Abstract
Chronic consumption of a large quantity of alcohol often results in the disruption of the communication between the nervous, endocrine and immune systems leading to profound and serious consequences at the physiological and behavioral levels. The overall impact of excessive alcohol consumption on bone health, metabolic profile and body composition, especially at moderate levels, is not well understood. Chronic excessive alcohol consumption adversely affects bone health through multiple mechanisms leading to low bone mass. It may also be significantly associated with various components on the metabolic syndrome. This review summarizes the findings from published studies that provide consistent evidence on the various effects of alcohol abuse on the bone health and metabolism.
Keywords: Chronic alcohol consumption, low bone mass, metabolic syndrome
INTRODUCTION: THE “ALCOHOL BURDEN”
Alcohol, a non-essential component of diet referred as “ethyl alcohol”, is consumed as an alcoholic beverage in diluted concentrations of absolute (i.e., 100%) ethyl alcohol. One standard alcoholic beverage corresponds to 10 g of absolute alcohol. The quantity differs among the different types of available alcoholic beverages. The most commonly used alcoholic beverages in India include beer, wine, whiskey, rum, vodka, gin, brandy and locally brewed beverages such as arrack and toddy. Some of the definitions associated with alcohol consumption are as follows:
Alcohol use disorder (AUD): This is a pattern of alcohol use that involves problems controlling drinking, being preoccupied with alcohol, continuing to use alcohol even when it causes problems, having to drink more to get the same effect, or having withdrawal symptoms when the dose is rapidly decreased or stopped.[1]
Hazardous drinking: The World Health Organisation (WHO) defines hazardous drinking as “a quantity or pattern of alcohol consumption that places individuals at risk for adverse health events”.
Harmful drinking: This is defined as “alcohol consumption that results in physical or psychological harm”.
Alcohol dependence: This is defined as “a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated alcohol use”.[2]
In India, according to the Sample Registration Survey conducted in 2014, the prevalence of alcohol consumption among adult males was found to be 10%.[3] In a similar study done in southern part of India, it was found that 67% of alcohol consumers had a problematic drinking pattern with 52.5% having a hazardous/harmful drinking pattern and 14.7% being dependent alcoholics.[4] A community based, cross-sectional study conducted among 946 subjects who were aged 18 years and above, in rural Tamil Nadu, India using “Alcohol Use Disorder Identification Test” (AUDIT) scale showed a prevalence of alcohol use of 9.4%.[5]
Assessment of alcohol consumption
The most common and validated tool for screening the pattern of alcohol use among individuals is the AUDIT test which was developed by the WHO. It is a ten-item screening tool with scores ranging 0–40 to assess alcohol consumption, drinking behaviors, and alcohol-related problems. A cut-off score of ≥8 provides guidance to whether a person has a problematic drinking behaviour or not. Those individuals with a problematic drinking pattern are further classified into hazardous drinkers (score 8–15), harmful drinkers (score 16–20), and possible dependent alcohol consumers (score >20–40). On the basis of the amount of alcohol consumption, three groups are defined as follows:[6]
Light alcohol consumption: consumption of 1–10 g of ethanol/day
Moderate alcohol consumption: 11–30 g of ethanol/day
Heavy alcohol consumption: >30 g of ethanol/day
Methodology
A detailed literature search was conducted utilising Pubmed and the Google search engines using the keywords “Alcohol”, “Bone”, “osteoporosis”, “vertebral fractures” “mechanisms of bone loss”, “body composition”, “obesity”, “diabetes”, “obesity”. All review articles and original studies describing the effect of alcohol and body composition were appraised in synthesising this review.
Alcohol consumption and bone health
Chronic excessive alcohol consumption could result in low bone mass and this may predispose to fragility fractures and an increased morbidity and poor health-related quality of life. Regular alcohol consumption is most common following skeletal maturity, emphasising the importance of understanding the skeletal consequences of drinking in adults.
Normal bone physiology: In adults, bone tissue is continuously broken down and built up by a coupled process known as bone remodelling. During bone remodelling, osteoclasts are initially recruited to areas of quiescent bone surfaces (under the control of osteocytes) where they degrade bone tissue. Following this, osteoblasts are recruited to the site of resorption where they secrete a collagenous matrix that subsequently undergoes mineralisation to form mature bone. This bone remodelling cycle takes about 4–6 months to complete. A basic multicellular unit which is the functional unit of bone remodelling, includes not only bone cells (osteocytes, osteoblasts and osteoclasts) but also cells that support other bone functions, including endothelial and immune cells. An increased rate of bone resorption relative to formation will eventually result in lower bone mineral density (BMD).[7] On the one hand, excessive rates of bone remodelling will impair bone quality by increasing cortical porosity and/or decreasing trabecular connectivity. On the other hand, bone remodelling is necessary for repair of microfractures generated by activities of daily living. Moreover, reduced bone remodelling might lead to accumulation of microfractures, which could potentially reduce bone strength.[8]
Mechanisms of adverse effects of alcohol on bone health
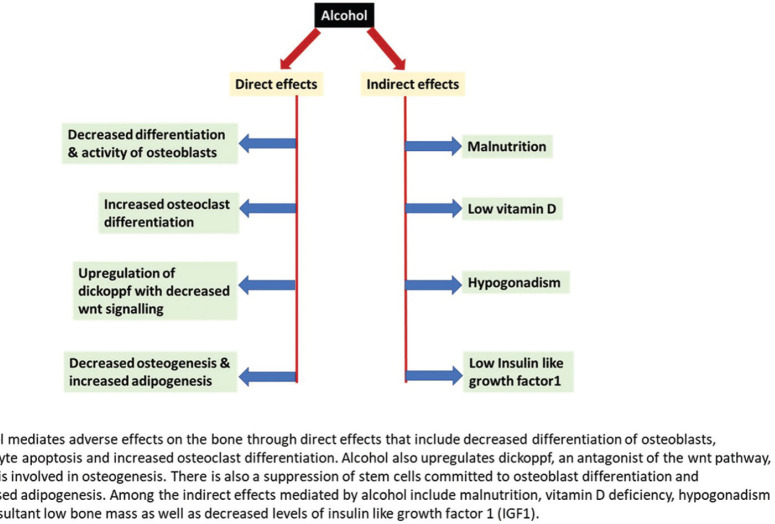
The mechanism of the production of ethanol associated osteopenia may be a direct effect of alcohol on bone cells or an indirect effect through mineral regulating hormones such as vitamin D metabolites, PTH, and calcitonin[9] [Figure 1].
Figure 1.
Mechanisms of adverse effects of alcohol on bone
Direct effects: Different mechanisms may be responsible for the direct effects of ethanol on bone as follows:
Changes in the number and activity of the osteoblasts and osteoclasts as well as an increase in osteocyte apoptosis. Suh et al. studied the differentiation ability of mesenchymal cells obtained from patients undergoing hip replacement for alcohol-induced osteonecrosis and patients with proximal femur fracture.[10] It was found that mesenchymal cells obtained from the marrow of alcohol-induced osteonecrosis displayed reduced differentiation ability of the osteogenic lineage as compared to mesenchymal cells obtained from the marrow of individuals with proximal femur fracture. Mesenchymal cells exposed to ethanol showed reduced proliferative capacity and reduced expression of alkaline phosphatase and osteocalcin. Additionally, high levels of ethanol could act directly on osteoclasts to increase their activity and their differentiation.[11]
Changes modulated by the Wnt/DKK1 signalling pathway due to increased oxidative stress. Wnts are secreted glycoproteins which act through four different pathways. The most commonly studied pathway is called the canonical pathway and designated as the Wnt/b- catenin pathway. This pathway is important for the differentiation of osteoblasts and the proliferation and synthesis of bone matrix. Osteocytes also use the Wnt/b-catenin pathway to transmit signals of mechanical loading to cells on the bone surface. There is also evidence to suggest that the Wnt/b-catenin pathway in osteocytes may be triggered by crosstalk with the prostaglandin pathway in response to mechanical and this leads to a decrease in the expression of negative regulators of the pathway such as sclerostin (sost) and dickoppf (dkk1). The Wnt/b-catenin pathway is activated by the binding of the appropriate Wnt to a co-receptor complex involving Lrp5 or Lrp6. b-catenin has the ability to accumulate in the cytoplasm and to translocate into the nucleus to affect gene transcription. Therefore, it can regulate a number of genes important in the differentiation, proliferation, apoptosis, and functionality of bone cells.[12] Dickoppf (DKK1) is an antagonist of the Wnt pathway. It has been shown to be upregulated by alcohol, which confirms that a decrease of the Wnt pathway may be linked to the suppression of bone formation observed with alcohol.[13]
Changes in cell differentiation may be responsible for low bone mass and is associated with an increase of fat accumulation in the bone marrow. Giuliani et al.[14] reported that alcohol (at concentrations of 0.04–0.6%) and acetaldehyde (at concentrations of 0.004% and 0.02%) could induce suppression in the recruitment of cells committed to the osteoblast lineage in bone marrow cultures from alcoholics compared with age-matched healthy controls. These concentrations are consistent with the serum ethanol concentrations observed in humans (0.02–0.3%). It has also been shown that alcohol treatment on cultured bone marrow stem cells obtained from mouse decreases osteogenesis and increases adipogenesis at gradually increasing concentrations of ethanol.[15] The number of adipocytes and the percentage of the area that contained the cells with fat vesicles increased significantly (P < 0.05), with longer durations and higher concentration of ethanol exposure. Moreover, an analysis of gene expression showed diminished expression of osteocalcin in these cells.
Indirect effects: The effects of alcohol consumption on bone may also be indirect and may be mediated by a decrease of calorie intake, alteration in hormone levels and signalling, and a change of body composition.
Nutrition: Malnutrition is often associated with chronic alcohol abuse[16] and low body weight is a well-established risk factor for low BMD. Acute weight loss has been shown to be detrimental to bone health and is associated with binge drinking.[17] Chronic ethanol use is also associated with micronutrient deficiencies. Although hypomagnesemia is the most common deficiency, hypocalcemia, hypercalciuria, hypophosphatemia, and hypokalemia have also been reported.[18] Also, a wide range of defects in renal tubular handling of minerals are noted to be prevalent in chronic alcohol consumers. Magnesium deficiency in humans, a known risk factor for bone loss, could result in hypocalcemia, impaired PTH secretion, and low-serum concentrations of 1,25-dihydroxyvitamin D.
Alterations in hormone levels and signalling: Excess alcohol consumption is associated with alterations in levels and/or signalling by several classes of hormones known to influence bone metabolism. These include hormones that regulate mineral homeostasis such as vitamin D and PTH, hormones that couple bone remodelling to energy metabolism like growth hormone and the reproductive hormones namely androgens and estrogens. Many individuals with chronic alcohol consumption have subnormal levels of 25-hydroxyvitamin D. Very low vitamin D levels could contribute to calcium and phosphate deficiency as well. Individuals with alcohol-related cirrhosis of the liver have been noted to have a decrease in the levels of vitamin D binding protein due to impaired hepatic protein synthesis with resultant low concentrations of total 1,25-dihydroxyvitamin D. The low 25-hydroxyvitamin D level could also be attributed to reduced hepatic 25-hydroxylase activity, inadequate sunlight exposure, poor nutritional intake, and malabsorption.[19] In a study done by Laitinen et al.,[20] it was noted that short-term alcohol administration resulted in transient hypoparathyroidism. Specifically, the parathyroid glands may not adequately respond to hypocalcemia by increasing PTH secretion in alcohol-intoxicated individuals. Many of the actions of growth hormone are mediated through circulating and/or locally produced insulin like growth factor-1 (IGF-1); acute as well as chronic alcohol consumption is associated with reduced serum IGF-1.[21] Moderate alcohol consumption is also associated with decreased plasma total and free testosterone[22] which is associated with low BMD in humans.[23,24]
Alcohol and bone: previous studies
In a study conducted by Peris et al.,[25] to assess whether vertebral fractures are associated with osteopenia in patients with chronic alcohol consumption, it was found that 36% had vertebral fractures, but only 6.5% had BMD below the fracture threshold. Jang et al.[26] studied the association between alcohol intake and postmenopausal women’s BMD by using data from the Korean National Health and Nutrition Examination Survey. A total of 3312 subjects were included and subjects were stratified into three groups: non-drinkers, light drinkers and heavy drinkers. Participants were also divided into three groups by AUDIT score: <5, 5–9, and >9. The mean femoral BMD for light drinkers was statistically significantly greater than that of heavy drinkers and non-drinkers. The mean BMD of participants with AUDIT scores 5–9 was equal to or greater than those of the other subgroups (AUDIT scores <5 and >9). Ganry et al.[27] studied the potential associations between alcohol consumption and BMD in women aged >75 years. It was found that compared with non-users, women who drank 11–29 g of alcohol/day had higher BMD values at the trochanteric site (P = 0.0017). However, alcohol intake was not associated with BMD at the femoral neck. Data suggested that moderate drinking is associated with an increase in trochanteric BMD in elderly ambulatory women. However, higher levels of alcohol intake may have detrimental effects on bone mass. Marrone et al.[28] conducted a study to ascertain the underlying possible cellular mechanisms responsible for the putative beneficial effects of moderate alcohol intake on bone in postmenopausal women. BMD was determined by dual energy x-ray absorptiometry (DXA) in 40 healthy postmenopausal women. Serum levels of the bone formation marker osteocalcin and resorption marker C-terminal telopeptide of type 1 collagen (CTx) were measured at baseline (day 0) and following alcohol withdrawal for 14 days. Participants then consumed alcohol and were assayed the following morning. Serum osteocalcin and CTx increased following abstinence. Osteocalcin and CTx decreased following alcohol re-administration compared to the previous day. They concluded that abstinence from alcohol resulted in increased markers of bone turnover whereas resumption of alcohol reduced bone turnover markers. Diamond et al.[29] found that ethanol may be responsible for osteoblastic dysfunction resulting in diminished bone formation and reduced bone mineralisation. Moreover, in a meta-analysis done by Cheraghi et al.,[30] it was found that compared with abstainers of alcohol, persons consuming 0.5–1 drinks/day had 1.38 times the risk of developing osteoporosis (adjusted RR = 1.38, 95% CI: 0.90–2.12); persons consuming 1–2 drinks/day had 1.34 times the risk of developing osteoporosis (adjusted RR = 1.34, 95% CI: 1.11–1.62), and persons consuming two drinks or more per day had 1.63 times the risk of developing osteoporosis (adjusted RR = 1.63, 95% CI: 1.01–2.65).
Alcohol consumption, metabolism and body composition
Studies have shown that alcohol consumption was significantly associated with the prevalence of various components of metabolic syndrome. Moreover, a global transition in disease pattern has been observed, where the relative impact of infectious diseases is decreasing while chronic diseases like cardiovascular disease (CVD) and diabetes are increasingly dominating the disease pattern. Metabolic syndrome is characterized by a constellation of individual risk factors of CVD. Central obesity is associated with changes in many biochemical variables, especially with the various adipokines like leptin and adiponectin.
Criteria for identifying metabolic syndrome (International Diabetes federation):
According to the IDF definition, a person is said to have metabolic syndrome if they have[31] the following criteria:
Central obesity: (defined as a waist circumference ≥94 cm for European men and ≥80 cm for European women with ethnicity specific values for other groups *
Plus any two of the following four factors.
Raised Triglyceride levels: ≥150 mg/dL or specific treatment for this lipid abnormality
Reduced HDL cholesterol <40 mg/dL in males and <50 mg/dL in females or specific treatment for this lipid abnormality
Raised blood pressure: systolic BP ≥130 or diastolic BP ≥85 mm Hg or treatment for previously diagnosed hypertension.
Raised fasting plasma glucose (FPG) >100 mg/dL or previously diagnosed type 2 diabetes mellitus. If FPG is >100 mg/dL, an oral glucose tolerance test is strongly recommended, but is not required for the diagnosis of the syndrome.
Note: The defining limit of waist circumference in Asian is 80 cm for women and 90 cm for men.
Body composition and visceral adiposity: Abdominal adiposity is characterized by increased adipose tissue surrounding the intra-abdominal organs. It is also referred to as visceral or central obesity. While abdominal obesity is determined by the accumulation of both subcutaneous adipose tissue (SCAT) and visceral adipose tissue (VAT), it has been distinctly linked to several pathological conditions including impaired glucose and lipid metabolism, and insulin resistance.[1] Body mass index (BMI) is not always a measure of fatness and individuals with high muscle mass may be incorrectly classified as obese which is defined as excess body fat that has accumulated to the extent that it may have a negative effect on health, leading to reduced life expectancy and/or increased health problems. The difference between visceral and subcutaneous adipose tissue is as shown in Table 1.[32]
Table 1.
Comparison of VAT and SCAT
| VAT | SCAT |
|---|---|
| VAT is present mainly in the mesentery and omentum, and drains directly through the portal circulation to the liver. Present mainly in omentum and mesentry | Under the skin in subcutaneous areas |
| Predictor of insulin resistance | Probably is protective |
| More cellular and contains large number of inflammatory and immune cells | Less cellular |
| More androgen receptors | Very less androgen receptors |
| Metabolically more active | Less metabolically active |
| More Lipolytic | Less lipolytic |
| Greater predictor of mortality | Lesser predictor of mortality |
ALCOHOL CONSUMPTION, METABOLISM AND BODY COMPOSITION: PREVIOUS STUDIES
Freiberg et al.[33] conducted a study with an aim to examine the association between alcohol consumption and the prevalence of the metabolic syndrome and its components in the US population. Alcohol consumption was significantly and inversely associated with the prevalence of the following three components of the metabolic syndrome: low serum HDL cholesterol, elevated serum triglycerides, high waist circumference, as well as hyperinsulinemia (P – 0.05 for all). It was concluded that mild to moderate alcohol consumption is associated with a lower prevalence of the metabolic syndrome, with favourable influence on lipids, waist circumference, and fasting insulin. Kim et al.[34] investigated the relationship between alcohol consumption and the components of metabolic syndrome and its prevalence among individuals in Republic of Korea. In both men and women, alcohol consumption of 0.1–5.0 g/day was associated with a low prevalence of metabolic syndrome and its components. However, alcohol consumption >5 g/day may contribute to abnormalities in the components of the metabolic syndrome, including high glucose, high blood pressure, hypertriglyceridemia, and low HDL cholesterol.
Once ingested, ethanol is immediately metabolised and yields 7.1 kcal per each gram of ethanol. Apart from fat, ethanol is the macronutrient with the highest energy density and it affects the individual’s total daily energy intake. In heavy alcohol drinkers, energy derived from alcoholic beverages might replace the energy from other macronutrients such as carbohydrate and fat. There is a paradoxical relationship between the amount of alcohol consumed and body weight in chronic or heavy alcohol drinkers.[35] Weight loss, loss of temporal fat, peculiar body composition, and malnutrition are commonly observed in chronic drinkers.[36]
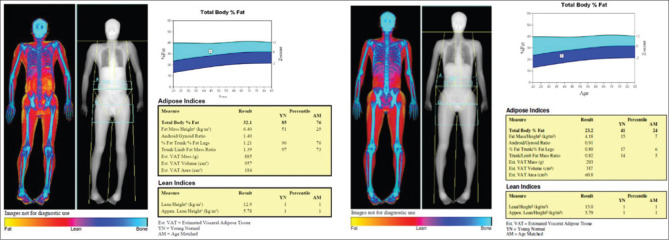
Addolorato et al.[36] studied the influence of chronic alcohol consumption on body composition in chronic alcohol consumers compared to healthy social drinkers. Body composition of 34 male alcohol consumers was compared with weight-matched controls and the study showed that alcoholics had a significantly lower body fat mass (although a higher waist to hip ratio), suggesting that they were less capable of storing ethanol-derived calories as fat compared with controls. Liangpunsakul et al.[37] studied the relationship between alcohol intake, body composition, and physical activity in the US population using the third National Health and Nutrition Examination Survey (NHANES III). In both genders, moderate and hazardous alcohol drinkers were younger (P < 0.05), had significantly lower BMI (P < 0.01) and body weight (P < 0.01) than controls (non-drinkers). Females had significantly higher percent body fat than males. In the multivariate linear regression analyses, the levels of alcohol consumption were found to be an independent predictor associated with lower percent body fat in male subjects. In yet another study done by Coulson et al.,[38] it was found that increasing levels of alcohol intake was associated with greater total and central body adiposity. The body composition analysis by DXA may be an additional tool in assessing visceral adiposity and total body fat especially in subjects with normal BMI. Figure 2 shows the body composition analysis performed by a DXA scan, of a 44-year-old man, a chronic alcohol consumer with a total body fat of 32% and low lean muscle mass despite a normal BMI of 19 kg/m2. The body composition of a healthy adult non-alcoholic male is also shown alongside for comparison.
Figure 2.
Body composition analysis in a chronic alcohol consumer (left panel). In comparison, the body composition analysis from a health adult male who is not an alcohol consumer is provided (right panel). The body composition has been performed using a DXA scan. The total body fat is 32.1% in the alcohol consumer as opposed to 23.2% in the non-alcohol consumer
Studies relating to alcohol consumption and diabetes have yielded conflicting results. In a recent study on 39259 participants from rural China, it was observed that increased risk of diabetes was associated with lower age at initiation of alcohol consumption as well as increased duration and quantity of alcohol intake. On the other hand, as the duration of abstinence from alcohol consumption increased, there was a reduction in the risk of type 2 diabetes mellitus.[39] A systematic review and meta-analyses done by Hirst et al.[40] however, failed to show that light to moderate consumption of alcohol adversely affected any measure of glycemic control.
CONCLUSION
Chronic excessive alcohol consumption may have deleterious effects on bone and might result in low bone mass which may predispose to fragility fractures leading to increased morbidity. Understanding the long-term impact of regular alcohol consumption on the skeleton is important because fractures represent a major cause for morbidity and mortality. Chronic excess alcohol consumption may also be significantly associated with various components of metabolic syndrome with conflicting reports in type 2 diabetes mellitus. Chronic alcohol consumption is also associated with alteration in body composition and fat which in turn might have effect on bone health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder:A Review. JAMA. 2018;320:815–24. doi: 10.1001/jama.2018.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT):WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addict Abingdon Engl. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 3.BASELINE TABLES 07062016.pdf. [Last accessed on 2019 Dec 15]. Available from:http://www.censusindia.gov.in/vital_statistics/BASELINE%20TABLES. 07062016.pdf.
- 4.Eashwar VMA, Gopalakrishnan S, Umadevi R, Geetha A. Pattern of alcohol consumption and its associated morbidity among alcohol consumers in an urban area of Tamil Nadu. J Fam Med Prim Care. 2019;8:2029–35. doi: 10.4103/jfmpc.jfmpc_226_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar SG, K CP, L S, E S, Vinayagamoorthy, Kumar V. Prevalence and pattern of alcohol consumption using alcohol use disorders identification test (AUDIT) in Rural Tamil Nadu, India. J Clin Diagn Res. 2013;7:1637–9. doi: 10.7860/JCDR/2013/5521.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva MC, Gaunekar G, Patel V, Kukalekar DS, Fernandes J. The prevalence and correlates of hazardous drinking in industrial workers:A study from Goa, India. Alcohol Alcohol. 2003;38:79–83. doi: 10.1093/alcalc/agg016. [DOI] [PubMed] [Google Scholar]
- 7.Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. Low bone mass and fast rate of bone loss at menopause:Equal risk factors for future fracture:A 15-year follow-up Study. Bone. 1996;19:9–12. doi: 10.1016/8756-3282(96)00102-0. [DOI] [PubMed] [Google Scholar]
- 8.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HW. Alcohol, osteoporosis, and bone regulating hormones. Alcohol Clin Exp Res. 1997;21:400–3. doi: 10.1111/j.1530-0277.1997.tb03782.x. [DOI] [PubMed] [Google Scholar]
- 10.Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop. 2005:220–5. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 11.Cheung RCY, Gray C, Boyde A, Jones SJ. Effects of ethanol on bone cells in vitro resulting in increased resorption. Bone. 1995;16:143–7. [PubMed] [Google Scholar]
- 12.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117–27. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani N, Girasole G, Vescovi PP, Passeri G, Pedrazzoni M. Ethanol and acetaldehyde inhibit the formation of early osteoblast progenitors in murine and human bone marrow cultures. Alcohol Clin Exp Res. 1999;23:381–5. [PubMed] [Google Scholar]
- 15.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88(Suppl 3):148–54. doi: 10.2106/JBJS.F.00534. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health J Natl Inst Alcohol Abuse Alcohol. 2003;27:220–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Shapses SA, Riedt CS. Bone, body weight, and weight reduction:What are the concerns? J Nutr. 2006;136:1453–6. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Marchi S, Cecchin E, Basile A, Bertotti A, Nardini R, Bartoli E. Renal tubular dysfunction in chronic alcohol abuse –effects of abstinence. N Engl J Med. 1993;329:1927–34. doi: 10.1056/NEJM199312233292605. [DOI] [PubMed] [Google Scholar]
- 19.Pitts TO.VanThiel DH. Disorders of divalent ions and vitamin D metabolism in chronic alcoholism. Recent Dev Alcohol. 1986;4:357–77. doi: 10.1007/978-1-4899-1695-2_16. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen K, Lamberg-Allardt C, Tunninen R, Karonen SL, Tähtelä R, Ylikahri R, et al. Transient hypoparathyroidism during acute alcohol intoxication. N Engl J Med. 1991;324:721–7. doi: 10.1056/NEJM199103143241103. [DOI] [PubMed] [Google Scholar]
- 21.Röjdmark S, Rydvald Y, Aquilonius A, Brismar K. Insulin-like growth factor (IGF)-1 and IGF-binding protein-1 concentrations in serum of normal subjects after alcohol ingestion:Evidence for decreased IGF-1 bioavailability. Clin Endocrinol (Oxf) 2000;52:313–8. doi: 10.1046/j.1365-2265.2000.00908.x. [DOI] [PubMed] [Google Scholar]
- 22.Cigolini M, Targher G, Bergamo Andreis IA, Tonoli M, Filippi F, Muggeo M, et al. Moderate alcohol consumption and its relation to visceral fat and plasma androgens in healthy women. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1996;20:206–12. [PubMed] [Google Scholar]
- 23.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–10. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 24.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone:Review of dose effects and mechanisms. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 25.Peris P, Guañabens N, Parés A, Pons F, del Rio L, Monegal A, et al. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif Tissue Int. 1995;57:111–4. doi: 10.1007/BF00298430. [DOI] [PubMed] [Google Scholar]
- 26.Jang HD, Hong JY, Han K, Lee JC, Shin BJ, Choi SW, et al. Relationship between bone mineral density and alcohol intake:A nationwide health survey analysis of postmenopausal women. PLoS One. 2017;12:e0180132. doi: 10.1371/journal.pone.0180132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women:The EPIDOS Study. Epidémiologie de l'Ostéoporose. Am J Epidemiol. 2000;151:773–80. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 28.Marrone JA, Maddalozzo GF, Branscum AJ, Hardin K, Cialdella-Kam L, Philbrick KA, et al. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause. 2012;19:974–9. doi: 10.1097/gme.0b013e31824ac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282–8. doi: 10.1016/0002-9343(89)90297-0. [DOI] [PubMed] [Google Scholar]
- 30.Cheraghi Z, Doosti-Irani A, Almasi-Hashiani A, Baigi V, Mansournia N, Etminan M, et al. The effect of alcohol on osteoporosis:A systematic review and meta-analysis. Drug Alcohol Depend. 2019;197:197–202. doi: 10.1016/j.drugalcdep.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Haverinen E, Paalanen L, Palmieri L, Padron-Monedero A, Noguer-Zambrano I, Sarmiento Suárez R, et al. Comparison of metabolic syndrome prevalence using four different definitions-A population-based study in Finland. Arch Public Health. 2021;79:231. doi: 10.1186/s13690-021-00749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal B. Subcutaneous adipose tissue &visceral adipose tissue. Indian J Med Res. 2019;149:571–3. doi: 10.4103/ijmr.IJMR_1910_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freiberg MS, Cabral HJ, Heeren TC, Vasan RS, Ellison RC. Alcohol Consumption and the Prevalence of the Metabolic Syndrome in the U.S.:A cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2954–9. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Hong SH, Chung JH, Cho KB. Association between alcohol consumption and metabolic syndrome in a community-based cohort of Korean adults. Med Sci Monit Int Med J Exp Clin Res. 2017;23:2104–10. doi: 10.12659/MSM.901309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colditz GA, Giovannucci E, Rimm EB, Stampfer MJ, Rosner B, Speizer FE, et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr. 1991;54:49–55. doi: 10.1093/ajcn/54.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Addolorato G, Capristo E, Marini M, Santini P, Scognamiglio U, Attilia ML, et al. Body composition changes induced by chronic ethanol abuse:Evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95:2323–7. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- 37.Liangpunsakul S, Crabb DW, Qi R. Relationship between alcohol intake, body fat, and physical activity –A population-based study. Ann Epidemiol. 2010;20:670–5. doi: 10.1016/j.annepidem.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulson CE, Williams LJ, Brennan SL, Berk M, Kotowicz MA, Lubman DI, et al. Alcohol consumption and body composition in a population-based sample of elderly Australian men. Aging Clin Exp Res. 2013;25:183–92. doi: 10.1007/s40520-013-0026-9. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Liu X, Liao W, Kang N, Dong X, Abdulai T, et al. Prevalence and characteristics of alcohol consumption and risk of type 2 diabetes mellitus in rural China. BMC Public Health. 2021;21:1644. doi: 10.1186/s12889-021-11681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirst JA, Aronson JK, Feakins BG, Ma C, Farmer AJ, Stevens RJ. Short- and medium-term effects of light to moderate alcohol intake on glycaemic control in diabetes mellitus:A systematic review and meta-analysis of randomized trials. Diabet Med. 2017;34:604–11. doi: 10.1111/dme.13259. [DOI] [PubMed] [Google Scholar]