Supplemental Digital Content is Available in the Text.
Key Words: anterior segment optical coherence tomography, in vivo confocal microscopy, fungal keratitis, bacterial keratitis, endothelial plaques
Purpose:
Endothelial plaque is an important sign of fungal keratitis and is related to diagnosis, surgical indications, and prognosis. However, bacterial keratitis sometimes involves fibrin formation on the back corneal surface, similar to endothelial plaques. Because corneal infiltration interferes with precise observation of the posterior corneal plaque, distinguishing pathogens with a slitlamp is difficult. We hope to assist clinicians in early diagnosis and timely treatment by observing the connection state of endothelial plaques and the corneal endothelium through anterior segment optical coherence tomography (AS-OCT) and the different forms of endothelial plaques in infectious keratopathy through in vivo confocal microscopy (IVCM).
Methods:
We analyzed 52 patients in the Eye Hospital of the First Affiliated Hospital of Harbin Medical University who were clearly diagnosed with fungal or bacterial keratitis with endothelial plaques. All patients underwent AS-OCT and IVCM on admission.
Results:
According to the smear, IVCM, or fungal and bacterial culture results, the patients were diagnosed with fungal (28 patients) or bacterial keratitis (24 patients). AS-OCT in 25 patients diagnosed with fungal keratitis revealed that the corneal endothelium–endothelial plaque boundary was unclear and wavy, and 24 patients had unclear cell boundaries and a large number of compactly distributed inflammatory cells in the endothelial layer according to IVCM. AS-OCT in 23 patients diagnosed with bacterial keratitis revealed clear corneal endothelium–endothelial plaque boundaries, and insufficient endothelial cell boundaries with a large number of visible and scattered inflammatory cell structures were observed through IVCM in 22 patients.
Conclusions:
Corneal endothelial plaque detection by AS-OCT and IVCM can be used for early diagnosis of infectious keratitis.
The human eye has a unique structure and is exposed to the environment for a long time, making it susceptible to external pathogenic microorganisms and infection.1 Infectious keratitis is a potentially vision-threatening eye disease caused by bacteria, fungi, and protists.2–4 The cornea lacks a vascular structure and has poor resistance to infection. Once keratitis is diagnosed, treatment for pathogens should be started immediately.5 If appropriate antibacterial treatment is delayed, corneal infection will be further aggravated and vision loss or even blindness will be caused.6 If the infected lesion is located only in the deep stroma or endodermal surface of the cornea when the patients are treated for infectious keratitis and when the corneal epithelium is complete and there is no lesion in the superficial stroma of the cornea, it is often difficult for doctors to make a correct diagnosis, and the rate of early misdiagnosis is high.7 Inappropriate medicine treatment can cause rapid aggravation of keratitis. For example, glucocorticoids can be used to treat bacterial keratitis, but they are contraindicated during the infection period of fungal keratitis8; therefore, the early diagnosis of keratitis infection is particularly important.
On the other hand, routine bacterial and fungal laboratory examinations include corneal lesion staining microscopy, bacterial and fungal culture, and drug susceptibility tests.9–11 Among them, the positive detection rate of corneal lesion staining microscopy is related to the size of the ulcer in the lesion area, the amount of material taken, and the professional level of the inspector.12 Repeated scraping may have a certain influence on corneal wound healing and the visual prognosis of patients.13 Bacterial culture requires 5 days or more, and fungal culture requires 1 week or more to determine the presence of pathogen growth, with low sensitivity and a long wait time.14 For infectious keratitis, early diagnosis and treatment can save the patient's vision.
Endothelial plaques are a typical feature of fungal keratitis.15 However, bacterial keratitis also produces endothelial-like plaques, which are related to the formation of fibrin on the posterior surface of the cornea.16 It is very difficult to distinguish the type of keratitis by slitlamp microscopy based on the morphology of the retrocorneal plaque because of the obstruction of the anterior lesion and corneal edema.17 It is particularly important to find other examination methods to observe endothelial plaques and determine the distribution pattern of endothelial plaques in different types of keratitis.
Anterior segment optical coherence tomography (AS-OCT) is a method for tomographic imaging of the biological tissues of the anterior segment of the eye using optical principles. It has the characteristics of high resolution and noncontact.18 It is currently used in keratitis, corneal thickness measurement, anterior chamber angle measurement, and corneal transplantation.19–21 It has been widely used in research. Sun et al22 demonstrated that it is safe and effective to remove the necrotic corneal tissue combined with a conjunctival flap under the guidance of AS-OCT in the treatment of fungal keratitis. Previous research has demonstrated that herpetic keratitis may present subepithelial infiltrates and specific stromal hyperreflectance signals in AS-OCT but nonspecifically.23 AS-OCT imaging may provide useful information for the diagnosis and monitoring of herpetic keratitis.23 AS-OCT showed the unclear corneal endothelium–endothelial plaque boundary in fungal keratitis in previous work.16 In summary, AS-OCT plays an important role in evaluating the diagnosis, treatment, and prognosis of infectious keratopathy.
In vivo confocal microscopy (IVCM) can be used to perform noninvasive and rapid inspection of the living cornea. Corneas infected by different pathogens show different characteristics. IVCM is a useful tool for diagnosing infectious keratitis.24 Previous clinical studies have shown that it has good sensitivity and specificity in the diagnosis of fungal and acanthamoeba infection keratitis.25 However, if the lesion is located in the deep layer of the cornea and corneal edema and ulcers affect the image quality, it is easy to miss the diagnosis.26 In our clinical work, we observed that the endothelial plaques of fungal and bacterial keratitis have different morphologies according to IVCM, but there has been no relevant report.
This study aimed to use AS-OCT and IVCM to detect fungal and bacterial plaques. Endothelial plaques of keratitis were observed to provide a reference for clinical diagnosis.
MATERIALS AND METHODS
Participants
A prospective analysis of the clinical records of patients with fungal and bacterial keratitis treated in the First Affiliated Hospital of Harbin Medical University from March 2018 to December 2020 was performed. The posterior corneal plaques were observed with AS-OCT and IVCM. The collected data included the patient's current medical history, anterior segment photography, corneal lesion scraping, and bacterial and fungal cultures.
According to the results of fungal and bacterial culture, this study included 52 eyes of 52 patients. The patients were diagnosed with fungal keratitis (28 patients) and bacterial keratitis (24 patients). The inclusion criteria were 1) patients with clearly diagnosed fungal and bacterial keratitis, methods of diagnosis included a) corneal lesion scraping with Giemsa stain reveals the bacterial or fungal structure, b) IVCM discovers fungal hyphae, c) positive bacterial or fungal culture, d) effective after antibacterial or fungal treatment, and e) satisfy any 2 of the above-mentioned 4 criteria, and 2) the presence of endothelial plaques. The exclusion criteria were as follows: a) viral keratitis, b) no endothelial plaques, c) a history of eye trauma, and d) corneal leukoplakia affecting endothelial plaque observation.
AS-OCT
Infected eyes were imaged by AngioVue OCT angiography (Optovue, Inc, Fremont, CA) (optical resolution: vertical 5 μm, horizontal 15 μm; scan condition: Cornea Cross Line model) to evaluate whether the boundary between the endothelial plaque and corneal endothelial cells was clear or wavy. Because severe corneal ulcers will affect the observation of endothelial plaques by AS-OCT, we choose the periphery of the patient's corneal ulcers to perform AS-OCT scanning to clearly observe the relationship between the endothelial plaques and the corneal endothelium. The results were evaluated by an experienced ophthalmologist who did not know the pathogen of the patient's corneal infection.
IVCM
Infected eyes were imaged by IVCM (Heidelberg Retinal Tomograph 3 with the Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany) (resolution: 1 μm) to evaluate whether inflammatory cell structures were visible or scattered in fungal and bacterial keratitis. Corneal lesions and the endothelial plaques were scanned by another experienced ophthalmologist.
Because corneal ulcers will affect the observation of endothelial plaques by IVCM, we choose the periphery of the patient's corneal ulcers to perform IVCM scanning to clearly observe the pathogen and inflammatory cell structures in the corneal endothelium.
Acquired Severely Infected Cornea and Hematoxylin-Eosin Staining
A patient with fungal keratitis underwent penetrating corneal transplantation because of his serious condition. This patient had a clear history of plant trauma, and fungal hyphae were found in IVCM, corneal curettage, and fungal culture and the hyphae invaded the deep stromal layer. After drug treatment, the condition was aggravated and a large number of anterior chambers appeared. To save the patient's eye, we took active treatment by performing penetrating corneal transplantation. We removed his corneal tissue and performed hematoxylin-eosin (HE) staining. After the corneal tissue was removed, it was immediately fixed in 4% paraformaldehyde. Paraffin sections were prepared. The changes in the cornea were observed and recorded under an optical microscope pathologically. The distribution of mycelia and corneal endodermis was compared with the AS-OCT and IVCM results.
RESULTS
According to the results of smears, IVCM, or fungal and bacterial culture, the patients were diagnosed with fungal keratitis (28 patients) and bacterial keratitis (24 patients), and there were no statistically significant differences in age or sex. Among the cases, fungal keratitis was diagnosed by IVCM in 26 of 28 patients (92.86%), smears in 20 of 28 (71.43%), and fungal culture in 12 of 28 (42.86%); bacterial keratitis was diagnosed by smears in 8 of 24 patients (33.33%) and bacterial culture in 18 of 24 (75%). The causes of fungal keratitis were as follows: plant trauma in 9 of 28 patients, unknown in 9 of 28, trauma (in addition to plant) in 4 of 28, eye surgery in 4 of 28, and contact lens in 2 of 28; the causes of bacterial keratitis were as follows: plant trauma in 2 of 24 patients, unknown in 8 of 24, trauma (in addition to plant) in 7 of 24, eye surgery in 4 of 24, and contact lens in 3 of 24. The fungal keratitis treatments were as follows: medicine in 6 of 28 patients, conjunctival flap occlusion surgery in 8 of 28, lesion clearance surgery in 13 of 28, and penetrating keratoplasty in 1 of 28. The bacterial keratitis treatments were as follows: medicine in 18 of 24 patients, conjunctival flap occlusion surgery in 2 of 24, lesion clearance surgery in 3 of 24, and penetrating keratoplasty in 1 of 24. All patients had corneal endothelium plaques, and for 52 patients, we could see clearly whether the boundary between endothelial plaque and endothelium was clear by AS-OCT, whereas for 3 of them, we could not observe the state of the endocorneal inflammatory cells through IVCM. AS-OCT in 25 of 28 patients diagnosed with fungal keratitis showed that the boundary between the corneal endothelium and endothelial plaques was unclear and wavy (Figs. 1B and 2B), and 24 of 28 patients had unclear cell boundaries and a large number of compactly distributed inflammatory cells in the endothelial layer according to IVCM (Figs. 1D and 2E). The corneal tissue from patient who had fungal keratitis was detected by HE, and our results showed that fungal hyphae entered the corneal tissue obliquely through the corneal endothelial cell layer, involving the deep stromal layer. A large number of endothelial plaques were attached to the endothelial cell layer, and the endothelium of the fungal hyphae was curved and irregular, which was consistent with the results of AS-OCT and IVCM (Fig. 1F). AS-OCT in 23 of 24 patients diagnosed with bacterial keratitis revealed clear boundaries between the corneal endothelium and endothelial plaques (Fig. 3B). The boundary of endothelial cells of 22 of 24 patients was insufficient, and a large number of inflammatory cell structures were visible and scattered according to IVCM (Fig. 3C). To prevent fungal ulcers tending to be more severe and therefore have more density and that is disrupting the AS-OCT and IVCM signal, a figure who had similar density/size fungal (Fig. 4A) and bacterial (Fig. 4C) ulcers side by side with AS-OCT (Figs. 4B, E) and IVCM (Figs. 4C, F) images to prove our conclusion was added (Fig. 4). Refer to Table 1 for clinical information of the patients, and also refer to Supplemental Digital Content 1 (see Table S1, http://links.lww.com/ICO/B313) for details of patients, content covered, case number, sex, age, cause, examination, diagnosis, treatment, AS-OCT findings, boundary between cornea and plaque, IVCM findings, inflammatory cell boundary, and distribution.
FIGURE1.
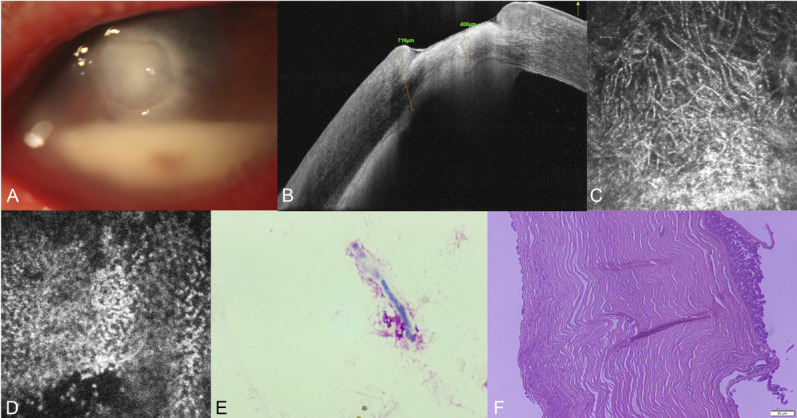
Imaging and histological examination of the patient (case 20, see Table S1, Supplemental Digital Content 1, http://links.lww.com/ICO/B313) with fungal keratitis. The ulcer is ill-defined with anteriormal pyorrhea by anterior segment photography (A), the corneal endothelium–endothelial plaque boundary was unclear and wavy by AS-OCT (B), fungal hyphae in the lesion, unclear cell boundaries, and a large number of compactly distributed inflammatory cells in the endothelial layer by IVCM (C, D), fungal hyphae by smears (E), and endothelial plaque and destruction of endothelium by HE staining (F).
FIGURE2.
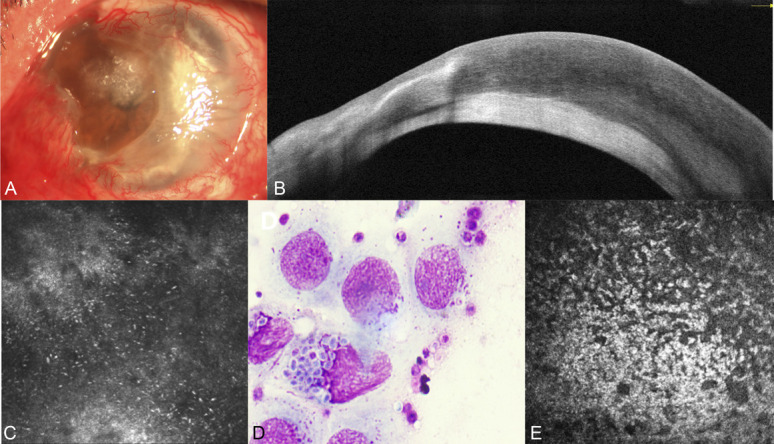
Imaging of the patient (case 14, see Table S1, Supplemental Digital Content 1, http://links.lww.com/ICO/B313) with fungal keratitis. The ulcer is ill-defined by anterior segment photography (A), the corneal endothelium–endothelial plaque boundary was unclear and wavy by AS-OCT (B), yeast in the lesion by IVCM (C), yeast by smears (D), and unclear cell boundaries and a large number of compactly distributed inflammatory cells in the endothelial layer (E).
FIGURE3.
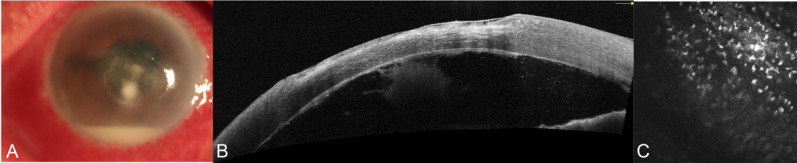
Imaging of the patient (case 34, see Table S1, Supplemental Digital Content 1, http://links.lww.com/ICO/B313) with bacterial keratitis. The ulcer boundary was clear, and anteriormal pyorrhea can be seen by anterior segment photography (A), the corneal endothelium–endothelial plaque boundary was clear by AS-OCT (B), and insufficient endothelial cell boundaries with a large number of visible and scattered inflammatory cell structures by IVCM (C).
FIGURE4.
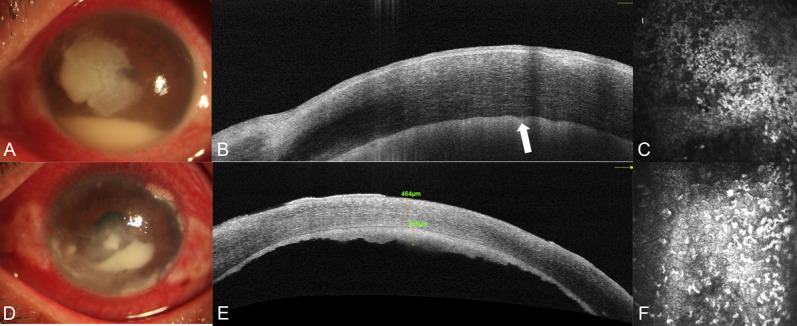
Imaging of the patient (cases 17 and 40, see Table S1, Supplemental Digital Content 1, http://links.lww.com/ICO/B313) with the similar severity of fungal and bacterial keratitis. The fungal ulcer is ill-defined with anteriormal pyorrhea by anterior segment photography (A), the corneal endothelium–endothelial plaque boundary was unclear and wavy shown by the white arrow (B), a large number of compactly distributed inflammatory cells in the endothelial layer by IVCM (C), the bacterial ulcer is ill-defined with anteriormal pyorrhea by anterior segment photography (D), the corneal endothelium–endothelial plaque boundary was clear by AS-OCT (E), and insufficient endothelial cell boundaries with a large number of visible and scattered inflammatory cell structures by IVCM (F).
TABLE 1.
Clinical Data
| Item | FK | BK |
| Sex | ||
| Male | 16/52 | 8/52 |
| Female | 11/52 | 17/52 |
| Cause | ||
| Plant trauma | 9/28 | 2/24 |
| Unknown | 9/28 | 8/24 |
| Trauma (in addition to plant) | 4/28 | 7/24 |
| Eye surgery | 4/28 | 4/24 |
| Contact lens | 2/28 | 3/24 |
| Treatment | ||
| Medicine | 6/28 | 18/24 |
| Conjunctival flap occlusion surgery | 8/28 | 2/24 |
| Lesion clearance surgery | 1/28 | 1/24 |
| A | ||
| Clear | 3/28 | 23/24 |
| Unclear | 25/28 | 1/24 |
| B | ||
| Clear and scattered | 3/28 | 22/24 |
| Unclear and clustered | 24/28 | 0/24 |
| Unclear image | 1/28 | 2/24 |
A = the boundary between endothelial plaque and endothelium by AS-OCT; B = inflammatory cell boundary and distribution.
BK, bacterial keratitis; FK, fungal keratitis.
DISCUSSION
Infectious keratitis is one of the important causes of corneal blindness in our country.27 There are many kinds of pathogens that cause corneal infections, and the clinical manifestations are complex and diverse.28 Only early, rapid, and clear diagnosis can facilitate the adoption of accurate and effective treatment plans for the infectious cause and control the infection in a timely manner. The traditional auxiliary examination of infectious keratitis is a laboratory examination of etiology, including corneal smearing and culture. This diagnostic method is limited by material selection, and the positive rate is not high.29–31 In recent years, endothelial plaques caused by infectious keratitis have gradually attracted the attention of ophthalmologists. Endothelial plaques were originally considered a characteristic sign of fungal keratitis.32 However, bacterial keratitis also exhibits endothelial-like plaques, which are related to the formation of fibrin on the posterior surface of the cornea.33 Because corneal edema caused by infectious keratitis obscures the observation of the morphology of corneal endothelial plaques, it is difficult to distinguish the type of infectious keratitis using a slit lamp in clinical practice.34 It is particularly important to find other examination methods to observe endothelial plaques and determine the distribution pattern of endothelial plaques in different types of keratitis.
AS-OCT is currently used in keratitis, corneal thickness measurement, anterior chamber angle measurement, and corneal transplantation.35 Park et al36 demonstrated that AS-OCT can distinguish acanthamoeba keratitis and herpetic epithelial keratitis, and highly reflective lesions were observed in the subepithelial area in herpetic epithelial keratitis and in the stroma in acanthamoeba keratitis. Clemence Bonnet et al demonstrated that AS-OCT can be applied to assess the activity level of eye diseases in patients with peripheral corneal ulcers.16 Takezawa et al16 showed that the application of AS-OCT to observe the posterior corneal plaque can be used to diagnose infectious keratitis. IVCM allows the direct observation of fungal hyphae, spores, and acanthamoeba cysts in living corneas.37,38 Previous clinical studies have shown that IVCM has good sensitivity and specificity in the diagnosis of fungal and acanthamoeba infection keratitis. IVCM may be missed or misdiagnosed in many cases, including corneal edema, excess necrotic tissue, or fungal hyphae in the deep stroma or on the endothelium surface.39–41 However, IVCM can be used to observe the morphology of endothelial plaques through the transparent cornea. We aimed to use AS-OCT and IVCM to detect fungal and bacterial plaques. Endothelial plaques of keratitis were observed to provide a reference for clinical diagnosis.
In our study, AS-OCT was applied to evaluate whether the boundary between the endothelial plaque and corneal endothelial cells was clear or wavy. Most of the cases (24/28) showed that the boundary between the endothelial plaque and corneal endothelial cells was unclear and wavy. The AS-OCT scans of 6 patients with fungal keratitis showed a clear boundary between the endothelial plaque and the corneal endothelium. Four of these 6 patients were treated with medicine to solve the infection problem, and the characteristics of the lesions of these 4 patients were relatively uniform. The lesions were confined to the corneal epithelial layer and superficial stroma, and there was no damage to endothelial cells. On the contrary, the HE staining results of a patient undergoing corneal transplantation for fungal keratitis showed that the fungus penetrated through the endothelial cell layer of the cornea, and the deep stromal layer of the cornea was destroyed. The reason for the ineffectiveness of drug therapy may be that the penetration of antifungal drugs is poor and the deep stromal layer cannot be reached. This means that the fungal damage to the endothelium extended from the corneal stroma to the anterior chamber. Bacterial keratitis can induce inflammation in the anterior chamber, and the fibrin formed is similar to the morphology of endothelial plaques. In our study, the boundary between the endothelial plaque and corneal endothelial cells in all patients who were diagnosed with bacterial keratitis was clear. The reason may be that these corneal endothelial plaques are actually made up of a large number of inflammatory cells and not caused by bacteria that disrupt the corneal endothelial cells in the anterior chamber. Eighteen of 24 patients with bacterial keratitis were well managed with medicine treatment. At the same time, the endothelial plaques observed by AS-OCT gradually shrank with effective treatment, which indicates that the endothelial plaques of bacterial keratitis are likely to be caused by the accumulation of inflammatory cells. The observation of corneal endothelial plaques by AS-OCT can be used for the early diagnosis of infectious keratitis.
IVCM is a noninvasive imaging method that can quickly and accurately diagnose fungal and acanthamoeba infectious keratitis. In our study, IVCM was applied to evaluate whether the cell boundaries of inflammatory cells were clear or unclear and scattered or clustered. Most of the cases (24/28) showed that the cell boundaries of inflammatory cells were unclear and clustered, 2 of 28 cases showed clear and scattered cell boundaries of inflammatory cells, and posterior endothelial inflammatory cells in 2 of 28 cases could not be observed because of the obscuration of the lesion. Twenty-one of 24 cases showed that the cell boundaries of inflammatory cells were clear and clustered or scattered, and posterior endothelial inflammatory cells in 3 of 24 cases could not be observed because of the obscuration of the lesion. This finding means that bacterial keratitis causes more inflammation of the endothelium, and the endothelial plaque is thicker than bacterial keratitis. With the application of antimicrobial agents, the disappearance of endothelial plaques in patients with bacterial keratitis was more obvious than that in patients with fungal keratitis, and the endothelial plaque in patients with bacterial keratitis was thinner.
In conclusion, fungal keratitis results in an unclear and wavy boundary between the corneal endothelium and endothelial plaques, and unclear cell boundaries and a large number of compactly distributed inflammatory cells in the endothelial layer were observed through IVCM. The boundary between the endothelial plaque and corneal endothelial cells in all patients who were diagnosed with bacterial keratitis was clear through AS-OCT, and most of the cases showed that the cell boundaries of inflammatory cells were clear and clustered or scattered through IVCM. The observation of corneal endothelial plaques by AS-OCT and IVCM can be used for the early diagnosis of infectious keratitis.
Supplementary Material
Footnotes
Supported by the National Natural Science Foundation of China (Grant No. U20A20363-81970776), the Natural Science Foundation of Heilongjiang Province, China (Grant No. LH2020H039), and Research Innovation Fund of the First Affiliated Hospital of Harbin Medical University (Grant Nos. 2019B04 and 2019B12).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
Contributor Information
Xin Jin, Email: zdshjinxinbisheng@163.com.
Hao Jin, Email: 280798492@qq.com.
Yan Shi, Email: dr_shiyan@163.com.
Nan Zhang, Email: 13766804226@163.com.
REFERENCES
- 1.Ozkan J, Willcox MD. The ocular microbiome: molecular characterisation of a unique and low microbial environment. Curr Eye Res. 2019;44:685–694. [DOI] [PubMed] [Google Scholar]
- 2.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kam KW, Yung W, Li GKH, et al. Infectious keratitis and orthokeratology lens use: a systematic review. Infection. 2017;45:727–735. [DOI] [PubMed] [Google Scholar]
- 4.Tena D, Rodríguez N, Toribio L, et al. Infectious keratitis: microbiological review of 297 cases. Jpn J Infect Dis. 2019;72:121–123. [DOI] [PubMed] [Google Scholar]
- 5.Szentmáry N, Módis L, Imre L, et al. Diagnostics and treatment of infectious keratitis. Orv Hetil. 2017;158:1203–1212. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Moramarco A, Mandarà E, et al. Interface infectious keratitis after anterior and posterior lamellar keratoplasty. Clinical features and treatment strategies. A review. Br J Ophthalmol. 2019;103:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting DSJ, Henein C, Said DG, et al. Effectiveness and safety of early adjuvant amniotic membrane transplant versus standard antimicrobial treatment for infectious keratitis: a systematic review protocol. JBI Evid Synth. 2020;18:1808–1814. [DOI] [PubMed] [Google Scholar]
- 8.Fan F, Huang X, Yuan K, et al. Glucocorticoids may exacerbate fungal keratitis by increasing fungal aggressivity and inhibiting the formation of neutrophil extracellular traps. Curr Eye Res. 2020;45:124–133. [DOI] [PubMed] [Google Scholar]
- 9.Lakhundi S, Siddiqui R, Khan NA. Pathogenesis of microbial keratitis. Microb Pathog. 2017;104:97–109. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoudi S, Masoomi A, Ahmadikia K, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. 2018;61:916–930. [DOI] [PubMed] [Google Scholar]
- 11.Maycock NJ, Jayaswal R. Update on acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35:713–720. [DOI] [PubMed] [Google Scholar]
- 12.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19:210–220. [DOI] [PubMed] [Google Scholar]
- 13.Niu L, Liu X, Ma Z, et al. Fungal keratitis: pathogenesis, diagnosis and prevention. Microb Pathog. 2020;138:103802. [DOI] [PubMed] [Google Scholar]
- 14.Carnt N, Samarawickrama C, White A, et al. The diagnosis and management of contact lens-related microbial keratitis. Clin Exp Optom. 2017;100:482–493. [DOI] [PubMed] [Google Scholar]
- 15.Qi X, Liu T, Du M, et al. Endothelial plaques as sign of hyphae infiltration of Descemet's membrane in fungal keratitis. J Ophthalmol. 2020;2020:6083854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takezawa Y, Suzuki T, Shiraishi A. Observation of retrocorneal plaques in patients with infectious keratitis using anterior segment optical coherence tomography. Cornea. 2017;36:1237–1242. [DOI] [PubMed] [Google Scholar]
- 17.Palioura S, Tsiampali C, Dubovy SR, et al. Endothelial biopsy for the diagnosis and management of culture-negative retrocorneal fungal keratitis with the assistance of optical coherence tomography imaging. Cornea. 2021;40:1193–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SB, Cornish EE, Grigg JR, et al. Anterior segment optical coherence tomography and its clinical applications. Clin Exp Optom. 2019;102:195–207. [DOI] [PubMed] [Google Scholar]
- 19.Shipton C, Hind J, Biagi J, et al. Anterior segment optical coherence tomographic characterisation of keratic precipitates. Cont Lens Anterior Eye. 2020;43:465–468. [DOI] [PubMed] [Google Scholar]
- 20.Thanathanee O, Laohapitakvorn S, Anutarapongpan O, et al. Anterior segment optical coherence tomography images in microsporidial keratoconjunctivitis. Cornea. 2019;38:943–947. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi A, Shimizu K, Kato S, et al. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg. 2019;45:1099–1104. [DOI] [PubMed] [Google Scholar]
- 22.Sun GH, Li SX, Gao H, et al. Clinical observation of removal of the necrotic corneal tissue combined with conjunctival flap covering surgery under the guidance of the AS-OCT in treatment of fungal keratitis. Int J Ophthalmol. 2012;5:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman W, Nassr MA, Abdelazeem K, et al. Appearance of herpes simplex keratitis on anterior segment optical coherence tomography. Int Ophthalmol. 2019;39:2923–2928. [DOI] [PubMed] [Google Scholar]
- 24.Wang YE, Tepelus TC, Vickers LA, et al. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol. 2019;39:2865–2874. [DOI] [PubMed] [Google Scholar]
- 25.Labbé A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf. 2009;7:41–52. [DOI] [PubMed] [Google Scholar]
- 26.Roth M, Daas L, MacKenzie CR, et al. Development and assessment of a simulator for in vivo confocal microscopy in fungal and Acanthamoeba keratitis. Curr Eye Res. 2020;45:1484–1489. [DOI] [PubMed] [Google Scholar]
- 27.Müller RT, Abedi F, Cruzat A, et al. Degeneration and regeneration of subbasal corneal nerves after infectious keratitis: a longitudinal in vivo confocal microscopy study. Ophthalmology. 2015;122:2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seck SM, Diakhaté M, Oulfath A, et al. Severe infectious keratitis in tropical environments: 118 cases collected over 10 years. Med Sante Trop. 2019;29:151–154. [DOI] [PubMed] [Google Scholar]
- 29.Henry CR, Flynn HW, Jr, Miller D, et al. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012;119:2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta N, Tandon R. Investigative modalities in infectious keratitis. Indian J Ophthalmol. 2008;56:209–213. [PMC free article] [PubMed] [Google Scholar]
- 31.Kasparova EA, Sobkova OI, Yang B. Corneal collagen cross-linking in the treatment of infectious keratitis and corneal ulcers. Vestn Oftalmol. 2017;133:113–119. [DOI] [PubMed] [Google Scholar]
- 32.Mills B, Radhakrishnan N, Karthikeyan Rajapandian SG, et al. The role of fungi in fungal keratitis. Exp Eye Res. 2021;202:108372. [DOI] [PubMed] [Google Scholar]
- 33.Green M, Hughes I, Hogden J, et al. High-dose steroid treatment of bacterial keratitis. Cornea. 2019;38:135–140. [DOI] [PubMed] [Google Scholar]
- 34.Bograd A, Seiler T, Droz S, et al. Bacterial and fungal keratitis: a retrospective analysis at a university hospital in Switzerland. Klin Monbl Augenheilkd. 2019;236:358–365. [DOI] [PubMed] [Google Scholar]
- 35.Nubile M, Dua HS, Lanzini M, et al. In vivo analysis of stromal integration of multilayer amniotic membrane transplantation in corneal ulcers. Am J Ophthalmol. 2011;151:809–822.e1. [DOI] [PubMed] [Google Scholar]
- 36.Park YM, Lee JS, Yoo JM, et al. Comparison of anterior segment optical coherence tomography findings in Acanthamoeba keratitis and herpetic epithelial keratitis. Int J Ophthalmol. 2018;11:1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YE, Tepelus TC, Gui W, et al. Reduction of acanthamoeba cyst density associated with treatment detected by in vivo confocal microscopy in Acanthamoeba keratitis. Cornea. 2019;38:463–468. [DOI] [PubMed] [Google Scholar]
- 38.Kheirkhah A, Satitpitakul V, Syed ZA, et al. Factors influencing the diagnostic accuracy of laser-scanning in vivo confocal microscopy for Acanthamoeba keratitis.Cornea. 2018;37:818–823. [DOI] [PubMed] [Google Scholar]
- 39.Alomar TS, Al-Aqaba M, Gray T, et al. Histological and confocal microscopy changes in chronic corneal edema: implications for endothelial transplantation. Invest Ophthalmol Vis Sci. 2011;52:8193–8207. [DOI] [PubMed] [Google Scholar]
- 40.Lv J, Zhang K, Chen Q, et al. Deep learning-based automated diagnosis of fungal keratitis with in vivo confocal microscopy images. Ann Transl Med. 2020;8:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei A, Wang K, Wang Y, et al. Evaluation of corneal cross-linking as adjuvant therapy for the management of fungal keratitis. Graefes Arch Clin Exp Ophthalmol. 2019;257:1443–1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


