Abstract
Background:
Information on HIV drug resistance (HIVDR) prevalence in people newly diagnosed with HIV is limited. We implemented a cross-sectional study to estimate HIVDR prevalence among pregnant women recently infected with HIV in Malawi.
Methods:
The HIVDR study was nested within a routine antenatal clinic (ANC) sentinel surveillance survey. Dried blood spot samples were tested for recent infection using a limiting antigen antibody assay together with HIV viral load testing. HIV-1 protease and reverse transcriptase were sequenced using Sanger sequencing. Drug susceptibility was predicted using Stanford HIVdb algorithm (version 8.9). Weighted analysis was performed in Stata 15.1.
Results:
Of the 21,642 pregnant women enrolled in the ANC survey, 8.4% (1826/21,642) tested HIV positive. Of these, 5.0% (92/1826) had recent HIV infection, and 90.2% (83/92) were tested by PCR. The amplification and sequencing success rate was 57.8% (48/83). The prevalence of any HIVDR was 14.6% (5/45) (95% CI: 4.7–36.8%), all of which indicated HIVDR to nonnucleoside reverse transcriptase inhibitors (NNRTIs). HIVDR to nucleoside reverse transcriptase inhibitors was 7.9% (2/45) (95% CI: 1.4–34.6%). Resistance to protease inhibitors currently in use in Malawi was not observed.
Conclusions:
Despite the low number of cases with presumed TDR, our study hints that resistance to NNRTIs was high, above the 10% target for regimen change. Further investigation is needed to establish the exact magnitude of presumed TDR among women recently infected with HIV. These findings support the transition to an integrase inhibitor-based first-line regimen for patients initiating or on ART.
Introduction
With the scale-up of antiretroviral therapy (ART) in countries with generalised HIV epidemics, regular monitoring of HIV drug resistance (HIVDR) is needed [1]. Malawi has implemented HIVDR surveillance using methods derived from the World Health Organisation (WHO) since 2004.
Documented levels of pre-treatment HIVDR in the past decade range from <5% to over 25% [2–4]. In 2009, an HIVDR survey was conducted in Malawi’s largest cities, Lilongwe and Blantyre, using WHO methods to classify presumed transmitted drug resistance (TDR) to nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) among primigravida women aged <25 years. Results from Lilongwe showed that while presumed TDR to NRTIs and PIs was <5%, presumed TDR to NNRTIs was 5%–15%; in Blantyre, presumed TDR was <5% to all drug classes [5]. A more recent study showed higher levels of transmitted NNRTI resistance (11%) according to surveillance drug resistance mutations (SDRM) definitions, and 20% when additional NNRTI polymorphisms that may affect treatment response were included [6].
With widespread use of ART, there is an increased likelihood of TDR in individuals recently infected with HIV, which may lead to a rapid decline of CD4 cell counts, limiting the magnitude and duration of treatment response [7,8]. ART initiation during the acute/recent phase of HIV infection limits HIV reservoirs and improves immune response [9,10]. The assessment of TDR monitors the emergence and spread of drug-resistant strains and informs HIV treatment guidelines. We implemented a cross-sectional study to estimate HIVDR prevalence among recently HIV-infected pregnant women attending antenatal (ANC) services in Malawi.
Methods
This study was nested within the national 2016 HIV sentinel surveillance survey, conducted in Malawi’s 54 sentinel facilities. Pregnant women who provided informed consent at their first ANC visit of the current pregnancy were included. Venous blood specimens were collected and spotted on two dried blood spot cards, one for the sentinel surveillance survey and the other for the current HIVDR survey, and then frozen at ≤ −20°C. All HIV positive samples from ART naïve women with no known prior HIV diagnosis were tested based on Murex HIV Ag/Ab Combination Enzyme immunoassay (DIASORIN South Africa (PTY) Ltd, Gauteng, South Africa) and genius kits (Bio-Rad Laboratories, Inc, Johannesburg, South Africa) with Western Blot (Bio-Rad Laboratories, Inc, Johannesburg, South Africa) as a tie breaker. HIV positive samples were then tested for recent infection using the Maxim Bio-medical Limiting Antigen assay (LAg) Avidity DBS Enzyme Immunoassay (Maxim Biomedical, Inc, Rockville, USA). Those with normalized optical density (ODn) values less than 1.5 were tested for viral load (VL). Consistent with WHO definitions [11], all women with VL ≥ 1000 copies/ml were categorized as recent infections and tested for HIVDR. HIV-1 protease and reverse transcriptase were analysed at the CDC International Laboratory Branch of the Division of Global HIV and Tuberculosis using Sanger sequencing [HIV-1 Genotyping Kit (Thermo Fisher Scientific, Waltham, MA USA)] per manufacture instructions. HIVDR post-testing quality assurance was performed using the WHO/BCCfE HIVDR quality control tool [12]. Drug susceptibility was predicted using the Calibrated Population Resistance tool for SDRMs from the Stanford HIVdb algorithm (version 8.9). HIV-1 subtypes were assigned using the Stanford method. Any mutation or combination of mutations that produced low level-, intermediate- or high-level resistance was defined as having HIV drug resistance to that drug or drug class.
All analyses were weighted for selection probability. Individual sampling weights were calculated based on the number of pregnant women sampled per clinic site divided by the clinic size and were then inversed to calculate probability weights. All analyses were conducted in Stata (Version 15.1, Stata Corporation, College Station, TX, USA).
Ethical approval was given by the National Health Sciences Research Committee in Malawi. This project was reviewed in accordance with CDC human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. All participants provided written informed consent and were anonymized.
Results
Of the 21,642 pregnant women enrolled in the sentinel surveillance survey, 8.4% (1826/21,642) tested positive for HIV. Of these, 73.4% (1340/1826) had an ODn ≥1.5 and 26.6% (486/1826) had an ODn<1.5. Of the 486 with an ODn < 1.5, 81% (394/486) had a VL <1000 copies/mL and were excluded from resistance testing. The remaining 92 women (5.0%) had VL≥1000 copies per mL and were defined as recent HIV infections (Figure 1).
Figure 1.
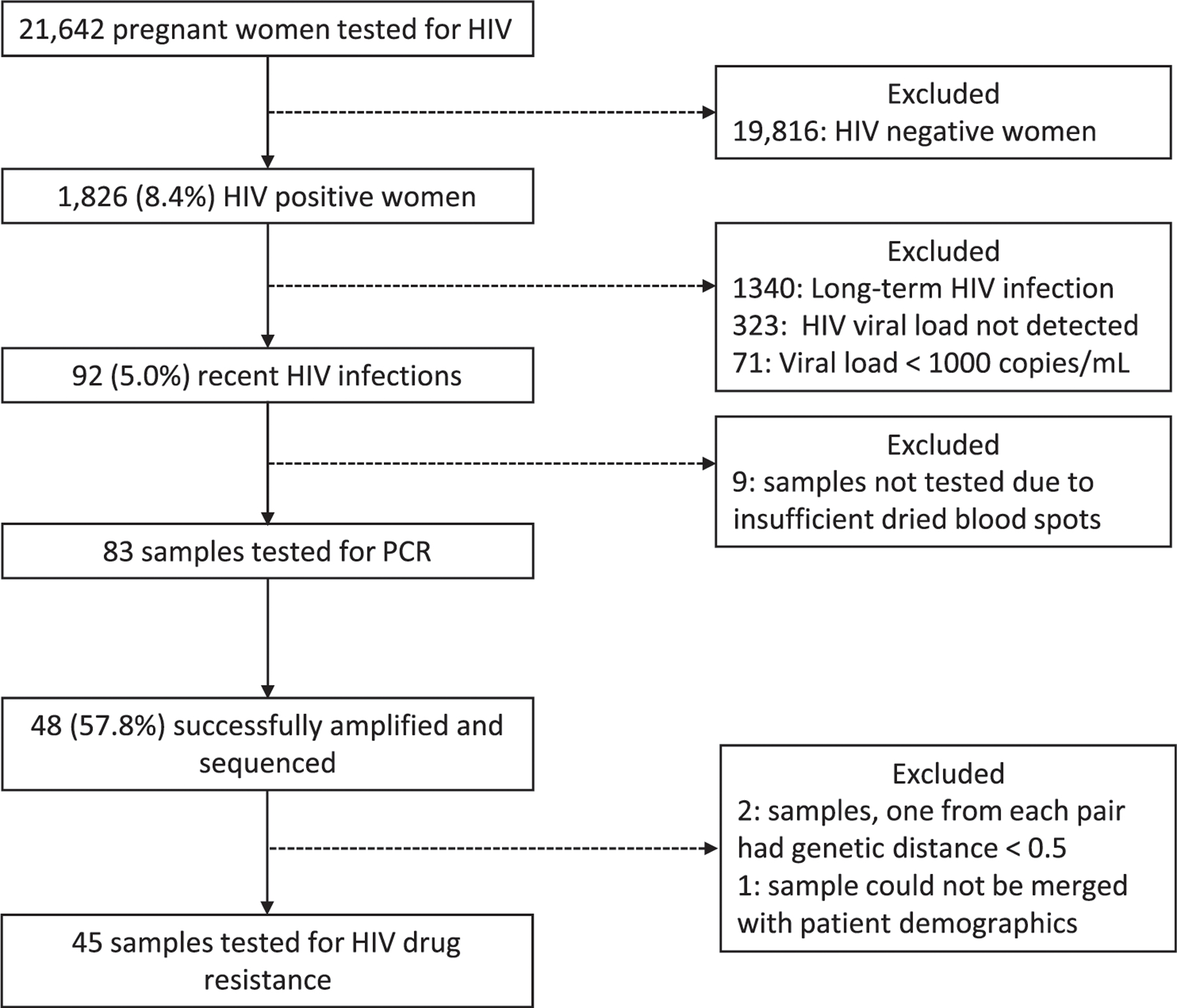
Study participant flow diagram.
Nine samples could not be tested due to insufficient dried blood spots remaining after HIV testing. Of the 83 samples tested by PCR, 57.8% (48/83), were successfully amplified and sequenced. Three additional samples were subsequently excluded; the genetic distances were <0.5% in two sequence pairs of four samples and one sample could not be linked with participant data. A total of 45 samples were analysed to estimate the proportion of HIVDR among pregnant women recently infected with HIV (Figure 1).
Of the 45 samples analysed for HIVDR, 97.8% (44/45) (95% confidence interval [CI]: 85.1–99.7%) were infected with HIV-1 subtype C and 2.2% (1/45) (95% CI: 0.3–14.9%) with subtype B. (Table 1) Five women had HIVDR (14.6%, 95% CI: 4.7–36.8%) all of whom presented with NNRTI drug resistance to nevirapine or efavirenz. Two women had also NRTI drug resistance mutations (DRMs) (7.9%, 95% CI: 1.4–34.6%). Resistance to the PIs darunavir, lopinavir, atazanavir or ritonavir was not observed. Prevalence of resistance to tenofovir disoproxil fumarate (TDF) or lamivudine (FTC/3 TC) was 1.1% (1/45) (95% CI: 0.1–7.8%) (Figure 2). The common mutations observed were V106 M, E138AG and Y181 C.
Table 1.
Characteristics of recently HIV-infected pregnant women in 54 antenatal care sites in Malawi, 2016 (N = 45).
| Characteristics | n/N | % (95% CI) |
|---|---|---|
| HIV subtype | ||
| HIV-1 subtype C | 44 | 97.8 (85.1–99.7) |
| HIV-1 subtype B | 1 | 2.2 (0.3–14.9) |
| Location | ||
| Rural | 11 | 24.4 (13.9–39.4) |
| Urban | 34 | 75.6 (60.6–86.1) |
| Age | ||
| 15–24 years | 24 | 53.3 (38.5–67.6) |
| 25–49 years | 21 | 46.7 (32.4–61.5) |
| Gravidity | ||
| Primigravida | 13 | 28.9 (17.3–44.1) |
| Non primigravida | 32 | 71.1 (55.9–82.7) |
Figure 2.

Weighted prevalence of pre-treatment HIVDR by drug among recently HIV-infected pregnant women in Malawi, 2016. ABC, abacavir; d4T, stavudine; ddI, didanosine; 3 TC/FTC, lamivudine/emtricitabine; TDF, tenofovir; ZDV, zidovudine; DOR, doravirine; EFV, efavirenz; ETV, etravirine; NVP, nevirapine; RPV, rilpivirine; ATV/r, atazanavir/ritonavir; DRV/r, darunavir/ritonavir; FPV/r, fosamprenavir/ritonavir; IDV/r, indinavir/ritonavir; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; SQV/r, saquinavir/ritonavir; TPV/r, tipranavir/ritonavir; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Discussion
Our study identified 5% of HIV positive ANC women with evidence of recent infection, about half of whom could be analysed for drug resistance, with low amplification rate likely due, in part, to the long storage time. Among this group, prevalence of NNRTI resistance was >10% while resistance to TDF and/or FTC/3 TC was low. PI resistance mutations were absent. This is consistent with the high population exposure to NNRTIs, as nevirapine (NVP) and efavirenz (EFV) were used in all first-line ART regimens since 2004. The absence of PI resistance mutations is due to the almost exclusive use of PIs in second-line regimens and only 1% of patients on ART in 2016 were on second-line regimens. The level of presumed TDR (14.6%) is consistent with other recently published data in Malawi, where NNRTI resistance was estimated at 11% among persons with recent HIV infection [6]. The results also align with recent WHO surveillance in southern and eastern Africa estimating pre-treatment drug resistance at 10% [13]. Our HIVDR results support the WHO recommendations to transition to first-line ART regimens that include integrase inhibitors such as dolutegravir (DTG), in the context of public health-driven treatment programmes [14].
Although DTG is a preferred first-line backbone ART for its favourable safety profile, clinical tolerability, high resistance barrier and cost-effectiveness [15], surveillance activities to monitor the safety and drug resistance among pregnant women initiating DTG-based regimens, should be pursued. Pregnant women represent a unique population for monitoring potential emergence of resistance in infants considering the transplacental and breastmilk passage of DTG [16]. It is also unknown if the transition to DTG-based ART regimens in Malawi, which was completed for adults and children in 2021, will mitigate the treatment failure risk from TDR from maternal ART use and acquired DR from NVP prophylaxis for HIV-exposed infants. Future studies are needed to estimate the prevalence of NRTI resistance in infants and mothers taking DTG-based regimens. In an analysis of data from laboratories performing viral load testing in Malawi, the rate of virologic suppression among pregnant women receiving TDF/3 TC/dolutegravir was 94% (95% CI: 92.8–95.0%; N = 1947) in 2020 and 92% (95% CI: 90.7–93.1%, N = 1932) in 2021 (unpublished data). Additional studies on drug resistance should be performed.
Our definition of “recent” included a default threshold for LAg (ODn < 1.5); however, there is uncertainty on how long this measurement stays below 1.5 in pregnant women. The estimated window of infection for recent infections in the general population could be up to 12 months. The low VL in DBS can be artefactual if the DBS were not stored properly, leading to a biased under-identification of recent infection samples for resistance testing. However, storage conditions in this study were actively monitored to ensure sample quality. Our recent infection testing algorithm may have misclassified some people who recently interrupted treatment or had erratic adherence and developed viral rebound ≥1000 copies/ml, but whose antibody response had not recovered. Our finding of 5% recent infection is consistent with other surveillance data that show recent infection of 4.98% and 7.76% among 15–24 and 25–34 years old Malawian women, respectively [17]. However, the small number of presumed TDR cases observed due to the low rate of recent infections limits the generalizability of our findings, especially to populations with higher recent infection rates. The age of the samples that were tested for drug resistance could have affected the amplification rate. However, a sensitivity analysis found no statistically significant differences between samples that were and were not successfully amplified with regard to demographic characteristics and time elapsed between sample collection and amplification.
Conclusion
Despite the low number of overall cases with presumed TDR, our study found 14.6% resistance to NNRTIs, which is above the 10% threshold for regimen change recommended by the WHO. Further investigation is needed to establish the exact magnitude of presumed TDR among women recently infected with HIV. This high prevalence of NNRTI resistance suggests that EFV-based regimens are not ideal for PMTCT programmes and treatment of HIV positive adults. Our findings support the transition to integrase inhibitors as the backbone of first-line regimens for the treatment of newly diagnosed HIV-infected individuals initiating ART in Malawi.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement # GH000649.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bertagnolio S, Beanland RL, Jordan MR, Doherty M, Hirnschall G. The world health organization’s response to emerging human immunodeficiency virus drug resistance and a call for global action. J Infect Dis 2017; 216(suppl_9): S801–S804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerma RS, Sigaloff KC, Akanmu AS, et al. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: a systematic review and meta-analysis. J Antimicrob Chemother 2017; 72(2): 365–371. [DOI] [PubMed] [Google Scholar]
- 3.Avila-Rios S, Garcia-Morales C, Matias-Florentino M, et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV 2016; 3(12):e579–e591. [DOI] [PubMed] [Google Scholar]
- 4.Kang RH, Liang SJ, Ma YL, et al. Pretreatment HIV drug resistance in adults initiating antiretroviral therapy in China, 2017. Infect Dis Poverty 2020; 9(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadonda-Kabondo N, Banda R, Moyo K, et al. Prevalence of transmitted HIV drug resistance among newly diagnosed anti-retroviral therapy-naive pregnant women in Lilongwe and Blantyre, Malawi. Clin Infect Dis 2012; 54(Suppl 4):S324–S327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutstein SE, Chen JS, Nelson JAE, Phiri S, Miller WC, Hosseinipour MC. High rates of transmitted NNRTI resistance among persons with acute HIV infection in Malawi: implications for first-line dolutegravir scale-up. AIDS Res Ther 2019; 16(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med 2015; 175(1):88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phanuphak P, Sirivichayakul S, Jiamsakul A, et al. Transmitted drug resistance and antiretroviral treatment outcomes in non-subtype B HIV-1-infected patients in South East Asia. J Acquir Immune Defic Syndr 2014; 66(1):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wittkop L, Gunthard HF, de Wolf F, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis 2011; 11(5):363–371. [DOI] [PubMed] [Google Scholar]
- 10.Laanani M, Ghosn J, Essat A, et al. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 Infection on the Decay of Cell-Associated HIV-DNA. Clin Infect Dis 2015; 60(11):1715–1721. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Working Group on HIV incidence measurement and data use. 2018. Contract No: (WHO/CDS/HIV/18.9) Licence: CC BY-NC-SA 3.0 IGO
- 12.WHO HIVResNet. HIV Drug Resistance Laboratory Operational Framework Geneva, Switzerland: World Health Organization, 2017. Report No: 73. [Google Scholar]
- 13.World Health Organization. HIV Drug Resistance Report 2017 Geneva, Switzerland: Wolrd Health Organization, 2017. [Google Scholar]
- 14.World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens Geneva, Switzerland: World Health Organization, 2019. Report No: Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 15.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health 2018; 6(7):e804–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson L, Walimbwa S, Singh Y, et al. Infant Exposure to dolutegravir through placental and breast milk transfer: a population pharmacokinetic analysis of DolPHIN-1. Clin Infect Dis 2021; 73(5):e1200–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health Malawi. Malawi Population-Based HIV Impact Assessment (MPHIA) 2015–2016: Final Report Lilongwe: Ministry of Health Malawi, 2018. [Google Scholar]


