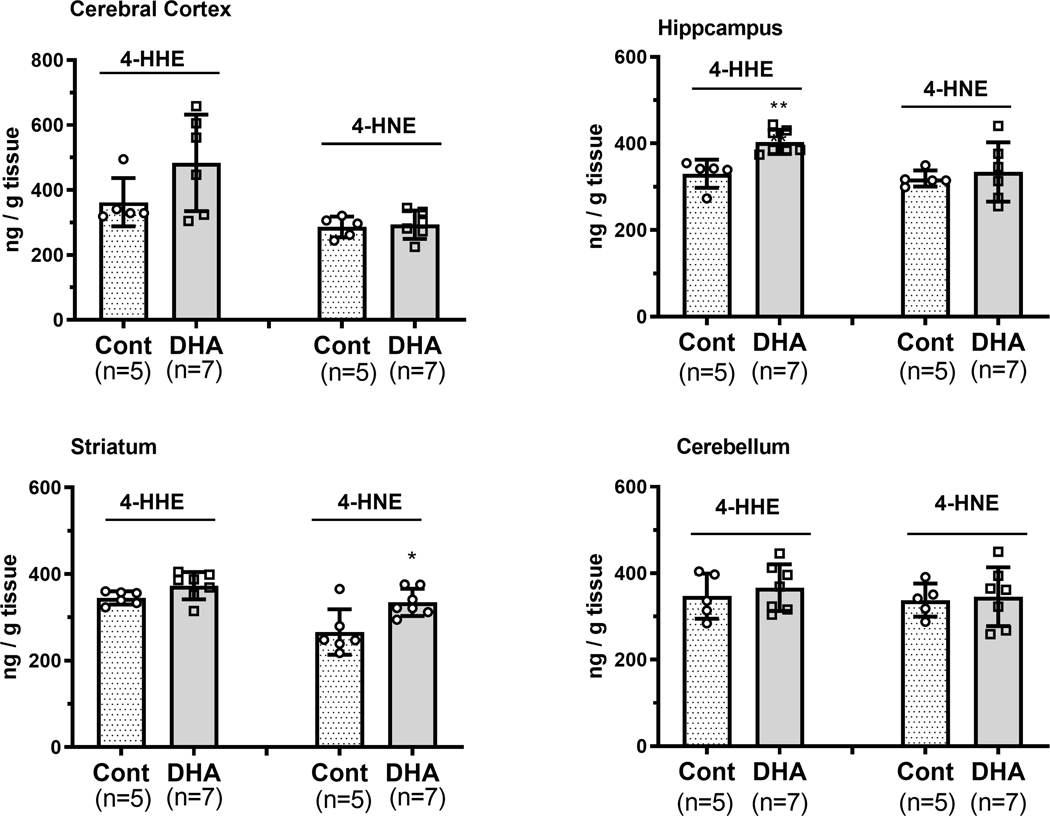
Levels of 4-HHE and 4-HNE in cerebral cortex, hippocampus, striatum and cerebellum in control and DHA supplement groups were determined. Among the different brain regions, only hippocampus showed a small but significant (p < 0.01) increase in 4-HHE (Figure 2). Although there was an apparent increase in 4-HHE in the cerebral cortex, this change was not significant (p = 0.12) due to the large variances. Except for the striatum which showed a small but significant (p < 0.05) increase in 4-HNE levels in the DHA-enriched diet group, no apparent changes in 4-HNE levels were found in other brain regions (Figure 2).
Figure 2.
Levels of 4-HHE and 4-HNE in mouse brain regions after feeding with a control or DHA-enriched (1%) diet for three weeks. Brain tissues including cerebral cortex, hippocampus, striatum, and cerebellum, were dissected and homogenized as described in text. Levels of 4-HHE and 4-HNE were determined by LC-MS/MS protocol as described in text. Data are normalized to tissue weight. Results represent the mean ± SEM of control (n = 5) and DHA (n = 7) samples. Analysis using a two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01.
In the heart tissue, levels of 4-HNE were higher than levels of 4-HHE in the control group (Figure 3A). Nevertheless, supplement with the DHA-enriched diet resulted in an almost two-fold increase in 4-HHE (p < 0.05) as compared to the control group (Figure 3A). Again, DHA-enriched diet did not alter 4-HNE levels in the heart tissue (Figure 3A).
Figure 3.
Levels of 4-HHE and 4-HNE in mouse (A) heart and (B) plasma after feeding with a control or DHA-enriched diet for three weeks. Procedures for processing the heart and plasma, and protocol for LC-MS/MS determination of 4-HHE and 4-HNE are described in text. Results represent the mean ± SEM of control (n = 5) and DHA (n = 7) samples. Analysis using two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01; *** p < 0.001.
In the plasma samples, there were higher levels of 4-HHE as compared to 4-HNE in the control group (Figure 3B). Similarly, levels of 4-HHE in the DHA-enriched group were increased significantly (p < 0.001) as compared to the controls. In the plasma, the level of 4-HNE in the DHA-enriched group showed a small but significant increase (p < 0.01) (Figure 3B).
3.3. Analysis of phospholipid species
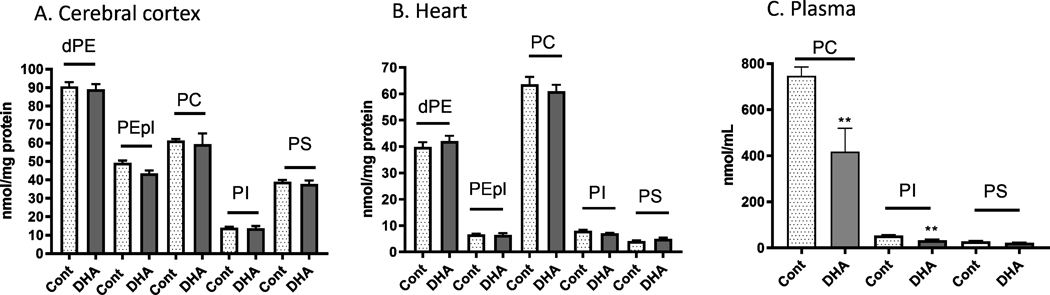
The major phospholipid classes in the cerebral cortex are diacyl-PE, alkenylacyl-PE (PE-pl, also PE plamalogen), PC, PS and PI (Figure 4A). Analysis also detected small amounts of alkylacyl-PE and alkenylacyl-PC species (data not shown). The major phospholipids in the heart tissue are PC and diacyl-PE, and unlike those in the cerebral cortex, only low levels of PEpl, PI and PS were detected (Figure 4B). In the plasma, although PC was the major phospholipid, low levels of PI and PS were detected (Figure 4C). However, the levels of diacyl-PE in plasma were too low to allow proper analysis of its molecular species. Levels of individual phospholipid classes in the cerebral cortex and heart were not different when comparing the DHA supplement group with the control group (Figure 4). Nevertheless, levels of PC and PI in plasma were significantly lower (p < 0.01) in the DHA supplement group as compared with the control group (Figure 4C).
Figure 4.
Levels of phospholipids (diacyl-PE, PEpl, PC, PI, and PS) in mouse (A) cerebral cortex, (B) heart, and (C) plasma after feeding a control and DHA-enriched diet for three weeks. Lipids were extracted and analyzed by the shotgun lipidomics platform as described in text. Data are expressed as nmol/mg protein, and are mean ± SEM of control (n=4) and DHA (n=4) samples. Analysis using two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01; *** p < 0.001. Abbreviations: dPE, diacyl-phosphatidylethanolamine, PEpl, alkenylacyl-phosphatidylethanolamine or ethanolamine plasmalogen, PC, phosphatidylcholine, PI, phosphatidylinositol, PS, phosphatidylserine.
In the cerebral cortex, analysis of the diacyl-PE species containing (n-6) PUFAs showed primarily the 18:0/20:4 species, and those with (n-3) were primarily 18:0/22:6 (Table S3 in the supplemental material). For PE-pl, where linkage in the sn-1 position is in alkenyl (ake) form, the species with (n-6) were comprised mainly of 18:0 ake/20:4 and 18:0 ake/22:4, and the (n-3) species were mainly 16:0 ake/22:6. For PC, the (n-6) species were primarily 16:0/20:4 and 18:0/20:4, and the (n-3) species were mainly 16:0/22:6 and 18:0/22:6. For PI, there were high levels of (n-6) species, mainly 18:0/20:4, and low levels of the (n-3) species, mainly 16:0/22:6. For PS, there were low levels of (n-6) species, mainly 18:0/20:4, and high levels of (n-3) species, mainly 18:0/22:6.
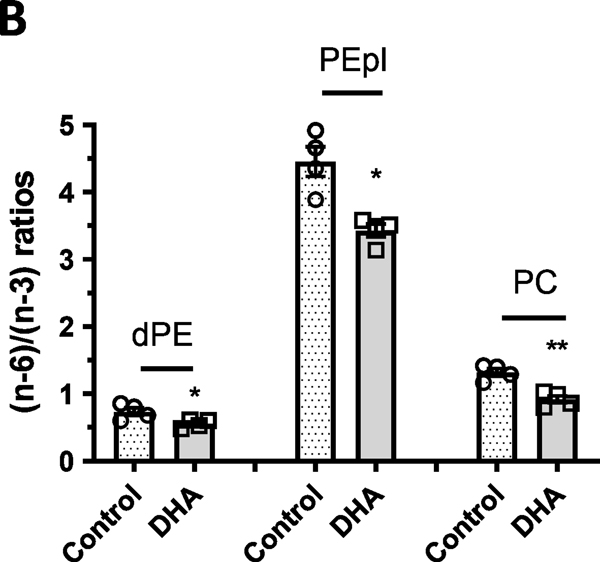
Among the diacyl-PE species in the cerebral cortex, there were more (n-3) species than (n-6) species, whereas in PE-pl, the (n-6) species were higher than (n-3) species (Figure 5A). PC had similar levels of (n-6) and (n-3) species, whereas PI contained high (n-6) species and PS contained high (n-3) species (Figure 5A). In all phospholipid species analyzed in the cerebral cortex, the DHA-enriched group showed a trend for increase in (n-3) species and a decrease in (n-6) species. In fact, significant (p < 0.05) decreases in the (n-6) species were observed in dPE, PE-pl and PS. When the ratios for (n-6)/(n-3) species in the control and DHA-enriched group were determined, all three major phospholipid classes (diacyl-PE, PE-pl, and PC) showed significant decreases (p < 0.05, p < 0.01) in (n-6) to (n-3) ratios in the DHA-enriched group as compared with the control group (Figure 5B).
Figure 5.
(A) Levels of (n-3) and (n-6) phospholipid species (dPE, PEpl, PC, PI and PS) in mouse cerebral cortex after feeding a control and DHA-enriched diet for three weeks. Lipids were extracted and analyzed by the shotgun lipidomics platform as described in text. In each phospholipid class, species with fatty acids containing (n-3) or (n-6) were grouped and expressed as nmol/mg protein. (B) Ratios of (n-6)/(n-3) phospholipid species of dPE, PEpl and PC from control and DHA group in the cortex using the data from (A). Data are mean ± SEM of control (n=4) and DHA (n=4) samples. Analysis using two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01.
Analysis of phospholipid species in the heart tissue showed significant larger effects of DHA supplementation to all phospholipid classes. Among the phospholipid classes analyzed (diacyl-PE, PC, PI and PS), the DHA-enriched group showed significant decreases in (n-6) species (p < 0.001), and significant increases in the (n-3) species (p < 0.05) in all classes examined (Figure 6). These results clearly indicate that a 3-week DHA-enriched diet is sufficient to cause alterations of membrane phospholipids in the heart tissue.
Figure 6.
Levels of (n-3) and (n-6) phospholipid species (dPE, PC, PI and PS) in mouse heart after feeding a control and DHA-enriched diet for three weeks. In each phospholipid class, species with fatty acids containing (n-3) or (n-6) were grouped and expressed as nmol/mg protein. Data are mean ± SEM of control (n=4) and DHA (n=4) samples. Analysis using two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01; *** p < 0.001.
In the plasma, PC with (n-6) PUFA is the predominant phospholipid species, and DHA-enriched diet resulted in a large and significant decrease (p < 0.001) in this phospholipid species (Figure 7). For PI in plasma, DHA-enriched diet resulted a large decrease in (n-6) and a small increase in (n-3) species (p < 0.001, p < 0.01, respectively) (Figure 7). Despite low levels, PS in plasma also showed a decrease in (n-6) and only a small increase in (n-3) species in this phospholipid (p < 0.01, p < 0.05, respectively) (Figure 7).
Figure 7.
Levels of (n-3) and (n-6) phospholipid species (PC, PI, and PS) in mouse plasma after feeding a control and DHA-enriched diet for three weeks. In each phospholipid class, species with fatty acids containing (n-3) or (n-6) were grouped and expressed as nmol/mg protein. Data are mean ± SEM of control (n=4) and DHA (n=4) samples. Analysis using two-tail unpaired t-test indicated significance between DHA group and controls. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. DISCUSSION
In many studies, including our own, there is evidence for the ability for DHA-enriched or diet with (n-3) fatty acids to alter fatty acid composition in brain and other body organs (Barceló-Coblijn, Collison, Jolly, & Murphy, 2005; Nakagawa et al., 2014; B. Yang, Li, et al., 2019). In a study by Murphy’s group, they further demonstrated increases in (n-3) and decreases in (n-6) fatty acids in different phospholipid classes in brain, heart and liver upon feeding rats with fish oil diet for 8 weeks (Barceló-Coblijn et al., 2005). Unlike the peripheral organs, linoleic acid (18:2n-6) is low in the brain tissue, and thus ARA and DHA contributed to the major (n-6) and (n-3) PUFAs linked to the sn-2 position of phospholipids. Despite that both PUFAs are abundant in the brain tissue, it is well recognized that they undergo different metabolic pathways and participate in different reactions: ARA is the precursor for multiple reactions leading to lipid mediators that are involved in pro-inflammatory functions, whereas DHA are known to production of oxylipins that are pro-resolving (B. Yang, Fritsche, et al., 2019). Recent studies also demonstrated involvement of both PUFAs in lipid peroxidation and production of 4-HNE from ARA and 4-HHE from DHA. Although the physiological role of these reactive aldehydes has not been clearly understood, there is increasing evidence for their engagement in specific cellular metabolism (Long & Picklo, 2010). In our previous study with the maternal mouse model, weaning pups nursed by mothers with the DHA-enriched diet showed increase in 4-HHE mainly in the cerebral cortex and hippocampus, despite that changes in fatty acid composition were observed in all brain regions, including cerebellum (B. Yang, Li, et al., 2019). In this study with adult mice, a three-week supplement with DHA-enriched diet again showed increase in 4-HHE in the hippocampus. These results are in agreement with the contention that the hippocampus is responsive to the DHA-enriched diet in producing more 4-HHE. Interestingly, despite that supplement of DHA-enriched or fish oil diet resulted in a decrease in ARA in all brain regions, there was no obvious decrease in 4-HNE in these brain regions. The study by Nakagawa’s group reported changes in fatty acids and peroxidation products (4-HHE and 4-HNE) in plasma and in multiple organs after feeding adult mice with fish oil for three weeks (Nakagawa et al., 2014). In their study, besides increase in 4-HHE, a significant decrease in 4-HNE was observed in the plasma. The discrepancy between our results and those by Nakagawa’s group is not known. In a study by Calzada et al., healthy human subjects given DHA supplement ranging from 200 to 1600 mg/day for two weeks also showed a dose-dependent increase in 4-HHE (but not 4-HNE) in plasma (Calzada et al., 2010). Therefore, these studies with animal models and humans provided consensus that DHA-enriched and fish oil diets are associated with increase in 4-HHE. Taken together, these results also show that determination of 4-HHE levels in plasma can be a useful biomarker for assessing DHA consumption. Since there is a concern that the increase in 4-HHE due to DHA-enriched or fish oil diets may be due to contamination of this peroxidation product in the diet, an attempt was made to determine the 4-HHE and 4-HNE content in the control and DHA-enriched diets used in our experiments. Our results indicated no detectable 4-HHE in the diets although trace amounts of 4-HNE (control 7.62 ± 0.21versus DHA 5.73 ± 0.15 ng/g) were detected in both control and DHA-enriched diet. The rationale for detecting 4-HNE in both control and DHA-enriched diets is not clear but since we could not detect 4-HHE, these results clearly show that the increase in 4-HHE levels in brain and other organs upon feeding the DHA-enriched diet is a physiological phenomenon. Several studies with cell and animal models have demonstrated the hormetic effects of 4-HHE through upregulation of the Nrf2 pathway and production of heme oxygenase-1, a potent antioxidant enzyme (Geng et al., 2020; Ishikado et al., 2010; Nakagawa et al., 2014; B. Yang et al., 2018). However, more studies are needed to examine possible physiological roles of these lipid peroxidation products in the heart and other body systems (Long & Picklo, 2010).
In this study, we examine changes in phospholipid species in the brain, heart and plasma after feeding with DHA-enriched diet for three weeks. In agreement with the presence of the blood brain barrier, uptake of fatty acids to the brain and their turnover are slower as compared to peripheral tissues such as heart (Patrick, McHowat, Rosenberger, Rapoport, & Murphy, 2005). Phospholipid classes in the cerebral cortex are comprised primarily of dPE, PE-pl, PC, PI and PS (Figure 4). Analysis of the molecular species of dPE, PE-pl, and PC in this brain region indicate small increase in (n-3) and decrease in (n-6) species for these phospholipids (Figure 5A). Although determination of (n-6)/(n-3) ratios showed significant decrease for dPE, PEpl and PC, it is obvious that a longer feeding time is required to obtain significant outcome (Bascoul-Colombo et al., 2016; Lamaziere et al., 2011). In this study, it is of interest that PE-pl showed a high level of (n-6) species, and the decrease in (n-3)/(n-6) ratios in PE-pl was greater for diacyl-PE and PC. In a study by Rosenberger et al., a two-hour i.v. injection of labeled hexadecanol (precursor for the alkyl group) showed a distribution of 97% in the gray matter and only 3% in myelin (Rosenberger, Oki, Purdon, Rapoport, & Murphy, 2002). This study demonstrated at least two pools of ether phospholipids, a static pool in the myelin and a dynamic pool in the synaptic area. Their findings together with the present results highlight the active metabolic function of plasmalogens in brain as well as their ability to participate in membrane signaling activity (Su, Wang, & Sinclair, 2019). Future investigation should examine whether dietary DHA may alter molecular species of this PE-pl in different brain regions and subcellular fractions.
Upon examining the molecular species of dPE and PC in heart, results showed significant decreases (p < 0.001) in (n-6) and concomitant increases (p < 0.05, 0.001, respectively) in (n-3) species in both phospholipids comparing the DHA-enriched diet group with controls (Figure 6). In a previous study by Murphy’s group, analysis of fatty acids in phospholipid classes (PC, PE, PI and PS) in rat heart after an 8-week feeding with fish oil also indicated increase in (n-3) and decrease in (n-6) fatty acids in all phospholipid classes (Barceló-Coblijn et al., 2005). In another study, feeding rats with different levels of fish oil diet also showed a time- and dose-dependent decrease in (n-6) and increase in (n-3) phospholipids in the heart tissue (Owen, Peter-Przyborowska, Hoy, & McLennan, 2004). As indicated in the review by Oppedisano et al., (n-3) fatty acids could offer vaso- and cardio-protective effects through modulation of membrane phospholipids and improve cardiac mitochondrial functions (Oppedisano et al., 2020). In our study, it seems that a three-week DHA-enriched diet is sufficient to change phospholipid species and peroxidation products in the heart tissue.
PC is the major phospholipid in plasma, and in the control group, plasma PC are comprised with high levels of (n-6) species and only low levels of (n-3) species (Figure 7). In this study, plasma obtained from DHA-enriched diet showed more than 70% decrease in the PC (n-6) species but only a small increase in the (n-3) species. As a result, the total level of plasma PC in the plasma from the DHA-enriched diet group was declined by almost 50% as compared with the control group (Figure 4C).
In agreement with earlier studies (Bascoul-Colombo et al., 2016; G. Y. Sun, Huang, Kelleher, Stubbs, & Sun, 1988), PI in brain showed high proportions of 20:4n-6, and PS with high proportions of 22:6 n-3 (Figure 5A). It is also recognized that PI and PS have distinctive functions, i.e., PI is involved in inositol metabolism associated with second messengers and intracellular calcium mobilization (Lin, Zhang, & Sun, 1993), whereas PS plays a role in membrane trafficking and endocytosis (Varga, Jiang, & Gong, 2020). In the study here, the three-week DHA-enriched diet did not greatly alter the (n-6) and (n-3) species in PI and PS in the cerebral cortex (Figure 5A), but this dietary regimen resulted in more than 50% decrease in (n-6) species and a concomitant increase in (n-3) species in both PI and PS in the heart (Figure 6). Considering the diverse functions of these two phospholipids, it is possible that a change in their fatty acid composition may link to its interaction to different enzymes and thus resulting in altered ability to regulate myocardial functions.
Our results indicate that DHA-enriched diet not only decrease phospholipid species containing 20:4(n-6), but also other (n-6) fatty acids, such as 20:4 (n-6) and 22:4 (n-6). These results suggest that changes in the (n-6) fatty acid pool are likely to occur at the initiation point of fatty acid elongation and desaturation. This is in agreement with the study showing that DHA could suppress metabolism of linoleic acid (18:2 n-6) and its ability to form elongation products, including 20:4(n-6) and 22:4(n-6) (Sullivan et al., 2018). Nevertheless, considering that phospholipid species with 20:4 (n-6) undergo active metabolism through the diacylation-reacylation cycle, and its release by cytosolic phospholipase A2 is linked to inflammatory pathways, the ability for DHA-enriched diet to decrease phospholipids with (n-6) species may be advantageous in suppressing cell membranes against inflammatory responses.
5. CONCLUSIONS
Albeit to different extents, feeding adult mice a DHA-enriched diet for three weeks resulted in changes in (n-6) and (n-3) phospholipid species and alteration of lipid peroxidation products in the brain, heart and plasma. The increase in (n-3) phospholipids due to DHA-enriched diet is associated with the increase in 4-HHE but not 4-HNE. In brain, the increase in 4-HHE appears to be mainly observed in the hippocampus, suggesting higher lipid peroxidation activity in this brain region. The decrease in (n-6) species in the heart tissue suggests the possibility that the DHA dietary regimen can mitigate ARA metabolism associated with inflammatory and oxidative activity.
Supplementary Material
Supplemental Table S1. Experimental mouse diets
Supplemental Table S2. Fatty acid composition of diets
Supplemental Table S3. Lipidomic analysis of mouse cerebral cortex samples
Funding:
This study is supported by the MU Research Council grant (17-009) and support from Department of Radiology Mission Enhancement funds from University of Missouri to DQB, and NIH/NIA RF1 AG061872 to XH. We wish to thank DSM Nutritional Products (USA) for donating DHASCO, the source of dietary DHA used in this study. MC’s visit to University of Missouri was funded in part by a scholarship for young scientists from the Polish Academy of Sciences and Mossakowski Medical Research Centre, Poland.
Abbreviations
- 4-HHE
4-hydroxyhexenal
- 4-HNE
4-hydroxynonenal
- ARA
arachidonic acid
- CHD
1,3-cyclohexanedione
- cPLA2
cytosolic phospholipases A2
- CTL
control
- DHA
docosahexaenoic acid
- HO-1
heme oxygenase-1
- HPLC
high performance liquid chromatography
- iPLA2
Ca+2-independent phospholipases A2
- LC-MS/MS
liquid chromatography-mass spectrometry/mass spectrometry
- MS
mass spectrometry
- Nrf2
nuclear factor erythroid 2-related factor 2
- PBS
phosphate-buffered saline
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- dPE
diacyl-phosphatidylethanolamine
- PE-p1
alkenylacyl-phosphatidylethanolamine or phosphatidylethanolamine plasmalogen
- PI
phosphatidylinositol
- PL
phospholipids
- PLA2
phospholipases A2
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acid
- UHPLC
ultra-high performance liquid chromatography
Footnotes
DECLARATIONS
Conflicts of Interest: The authors declare no conflict of interest.
Compliance with Ethical Standards: All experiments were approved by the University of Missouri Animal Care and Use Committee (#8945) and were performed in compliance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Availability of data and material:
Original data for the manuscript is available upon written request of the corresponding author.
REFERENCES
- Agostoni C, Nobile M, Ciappolino V, Delvecchio G, Tesei A, Turolo S, et al. (2017). The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int J Mol Sci, 18(12). doi: 10.3390/ijms18122608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R, Vitale G, & Bertolotti M.(2019). Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int J Mol Sci, 20(17). doi: 10.3390/ijms20174256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló-Coblijn Gwendolyn, Collison Lauren W., Jolly Christopher A., & Murphy Eric J. (2005). Dietary α-linolenic acid increases brain but not heart and liver docosahexaenoic acid levels. Lipids, 40(8), 787–798. doi: 10.1007/s11745-005-1440-y [DOI] [PubMed] [Google Scholar]
- Bascoul-Colombo C, Guschina IA, Maskrey BH, Good M, O’Donnell VB, & Harwood JL (2016). Dietary DHA supplementation causes selective changes in phospholipids from different brain regions in both wild type mice and the Tg2576 mouse model of Alzheimer’s disease. Biochim Biophys Acta, 1861(6), 524–537. doi: 10.1016/j.bbalip.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG (2018). Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol Aspects Med, 64, 18–33. doi: 10.1016/j.mam.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan Nicolas G., Molina Miguel F., & Gordon William C. (2011). Docosahexaenoic Acid Signalolipidomics in Nutrition: Significance in Aging, Neuroinflammation, Macular Degeneration, Alzheimer’s, and Other Neurodegenerative Diseases. Annual Review of Nutrition, 31(1), 321–351. doi: 10.1146/annurev.nutr.012809.104635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkouch M, Hachem M, Elgot A, Lo Van A, Picq M, Guichardant M, et al. (2016). The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J Nutr Biochem, 38, 1–11. doi: 10.1016/j.jnutbio.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Calzada C, Colas R, Guillot N, Guichardant M, Laville M, Vericel E, et al. (2010). Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis, 208(2), 467–472. doi: 10.1016/j.atherosclerosis.2009.07.049 [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Garg ML, & Hazell PL (2007). Long chain omega-3 polyunsaturated fatty acids in the treatment of psychiatric illnesses in children and adolescents. Acta Neuropsychiatr, 19(2), 92–103. doi: 10.1111/j.1601-5215.2007.00189.x [DOI] [PubMed] [Google Scholar]
- Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, et al. (2013). Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med, 65, 978–987. doi: 10.1016/j.freeradbiomed.2013.08.163 [DOI] [PubMed] [Google Scholar]
- Domenichiello Anthony F., Kitson Alex P., & Bazinet Richard P. (2015). Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Progress in Lipid Research, 59, 54–66. doi: 10.1016/j.plipres.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Duda MK, O’Shea KM, & Stanley WC (2009). omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res, 84(1), 33–41. doi: 10.1093/cvr/cvp169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Yang B, Li R, Teng T, Ladu MJ, Sun GY, et al. (2020). Effects of Docosahexaenoic Acid and Its Peroxidation Product on Amyloid-beta Peptide-Stimulated Microglia. Mol Neurobiol, 57(2), 1085–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger MW, Liu H, Fowler CF, Blanchard AP, Taylor M, Sherman SPM, et al. (2019). Distinct disruptions in Land’s cycle remodeling of glycerophosphocholines in murine cortex mark symptomatic onset and progression in two Alzheimer’s Disease mouse models. J Neurochem. 149, 499–517, doi: 10.1111/jnc.14560. [DOI] [PubMed] [Google Scholar]
- Han X, Yang K, & Gross RW (2008). Microfluidics-based electrospray ionization enhances the intrasource separation of lipid classes and extends identification of individual molecular species through multi-dimensional mass spectrometry: development of an automated high-throughput platform for shotgun lipidomics. Rapid Commun Mass Spectrom, 22(13), 2115–2124. doi: 10.1002/rcm.3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Maekawa M, Katakura M, Hamazaki K, & Matsuoka Y.(2014). Possibility of polyunsaturated fatty acids for the prevention and treatment of neuropsychiatric illnesses. J Pharmacol Sci, 124(3), 294–300. doi: 10.1254/jphs.13r14cp [DOI] [PubMed] [Google Scholar]
- Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, et al. (2010). Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun, 402(1), 99–104. [DOI] [PubMed] [Google Scholar]
- Jump DB, Depner CM, & Tripathy S.(2012). Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res, 53(12), 2525–2545. doi: 10.1194/jlr.R027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaziere A, Richard D, Barbe U, Kefi K, Bausero P, Wolf C, et al. (2011). Differential distribution of DHA-phospholipids in rat brain after feeding: A lipidomic approach. Prostaglandins Leukot Essent Fatty Acids, 84(1–2), 7–11. doi: 10.1016/j.plefa.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Brambilla P, Mazzocchi A, Harslof LB, Ciappolino V, & Agostoni C.(2016). DHA Effects in Brain Development and Function. Nutrients, 8(1). doi: 10.3390/nu8010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Wong HY, Chai YY, Shi CW, Amino N, Kikuchi S, et al. (2012). Lipid peroxidation dysregulation in ischemic stroke: plasma 4-HNE as a potential biomarker? Biochem Biophys Res Commun, 425(4), 842–847. doi: 10.1016/j.bbrc.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Lin TA, Zhang JP, & Sun GY (1993). Metabolism of inositol 1,4,5-trisphosphate in mouse brain due to decapitation ischemic insult: effects of acute lithium administration and temporal relationship to diacylglycerols, free fatty acids and energy metabolites. Brain Res, 606(2), 200–206. doi: 10.1016/0006-8993(93)90985-v [DOI] [PubMed] [Google Scholar]
- Long EK, & Picklo MJ Sr. (2010). Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radic Biol Med, 49(1), 1–8. doi: 10.1016/j.freeradbiomed.2010.03.015 [DOI] [PubMed] [Google Scholar]
- Matsui F, Hecht P, Yoshimoto K, Watanabe Y, Morimoto M, Fritsche K, et al. (2018). DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model. Neuroscience, 371, 407–419. doi: 10.1016/j.neuroscience.2017.12.029 [DOI] [PubMed] [Google Scholar]
- Maulucci G, Cohen O, Daniel B, Sansone A, Petropoulou PI, Filou S, et al. (2016). Fatty acid-related modulations of membrane fluidity in cells: detection and implications. Free Radic Res, 50(sup1), S40–S50. doi: 10.1080/10715762.2016.1231403 [DOI] [PubMed] [Google Scholar]
- Mita T, Mayanagi T, Ichijo H, Fukumoto K, Otsuka K, Sakai A, et al. (2016). Docosahexaenoic Acid Promotes Axon Outgrowth by Translational Regulation of Tau and Collapsin Response Mediator Protein 2 Expression. J Biol Chem, 291(10), 4955–4965. doi: 10.1074/jbc.M115.693499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Reichel M, Muhle C, Rhein C, Gulbins E, & Kornhuber J.(2015). Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta, 1851(8), 1052–1065. doi: 10.1016/j.bbalip.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Nakagawa F, Morino K, Ugi S, Ishikado A, Kondo K, Sato D, et al. (2014). 4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem Biophys Res Commun, 443(3), 991–996. doi: 10.1016/j.bbrc.2013.12.085 [DOI] [PubMed] [Google Scholar]
- Oppedisano Francesca, Macrì Roberta, Gliozzi Micaela, Musolino Vincenzo, Carresi Cristina, Maiuolo Jessica, et al. (2020). The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines, 8(9), 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AJ, Peter-Przyborowska BA, Hoy AJ, & McLennan PL (2004). Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids, 39(10), 955–961. doi: 10.1007/s11745-004-1317-0 [DOI] [PubMed] [Google Scholar]
- Patrick Casey B., McHowat Jane, Rosenberger Thad A., Rapoport Stanley I., & Murphy Eric J. (2005). Arachidonic acid incorporation and turnover is decreased in sympathetically denervated rat heart. American Journal of Physiology-Heart and Circulatory Physiology, 288(6), H2611–H2619. doi: 10.1152/ajpheart.00549.2004 [DOI] [PubMed] [Google Scholar]
- Riahi Y, Cohen G, Shamni O, & Sasson S.(2010). Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab, 299(6), E879–886. doi: 10.1152/ajpendo.00508.2010 [DOI] [PubMed] [Google Scholar]
- Rosenberger Thad A., Oki Jun, Purdon A. David, Rapoport Stanley I., & Murphy Eric J. (2002). Rapid synthesis and turnover of brain microsomal ether phospholipids in the adult rat. Journal of Lipid Research, 43(1), 59–68. [PubMed] [Google Scholar]
- Sakamoto A, Saotome M, Iguchi K, & Maekawa Y.(2019). Marine-Derived Omega-3 Polyunsaturated Fatty Acids and Heart Failure: Current Understanding for Basic to Clinical Relevance. Int J Mol Sci, 20(16). doi: 10.3390/ijms20164025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, & Dalli J.(2015). The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol, 27(3), 200–215. doi: 10.1016/j.smim.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XQ, Wang J, & Sinclair AJ (2019). Plasmalogens and Alzheimer’s disease: a review. Lipids Health Dis, 18(1), 100. doi: 10.1186/s12944-019-1044-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EM, Pennington ER, Sparagna GC, Torres MJ, Neufer PD, Harris M, et al. (2018). Docosahexaenoic acid lowers cardiac mitochondrial enzyme activity by replacing linoleic acid in the phospholipidome. J Biol Chem, 293(2), 466–483. doi: 10.1074/jbc.M117.812834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Chuang DY, Zong Y, Jiang J, Lee JC, Gu Z, et al. (2014). Role of cytosolic phospholipase A2 in oxidative and inflammatory signaling pathways in different cell types in the central nervous system. Mol Neurobiol, 50(1), 6–14. doi: 10.1007/s12035-014-8662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Huang HM, Kelleher JA, Stubbs EB Jr., & Sun AY (1988). Marker enzymes, phospholipids and acyl group composition of a somal plasma membrane fraction isolated from rat cerebral cortex: a comparison with microsomes and synaptic plasma membranes. Neurochem Int, 12(1), 69–77. doi: 10.1016/0197-0186(88)90150-7 [DOI] [PubMed] [Google Scholar]
- Sun GY, & MacQuarrie RA (1989). Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci, 559, 37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x [DOI] [PubMed] [Google Scholar]
- Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, et al. (2018). Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids, 136, 3–13. doi: 10.1016/j.plefa.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Li R, Yang B, Fritsche KL, Beversdorf DQ, Lubahn DB, et al. (2019). Quercetin Potentiates Docosahexaenoic Acid to Suppress Lipopolysaccharide-induced Oxidative/Inflammatory Responses, Alter Lipid Peroxidation Products, and Enhance the Adaptive Stress Pathways in BV-2 Microglial Cells. Int. J. Mol. Sci, 20, 932. doi: 10.3390/ijms20040932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer G, Cui J, Ndam T, Bekemeier M, Song H, Li R, et al. (2020). Harpagophytum procumbens Extract Ameliorates Allodynia and Modulates Oxidative and Antioxidant Stress Pathways in a Rat Model of Spinal Cord Injury. Neuromolecular Med. 22, 278–292, doi: 10.1007/s12017-019-08585-z. [DOI] [PubMed] [Google Scholar]
- Varga K, Jiang ZJ, & Gong LW (2020). Phosphatidylserine is critical for vesicle fission during clathrin-mediated endocytosis. J Neurochem, 152(1), 48–60. doi: 10.1111/jnc.14886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, & Han X.(2014). Multidimensional mass spectrometry-based shotgun lipidomics. Methods Mol Biol, 1198, 203–220. doi: 10.1007/978-1-4939-1258-2_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang C, & Han X.(2017). Selection of internal standards for accurate quantification of complex lipid species in biological extracts by electrospray ionization mass spectrometry-What, how and why? Mass Spectrom Rev, 36(6), 693–714. doi: 10.1002/mas.21492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Fritsche KL, Beversdorf DQ, Gu Z, Lee JC, Folk WR, et al. (2019). Yin-Yang Mechanisms Regulating Lipid Peroxidation of Docosahexaenoic Acid and Arachidonic Acid in the Central Nervous System. Front Neurol, 10, 642. doi: 10.3389/fneur.2019.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Li R, Greenlief CM, Fritsche KL, Gu Z, Cui J, et al. (2018). Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J Neuroinflammation, 15(1), 202. doi: 10.1186/s12974-018-1232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Li R, Woo T, Browning JD Jr., Song H, Gu Z, et al. (2019). Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice. Metabolites, 9(3), 40. doi: 10.3390/metabo9030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Cheng H, Gross RW, & Han X.(2009). Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem, 81(11), 4356–4368. doi: 10.1021/ac900241u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, et al. (2014). Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J Neurosci, 34(5), 1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Ding Y, Kong W, Li T, & Chen H.(2018). Docosahexaenoic Acid (DHA) Provides Neuroprotection in Traumatic Brain Injury Models via Activating Nrf2-ARE Signaling. Inflammation, 41(4), 1182–1193. doi: 10.1007/s10753-018-0765-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Experimental mouse diets
Supplemental Table S2. Fatty acid composition of diets
Supplemental Table S3. Lipidomic analysis of mouse cerebral cortex samples