Abstract
Background:
Adiponectin and leptin play a major role in metabolic homeostasis. Adiponectin to Leptin ratio can be used as an indicator of insulin resistance and a marker of polycystic ovarian syndrome (PCOS). The study was planned to compare serum adiponectin, leptin, and adiponectin to leptin ratio in age and BMI matched women with and without PCOS and to find out the association of adiponectin to leptin ratio with Insulin resistance in these women.
Methods:
It was a cross-sectional study done in the Gynecology outpatient clinic in a tertiary care center. A total of 120 women, 60 with PCOS and 60 age and BMI matched women without PCOS, who presented in the clinic after the index cases were enrolled and tested for serum adiponectin, leptin, and insulin sensitivity. The main outcome measures were serum levels of adiponectin, leptin, adiponectin to leptin ratio, oral glucose tolerance test, serum insulin and HOMA-IR.
Results:
PCOS women had lower serum Adiponectin, higher serum Leptin level and lower Adiponectin to Leptin ratio compared to non PCOS women, 2.15 ± 3.07 ng/ml vs 10.7 ± 27.91 ng/ml, P < 0.0001; 24.25 ± 16.5 ng/ml vs 13.89 ± 11.19 ng/ml, P = 0.0003 and 0.15 ± 0.24 vs 3.03 ± 15.04, P < 0.0001, respectively. Plasma glucose 2 hours after 75 gm glucose and serum Insulin was significantly increased in PCOS women (108.78 ± 10.22 mg/dl vs 100.18 ± 4.89 mg/dl, P = 0.001 and 5.7 ± 9.53 mU/ml vs 3.02 ± 5.34 mU/ml, P = 0.02, respectively). The mean values of fasting plasma glucose and HOMA-IR were comparable in both groups, P = 0.145, P = 0.719, respectively. There was no significant association of A/L Ratio with BMI, plasma glucose 2 hours after 75 gm glucose, serum Insulin and HOMA-IR, in these women, r = -0.074, P = 0.5754; r = -0.203, P = 0.12; r = -0.018, P = 0.8915; and r = -0.041, P = 0.757, respectively.
Conclusion:
Adiponectin to leptin ratio is significantly reduced in women with PCOS but has no association with insulin resistance.
Keywords: Adiponectin, adiponectin to leptin ratio, insulin resistance, leptin, PCOS, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common reproductive disorder affecting 15-18% of women of the reproductive age group.[1] It is characterized by hyperandrogenism, ovulatory dysfunction and polycystic ovaries, and is associated with obesity/central adiposity, dyslipidemia, Insulin resistance (IR) and an increased risk of type 2 diabetes (T2DM).[2,3]
PCOS is a complex heterogeneous disease in which obesity, IR, and metabolic syndrome are common presentations.[4,5] Morphological signs are manifested by the ultrasonographic appearance of polycystic ovaries.[6] According to the Rotterdam criteria and National Institute of Health criteria, hyperandrogenism, chronic anovulation, and polycystic ovaries on ultrasonography are the three main criteria for diagnosis of PCOS patients.[7] Insulin resistance is proposed to be one of the most important factors that predispose women to PCOS because it promotes hyperandrogenism and chronic anovulation by multiple mechanisms.[8] Therefore, individuals with PCOS have an increased lifetime risk of T2DM and cardiovascular diseases.[9] However, the mechanism linking PCOS to metabolic abnormality is not completely understood. It increases LH secretion and decreases serum levels of sex hormone binding globulins (SHBGs), which in turn increase the fraction of unbound circulating androgens.[10] These major metabolic and reproductive abnormalities are coupled with altered secretion of several adipose derived peptide hormones. These bioactive proteins secreted into the circulation by adipose tissues are associated with dysregulated adipokine expression and the onset of PCOS.[11]
Adiponectin and Leptin are important adipocytokines that play a major role in the regulation of metabolic homeostasis and also affect insulin sensitivity.[12] Adiponectin levels are inversely related to IR, and reduced adiponectin levels are linked with IR-associated conditions, including obesity, T2DM, and PCOS.[12,13] Adiponectin levels are found to be lower in PCOS women as compared to controls.[14,15,16,17] A meta-analysis found that in studies with few participants, or including women with PCOS who were markedly more insulin resistant than controls, total serum adiponectin was significantly lower in PCOS whereas in larger studies, or in populations presenting moderate degrees of IR, there was no difference in serum adiponectin from controls.[18] Recent evidence is suggestive of high molecular weight adiponectin (active form) is more useful than total adiponectin to evaluate insulin sensitivity and glucose tolerance in PCOS.[19] Leptin is adipocyte derived hormone that reflects the amount of body fat in the human being, also involved in insulin resistance and energy homeostasis.[20] Insulin has been shown to increase leptin messenger RNA in adipocytes, suggesting its possible role in stimulating leptin secretion. Leptin is inversely related to adiponectin levels and positively correlated with body fat independent of PCOS.[21] In some studies, Leptin has been found to be higher in PCOS women independent of obesity.[22] Whereas, in some no difference in leptin level was found after accounting for BMI.[23,24]
Adipokine ratio (adiponectin to leptin ratio) is implicated as a stronger indicator of IR than individual adipokines, and is speculated as a measure of IR and a marker of PCOS.[25,26,27] Adiponectin Leptin ratio (A/L ratio) might help to identify women at risk of developing metabolic abnormalities like IR, dyslipidemia, type 2 DM and PCOS.[28] Interventions to reverse the ratio either by lifestyle changes or medical management may prevent or delay the development of metabolic derangements in these women.
The aim of the present study was to compare adiponectin, leptin and adiponectin to leptin ratio (A/L ratio) in age and BMI matched PCOS and non PCOS women, therefore eliminating the role of BMI in altering these adipokines. We also calculated the association of the A/L ratio with insulin resistance in these women to find out the role of altered A/L ratio in the development of insulin resistance.
MATERIAL AND METHODS
This was a hospital-based cross-sectional study conducted in the Department of Obstetrics and Gynaecology in collaboration with the Department of Biochemistry, at the tertiary level hospital of New Delhi. The sample size was calculated from the study by Ali et al. who observed mean values of adiponectin to leptin ratio in women with PCOS and Controls as 1.32 ± 0.32 and 1.63 ± 0.33 respectively.[27] Taking these values as a reference, the minimum required sample size calculated with 80% power of study and 5% level of significance was 120.
120 women were allocated into two groups, 60 PCOS women (diagnosed on the basis of revised Rotterdam criteria for PCOS), taken as a study group, and 60 age and BMI matched non-PCOS women presenting in an outpatient department after recruitment of PCOS women, taken as the control group. Pregnant, lactating and women with diabetes, liver disease, Cushing syndrome, late onset of CAH or other serious medical condition, history of intake of oral contraceptives in the last 3 months, glucocorticoids, antiandrogens, ovulation induction agents, antidiabetic, antipsychotic, or antihypertensive or hormone replacement therapy were not included in the study. Informed consent was obtained from all the subjects participating in the study. Ethical clearance from the Institutional ethics committee was obtained and “we certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research”.
Information regarding medical, personal, family, dietary and menstrual history was obtained in the form of a questionnaire and women in both the groups were examined in the follicular phase of the menstrual cycle. Clinical and anthropometric variables, body mass index, waist-hip ratio, waist circumference and blood pressure were determined, and hirsutism was quantified with a modified Ferriman-Gallwey (mFG) score and mFG score >7 was taken as clinical hyperandrogenism in all the patients.
For the biochemical and hormonal profile 10 ml fasting venous blood sample after 12 hours of overnight fasting, was obtained in the morning from each subject on the 2nd -7th day of the menstrual cycle or after progesterone withdrawal. Fasting and 2 hours post 75 grams serum glucose levels were measured using glucose oxidase method. Serum FSH, LH, prolactin and total testosterone were measured using the Radioimmunoassay test kit. Serum Adiponectin and serum Leptin concentrations were measured by using ELISA (Leptin ELISA Version 8.2 by Diagnostics Biochem Canada Inc., Cat. No.: CAN-L-4260). Fasting Serum insulin was measured using ELISA by Calbiotech Insulin ELISA kit Catalogue No. IS130. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR = fasting plasma insulin (mU/ml) × fasting plasma glucose (mg/dl)/405) was calculated as a marker of insulin resistance.
Statistical analysis: Statistical tests were applied, Quantitative variables were compared using Unpaired t-test.
Receiver operating characteristic curve was used to find out the cutoff point of the adiponectin to Leptin ratio for the prediction of PCOS. Diagnostic tests were used to find out sensitivity, Specificity, PPV and NPV of adiponectin, leptin and Adiponectin to Leptin ratio. Pearson correlation coefficient was used to correlate quantitative parameters with each other. A P value of <0.05 was considered statistically significant. The analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
RESULTS
The mean age, BMI, clinical, biochemical and hormonal profile of women in the study and control group is shown in Table 1. Levels of Serum total testosterone, 2 hours 75 gm oral glucose tolerance test, Adiponectin, Leptin and A/L ratio were significantly different in PCOS and non-PCOS women [Table 1]. ROC curve showed a significant correlation between adiponectin to leptin ratio and PCOS with area under the curve of 0.7873 and the cut off value of adiponectin to leptin ratio of ≤ 0.1154. [Figure 3].
Table 1.
Demographic and clinical, hormonal and metabolic characteristics of women with PCOS and healthy controls
| PCOS (n=60) | Non PCOS (n=60) | P | |
|---|---|---|---|
| Age | 27.5±2.83 | 27.83±3.03 | 0.886 |
| BMI | 25.06±2.42 | 25.15±2.32 | 0.593 |
| Waist Hip ratio | 0.88±0.3 | 0.87±0.03 | 0.922 |
| Fasting glucose | 88±0.93 | 83±0.78 | 0.145 |
| S. Insulin | 5.7±9.53 | 3.02±5.34 | 0.023 |
| OGTT | 108.78±10.22 | 100.18±4.89 | 0.001 |
| HOMA-IR | 1.38±2.27 | 5.39±3.18 | 0.719 |
| S. FSH | 4.74±1.24 | 4.22±0.67 | 0.121 |
| S. LH | 3.7±1 | 3.6±0.74 | 0.24 |
| S. Testosterone | 0.56±0.38 | 0.28±0.11 | 0.0001 |
| S. Adiponectin | 2.15±3.07 | 10.7±27.19 | 0.0001 |
| S. Leptin | 24.25±16.5 | 13.89±11.19 | 0.003 |
| Adiponectin/Leptin ratio | 0.15±0.24 | 3.03±15.04 | <.0001 |
Independent t-test
Figure 3.
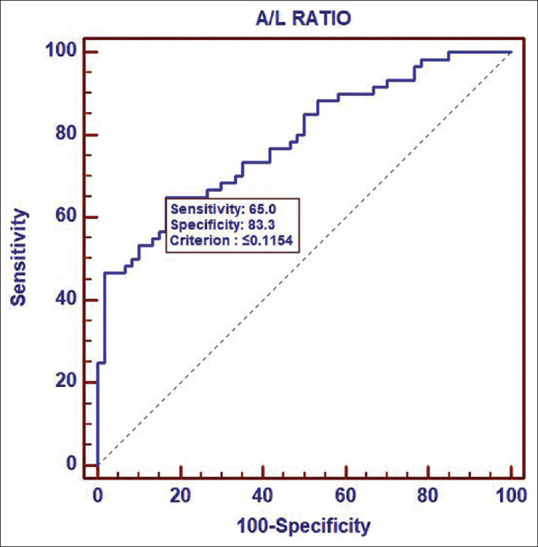
Receiver operating characteristic curve analysis of adiponectin/leptin ratio
Figure 2.
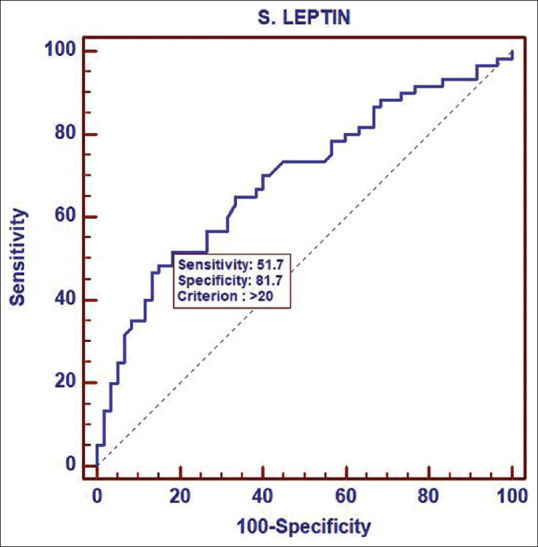
Receiver operating characteristic curve analysis of leptin
Adiponectin, leptin and A/L ratio were evaluated as diagnostic markers for PCOS. Sensitivity, specificity and PPV of Adiponectin, Leptin & A/L ratio are shown in Table 2 and Figures 1- 3. A/L ratio has the highest sensitivity for predicting PCOS as compared to Adiponectin and Leptin alone. There was no correlation of Adiponectin to Leptin Ratio with BMI and IR in PCOS and non PCOS women [Table 3].
Table 2.
Evaluation of adiponectin, leptin and adiponectin to leptin ratio as a diagnostic test for PCOS
| Adiponectin (95% CI) | Leptin (95% CI) | A/L ratio (95% CI) | |
|---|---|---|---|
| Sensitivity | 46.07% (33.7-60) | 51.67% (38.4-64.8) | 65%(51.6-76.9) |
| Specificity | 82.33% (83.8-92.2) | 81.67% (69.6-90.5) | 83.33% (71.5-91.76) |
| PPV | 87.5% (71.0-96.5) | 73.8% (58-86.1) | 79.6% (65.7-89.8) |
| NPV | 63.6% (52.7-73.6) | 62.8% (51.1-73.5) | 70.4% (58.4-80.7) |
| Diagnostic Accuracy | 70% (58.75-79.10) | 66.67%(54-77.65) | 73.33%(61.55-84.3) |
| LR of a Positive Test | 7 (2.6-18.7) | 2.82 (1.6-5.1) | 3.9 (2.2-7.1) |
Figure 1.
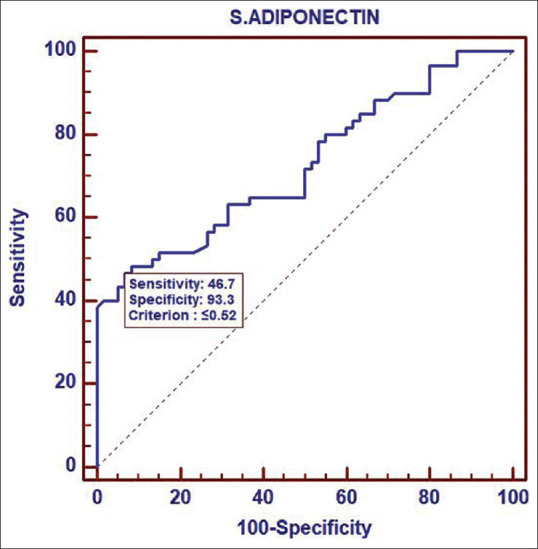
Receiver operating characteristic curve analysis of adiponectin
Table 3.
Correlation of adiponectin to leptin ratio with BMI and IR in PCOS and Non PCOS Women
| Parameters | A/L ratio |
|---|---|
| BMI | |
| Correlation Coefficient (r) | -0.074 |
| P | 0.5754 |
| n | 60 |
| OGTT | |
| Correlation Coefficient (r) | -0.203 |
| P | 0.12 |
| n | 60 |
| S. INSULIN | |
| Correlation Coefficient (r) | -0.018 |
| P | 0.8915 |
| n | 60 |
| HOMA IR | |
| Correlation Coefficient (r) | -0.041 |
| P | 0.757 |
| n | 60 |
Pearson correlation coefficient
DISCUSSION
PCOS is the most common endocrine disorder amongst women of reproductive age. Even though conditions such as insulin resistance and obesity are considered intrinsic to PCOS, none of them is included in the diagnostic criteria.[7]
Recently low A/L ratio has been reported to be independently associated with PCOS and is proposed to be a novel marker for obesity and IR.[26,27,28] Therefore, it might help to identify PCOS women who are at risk of developing metabolic abnormalities like IR, dyslipidemia, type 2 DM.[28]
In our study, a total of 120 women were enrolled and divided into two groups. The study group (n = 60) comprised of women diagnosed with PCOS, according to Rotterdam criteria and the Control group (n = 60) comprised of aged and BMI matched non PCOS women.
Among PCOS women Adiponectin levels were significantly lower than in non-PCOS women with mean value of 2.15 ± 3.07 ng/ml vs 10.7 ± 27.91 ng/ml, respectively, P ≤ 0.0001. Our result was in accordance with previous studies that also reported significantly lower adiponectin levels in both overweight and lean PCOS women.[14,15,16,17] However, many authors have failed to demonstrate lower serum Adiponectin levels in lean PCOS women.[18] In a meta-analysis by Li et al.,[17] despite significant heterogeneity (I² = 95.9%, P = 0.000) across studies, the pooled serum Adiponectin levels in PCOS women were significantly lower than in non PCOS women, WMD − 2.67, 95%, CI − 3.22 to − 2.13; P = 0.000.
We observed significantly higher levels of Serum Leptin in PCOS women as compared to non PCOS women, 24.25 ± 16.5 ng/ml vs 13.89 ± 11.9 ng/ml, P = 0.0003. ROC curve showed a significant correlation between Leptin levels and PCOS with Area under the curve of 0.693 at the cut off value of 20 ng/ml. Previous studies on serum Leptin in PCOS women have conflicting results with some studies showing significantly higher levels of leptin in PCOS women as compared to non-PCOS women.[22] While in some studies there was no difference in the serum leptin levels in PCOS and non-PCOS women.[23,24] A meta analysis of all relevant studies revealed that leptin levels were significantly higher in patients with PCOS than in controls, with a standardized mean difference of 1.62 (95% confidence interval: 1.01-2.23).[21]
A/L ratio, a novel predictor for PCOS is proposed to target newer therapeutic modalities in PCOS.[26,27,28]A/L ratio has also been reported to be a better predictor of IR than adiponectin or leptin alone.[25,26,27,28,29] In our study, serum adiponectin to leptin ratio levels were significantly lower in PCOS women as compared to non PCOS women, mean of 0.15 ± 0.24 ng/ml vs 3.03 ± 15.04 ng/ml, P < 0.0001. Our results are similar to previous studies showing significantly lower levels of A/L Ratio in PCOS as compared to non-PCOS women.[25,26,27,28,29] Many other studies have evaluated the L/A ratio instead of the A/L ratio which was reported higher in PCOS women compared to controls similar to our study.[28,29] The result of the present study is in contrast with the findings of Moran et al.[30] who found no difference in adiponectin and L/A ratios in women with and without PCOS.
Receiver Operator Characteristic (ROC) curve showed higher sensitivity and specificity of Adiponectin to Leptin ratio for diagnosis of PCOS with Area under the curve of 0.7873 at the cut off value of ≤0.1154 [Figure 3]. Our result was similar to that observed by Sarray et al.,[26] with AUC for A/L ratio 0.650 (std. error- 0.039), P < 0.001, whereas reported AUC for Adiponectin and Leptin were 0.546, P = 0.256 and 0.445, P = 0.178. Thus they concluded that the A/L ratio is a better predictor of PCOS as compared to individual adiponectin and leptin levels. ROC analysis in the previous studies have shown that A/L and L/A had significantly higher AUC values as compared with Adiponectin or leptin for the detection of PCOS, with a better predicting power of these ratios than leptin and adiponectin alone.[26,27,28,29]
Gupta et al.,[28] have proposed that Leptin/Adiponectin ratio (L/A ratio), rather than adiponectin or leptin alone, relates to insulin resistance in women with PCOS. But, in our study, there was no significant association of A/L Ratio with BMI, OGTT, serum Insulin and HOMA-IR, r = -0.074 and P = 0.5754, r= -0.203, P = 0.12, r = -0.018 and P = 0.8915 and r = - 0.041 and P = 0.757 respectively.
Lecke et al.[20] has reported that the percentage of body fat contributed significantly to the L/A ratio in PCOS, independently of BMI. They found a positive association of serum L/A ratio with HOMA-IR and reported strikingly higher serum L/A ratio in overweight/obese control women and women with PCOS vs normal weight controls, 5.27 (2.66 -13.58) and 7.73 (3.81-15.04) vs. 1.80 (0.94-3.72); P <.001). Sarray et al.[26] and Ali et al., observed a negative correlation of Adiponectin/leptin ratio while Gupta et al.[28] observed a positive correlation of L/A ratio with body mass index, HOMA-IR, insulin resistance, free insulin, testosterone, and sex hormone-binding globulin in PCOS women.[27] However, in our study no such correlation was observed. We also found significantly lower adiponectin, higher leptin and lower A/L ratio independent of Insulin resistance in PCOS women.
Alteration in the Adiponectin Leptin ratio can be used as a screening method for the diagnosis of PCOS. Further population based studies including large sample sizes are needed to find out if the A/L ratio at 0.1154 cut off can be used as a prognostic marker in women with PCOS.
A major limitation of this study is the small sample size and the women were recruited from the outpatient clinic of a tertiary care centre and may not reflect the true distribution of the general population. The main strength of the study is the inclusion of all recruited women for analysis. All women recruited in the study were able to give a sample in the follicular phase (Day 2-7 of the menstrual cycle).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam ESHRE/ASRM-Sponsored 3rdPCOS Consensus Workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. doi: 10.1093/humrep/der396. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome:An androgen excess society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 4.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovarian syndrom. Fertil Steril. 2016;106:6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 6.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries:Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–9. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 7.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus. Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Amato MC, Vesco R, Vigneri E, Ciresi A, Giordano C. Hyperinsulinism and polycystic ovary syndrome (PCOS):Role of insulin clearance. J Endocrinol Invest. 2015;38:1319–26. doi: 10.1007/s40618-015-0372-x. [DOI] [PubMed] [Google Scholar]
- 9.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome:A systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–63. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited:An update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–41. doi: 10.1210/er.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS:Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–11. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 14.Ardawi MSM, Rouzi AA. Plasma adiponectin and insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1708–16. doi: 10.1016/j.fertnstert.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 15.Olszanecka-Glinianowicz M, Kuglin D, Dąbkowska-Huć A, Skałba P. Serum adiponectin and resistin in relation to insulin resistance and markers of hyperandrogenism in lean and obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;154:51–6. doi: 10.1016/j.ejogrb.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J, Möhlig M, Wegewitz U, Ristow M, Pfeiffer AF, Schill T, et al. Adiponectin is independently associated with insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;61:738–46. doi: 10.1111/j.1365-2265.2004.02159.x. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Huang X, Zhong H, Peng Q, Chen S, Xie Y, et al. Low circulating adiponectin levels in women with polycystic ovary syndrome:An updated meta-analysis. Tumor Biol. 2014;35:3961–73. doi: 10.1007/s13277-013-1595-0. [DOI] [PubMed] [Google Scholar]
- 18.Toulis KA, Goulis DG, Farmakiotis D, Georgopoulos NA, Katsikis I, Tarlatzis BC, et al. Adiponectin levels in women with polycystic ovary syndrome:A systematic review and a meta-analysis. Hum Reprod Update. 2009;15:297–307. doi: 10.1093/humupd/dmp006. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor A, Phelan N, Tun TK, Boran G, Gibney J, Roche HM. High-molecular-weight adiponectin is selectively reduced in women with polycystic ovary syndrome independent of body mass index and severity of insulin resistance. J Clin Endocrinol Metab. 2010;95:1378–85. doi: 10.1210/jc.2009-1557. [DOI] [PubMed] [Google Scholar]
- 20.Lecke SB, Mattei F, Morsch DM, Spritzer PM. Abdominal subcutaneous fat gene expression and circulating levels of leptin and adiponectin in polycystic ovary syndrome. Fertil Steril. 2011;95:2044–9. doi: 10.1016/j.fertnstert.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Zheng SH, Du DF, Li XL. Leptin levels in women with polycystic ovary syndrome:A systematic review and a meta-analysis. Reprod Sci. 2017;24:656–70. doi: 10.1177/1933719116670265. [DOI] [PubMed] [Google Scholar]
- 22.Pusalkar M, Meherji P, Gokral J, Savardekar L, Chinnaraj S, Maitra A. Obesity and polycystic ovary syndrome:Association with androgens, leptin and its genotypes. Gynecol Endocrinol. 2010;26:874–82. doi: 10.3109/09513590.2010.487586. [DOI] [PubMed] [Google Scholar]
- 23.Telli MH, Yildirim M, Noyan V. Serum leptin levels in patients with polycystic ovary syndrome. Fertil Steril. 2002;77:932–5. doi: 10.1016/s0015-0282(02)02995-3. [DOI] [PubMed] [Google Scholar]
- 24.Pirwany IR, Fleming R, Sattar N, Greer IA, Wallace AM. Circulating leptin concentrations and ovarian function in polycystic ovary syndrome. Eur J Endocrinol. 2001;145:289–94. doi: 10.1530/eje.0.1450289. [DOI] [PubMed] [Google Scholar]
- 25.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio:A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7:57–62. doi: 10.1080/21623945.2017.1402151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarray S, Madan S, Saleh LR, Mahmoud N, Almawi WY. Validity of adiponectin-to-leptin and adiponectin-to-resistin ratios as predictors of polycystic ovary syndrome. Fertil Steril. 2015;104:460–6. doi: 10.1016/j.fertnstert.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Ali SH, Ali R AA, Al-Mosawy BJ. Adiponectin to leptin ratio as a marker of Insulin resistance in women with PCOS in relation to BMI. Int J Res Dev Pharm L Sci. 2015;5:1921–8. [Google Scholar]
- 28.Gupta V, Mishra S, Mishra S, Gupta V. L:A ratio, insulin resistance and metabolic risk in women with polycystic ovarian syndrome. Diabetes Metab Syndr. 2017;11(Suppl 2):S697–701. doi: 10.1016/j.dsx.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Golbahar J, Das NM, Al-Ayadhi MA, Gumaa K. Leptin-to-adiponectin, adiponectin-to-leptin ratios, and insulin are specific and sensitive markers associated with polycystic ovary syndrome:A case-control study from Bahrain. Metab Syndr Relat Disord. 2012;10:98–102. doi: 10.1089/met.2011.0075. [DOI] [PubMed] [Google Scholar]
- 30.Moran LJ, Meyer C, Hutchison SK, Zoungas S, Teede HJ. Novel inflammatory markers in overweight women with and without polycystic ovary syndrome and following pharmacological intervention. J Endocrinol Invest. 2010;33:258–65. doi: 10.1007/BF03345790. [DOI] [PubMed] [Google Scholar]


