Abstract
Background and Objectives:
Polycystic ovarian syndrome (PCOS) is one of the most common endocrinopathies in women frequently presenting with anovulatory infertility. Low successful pregnancy and live birth rates even after successful ovulation induction (OI) and in vitro fertilization (IVF) in these patients indicate that endometrial dysfunction may be another important factor contributing to infertility. Vitamin D acting through nuclear receptors induces the expression of various genes required for cell growth and differentiation and plays a crucial role in reproduction. Homeobox 10 (HOXA10) may be one of the potential targets for vitamin D action. HOXA10 gene product promotes the differentiation of endometrial cells, making the endometrium receptive for implantation. The present study was undertaken to determine the effect of circulating vitamin D levels on HOXA10 gene expression in endometrial tissues and its possible influence on the reproductive outcome of PCOS patients undergoing OI procedure.
Materials and Methods:
A prospective cohort study was conducted on 110 infertile PCOS patients. The patients were divided into two groups: Group 1: Vitamin D ³20 ng/ml, Group 2: Vitamin D <20 ng/ml. Endometrial samples were obtained from 22 patients using pipelle biopsy, used to determine HOXA10 mRNA (messenger ribonucleic acid) expression by quantitative RT-PCR (reverse transcription-polymerase chain reaction) and protein expression by Western blotting. OI was performed using Clomiphene citrate or Letrozole from the 3rd day of the cycle, and patients were followed up for a maximum of five cycles. Attainment of successful pregnancy was considered a positive outcome.
Results:
Both the groups were similar in mean age and other endocrine parameters. Serum vitamin D levels were significantly low (P < 0.001), and BMI (body mass index) was significantly high (P = 0.032) in group 2 compared to group 1. Endometrial HOXA10 mRNA (by quantitative rtPCR) and protein expression (by western blotting) were significantly low in group 2 compared to group 1. The clinical pregnancy rate was low in group 2 (28.6%) compared to group 1 (42.3%), but this difference was not significant (P = 0.22). On regression analysis adjusted for age and BMI, vitamin D was an independent predictor of successful pregnancy after OI (P = 0.09).
Conclusion:
Circulating vitamin D levels influence the endometrial HOXA10 gene expression, and this may be reflected on the reproductive outcome of infertile PCOS patients undergoing OI.
Keywords: HOXA 10, ovulation induction, polycystic ovarian syndrome, Vitamin D
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is a heterogeneous disorder with varying degrees of reproductive and metabolic disturbances. Being one of the most common endocrinopathies in women, PCOS affects 6.5–8% of women in the reproductive age group.[1] Patients may present with ovulatory dysfunction manifesting as oligo/amenorrhea, features of hyperandrogenism such as hirsutism/acne/alopecia, and signs of metabolic disturbances like impaired glucose tolerance, insulin resistance, and compensatory hyperinsulinemia.[2] Anovulatory infertility is the most common presenting feature, and ovulation induction (OI) with clomiphene citrate or gonadotropins is the first line of treatment.[3] Successful implantation after OI is a crucial step to achieve pregnancy, which requires synchrony between a healthy embryo and a functionally competent or receptive endometrium.[4] Infertility in PCOS appears to be due to more than anovulation and is likely to involve abnormalities in the endometrium.[5] Nowadays, even though high-quality oocytes and fertilised eggs are produced and transferred during assisted reproduction techniques (ART), successful implantation, pregnancy, and live birth rates remain low in these patients.[6,7] Microarray analysis has revealed differential expression of various genes related to the cell membrane, extracellular matrix, cell adhesion, invasion, and cytoskeleton in the endometrium of PCOS patients, which are essential for the establishment of endometrial receptivity.[6] This may be due to the endocrine and metabolic derangements observed in PCOS, such as elevated oestrogen (without the opposing effects of progesterone in the absence of ovulation), free Insulin like growth factor-1 (IGF-I), androgens, and hyperinsulinemia. These may have complex effects on the endometrium, resulting in endometrial dysfunction.[5,8]
Homeobox (HOXA) genes are expressed in the embryonic life necessary for body axis patterning. HOXA genes are also expressed in some adult tissues, such as the female reproductive tract and haematopoietic system, which enable these tissues to undergo continuous proliferation and differentiation necessary for their functional capability.[9] HOXA10 gene demonstrates a dynamic temporal pattern of expression in adult female endometrium with the highest expression in the mid and late secretory phase, which coincides with peak oestrogen and progesterone levels. The HOXA gene product acting as a nuclear transcriptional factor promotes the differentiation of endometrial cells to specialised decidual cells, making the endometrium receptive for implantation.[10] Several studies have shown altered expression of endometrial HOXA10 in patients with PCOS, recurrent implantation failure, and recurrent miscarriage, which could account for defective implantation observed in these patients.[11,12] Aberrant HOXA10 gene expression may also result in the misplaced endometrial cells during embryonic life resulting in endometriosis.[13,14] Along with HOXA10, many other factors such as HOXA11, leukaemia inhibitory factor (LIF), pinopodes, and avb3-integrins also play a role in determining endometrial receptivity during the window of implantation.[6,11] In animal models, targeted disruption of the HOXA10 gene exhibited uterine factor infertility.[15] Oestrogen, progesterone, and testosterone act through their nuclear receptors and regulate HOXA10 gene expression in the endometrium.[9] Vitamin D, also being a member of a nuclear receptor superfamily, plays a crucial role in cell growth, differentiation and reproduction. In animal models, vitamin D deficiency resulted in a reduced fertility rate, and intraluminal injection of 1,25 Dihydroxy vitamin D significantly increased the uterine weight and decidual reaction.[16,17] According to the studies done in patients undergoing ART, sufficient vitamin D levels were associated with better pregnancy outcomes which were attributed to its effects on the endometrium.[18,19,20,21] It is postulated that vitamin D is required for successful implantation, as activating enzymes and receptors of vitamin D have been found in the endometrium.[22,23] Most of the studies on PCOS patients have considered body mass index (BMI), age, Free androgen index (FAI), proinsulin levels, smoking, and hirsutism score as significant predictors of successful pregnancy after OI,[24] but very few studies have focused on endometrial factors and vitamin D levels. The present study was undertaken to determine the effect of circulating vitamin D levels on HOXA10 gene expression in primary human endometrial tissues and its possible influence on the reproductive outcome of PCOS patients undergoing OI procedures.
MATERIALS AND METHODS
A prospective cohort study was conducted in a tertiary care hospital for a period of two years. After receiving ethical clearance from the institutional ethical committee, PCOS patients in the age group of 18–39 years, presenting with primary infertility, were recruited for the study. As per the endocrine society guidelines, the diagnosis of PCOS was made according to the Rotterdam criteria. The presence of two of the following three findings—hyperandrogenism, ovulatory dysfunction, and polycystic ovaries was considered diagnostic.[25] A total of 110 patients meeting the above-mentioned criteria were included. Patients on treatment with vitamin D, bilateral tubal block, endometriosis, and a male factor contributing to infertility were excluded from the study. The weight and height were measured, and BMI was calculated as weight (kg)/height (m) 2
Sample collection and laboratory investigations
After obtaining informed consent, 6 ml of venous blood was collected under aseptic precautions from a peripheral vein and used for the estimation of follicle-stimulating hormone (FSH), luteinising hormone (LH), testosterone, sex hormone binding globulin (SHBG), and total (25OH) vitamin D (in duplicates, sensitivity 4.2 ng/ml, intra-assay and inter-assay Coefficient of variation (CV) <20%) by fully-automated Siemens Advia Centaur XP instrument using chemiluminescence immunoassay technology. All reagents, calibrators, and controls were purchased from Siemens healthcare India Pvt Ltd. FAI was calculated as total testosterone (in nmol/l)/SHBG (in nmol/l) x 100. Ultrasound examination was carried out to confirm the presence of bilateral ovarian cysts. As per the guidelines of the endocrine society, 25OH vitamin D ³30 ng/ml was considered sufficient, 20–29.9 ng/ml was insufficient, and <20 ng/ml was deficient. After screening 110 patients with PCOS, we identified only three patients with 25OH vitamin D >30 ng/ml. Hence, we divided the patients into only two groups for further analysis.
Group 1: Vitamin D ³20 ng/ml
Group 2: Vitamin D <20 ng/ml
After explaining the procedure, endometrial samples were obtained from 22 patients (12 from group 1 and 10 from group 2) during the secretory phase using non-invasive endometrial pipelle biopsy. In patients with amenorrhoea, samples were collected randomly. The tissue was separated into two parts (one in TRIzol® for RNA extraction and the other in Phosphate buffered saline (PBS) for protein extraction) and stored in a deep freezer at -80° until the analysis was done.
RNA extraction and quantitative RT-PCR in primary human endometrial tissues
Total ribonucleic acid (RNA) was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific Inc.). RNA quantity and purity were measured by Epoch UV spectrophotometer after dissolving it in 40 uL RNAase free water. MOPS gel electrophoresis was carried out to identify 18 S and 28 S RNA bands. In order to obtain the complementary deoxyribonucleic acid (cDNA). 1 mg total RNA was reverse-transcribed using Primescript RT reagent kit (cat no RR037A) from TAKARA BIO INC as per the manufacturer’s recommendations.
Quantitative real-time RT-PCR was conducted for 40 cycles (initial denaturation 95° for 30 sec, followed by 95° for 5 sec, 65° for 30 sec, and 72° for 30 sec) using 2 ul cDNA product (in 25 ul reaction mixture containing 0.4 umol each of forward and reverse primers of HOXA10 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH)sequence of primers given in Table 1) in triplicates using TB green premix (RR820 A) from TAKARA BIO INC. The expression of the HOXA10 gene was normalised to that of GAPDH, which served as control. Relative quantification of HOXA10 messenger RNA (mRNA) expression in target tissues was done using the 2-ΔΔct method. HOXA10 mRNA expression in endometrial tissues of group 1 patients (vitamin D ³20 ng/ml) was compared with group 2 patients (vitamin D <20 ng/ml).
Table 1.
Sequence and length of Primers
| Primer sequence | Length (bp) | |
|---|---|---|
| HOXA10 | ||
| Forward | GCCCCTTCCGAGAGCAGCAAAG | 22 |
| Reverse | AGGTGGACGCTGCGGCTAATCTCTA | 25 |
| GAPDH | ||
| Forward | GAGCGAGATCCCTCCAAA | 18 |
| Reverse | ACTGTGGTCATGAGTCCTTC | 20 |
Protein extraction and Western blotting in primary human endometrial tissues
Endometrial samples were homogenised in tissue lysis buffer (50 mM TrisHCl pH 7.4, 0.5% NP40, 250 mM Nacl, 5 mM EDTA, 50 mM NaF, and 0.1 mM PMSF) followed by sonication to extract the proteins. Samples were then centrifuged at 10,000 rpm for 15 min at 4°C, and the supernatant was used for protein estimation by the Bicinchoninic acid (BCA) method. Equal amounts of proteins (50 ug from each sample) were electrophoresed in 10% polyacrylamide gel at 90 V for 90 min. Separated proteins were then transferred on nitrocellulose membranes (from HiMedia Laboratories Pvt. Ltd) at 65 V for 90 min in a transfer buffer. Blocking was done using 1% Bovine serum albumin (BSA) (from HiMedia Laboratories Pvt. Ltd) for one hour, followed by overnight incubation of blots in primary antibody diluted 1:1000 in blocking agent (HOXA10 sc-271954, mouse monoclonal Ab and GAPDH sc-47724, mouse monoclonal anti-GAPDH Ab from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4°C. After washing, the blots were incubated for one hour in Horse radish peroxidase (HRP)-labelled secondary antibody (goat-anti-mouse polyclonal Ab, cat no. 170-6516 from Bio-Rad laboratories, Inc) diluted 1:3000, in blocking agent. After the second series of washing, the membranes were developed using ECL Plus (Bio Rad Laboratories, Inc.), and images were obtained using a G: BOX ChemiXX9 system from Syngene Europe (Cambridge, UK). GAPDH was used as the internal control. HOXA10 protein expression in endometrial tissues of group 1 patients (vitamin D ³20 ng/ml) was compared with group 2 patients (vitamin D <20 ng/ml).
Ovulation induction was done in the in vitro fertilisation (IVF) centre using 50/100 mg of clomiphene citrate or 2.5/5 mg of Letrozole from the 3rd day of the cycle. The follicular growth was monitored by serial transvaginal ultrasonography. The dosages were modified based on the serial ultrasound findings. Most of the stimulation protocols were added with gonadotrophins: human menopausal gonadotrophin (HMG) or recombinant FSH. Ovulation trigger was done when at least one follicle size reached between 18 to 20 mm. Patients were followed up for a maximum of five treatment cycles. Attainment of a successful pregnancy (evidence of intrauterine gestation sac on ultrasound) was considered a positive outcome.
Statistical analysis
Continuous data are expressed as the mean ± standard deviation (SD), and categorical data as a percentage (%). The Student’s t-test was used to analyse continuous data and the Chi-square test to analyse categorical data. Pearson correlation analysis was performed to check the correlation of vitamin D levels with age and BMI. Regression analysis was performed to evaluate the relationship between serum vitamin D levels and successful pregnancy after adjusting for factors known to affect the pregnancy outcome (age) and vitamin D levels (BMI). Vitamin D is known to be affected by patients’ BMI and ethnicity. Since our study population included patients from the same ethnic group, we performed only age and BMI-adjusted analysis. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software version 17. Quantitative PCR (qPCR) analysis was done using the 2-ΔΔct method, and the results were presented as mean values, with error bars representing standard error. ImageJ 1.52h (National Institutes of Health, Bethesda, MD, USA) was used to analyse and quantify the western blot data after densitometric scanning. The results were presented as mean with error bars representing the standard error. The results were compared using the Student’s t-test with P < 0.05 as the level of significance. GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) was used to compare the data sets.
RESULTS
Baseline characteristics of study participants are given in Table 2. The prevalence of vitamin D deficiency, insufficiency, and sufficiency was 76%, 21%, and 3%, respectively.
Table 2.
Baseline characteristics of the study participants
| Number | 110 |
| Age in years | 26.9±3.1 |
| BMI in Kg/m2 | 26.3±4.8 |
| FSH in mIU/ml | 6.18±1.73 |
| LH in mIU/ml | 8.14±5.6 |
| Total 25 hydroxy vitamin D in ng/ml | 17.26±5.74 |
| SHBG in nmol/l | 51.33±60.26 |
| Testosterone in nmol/l | 1.52±0.73 |
| FAI | 4.5±3.1 |
| Vitamin D status in numbers and % | |
| Deficient (< 20 ng/ml) | 84 (76%) |
| Insufficient (20–29.9 ng/ml) | 23 (21%) |
| Sufficient (≥ 30 ng/ml) | 3 (3%) |
Continuous data are presented as mean±SD and categorical data as percentage (%)
Characteristics of PCOS patients undergoing OI by vitamin D status are shown in Table 3. Both the groups were similar in mean age and other endocrine parameters. Only BMI and vitamin D levels were significantly different between the groups. Serum vitamin D levels were significantly low (P < 0.001), and BMI was significantly high (P = 0.032) in group 2 compared to group 1. On Pearson correlation analysis [Table 4], vitamin D levels showed a significant negative correlation with BMI (P = 0.02), but there was no significant correlation between age and vitamin D levels (P = 0.42).
Table 3.
Characteristics of PCOS patients by vitamin D status
| Characteristic | Vitamin D status | P | |
|---|---|---|---|
|
| |||
| Group 1 (Vitamin D ≥20 ng/ml) | Group 2 (Vitamin D <20 ng/ml) | ||
| Number | 26 | 84 | |
| Serum vitamin D levels | 25.57±3.64 | 14.66±3.26 | <0.001* |
| Age | 26.58±3.57 | 26.95±2.96 | 0.59 |
| BMI | 24.57±5.14 | 26.85±4.56 | 0.032* |
| FSH | 5.72±2.13 | 6.33±1.58 | 0.12 |
| LH | 8.23±5.47 | 8.12±5.61 | 0.93 |
| Testosterone | 1.57±0.81 | 1.49±0.71 | 0.62 |
| SHBG | 63.14±79.6 | 47.63±52.8 | 0.25 |
| FAI | 4.14±3.05 | 4.62±3.12 | 0.49 |
| No. of patients with clinical pregnancy | 11 (42.3%) | 24 (28.6%) | 0.22 |
*significance with P<0.05
Table 4.
Pearson correlation analysis showing correlation of Vitamin D levels with age and BMI
| Age | BMI | |
|---|---|---|
| Vitamin D | ||
| Pearson Correlation | -0.078 | -0.232 |
| P | 0.42 | 0.02* |
| Significance | NS | S |
| n | 110 | 110 |
*Correlation is significant at P<0.05 (2-tailed)
The clinical pregnancy rate was low in group 2 patients (28.6%), having lower vitamin D levels and high BMI compared to group 1 (42.3%), but this difference was not statistically significant (P = 0.22) [Table 3].
Regression analysis to evaluate the relationship between serum vitamin D levels and successful pregnancy is shown in Table 5. On regression analysis adjusted for age and BMI, vitamin D level was an independent predictor of successful pregnancy after OI (P = 0.09).
Table 5.
Regression analysis to predict the occurrence of a successful pregnancy
| B | S.E. | Wald | Df | P | Significance | Exp (B) | |
|---|---|---|---|---|---|---|---|
| Age | 0.066 | 0.073 | 0.835 | 1 | 0.361 | NS | 1.069 |
| BMI | 0.046 | 0.049 | 0.901 | 1 | 0.343 | NS | 1.048 |
| Vitamin D | 0.105 | 0.040 | 6.847 | 1 | 0.009* | S | 1.110 |
| Constant | −5.630 | 2.453 | 5.267 | 1 | 0.022 | 0.004 |
*significance with P<0.05
Effect of serum 25OH vitamin D levels on HOXA10 gene/protein expression in primary human endometrial tissues
Results of quantitative RT-PCR in primary human endometrial tissues are shown in Figure 1. Mean HOXA10 mRNA expression was low in group 2 (vitamin D <20 ng/ml) patients compared to group 1 (vitamin D ³20 ng/ml), but the difference was not statistically significant.
Figure 1.
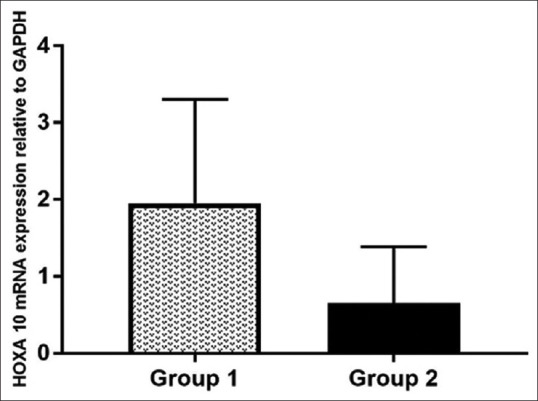
Quantitative RT-PCR showing HOXA10 mRNA expression relative to GAPDH in primary human endometrial tissues
On western blotting, mean HOXA10 protein expression was low in group 2 (vitamin D <20 ng/ml) patients compared to group 1 (vitamin D ³20 ng/ml), and the difference was statistically significant [Figures 2 and 3].
Figure 2.
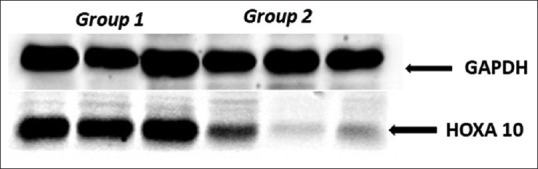
Western blot analysis showing the expression of HOXA10 protein in primary human endometrial tissues in group 1 and group 2 patients compared to GAPDH
Figure 3.
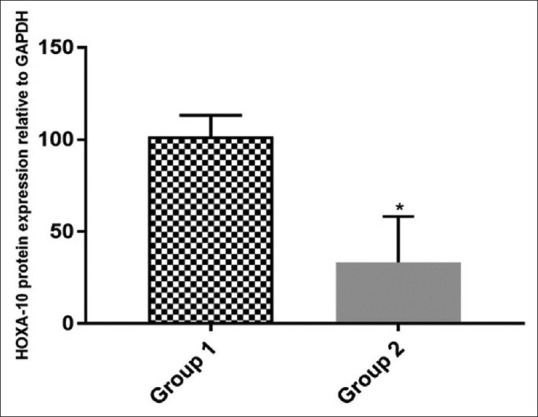
Quantitative analysis of WB data corresponding to HOXA10 protein expression in primary human endometrial tissues of both the groups relative to GAPDH
DISCUSSION
According to our findings, endometrial HOXA10 mRNA and protein expression are influenced by circulating vitamin D levels in PCOS patients. After the follow-up of PCOS patients for a maximum of five OI cycles, patients with high vitamin D levels had a higher rate of achieving clinical pregnancy compared to the patients with low vitamin D levels. Although the difference in clinical pregnancy rate was not statistically significant between the groups, vitamin D level was an independent predictor of a successful pregnancy. Previously, various studies have reported the beneficial effect of vitamin D on female reproductive outcomes. In a large multicentric Randomised controlled trial (RCT) involving 626 reproductive age women diagnosed with PCOS, serum 25OH vitamin D was an independent predictor of reproductive success following OI, and the threshold of serum 25OH vitamin D for achieving a successful pregnancy and having live birth was higher in these patients than recommended for the non-pregnant population.[19] However, the mechanism by which vitamin D affects the reproductive outcome was not clear. According to Garbedian and colleagues, women with sufficient vitamin D levels had significantly higher rates of clinical pregnancy following IVF compared to women with insufficient or deficient levels. This difference was attributed to higher embryo quality among women with sufficient 25(OH) D levels.[26] In contrast, in a retrospective study including 188 infertile patients of different races undergoing IVF, vitamin D levels were neither associated with ovarian stimulation parameters nor with markers of embryo quality.[22] Since the oocyte donor-recipient IVF model was able to distinguish the impact of vitamin D on oocyte vs. endometrium, in a study conducted by the same group of researchers, replete vitamin D level in recipients of donor eggs was associated with higher clinical pregnancy rates after adjusting for parameters of embryo quality. This confirmed that the effects of vitamin D are mainly mediated through endometrium.[27] However, in the same study, it was observed that beneficial effects of vitamin D on pregnancy outcomes were seen only in non-Hispanic whites but not in Asians. Pregnancy and live birth rates increased with worsening vitamin D status in Asians.[22] According to the authors, this was due to a relatively smaller number of Asians in the study or may be due to the other factors which may contribute to the lower pregnancy rate observed in Asian patients. In our study where patients were recruited from India, vitamin D level was the only significant predictor of clinical pregnancy after adjusting for confounders in PCOS patients undergoing OI, and the serum levels of vitamin D significantly influenced endometrial HOXA10 gene and protein expression. However, the difference in clinical pregnancy rates between the groups did not reach statistical significance. This may be due to the low sample size, or the cut-off values of vitamin D levels we have used to divide the patients into two groups may not be appropriate. This can be addressed by future studies, including a higher sample size and considering a higher threshold of vitamin D levels required by this set of population.
Apart from oestrogen, progesterone, and testosterone, vitamin D is the only ligand known to affect HOXA-10 gene expression in the human endometrium. Being fat-soluble, vitamin D easily crosses the nuclear membrane and exerts its effect through the Vitamin D receptor (VDR) after binding to the vitamin D response element (VDRE) and modulating the expression of various genes. One such novel VDRE was identified upstream of the HOXA10 promoter region.[10] Since the endometrial tissues we have collected are from clinically/biochemically diagnosed PCOS patients, and both the groups were similar with respect to age and other endocrine parameters except for vitamin D levels, we assume that the difference noticed in HOXA-10 gene expression is attributed to the difference in vitamin D levels only.
It is postulated that endometrial HOXA10 expression parallels that of the vitamin D signalling pathway; both increase midcycle shortly before the expected implantation, at the time of maximal endometrial differentiation. VDR and 1-alpha hydroxylase expression in the endometrium continue to increase in the first and second trimesters.[27] VDRE is located in the region -385 to -343 bp upstream of the HOXA10 transcription start site and directly binds the vitamin D-VDR complex. Such binding results in target gene activation and enhanced expression of the HOXA gene product. The HOXA gene product acting as a nuclear transcriptional factor promotes the differentiation of endometrial cells to specialised decidual cells, making the endometrium receptive for blastocyst implantation.[10] HOXA genes interact with other key developmental signalling molecules. In turn, these interactive pathways recruit genes that may be necessary for implantation, such as Msx-1.[9] From these observations, it is evident that endometrial receptivity is the potential target for a beneficial effect of vitamin D. Along with HOXA10, vitamin D is also known to upregulate the expression of other genes like osteopontin and calbindin, which are critical for embryo implantation.[27]
It has been observed that Decidua and placenta continue to secrete a large amount of active vitamin D throughout the pregnancy, with the highest concentration in the 1st trimester, which is known to regulate immune response at the maternal-fetal interface. The presence of blastocyst upregulates the secretion of active vitamin D in the endometrium through IL1b, and vitamin D, in turn, supports the embryo by attenuating the decidual T cell function and cytokine production from Natural killer (NK) cells.[22,28] By this, it can be concluded that vitamin D is not only required for implantation but also necessary for the continuation of the pregnancy. However, further studies are required to confirm the findings.
Our findings also revealed higher BMI in patients with lower vitamin D levels. An inverse correlation is observed between BMI and serum vitamin D concentrations. A possible explanation for this may be that in obese individuals, a higher proportion of vitamin D, which is fat-soluble, is sequestered in adipose tissues, lowering the bioavailability of the vitamin.[26] Lagunova and colleagues have reported the highest prevalence of vitamin D deficiency in patients with BMI >40 kg/m2.[29]
Major strength of our study is that it is conducted in the Indian population. Data available in the literature is mainly from the white/Caucasian population, and to date, very few studies have been carried out on Asians, and the results are still contradictory. Most of the previous studies addressing the association of serum vitamin D levels with clinical pregnancy rates were done in infertile women seeking IVF treatment, and these patients were on vitamin D supplements. Hence, the observed prevalence of vitamin D deficiency was very low in those studies[26] compared to our study, where we recruited infertile patients who were not on vitamin D therapy. In India, deficient and insufficient vitamin D levels were reported in 76% and 16.5% of women of reproductive age group, respectively.[30] The findings of our study hold therapeutic implications due to the high prevalence of vitamin D deficiency in the Indian population.
Major limitation of our study is the small sample size. Especially, we had only three patients with vitamin D >30 ng/ml and only 22 patients with vitamin D >20 ng/ml after screening 110 PCOS patients. Another limitation of the study is its observational nature. We could demonstrate the association between serum vitamin D levels and successful pregnancy after OI, but we were unable to conclude that by supplementing vitamin D to patients having low levels, we could improve the outcome. Only an interventional study can address this.
CONCLUSION
Circulating vitamin D levels influence the endometrial HOXA10 gene expression, and this might be reflected on the reproductive outcome of infertile PCOS patients undergoing OI. However, the lack of statistically significant difference in clinical pregnancy rates between the groups may be attributed to the cut-off values of vitamin D we have used to divide the patients into two groups not being appropriate. Future studies are required to systematically derive the appropriate threshold of vitamin D to achieve successful pregnancy in the reproductive-age women. Further, characterisation of downstream effects of the HOXA10 gene may assist in elucidating the complex molecular mechanism governing the implantation process.
Ethical approval
The Institutional ethics committee of SDM College of Medical Sciences and Hospital, Sattur, Dharwad, Karnataka, India, approved this study (SDMIEC: 0239:2017)
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The research was funded by Rajiv Gandhi University of Health Sciences, Karnataka, India (RGU/ADV.RES/BR/001/2017-2018).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Dr. Ravi Shirahatti for helping with the statistical analysis, and the staff from the Clinical biochemistry laboratory, SDM research institute of biomedical sciences, for technical assistance.
REFERENCES
- 1.Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome:Pitfalls and controversies. J Obstet Gynaecol Can. 2008;30:671–9. doi: 10.1016/S1701-2163(16)32915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayek SE, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome:An updated overview. Front Physiol. 2016;7:1–15. doi: 10.3389/fphys.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahajan N. Endometrial receptivity array:Clinical application. J Hum Reprod Sci. 2015;8:121–9. doi: 10.4103/0974-1208.165153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice LC. Endometrium in PCOS:Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–44. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Jiang NX, Li XL. The disorders of endometrial receptivity in PCOS and its mechanisms. Reprod Sci. 2021 doi: 10.1007/s43032-021-00629-9. doi:10.1007/s43032-021-00629-9. [DOI] [PubMed] [Google Scholar]
- 7.Guo F, Huang Y, Fernando T, Shi Y. Altered molecular pathways and biomarkers of endometrial receptivity in infertile women with polycystic ovary syndrome. Reprod Sci. 2022 doi: 10.1007/s43032-022-00845-x. doi:10.1007/s43032-022-00845-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Wen YX, Mai QY. Impact of metabolic disorders on endometrial receptivity in patients with polycystic ovary syndrome. Exp Ther Med. 2022;23:221. doi: 10.3892/etm.2022.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–55. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 10.Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH) 2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19:2222–33. doi: 10.1210/me.2004-0336. [DOI] [PubMed] [Google Scholar]
- 11.Kara M, Ozcan SS, Aran T, Kara O, Yilmaz N. Evaluation of endometrial receptivity by measuring HOXA-10, HOXA-11, and leukemia inhibitory factor expression in patients with polycystic ovary syndrome. Gynecol Minim Invasive Ther. 2019;8:118–22. doi: 10.4103/GMIT.GMIT_112_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Chen X, Saravelos SH, Liu Y, Huang J, Zhang J, Li TC. HOXA-10 and E-cadherin expression in the endometrium of women with recurrent implantation failure and recurrent miscarriage. Fertil Steril. 2017;107:136–43. doi: 10.1016/j.fertnstert.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Zanatta A, Rocha AM, Carvalho FM, Pereira RM, Taylor HS, Motta EL, et al. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility:A review. J Assist Reprod Genet. 2010;27:701–10. doi: 10.1007/s10815-010-9471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24:3180–7. doi: 10.1093/humrep/dep306. [DOI] [PubMed] [Google Scholar]
- 15.Vitiello D. Gene expression profiling reveals putative hoxa10 downstream targets in the periimplantation mouse uterus. Reprod Sci. 2008;15:529–35. doi: 10.1177/1933719108316911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halhali A, Acker GM, Garabedian M. 1,25-Dihydroxyvitamin D3 induces in vivo the decidualization of rat endometrial cells. J Reprod Fertil. 1991;91:59–64. doi: 10.1530/jrf.0.0910059. [DOI] [PubMed] [Google Scholar]
- 17.Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol. 1989;256:E483–7. doi: 10.1152/ajpendo.1989.256.4.E483. [DOI] [PubMed] [Google Scholar]
- 18.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following IVF. Fertil Steril. 2010;94:1314–9. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome:Secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2016;101:3027–35. doi: 10.1210/jc.2015-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott J, Wattar L, Kurz C, Seemann R, Huber JC, Mayerhofer K, et al. Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo clomiphene citrate stimulation:A prospective cohort study. Eur J Endocrinol. 2012;166:897–902. doi: 10.1530/EJE-11-1070. [DOI] [PubMed] [Google Scholar]
- 21.Lerchbauma E, Rabe T. Vitamin D and female fertility. Curr Opin Obstet Gynecol. 2014;26:145–50. doi: 10.1097/GCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Human Reprod. 2012;27:3321–7. doi: 10.1093/humrep/des280. [DOI] [PubMed] [Google Scholar]
- 23.Chu J, Gallos L, Tobias A, Robinson L, Kirkman-Brown J, Dhillon-Smith R, et al. Vitamin D and assisted reproductive treatment outcome:A prospective cohort study. Reprod Health. 2019;16:106. doi: 10.1186/s12978-019-0769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rausch ME, Legro RS, Barnhart HX, Schlaff WD, Carr BR, et al. Predictors of Pregnancy in Women with Polycystic Ovary Syndrome. JCEM. 2009;94:3458–66. doi: 10.1210/jc.2009-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106–13. [PubMed] [Google Scholar]
- 26.Garbedian K, Boggild M, Moody J, Liu KE. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open. 2013;1:E77–82. doi: 10.9778/cmajo.20120032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril. 2014;101:447–52. doi: 10.1016/j.fertnstert.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Dabrowski FA, Grzechocinska B, Wielgos M. The Role of Vitamin D in Reproductive Health—A trojan horse or the golden fleece? Nutrients. 2015;7:4139–53. doi: 10.3390/nu7064139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–20. [PubMed] [Google Scholar]
- 30.Harinarayan CV, Sachan A, Reddy PA, Satish KM, Prasad UV, Srivani P. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups:A cross-sectional study from South India. J Assoc Physicians India. 2011;59:695–701. [PubMed] [Google Scholar]


