Abstract
Background
The coronavirus disease 2019 (COVID-19) has affected approximately 2 million individuals worldwide; however, data regarding fatal cases have been limited.
Objective
To report the clinical features of 162 fatal cases of COVID-19 from 5 hospitals in Wuhan between December 30, 2019 and March 12, 2020.
Methods
The demographic data, signs and symptoms, clinical course, comorbidities, laboratory findings, computed tomographic (CT) scans, treatments, and complications of the patients with fatal cases were retrieved from electronic medical records.
Results
The median patient age was 69.5 (interquartile range: 63.0–77.25) years, and 80% of the patients were over 61 years. A total of 112 (69.1%) patients were men. Hypertension (45.1%) was the most common comorbidity, while 59 (36.4%) patients had no comorbidity. At admission, 131 (81.9%) patients had severe or critical COVID-19, whereas 39 (18.1%) patients with hypertension or chronic lung disease had moderate COVID-19. In total, 126 (77.8%) patients received antiviral treatment, while 132(81.5%) patients received glucocorticoid treatment. A total of 116 (71.6%) patients were admitted to the intensive care unit (ICU), and 137 (85.1%) patients received mechanical ventilation. Most patients received mechanical ventilation before ICU admission. Approximately 93.2% of the patients developed respiratory failure or acute respiratory distress syndrome. There were no significant differences in the inhospital survival time among the hospitals (P=0.14).
Conclusion
Young patients with moderate COVID-19 without comorbidity at admission could also develop fatal outcomes. The in-hospital survival time of the fatal cases was similar among the hospitals of different levels in Wuhan.
Keywords: Clinical features, Coronavirus disease 2019, Fatal cases, Survival time
Introduction
The coronavirus disease 2019 (COVID-19) has affected approximately 0.21 billion individuals and has caused more than 43 million deaths to date.[1] As COVID-19 is an outbreak caused by an emerging viral infection, the clinical features, and treatment methods are still under continuous study. Since the effectiveness of the current antiviral treatments is uncertain, the management of COVID-19 is still essentially supportive and symptomatic-based.[2] In most affected regions, the reported fatality rate for COVID-19 was approximately 5%, which is markedly lower than that for severe acute respiratory syndrome.[3,4] Recently, the fatality rate has increased in highly endemic countries. Based on data up to April 15, 2020, the fatality rates in Italy and the UK were 13.0% and 12.9%, respectively.[1] Although COVID-19 has caused nearly 120,000 deaths worldwide, limited data concerning fatal cases have been reported. Current evidence according to a small series of fatal cases indicated that men, elderly individuals, and patients with comorbidities have a potentially higher risk for death than their counter-parts.[5,6] However, this was not sufficient to allow clinical physicians to improve the case management strategy and recognize patients at risk for death in the early stage of hospital admission. Therefore, we aimed to further analyze the epidemiological and clinical characteristics of 162 fatal cases from 5 hospitals in Wuhan, China.
Materials and methods
Study design, setting, and participants
This retrospective study was conducted in 5 hospitals in Wuhan, China: Zhongnan Hospital of Wuhan University, the First People’s Hospital of Jiangxia District, Wuhan Third Hospital, Union Jiangbei Hospital, and Wuhan No. 7 Hospital. All hospitals were designated for treating COVID-19 cases. We included fatal cases of COVID-19 assessed between December 30, 2019 and March 12, 2020 from these hospitals in our analysis. COVID-19 was diagnosed in accordance with the guidelines of the National Health Commission (NHC) of China.[7] This study was approved by the Ethics Committees of Zhongnan Hospital of Wuhan University (No. 2020005), the First People’s Hospital of Jiangxia District (No. EN1003), Wuhan Third Hospital (No. 2020H001), Union Jiangbei Hospital (No. 10012), and Wuhan No. 7 Hospital (SQ1011).
Data collection
The demographic data, signs and symptoms, vital signs, smoking history, date of illness onset, date of hospital and intensive care unit (ICU) admission, comorbidities, laboratory findings, CT scans, treatments, and complications of the patients with fatal cases were retrieved from electronic medical records. The data were documented using a predesigned data collection form. All data were checked by two independent physicians, and a third expert made the final decision when disagreements occurred.
Fever was defined as an axillary temperature of at least 37.3°C. Acute respiratory distress syndrome (ARDS) was determined in accordance with the Berlin Definition.[8] Sepsis and septic shock were diagnosed in accordance with the 2016 Third International Consensus Definition for Sepsis and Septic Shock.[9] Acute kidney injury (AKI) was diagnosed in accordance with the KDIGO clinical practice guidelines.[10] Acute cardiac injury was diagnosed on the basis of increased serum cardiac biomarker levels. Disseminated intravascular coagulation was defined in accordance with the guidelines of the Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis.[11] The disease severity of COVID-19 at hospital admission was defined as mild, moderate, severe, and critical according to the guidelines of the NHC of China (trial version 6.0).[7] The date of illness onset was defined as the first day in which symptoms (eg, fever, cough, chest distress, and fatigue) appeared. Glucocorticoid treatment was defined as the use of glucocorticoid drugs, such as hydrocortisone, prednisone, methylprednisolone, and dexamethasone, during the treatment of COVID-19.
Laboratory testing for severe acute respiratory syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) laboratory test assays were based on the recommendations of the World Health Organization.[12] Throat swab samples were collected from patients with suspected COVID-19 and immediately placed into sterile tubes containing 3 mL of viral transport media. Throat swab RNA was extracted and tested using real-time reverse-transcription polymerase chain reaction (RT-PCR) with SARS-CoV-2 specific primers and probes. Two target genes (ie, open reading frame 1ab and nucleocapsid protein) were simultaneously amplified and tested using real-time RT-PCR. The real-time RT-PCR assay was performed using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer’s instructions (DAAN Gene Co., Ltd., Sun Yat-Sen University, Guangzhou, China), as previously described.[13]
Statistical analysis
Medians and interquartile ranges (IQRs) were used to describe the continuous variables. Numbers and percentages were used to describe the categorical variables. Survival analysis techniques were used to estimate time-delay distributions, including illness onset to hospital admission, illness onset to ICU admission, hospital admission to ICU admission, illness onset to death, hospital admission to death, and ICU admission to death. We compared alternative parametric distributions, including gamma, Weibull, and lognormal distributions, with nonparametric estimates and selected the best parametric distribution using the chi-square goodness-of-fittest. Kaplan–Meier analysis was performed to evaluate the survival time from illness onset, hospital admission, or ICU admission. The Kruskal–Wallis test was used to compare the time intervals between the different events and death and the time intervals between hospital admission and death in the different hospitals. The Kruskal–Wallis test was also used to examine the differences among the disease severities at admission for the continuous and ranked variables. The frequency rates of the categorical variables were compared using the chi-square test. All analyses were performed using the R software (version 3.6.3, R Foundation for Statistical Computing).
Results
General characteristics
In total, 162 fatal cases of COVID-19 from the 5 hospitals in Wuhan before March 12, 2020 were included in the analysis (Table 1). The patient age ranged from 29 to 96 years. The median patient age was 69.5 (IQR: 63.0–77.25) years, and 80% of the patients were at least 61 years (Fig. 1). Nearly two-thirds (112/162, 69.1%) of the patients were men, and the male-to-female sex ratio was 2.2:1 (Table 1). Only 12 (7.4%) patients were current smokers, while 146 (90.1%) had never smoked. A total of 103 (63.6%) patients had coexisting disorders at admission; specifically, 43 (26.5%) had a single comorbidity, and 60 (37.0%) had two or more comorbidities. Meanwhile, 59 (36.4%) patients had no comorbidities before SARS-CoV-2 infection. Hypertension was the most common comorbidity (45.1%), followed by diabetes (19.1%), coronary heart disease (18.5%), renal failure (14.8%), and cerebrovascular disease (10.5%). The most common symptoms at illness onset were fever, cough, expectoration, chest distress, dyspnea, and fatigue (Table 1). A large proportion of cases (n = 131, 80.9%) were assessed as severe or critical at hospital admission, and the remaining 29 (18.1%) cases were assessed as moderate. The comparison of the general characteristics by disease severity is shown in Table S1, http://links.lww.com/ECCM/A14. The patients with moderate COVID-19 had a higher prevalence of hypertension and chronic lung disease than those with severe and critical COVID-19 (P < 0.05).
Table 1.
General Characteristics of the Patients with Fatal COVID-19
| Characteristics | Patients with Fatal COVID-19 (n=162) n (%), Median (IQR) |
|---|---|
| Hospitals | |
| Zhongnan Hospital of Wuhan University (III-A)* | 30 (18.5) |
| The First People’s Hospital of Jiangxia | 22 (13.6) |
| District (III-B)* | |
| Wuhan Third Hospital (III-A)* | 26 (16.0) |
| Union Jiangbei Hospital (II-A)* | 33 (20.4) |
| Wuhan No. 7 Hospital (II-A)* | 51 (31.5) |
| Sex | |
| Female | 50 (30.9) |
| Male | 112 (69.1) |
| Smoking status | |
| Never smoked | 146 (90.1) |
| Former smoker | 4 (2.5) |
| Current smoker | 12 (7.4) |
| Comorbidities | |
| Hypertension | 73 (45.1) |
| Diabetes | 31 (19.1) |
| Coronary heart disease | 30 (18.5) |
| Renal insufficiency | 24 (14.8) |
| Cerebrovascular disease | 17 (10.5) |
| Chronic lung disease | 12 (7.4) |
| Malignant tumor | 12 (7.4) |
| Presence of comorbidities | |
| 0 | 59 (36.4) |
| 1 | 43 (26.5) |
| 2 | 36 (22.2) |
| ≥3 | 24 (14.8) |
| Surgery history within 6 months | 8 (4.9) |
| Signs and symptoms | |
| Any | 159 (98.1) |
| Fever | 128 (79.0) |
| Highest temperature | |
| 37.3–38.0°C | 75 (46.3) |
| 38.1–39.0°C | 74 (45.7) |
| >39.0°C | 13 (8.0) |
| Duration of fever, d | 7 (5–10) |
| Cough or sputum production | 111 (68.5) |
| Chest distress/dyspnea | 101 (62.3) |
| Fatigue | 92 (56.8) |
| Nausea or vomiting | 23 (14.2) |
| Diarrhea | 22 (13.6) |
| Disease severity at admission† | |
| Moderate | 29 (18.1) |
| Severe | 51 (31.9) |
| Critical | 80 (50.0) |
Data are n (%) or median (IQR). The denominator used for calculating the percentage may not be the total number because of missing data.
COVID-19, coronavirus disease 2019; IQR, interquartile range.
*Hospital grades. The order of grade from the best was grade III-A, grade III-B, and grade II-A.
† Data are missing for two cases.
Figure 1.
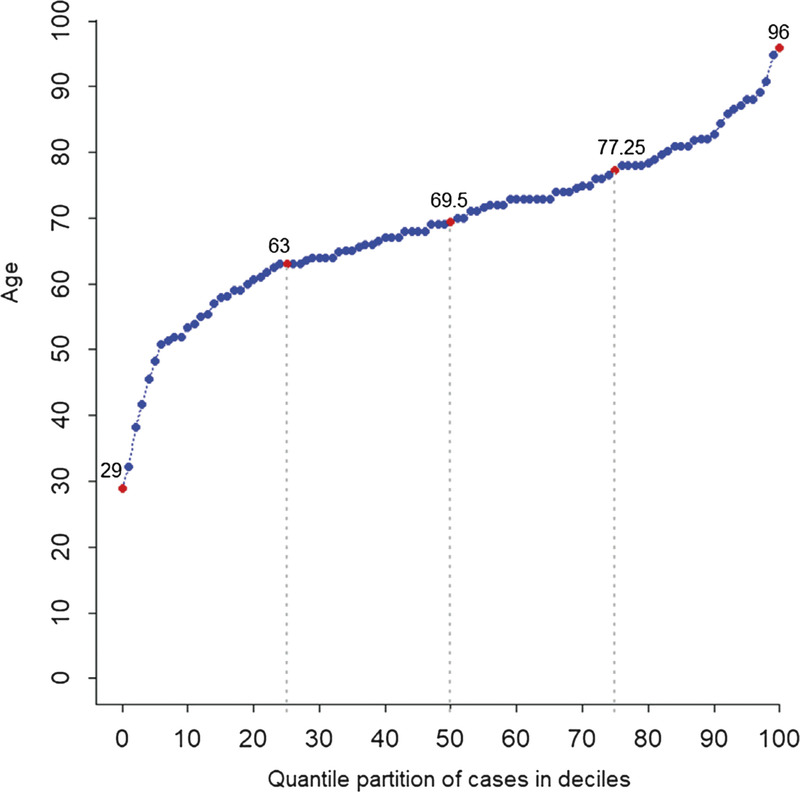
Age distribution of the patients with fatal COVID-19. COVID-19, coronavirus disease2019.
Laboratory and radiological findings
Most patients had normal vital signs at admission, including blood pressure (n = 108, 67.9%), heart rate (n = 116, 73.0%), and temperature (n = 96, 59.3%) (Table 2). Three (1.9%) patients had a decreased systolic pressure, while 18 (11.3%) patients had a decreased diastolic pressure. Seventy-nine (50.6%) patients had an increased respiratory rate, and only 15 (9.6%) patients had a respiratory rate of >30 breaths/min. A decreased oxygen saturation was observed in 110 (67.9%) patients. The neutrophil count was increased in 76 (47.8%) patients, while the lymphocyte count was decreased in 119 (74.4%) patients. We found increased procalcitonin and interleukin (IL)-6 levels in 51 (37.2%) and 12 (31.6%) patients, respectively. In addition, the erythrocyte sedimentation rate was increased in 46 (60.5%) patients. Thirty-nine (24.1%) patients had a longer activated partial prothrombin time and prothrombin time. The D-dimer level was increased in 77 (64.7%) patients. At admission, 107 (80.5%) patients showed a decreased partial pressure of oxygen (PaO2), and 72 (67.3%) of them had a PaO2 of <60mmHg. Acid-base imbalance occurred in 79 (59.4%) patients, and the lactate level was increased in 92 (76.0%) patients. There was radiological evidence of pneumonia in all patients at admission. Bilateral pneumonia was observed in the majority of the patients (155, 95.7%), while unilateral pneumonia was observed in 7 (4.3%) patients. Moreover, 112 (69.1%) patients showed multiple mottling and ground-glass opacities (Table 2).
Table 2.
Vital Signs and Laboratory and Radiological Findings of the Patients with Fatal COVID-19
| Items | Patients with Fatal COVID-19 (n=162) n (%), Median (IQR) |
|---|---|
| Vital signs | |
| Systolic blood pressure (mmHg; normal range: 90–140) | 130 (119–141) |
| Increased | 40 (25.2) |
| Decreased | 3 (1.9) |
| Diastolic blood pressure (mm Hg; normal range: 60–90) | 76 (66–82) |
| Increased | 10 (6.3) |
| Decreased | 18 (11.3) |
| Heart rate (beats per minute; normal range: 60–100) | 90 (80–101) |
| Increased | 40 (25.2) |
| Axillary temperature (°C; normal range: 36.2–37.3) | 36.8 (36.5–37.6) |
| Increased | 50 (30.9) |
| Respiratory rate (breaths per minute; normal range: 12–20) | 21 (20–25) |
| 21–29 | 64 (41.0) |
| ≥30 | 15 (9.6) |
| Oxygen saturation (%; normal range: ≥94) | 90 (83–95) |
| Decreased | 110 (67.9) |
| Laboratory findings | |
| Leukocyte count (109/L; normal range: 3.5–9.5) | 7.2 (4.7–11.5) |
| Increased | 60 (37.7) |
| Decreased | 13 (8.2) |
| Neutrophil count (109/L; normal range: 1.8–6.3) | 6.1 (3.6–10.3) |
| Increased | 76 (47.8) |
| Lymphocyte count (109/L; normal range: 1.1–3.2) | 0.6 (0.4–1.0) |
| <1.0 | 119 (74.4) |
| ≥1.0 | 41 (25.6) |
| Erythrocyte count (1012 /L; normal range: 4.3–5.8) | 4.1 (3.7–4.6) |
| Decreased | 93 (58.9) |
| Hemoglobin level (g/L; normal range: 130–175) | 125 (109–134) |
| Decreased | 103 (64.8) |
| Platelet count (109/L; normal range: 1 5–350) | 145 (104–191) |
| Decreased | 58 (36.7) |
| Procalcitonin level (ng/mL; normal range: < 0.5) | 0.27 (0.11–0.92) |
| Increased | 51 (37.2) |
| IL-6 level (pg/mL; normal range: 0.0–7.0) | 0 (0–45.4) |
| Increased | 12 (31.6 |
| ESR (mm/h; normal range: 0.0–15.0) | 27.5 (0–48.0) |
| Increased | 46 (60.5) |
| APTT (s; normal range: 8.0–43.5) | 41.2 (26.4–51.7) |
| Increased | 39 (24.1) |
| PT (s; normal range: 11.0–16.0) | 14.2 (8.7–19.6) |
| Increased | 39 (24.1) |
| D-dimer level (ng/mL; normal range: 0.0–500.0) | 740 (364–4270) |
| Increased | 77 (64.7) |
| ALT level (U/L; normal range: 9.0–50.0) | 31 (18–42) |
| Increased | 28 (17.8) |
| AST level (U/L; normal range: 15.0–40.0) | 48.0 (31.3–71.5) |
| Increased | 91 (58.0) |
| TBLI level (µmol/L; normal range: 5.0–21.0) | 11.4 (8.0–17.4) |
| Increased | 25 (15.9) |
| Albumin level (g/L; normal range: 40.0–55.0) | 32.9 (29.5–35.7) |
| Decreased | 148 (96.1) |
| Creatinine level (µmol/L; normal range: 64.0–104.0) | 78.0 (63.6–115.5) |
| Increased | 50 (31.8) |
| BUN level (mmol/L; normal range: 2.8–7.6) | 7.9 (5.5–11.9) |
| Increased | 80 (51.0) |
| hs-cTnI level (pg/mL; normal range: 0.0– 26.2) | 0.07 (0.02–15.28) |
| Increased | 14 (17.9) |
| NT-proBNP level (pg/mL; normal range: 0.0–900.0) | 125 (0–1190) |
| Increased | 18 (27.7) |
| Blood glucose level (mmol/L; normal range: 3.9–6.1) | 8.3 (6.4–10.1) |
| Increased | 43 (82.7) |
| pH (normal range: 7.35–7.45) | 7.43 (7.35–7.48) |
| Increased | 43 (32.3) |
| Decreased | 36 (27.1) |
| PaO2 (mmHg; normal range: 80–100) | 56 (44–72) |
| Decreased | 107 (80.5) |
| PaCO2 (mm Hg; normal range: 35–45) | 35 (28–44) |
| Increased | 29 (21.8) |
| HCO3¯ level (mmol/L; normal range: 21.4–27.3) | 22.1 (18.7–26.0) |
| Increased | 27 (20.6) |
| Decreased | 56 (42.7) |
| Lactate level (mmol/L; normal range: 0.5–1.6) | 2.2 (1.7–3.3) |
| Increased | 92 (76.0) |
| Radiological findings | |
| Unilateral pneumonia | 7 (4.3) |
| Bilateral pneumonia | 155 (95.7) |
| Multiple mottling and ground-glass opacities | 112 (69.1) |
Data are median (IQR) or n (%). Increased means values over the upper limit of the normal range, and decreased means values below the lower limit of the normal range. The denominator used for calculating the percentage may not be the total number because of missing data.
ALT, alanine aminotransferase; APTT, activated partial prothrombin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; COVID-19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; HCO3¯ bicarbonate; hs-cTnI, high-sensitivity troponin; IL-6, interleukin-6; IQR, interquartile range; NT-proBNP, N-terminal pro-brain natriuretic peptide; PaO2, partial pressure of oxygen in the artery; PaCO2, partial pressure of carbon dioxide in the artery; pH, potential of hydrogen; PT, prothrombin time; TBLI, total bilirubin.
Treatments and complications
Among the 162 patients with fatal COVID-19, 126 (77.8%) received antiviral treatment, including oseltami-vir (43.2%), ribavirin (29.6%), abidor (21.6%), interferon (13.6%), and lopinavir/ritonavir (4.3%). In addition, 81 (50.0%) patients received a single antiviral drug, while 45 (27.8%) received combined antiviral treatment. Gluco-corticoid treatment was administered to 132 (81.5%) patients. The median duration of glucocorticoid treatment was 5 (IQR: 3–9; range: 1–26) days, with a median initial dose of 80 (IQR: 40–80) mg/day. Vasoactive drugs were prescribed in 104 (80.0%) patients, and immunoglobulin was used in 63 (38.9%) patients. Moreover, 17 (10.6%) patients received continuous renal replacement therapy. Extracorporeal membrane oxygenation was performed in only 2 (1.2%) patients. Finally, 116 (71.6%) patients were admitted to the ICU, and 137 (85.1%) patients received mechanical ventilation. Respiratory failure or ARDS (93.2%) was the most common complication, followed by acute cardiac injury (51.9%), sepsis (37.0%), AKI (32.1%), septic shock (30.2%), and acute liver injury (22.2%) (Table 3). A total of 130 patients (80.2%) had multiple complications.
Table 3.
Treatments and Complications of the Patients with Fatal COVID-19
| Treatments and complications | Patients with Fatal COVID-19 (n=162) n (%), Median (IQR) |
|---|---|
| Antiviral drugs | |
| Oseltamivir | 70 (43.2) |
| Ribavirin | 48 (29.6) |
| Abidor | 35 (21.6) |
| Interferon | 22 (13.6) |
| Lopinavir/ritonavir | 7 (4.3) |
| Antiviral treatment | 126 (77.8) |
| Monotherapy | 81 (50.0) |
| Combined therapy | 45 (27.8) |
| Antibiotics | 154 (95.1) |
| Glucocorticoids | 132 (81.5) |
| Initial dose, mg/qd | 80 (40–80) |
| Duration of treatment, d | 5 (3–9) |
| Intravenous immunoglobin therapy | 63 (38.9) |
| Vasoactive drugs | 104 (80.0) |
| Oxygen therapy | 155 (96.9) |
| Noninvasive ventilation | 76 (47.2) |
| Invasive mechanical ventilation | 92 (57.1) |
| Duration of mechanical ventilation, d | 6 (2–13) |
| ECMO | 2 (1.2) |
| CRRT | 17 (10.6) |
| ICU admission | 116 (71.6) |
| Length of stay, d | 2 (1 –5) |
| Complications | |
| Any | 160 (98.8) |
| ARDS or respiratory failure | 151 (93.2) |
| Acute cardiac injury | 84 (51.9) |
| Sepsis | 60 (37.0) |
| Acute kidney injury | 52 (32.1) |
| Septic shock | 49 (30.2) |
| Acute liver injury | 36 (22.2) |
| DIC | 25 (15.4) |
| Arrhythmia | 24 (14.8) |
| Gastrointestinal bleeding | 13 (8.0) |
| ACVD | 7 (4.3) |
Data are n (%) or median (IQR). The denominator used for calculating the percentage may not be the total number because of missing data.
ACVD, acute cerebrovascular disease; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Time-delay distributions
The time-delay distribution was estimated on the basis of the best-fitting Weibull distribution (Fig. 2A and B). The median onset to admission interval was 8.0 days (95% CI: 7.1–8.9).
Figure 2.
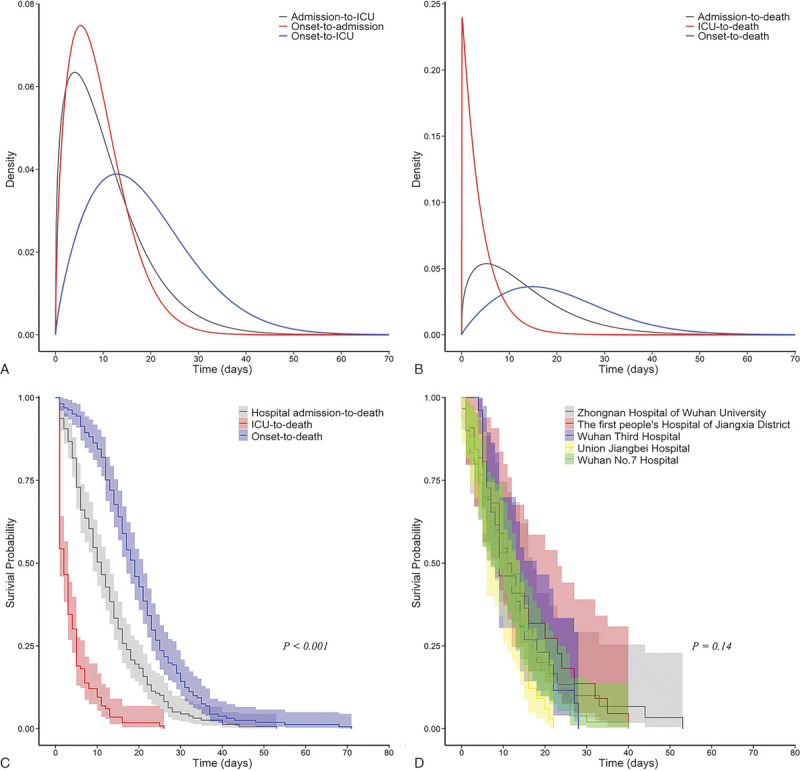
Time-delay distributions and Kaplan–Meier analysis of the time delay for the fatal cases of COVID-19. The estimated distributions of the time from illness onset to hospital or ICU admission and from hospital admission to ICU admission are shown in Panel A. The estimated distributions of the time from illness onset and hospital or ICU admission to death are shown in Panel B. The Kaplan–Meier analysis of the time from illness onset and hospital or ICU admission to death is shown in Panel C. The Kaplan–Meier analysis of the time from hospital admission to death is shown in Panel D. The shades correspond to the 95% confidence intervals of each curve. COVID-19, coronavirus disease 2019.
The median admission to ICU interval was 8.8 days (95% CI: 7.4–10.3) (Fig. 2A). The median admission to death and ICU to death intervals were 10.4 days (95% CI: 9.2–11.8) and 2.7 days (95% CI: 2.2–3.3)(Fig. 2B and C), respectively. The admission to death interval among the patients admitted to the different hospitals was similar (Fig. 2D). The observation time intervals between the different events are shown in Figure S1, http://links.lww.com/ECCM/A15.
Discussion
Elderly individuals and men have been reported to be vulnerable to death caused by COVID-19 in some studies.[5,6,14–17] In our study, we observed that the number of fatal cases of COVID-19 was higher among the men than among the women. The median age of the patients with fatal COVID-19 was 69.5 years, and the patients aged more than 60 years accounted for more than 80% of all patients, which is consistent with previous findings.[14] Nevertheless, the percentage of patients with fatal COVID-19 aged <60 years (20%) cannot be ignored, and death caused by COVID-19 might occur at any age. Additionally, more than 60% of the patients with fatal COVID-19 had chronic underlying diseases, including hypertension, diabetes, coronary heart disease, and renal insufficiency, which also agrees with previous find-ings.[6,14,16,17] Although comorbidities are risk factors for poor outcomes, 59 (36.4%) patients died without any comorbidity in our study. Another important finding is that a total of 29 (18.1%) patients with moderate COVID-19 at admission died during hospitalization. Compared with the patients with severe or critical COVID-19, most patients with moderate COVID-19 had a history of hypertension or chronic lung disease. Although it has been suggested that smoking history has a strong predictive ability for mortality from viral pneumonia,[18] our study found that more than 90% of the deaths were observed among the nonsmokers. In a recent study, 85.4% of patients with COVID-19 had never smoked.[19] Therefore, smoking history is not a typical risk factor for COVID-19 prognosis.
The most common laboratory abnormalities observed in our study were hypoalbuminemia, hyperglycemia, hypox-emia, hyperlactacidemia, lymphocytopenia, hypohemo-globin, and increased D-dimer levels. These laboratory abnormalities are similar to those previously observed in patients with SARS-CoV-2 infection.[5,6,13–17,19–22] We found that 96.1% of the patients with fatal COVID-19 had hypoalbuminemia, which has been proven to be associated with mortality in critical patients.[23] Lympho-cytopenia occurred in more than 70% of the patients in our study, a finding that is also consistent with the results of recent reports.[5,6,13,16,17,19] Furthermore, approximately 64.7% of the patients with COVID-19 had increased D-dimer levels. High D-dimer levels have been reported to be associated with 28-day mortality in patients with infection or sepsis identified in the emergency department.[24] Increased IL-16 levels may be related to cytokine storm induced by SARS-CoV-2 infection; however, only a few patients were tested for IL-6 expression in this study. In the later stages of the disease, the majority of the patients (98.8%) died of pulmonary and extrapulmonary organ damage. The major critical complications during hospi-talization included respiratory failure or ARDS, acute cardiac injury, sepsis, AKI, and septic shock in our study, which are slightly different from those reported in recent studies.[6,14,17]
The efficacy of antiviral treatment for SARS-CoV-2 infection is still under evaluation.[25,26] In our study, 126 (77.8%) patients received antiviral treatment, including oseltamivir (43.2%), ribavirin (29.6%), abidor (21.6%), interferon (13.6%), and lopinavir/ritonavir (4.3%). Nearly two-thirds of the patients were treated with a single antiviral drug, while more than one-third received combined antiviral treatment. Meanwhile, the efficacy of corticosteroids in the treatment of COVID-19 pneumonia remains uncertain.[27] Some experts recommended short courses of corticosteroid treatment at low to moderate doses, used prudently for critically ill patients with COVID-19 pneumonia.[28] In this study, most patients (81.5%) received glucocorticoid treatment. However, the recent RECOVERY trial from the UK has shown that a lower 28-day mortality was observed among patients receiving dexamethasone who were on oxygen/mechanical ventilation, but not among those receiving no respiratory support.[29] In addition, our study showed that most patients received vasoactive drug treatment and oxygen therapy, and the proportion of patients who received intravenous immunoglobulin therapy was relatively small, which is slightly different from that indicated in recent reports.[6,14,17]
The median interval between hospital admission and mechanical ventilation was 3 days. Nevertheless 50% of the patients were admitted to the ICU 8 days after hospital admission. The large time lag between mechanical ventilation and ICU admission might represent a shortage of beds in the ICU. A previous study conducted at the beginning of the COVID-19 outbreak has shown that the median duration from ICU admission to death was 7 days.[16] However our study showed a much shorter median duration using estimations of Weibull distribution (ie only 2.7days). This shorter median duration of ICU stay for COVID-19 cases before death also confirmed the shortage of ICU beds with a dramatic increase in the incidence of COVID-19. The patients could not be admitted to the ICU for best case management in the early stage of serious deterioration. The study by Du et al. on 85 fatal cases showed that the median duration from hospital admission to death was 5 days.[6] A longer duration of hospital stay for COVID-19 cases before death was observed in our study. Additionally the survival times for the COVID-19 cases from the 5 hospitals of different levels were similar. This could be explained by the standardized care for COVID-19 cases following the NHC guidelines in these hospitals. Moreover the assistance of medical teams and medical instruments from other cities may have minimized the differences in the human resources and equipment between the hospitals.
Our study has some limitations. First this study only analyzed fatal cases of COVID-19, which had limitations and one-sidedness and may have led to selection bias. Further studies including fatal and nonfatal cases of COVID-19 with a large sample size will provide more evidence for case management. Second the patients who died at the hospital after a long hospital stay had a lower probability of being observed at the date of last follow-up (ie March 10 2020) especially those who were admitted shortly before the date of the last follow-up than their counterparts. Therefore the delays reported in Figure 2 have been under-estimated. Meanwhile owing to the varying qualities of medical records and the large number of participating doctors there may be uncontrollable information bias in this study. Third we found in our descriptive analyses that the patients with moderate COVID-19 and younger patients had fatal outcomes even in those without comorbidity. A case–control or cohort study should be conducted in the future to better identify the predictive factors for recognizing patients at a high risk of death in the early stage of hospital admission.
Conclusion
In our study a considerable proportion of young patients with COVID-19 without comorbidity and those with moderate COVID-19 at admission developed fatal outcomes. In addition the in-hospital survival time for the fatal cases was similar between the hospitals of different levels in Wuhan. With the limited knowledge on COVID-19 it is a considerable challenge for clinical physicians to recognize patients at a high risk of death early.
Conflict of interest statement
The authors declare no conflict of interest. Yan Zhao is an Associate Editor of Emergency and Critical Care Medicine. The article was subject to the journal’s standard procedures with peer review handled independently of this Associate Editor and their research groups.
Author contributions
Zhao Z, Xing W, Hu Q, and Zhao Y conceived and designed the study. Fang Q, Guo J, Yang L Fu S, Li A, Xia J, Ma M, Hu Z, Huang L, and Jiang C performed data extraction from the electric medical records system. Ding G, Liu R, Yu J, Xia J, Li S, Yu M, Wang P, Xu X, and Zhou X performed the data analysis and figure illustration. Zhou X, Ding G, and Xing W discussed the data and wrote the manuscript. Hu Q, Zhao Y, and Zhao Z supervised the study. All authors approved the final version of the manuscript for submission.
Funding
This study was supported by the National Natural Science Foundation of China (81900097, 81903401), the Emergency Response Project of Hubei Science and Technology Department (2020FCA023), the Young Taishan Scholars Program of Shandong Province of China (tsqn20161046), the Shandong Province Higher Educational Young and Innovation Technology Supporting Program (2019KJL004), the Academic Promotion Program of Shandong First Medical University (2019RC010), and the Emergency Diagnostic and Therapeutic Center of Central China.
Ethical approval of studies and informed consent
This study was approved by the Ethics Committees of Zhongnan Hospital of Wuhan University (No. 2020005), the First People’s Hospital of Jiangxia District (No. EN1003), Wuhan Third Hospital (No. 2020H001), Union Jiangbei Hospital (No. 10012), and Wuhan No. 7 Hospital (SQ1011). Written informed consent was obtained from the patients’ legal representative in accordance with the ethical standards of the responsible committee on human research. All authors have read the manuscript and approved its submission to the journal.
Acknowledgments
We would like to thank Dr. Gilles Hejblum for the assistance in manuscript preparation.
Footnotes
How to cite this article: Zhou X, Ding G, Fang Q, et al. Clinical features of 162 fatal cases of COVID-19: a multi-center retrospective study. Emerg Crit Care Med. 2022;2(3):109–115. doi: 10.1097/EC9.0000000000000026
XZ, GD, QF, JG, and LY contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online: 25 February, 2022
Contributor Information
Xianlong Zhou, Email: xianlongzhou@whu.edu.cn.
Guoyong Ding, Email: gyding@sdfmu.edu.cn.
Qing Fang, Email: 382180392@qq.com.
Jun Guo, Email: 625246269@qq.com.
Luyu Yang, Email: yangluyu_114@163.com.
Ping Wang, Email: 807875383@qq.com.
Shou-Zhi Fu, Email: fszfsz188@163.com.
Ang Li, Email: medi_angel@126.com.
Jian Xia, Email: jianjian_1998@sina.com.
Jiangtao Yu, Email: yjt@whu.edu.cn.
Jianyou Xia, Email: dr.xiajianyou@foxmail.com.
Min Ma, Email: 1379014489@qq.com.
Zhuanzhuan Hu, Email: zhuanzhuanhu@whu.edu.cn.
Lei Huang, Email: 854600028@qq.com.
Ruining Liu, Email: rn.liu@whu.edu.cn.
Cheng Jiang, Email: chengjiang@whu.edu.cn.
Shaoping Li, Email: lishaoping@whu.edu.cn.
Mingxia Yu, Email: wb000778@whu.edu.cn.
Xizhu Xu, Email: xzxu@sdfmu.edu.cn.
Yan Zhao, Email: doctoryanzhao@whu.edu.cn.
Quan Hu, Email: huquan197799@sina.com.
Weijia Xing, Email: wjxing@sdfmu.edu.cn.
Zhigang Zhao, Email: drzhaozhigang@163.com.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed August 18, 2021. https://covid19.who.int/.
- 2.Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li LQ Huang T Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S Cao P Du P, et al. Early estimation of the case fatality rate of COVID-19 in mainland China: a data-driven analysis. Ann Transl Med. 2020;8(4):128. doi: 10.21037/atm.2020.02.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F Yu T Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Y Tu L Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–1379. doi: 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Health Commission of the People’s Republic of China. Guidelines for the Diagnosis and Treatment of Novel Coronavirus Infection (Trial Version 6). Accessed March 25, 2020. http://www.gov.cn/zhengce/zhengceku/2020-02/19/content_5480948.htm
- 8.Ranieri VM Rubenfeld GD Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 9.Singer M Deutschman CS Seymour CW, et al. The third International Consensus Definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada H Thachil J Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11(4):761–776. doi: 10.1111/jth.12155 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-nCoV in humans. Accessed April 10, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- 13.Wang D Hu B Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T Wu D Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N Zhou M Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X Yu Y Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du R.H Liu L.M Yin W, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17(7):839–846. doi: 10.1513/Annal-sATS.202003-225OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L Wei D Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.-J Ni Z.-Y Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young BE Ong SWX Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu XW Wu XX Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368: m606. doi: 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. 2017;130(12):1465.e11–1465.e19. doi: 10.1016/j.amjmed.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 24.Rodelo J.R De la Rosa G Valencia M.L, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30(9):1991–1999. doi: 10.1016/j.ajem.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 25.Cao B Wang Y Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grein J Ohmagari N Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horby P Lim WS Emberson J.R, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]


