Abstract
Social animals expend considerable energy to maintain social bonds throughout their life. Male and female mice show sexually dimorphic behaviors, yet the underlying neural mechanisms of sociability and their dysregulation during social disconnection remain unknown. Dopaminergic neurons in dorsal raphe nucleus (DRNTH) is known to contribute to a loneliness-like state and modulate sociability. We identified that activated subpopulations in DRNTH and nucleus accumbens shell (NAcsh) during 24 hours of social isolation underlie the increase in isolation-induced sociability in male but not in female mice. This effect was reversed by chemogenetically and optogenetically inhibiting the DRNTH-NAcsh circuit. Moreover, synaptic connectivity among the activated neuronal ensembles in this circuit was increased, primarily in D1 receptor–expressing neurons in NAcsh. The increase in synaptic density functionally correlated with elevated dopamine release into NAcsh. Overall, specific synaptic ensembles in DRNTH-NAcsh mediate sex differences in isolation-induced sociability, indicating that sex-dependent circuit dynamics underlie the expression of sexually dimorphic behaviors.
Synaptically connected raphe and accumbens shell neurons underlie isolation-induced sociability in males.
INTRODUCTION
Social species make great efforts to maintain their social connections and show strong motivation of need to belong (1, 2). Social isolation disconnects the social bond and may be a form of torture in humans (3). Chronic isolation is aversive for virtually all social species and is linked to antisocial behaviors, such as depression, anxiety, and aggression (4, 5). Even an acute period of isolation influences how behaviors are expressed. Affiliative social behaviors follow periods of acute social isolation in rodents to humans (6, 7). The effects of social isolation vary by gender (8, 9). Sexual dimorphisms exist not only in brain anatomy (10) and neurological disorders (11, 12) but also in behaviors like sociability (13–17). However, sex differences in the precise neural adaptations at the synaptic and circuit levels that follow acute social isolation remain poorly understood.
The dorsal raphe nucleus (DRN) is a brain region in the central dopamine system, containing a dopaminergic population outside the ventral tegmental area (VTA) and substantia nigra pars compacta (18–20). While VTA dopaminergic neurons show strong responses in positive and negative reinforcement (21–23) and in motivated behaviors (24, 25), the DRN dopaminergic neurons (DRNTH) can promote aversion, as well as social interactions (6). Recent studies further revealed additional functions of DRNTH neurons in memory formation and arousal (26–28). However, the mechanism in neuronal circuit levels of how the DRNTH exerts its behavioral effects, or whether it shows sexual dimorphic characteristics, remains unknown. One downstream candidate that is regulated by the dopamine system is the nucleus accumbens (NAc), which is anatomically divided into the NAc core and the NAc shell (NAcsh) (29). It is composed of two major cell types that expresses a specific family of dopamine receptors: D1 receptor (D1R)–expressing and dopamine D2 receptor (D2R)–expressing neurons (30). These two neuronal populations have distinct projections throughout the brain (31) and are associated with motivated behaviors, including sociability (25, 32, 33).
Because innate behaviorally activated neuronal ensembles show different intrinsic and functional properties (34), an immediate-early gene (IEG)–based tagging strategy can target these specific neuronal populations and define how the ensembles participate in behavioral responses. While the studies on specific activated populations with fear experience greatly advanced the understanding of learning and memory (35–37), many questions remain for their role with other behaviors. Recently, neuroscience has progressed to integrate activated neuronal ensembles in response to stimuli other than memory. Cocaine activates specific subpopulations of neurons in several brain regions (38–40), and these cocaine-activated neurons show enhanced connectivity in between (40), providing evidence to experience-dependent plasticity.
Now, we combined isolation experience with activity-based tagging strategy in the dopamine system. We hypothesized that such activated neuronal ensembles in DRNTH-NAcsh circuit regulate the behavioral and neurobiological responses to 24-hour social isolation in a sex-specific manner. Using optogenetics and chemogenetics combined with an IEG-based tagging system, we found that specific subpopulations of DRNTH and NAcsh neurons active during social isolation are responsible for promoting isolation-induced sociability in males but not in females. The behavioral change was accompanied by an increase in dopamine release into NAcsh. We further narrowed down this functional observation to the synaptic level and visualized the specific synapses between the isolation-activated neurons in DRNTH-NAcsh, which showed higher synaptic density only in isolated males. This synaptic strengthening occurred primarily at D1R-expressing neurons in the NAcsh. Overall, our investigations reveal sex-dependent circuit dynamics that underlie the expression of social isolation–induced sociability.
RESULTS
Acute social isolation alters sociability and DRNTH neuronal excitability in a sex-specific manner
To examine whether 24 hours of social isolation affects sociability differently in male and female mice, we performed the social approach task in a three-chamber arena (Fig. 1A). Socially isolated male mice showed a significant increase in sociability compared to group-housed males (Fig. 1B), but anxiety-related behavior in the open field test was not altered (fig. S1A). In contrast, female mice showed no significant difference in sociability after 24 hours of social isolation relative to group-housed females (Fig. 1C). Rather, female mice had an increase in anxiety level (fig. S1B). Because the three-chamber test might not be sensitive enough to detect the social interactions in the female mice, we further measured the level of sociability using the juvenile interaction test, in which the test mouse directly interacts with the stranger mouse (fig. S1C). Once more, increase in social interaction following 24-hour isolation was not observed in females while it was observed in males (fig. S1, D and E), consistent with the result of the three-chamber test (Fig. 1, B and C). Given that estrogen/progesterone may modulate dopaminergic function differently in brain regions (41, 42), we also tested with the ovariectomized females to determine whether female sex hormones influenced their response to isolation. However, time spent with the social target did not differ between isolated ovariectomized females and isolated sham-control females (fig. S1F). Thus, altered social interaction following 24 hours of isolation was displayed to be a sexual dimorphic phenotype, independent of sex hormones.
Fig. 1. Behavioral and electrophysiological changes in DRNTH neurons following 24 hours of social isolation.
(A) Experimental scheme of group-housing and social isolation for the three-chamber test. (B) In male mice, a significant increase in time spent with the social target is detected in the socially isolated mice compared to the group-housed mice (Grouped, n = 16, 75.52 ± 3.095; Isolated, n = 17, 86.01 ± 1.878%; **P = 0.0062). (C) In female mice, social isolation does not alter time spent with the social target (Grouped, n = 10, 72.16 ± 2.927; Isolated, n = 10, 75.56 ± 2.776; P = 0.4106). ns, not significant. (D) Dopaminergic neurons in DRN of TH::cre;Ai14 mouse. Scale bar, 50 μm. In male mice, DRNTH neuronal excitability (delivery of a 200-pA current pulse) shows a robust increase after 2 hours of isolation (Grouped, n = 17 from five mice, 2.529 ± 0.6131; Isolated, n = 19, from four mice, 4.789 ± 0.8257; *P = 0.0383). Example trace of grouped (black color) and isolated (blue color). Scale bar, 50 mV, 100 ms. (E) In female mice, DRNTH neurons exhibit no difference in excitability between group-housing and social isolation (Grouped, n = 24 from three mice, 3.250 ± 0.8281; Isolated, n = 18 from two mice, 1.833 ± 0.4591; P = 0.1798). Example trace of grouped (black color) and isolated (red color). Scale bar, 50 mV, 100 ms. (F) Experimental scheme for viral injection into the DRN of TH::cre mice and timeline of the behavioral task. (G) Chemogenetic inhibition of the DRNTH neurons during social isolation decreases the time spent with the social target following isolation (Saline, n = 6, 85.51 ± 2.237; CNO, n = 9, 74.35 ± 3.157; *P = 0.0224). All unpaired t test.
Neuronal excitability sets the threshold for action potential generation and regulates synaptic transmission (43). To demonstrate whether social isolation modulates neuronal excitability, we focused on DRNTH neurons, as their glutamatergic inputs and in vivo activity are sensitive to acute isolation (6). Using ex vivo whole-cell patch-clamp electrophysiology to record from DRNTH neurons, while delivering current steps, we observed a robust increase in the excitability in males following just 2 hours of isolation (Fig. 1D). However, this increase in the excitability was not observed both in nondopaminergic DRN neurons and in female mice (Fig. 1E and fig. S1G). These results suggest a mechanism that sex-dependent increased activity of DRNTH during the early phase of social isolation may trigger circuit level changes, thereby expressing the sexual dimorphic social behavior.
Isolation-induced sociability requires DRNTH activity during isolation
We next sought to determine whether this endogenous increase in activity of DRNTH neurons during 24-hour isolation was required for the expression in social behavior following isolation. So, we used tyrosine hydroxylase (TH)::cre mice and expressed the Gi-coupled DREADD (designer receptor exclusively activated by designer drugs) hM4Di in DRNTH neurons to chemogenetically inhibit the neurons at the onset of the group-housed or socially isolated period (Fig. 1F and fig. S2C). Because we found a robust increase in excitability after 2 hours of social isolation (Fig. 1D), we delivered clozapine N-oxide (CNO) at the onset of isolation during this time period (44, 45). When we inhibited DRNTH neurons during isolation, the isolated mice performed as if they have not been under isolation. They spent less time with the social target compared with mice that only received saline (Fig. 1G) and showed no differences in locomotion or anxiety-related behavior (fig. S2, A and B). In contrast, inhibiting DRNTH neurons during group-housing did not affect social interaction nor locomotion (fig. S2, D and E), implying that the necessity for DRNTH activity is specific to social isolation. Then, we tried to mimic the isolation-induced sharp increase in DRNTH activity by expressing hM3Dq-mCherry in group-housed males and examined whether it may artificially increase the social interactions (fig. S2, F and I). However, simply activating the DRNTH neurons did not induce any changes in the sociability and mobility levels (fig. S2, G and H). Saline-injected and CNO-injected mice without DREADD expression showed no changes in social interaction, ruling out the effect of CNO injection itself (fig. S2, J and K). Together, these results indicate that the expression of increased social behavior requires a concomitant increase in the endogenous activity of DRNTH neurons during the 24-hour isolation period.
Isolation-activated DRNTH neuronal ensembles drive increased sociability after social isolation
We next questioned whether the subpopulation of DRNTH ensembles that are active during isolation underlie the isolation-induced sociability. To address this, we combined IEG-based tagging systems with chemogenetics to specifically label and manipulate the activated population. We used a reverse tetracycline-controlled transactivator (rtTA) driven by the c-fos promoter to express various proteins in a doxycycline-dependent manner (46–49). First, we examined the neuronal excitability between isolation-activated and isolation-nonactivated DRNTH neurons. We selectively captured the activated population through adeno-associated virus (AAV)2/1-Fos-rtTA and AAV2/1-TRE3G-DIO-mEmeraldNuc (nucleus-targeted mEmerald) injection into DRN of TH::cre mice crossed with an Ai14 reporter mice and delivered doxycycline at the onset of the isolation period (Fig. 2, A and B). Protein expression driven by tetracycline-responsive element third generation (TRE3G) promoter was not detectable without AAV2/1-Fos-rtTA, showing the specificity of the tagging system (fig. S3, A and B). In male mice, isolation-activated DRNTH (mEmeraldNuc+/TH+) neurons showed higher excitability compared to isolation-nonactivated DRNTH (mEmeraldNuc−/TH+) neurons (Fig. 2C, left). Although isolation has been observed to induce a c-fos expression in female mice, we did not detect any changes in excitability between activated and nonactivated DRNTH neurons (Fig. 2C, right). These results suggest that the intrinsic properties of isolation-activated DRNTH ensembles demonstrate sexual dimorphism.
Fig. 2. Isolation-activated DRNTH neurons are required for increased sociability after 24 hours of isolation.
(A) Experimental scheme for ex vivo patch-clamp recording. DOX, doxycycline. (B) Confocal images showing TH-positive neurons in the DRN expressing tdTomato (magenta color) and isolation-activated DRNTH neurons expressing mEmeraldNuc (cyan color). Scale bar, 100 μm. (C) Isolation-activated DRNTH (mEmerald+) neurons show a higher number of spikes (on delivery of a 200-pA current pulse) compared to isolation-nonactivated DRNTH (mEmerald−) neurons in a male-specific manner (left; mEmerald−, n = 15 from six mice, 4.067 ± 0.8134; mEmerald+, n = 12 from six mice, 7.167 ± 1.325; *P = 0.0480; right; mEmerald−, n = 15 from five mice, 4.067 ± 1.419; mEmerald+, n = 15 from five mice, 4.600 ± 0.8772; unpaired t test, P = 0.7516). Example traces of mEmerald− (black color) and mEmerald+ (green color). Scale bars, 50 mV, 100 ms. (D and F) Schematic of virus injection and behavior schemes for labeling isolation-activated (D) and group-house–activated DRNTH neurons (F). (E) Inhibition of isolation-activated DRNTH neurons abolishes social interaction (Saline, n = 4, 82.01 ± 2.515; CNO, n = 7, 71.13 ± 3.071; *P = 0.0400). (G) Inhibiting the group-house–activated DRNTH neurons does not affect social interaction (Saline, n = 5, 75.48 ± 2.473; CNO, n = 5, 79.23 ± 4.934; P = 0.5160). (H) Schematic of the virus injection and behavioral paradigm for reactivation ratio. (I) Representative images of DRN in Iso-Iso mice. Activated DRNTH neurons through the first isolation experience are labeled with mCherry. The second isolation-activated neurons are immunostained with c-fos in Alexa Fluor 488+. Scale bar, 100 μm. (J) Reactivation ratio is significantly higher in Iso-Iso compared to Gro-Iso (Gro-Iso, n = 7, 5.969 ± 1.457; Iso-Iso, n = 5, 17.07 ± 2.814; **P = 0.0034). All unpaired t test.
Next, we examined whether activity in the isolation-activated DRNTH neurons was required for sociability in isolated male mice. We injected AAV2/1-Fos-rtTA and AAV2/1-TRE3G-DIO-hM4Di-mCherry into the DRN of TH::cre mice to express hM4Di selectively in isolation-activated DRNTH neurons (Fig. 2D). We verified that the activity of these labeled neurons could be successfully suppressed by application of CNO using ex vivo whole-cell patch-clamp recording (fig. S3, C and D). Following 24 hours of isolation, we found that CNO-injected mice showed reduced sociability relative to saline-injected controls (Fig. 2E). However, CNO injection did not alter velocity nor total distance traveled (fig. S3F). These results indicate that the activated DRNTH cells during isolation experience were necessary for the expression of increased sociability. Then, we wondered whether inhibiting the group-housed–activated DRNTH neurons would affect the level of sociability. The social interaction remained intact (Fig. 2, F and G) with no changes in locomotion (fig. S3I), suggesting that the dopaminergic population in the DRN has no role in sociability when group-housed (6). The number of activated DRNTH neurons labeled with hM4Di-mCherry were significantly higher in isolated mice than in group-housed mice (fig. S3E). To further confirm the functional specificity of isolation-activated DRNTH neurons, we performed auditory fear conditioning as DRNTH neurons were implicated in fear memory (28). We observed that fear memory fully formed although isolation-activated DRNTH neurons were inhibited (fig. S3, G and H). Together, these results suggest that the expression of isolation-induced social behavior depends on activation of this unique subpopulation of DRNTH.
To assess whether isolation-activated DRNTH population would be repeatedly activated when introduced to additional social isolation event, we conducted the isolation event two times with 1 week in between (Iso-Iso) or isolation event after group housing (Gro-Iso). Following viral expression, mice were group-housed or socially isolated for 24 hours (the first event), returned to the home cage for a week, and then exposed to social isolation (the second event) for 2 hours before perfusion (Fig. 2H). In this way, neurons active during the initial 24-hour grouped or isolated period would be labeled with mCherry, and neurons active (fos positive) during the final 2-hour social isolation would be immunolabeled with Alexa Fluor 488+ (Fig. 2I). The proportion of [Alexa Fluor 488+ mCherry+]/[mCherry+] cells (reactivation ratio) was significantly higher in the mice exposed to social isolation twice (Iso-Iso) than in the mice exposed to social isolation after group-housing (Gro-Iso; Fig. 2J), indicating that isolation-activated DRNTH neurons are not randomly activated.
DRNTH neurons project to the NAcsh
On the basis of our findings, we next explored which downstream neural circuit drove the isolation-induced sociability mediated by DRNTH neurons. We labeled DRNTH neurons by injecting AAV1-Ef1a-DIO–enhanced yellow fluorescent protein (eYFP) into the DRN of TH::cre mice to visualize their downstream projections. Along with the innervations to central amygdala and bed nucleus of stria terminalis, we observed an axonal projection to the NAcsh (Fig. 3A) (18, 26). To further confirm the spatial accuracy of the viral spread in DRN, we injected AAV1-Ef1a-DIO-eYFP into DRN and AAV2/1-Ef1a-DIO-hM4Di-mCherry into VTA of TH::cre mice. DRNTH cell bodies expressed eYFP while no eYFP-expressing cell bodies were observed in the VTA, indicating that our DRN targeting parameters did not spread and label the dopaminergic neurons in the VTA (fig. S4A). Then, we bilaterally injected CAV-cre into the NAcsh of Ai14 reporter mice to label upstream neurons and stained each DRN slice with TH to distinguish dopaminergic neurons from nondopaminergic neurons. This revealed that neurons projecting from the DRN to NAcsh are ~42% dopaminergic (Fig. 3B).
Fig. 3. Optical inhibition of the DRNTH-NAcsh circuit suppresses social interaction following 24-hour isolation.
(A) Visualization of the axonal projections of DRNTH neurons using SeeDB2 method (scale bar, 1000 μm). (B) Illustration of virus injection site in Ai14 mouse. Representative image of DRN showing the monosynaptically connected tdTomato neurons (magenta) and immunostained TH-positive neurons (cyan). Scale bar, 100 μm. Among tdTomato-labeled cells, approximately 42% were TH positive. (C) Illustration of virus-injected regions and implanted optic fibers. Changes in GRABDA fluorescence in response to DRNTH axon terminal activation. (D) Experimental scheme for the photoinhibition of DRNTH terminals in NAcsh with group-housed and socially isolated mice. Representative image of bilateral placement of optic fiber on the eYFP-labeled axons from DRNTH. Scale bar, 1000 μm. (E and F) In socially isolated male mice, inhibiting DRNTH to NAcsh circuit significantly suppresses social interaction time in the three-chamber test (E) (eYFP, n = 8, P = 0.3424; eNpHR, n = 10, *P = 0.0410) and in juvenile interaction test (F) (eYFP, n = 6, P = 0.6937; eNpHR, n = 7, *P = 0.0265). (G) In group-housed male mice, inhibition of DRNTH-NAcsh do not attenuate sociability (eYFP, n = 5, P = 0.0803; eNpHR, n = 6, P = 0.9370). (H and I) In socially isolated female mice, DRNTH-NAcsh circuit inhibition does not suppress the social interaction time both in the three-chamber test (H) (eYFP, n = 10, P = 0.7231; eNpHR, n = 9, P = 0.9905) and in the juvenile interaction test (I) (eYFP, n = 10, P = 0.9685; eNpHR, n = 7, P = 0.7661). (J) In group-housed female mice, inhibiting DRNTH to NAc terminals does not change the sociability level (eYFP, n = 3, P = 0.9740; eNpHR, n = 5, P = 0.4471). All paired t test.
The functional connectivity between DRNTH and NAcsh was further examined through optogenetics and in vivo fiber photometry. Photoactivation of channelrhodopsin 2 (ChR2)–expressing DRNTH axons in NAcsh slices evoked an excitatory postsynaptic current in 36 of 39 neurons, with an average response of 50 pA and 4.8-ms latency (fig. S4, B and C). As previously reported, dopaminergic neurons in DRN are labeled in a high proportion through TH::cre mice (6), and we also verified this result by immunostaining dopamine transporter (DAT) in DRN of TH::cre;Ai14 mice (fig. S4D). Using fiber photometry with G protein–coupled receptor activation–based dopamine (GRABDA) sensors, we examined dopamine transmissions in the DRNTH-NAcsh circuit. AAV2/1-Ef1a-DIO-Chronos-mCherry was injected into DRN of TH::cre mice and AAV2/1–human synapsin (hSyn)–GRABDA was injected into NAcsh with optic fiber implanted in each region. We observed an increase in GRABDA fluorescence upon blue-light illumination in DRN indicating that DRNTH neurons released dopamine into the NAcsh (Fig. 3C), consistent with prior reports (50, 51). Collectively, dopaminergic neurons in DRN corelease glutamate and dopamine into the NAcsh.
Optogenetic inhibition of DRNTH to NAcsh circuit suppresses isolation-induced sociability in male mice
We next examined whether the DRNTH-NAcsh circuit mediates the sociability after 24 hours of social isolation in both sexes. We injected AAV1-Ef1a-DIO-eYFP or AAV1-Ef1a-DIO-eNpHR-eYFP into the DRN of TH::cre mice and implanted optic fibers bilaterally over the NAcsh (Fig. 3D). In male mice, inhibition of DRNTH terminals in NAcsh significantly diminished the increased sociability induced by social isolation (Fig. 3, E and F). This modulation occurred both in the three-chamber test and in the juvenile interaction test without a concomitant change in anxiety (fig. S4, E and F). To confirm whether this optogenetic manipulation was specific to social isolation, we performed the same experiments with the group-housed mice. Group-housed males did not show any changes in social interaction time when DRNTH-NAcsh were inhibited (Fig. 3G). As 24 hours of social isolation did not affect the sociability in female mice, we wondered whether manipulating the DRNTH-NAcsh circuit may affect the social interactions. Photoinhibition of the DRNTH neuronal terminals in NAcsh did not alter social behavior nor anxiety-like behavior in socially isolated and group-housed female mice (Fig. 3, H to J, and fig. S4G). Then, we refined our question to whether the sociability may be enhanced through activating DRNTH-NAcsh with AAV1-Ef1a-DIO-ChR2-eYFP (fig. S4H). ChR2-expressing group-housed and isolated mice in both sexes performed like eYFP-expressing control mice in the sociability level, indicating that artificially activating DRNTH-NAcsh circuit does not increase the social interactions (fig. S4, I to L). In summary, the increased sociability following 24 hours of social isolation was attenuated in males specifically by optogenetic inhibition of DRNTH to NAcsh pathway, suggesting that the DRNTH-NAcsh circuit modulates social isolation–induced sociability in a sex-dependent manner.
Increased dopamine release in NAcsh of isolated males
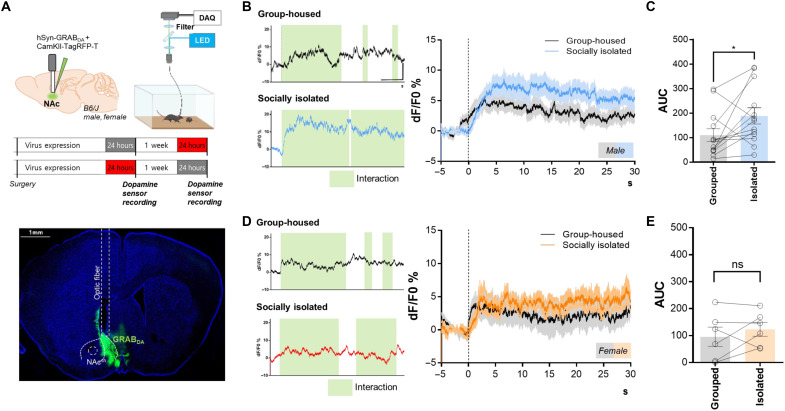
We questioned whether 24 hours of social isolation actually boosts dopamine release into the NAcsh. Using GRABDA, we recorded in vivo dopamine release in the NAcsh, while group-housed and socially isolated mice interacted with sex-matched juvenile stranger mice (Fig. 4A). We injected AAV2/1-hSyn-GRABDA with AAV2/1-CaMKII-TagRFP-T into the NAcsh to record dopamine release and detect movement-related artifacts. In males, we observed a significantly larger increase of GRABDA fluorescence when isolated mice undergo social interaction compared to the signals of group-housed mice (Fig. 4, B and C). However, female mice showed no difference in the amount of dopamine release into NAcsh between isolated and group-housed (Fig. 4, D and E). Consistent with our prior data on sexual-dimorphic behavioral responses and manipulations, dopamine release in the NAcsh further provides a functional link.
Fig. 4. Male-specific increase of in vivo dopamine transmission in NAcsh following 24-hour isolation.
(A) Experimental schematic for in vivo fiber photometry with dopamine sensors and the expression image of GRABDA. Scale bar, 1000 μm. DAQ, Data acquisition; LED, Light emitting diode. (B) Representative trace of group-housed and socially isolated male mouse interacting with juvenile stranger mouse. Interaction bouts are indicated by shaded green areas. Scale bar, 10 dF/F0%, 10 s. Socially isolated mice show higher GRABDA fluorescence compared to group-housed mice. The moment before testing when the interaction ensued was denoted as 0 s. (C) Significantly larger area under curve (AUC) in isolated mice (n = 13; paired t test; *P = 0.0435). (D) Representative trace of group-housed and socially isolated female mice interacting with sex-matched juvenile mouse. Interaction bouts are shown in green. Scale bars, 10 dF/F0%, 10 s. (E) Comparable AUC in group-housed and socially isolated females (n = 6; paired t test; P = 0.3688).
Increased synaptic density among isolation-activated DRNTH-NAcsh ensembles in isolated males
While the synaptic dynamics of glutamatergic neurons are well studied (36, 52), the synaptic dynamics of dopaminergic neurons have many open questions, especially in DRNTH neurons given the smaller dopaminergic population compared to the VTA. To examine the synaptic implications of the increased dopamine release into NAcsh, we hypothesized that social isolation may alter synaptic dynamics in the DRNTH-NAcsh circuitry to increase social interaction. Because capturing experience-activated synapses in DRNTH was difficult using traditional methods, we took advantage of our recently developed synapse labeling technique, dual-eGRASP [green fluorescent protein (GFP) reconstitution across synaptic partners] (35, 53). The dual-eGRASP technique is an advanced version of split fluorescent protein, which can emit cyan or yellow fluorescence signals when the pre- and post-eGRASP constructs are physically reconstructed in the synaptic cleft (Fig. 5A). Using dual-eGRASP, we visualized the dopaminergic synapses between DRNTH and NAcsh neurons that were activated during 24 hours of isolation.
Fig. 5. Isolated male mice show increased spine density between isolation-activated DRNTH-NAcsh neuronal ensembles.
(A) Schematics of dual-eGRASP that distinguishes the synapses depending on their inputs from DRNTH on a single NAcsh dendrite. (B) Illustration of AAV constructs and the combinations used in DRN and NAc. (C) Illustration of virus injection sites and timeline of the experimental protocol to label the synapses with dual-eGRASP. (D) Representative image of post-GRASP expressed in NAcsh. Scale bar, 1000 μm. Illustration of the four types of synapses between DRNTH and NAcsh neurons. The isolation-nonactivated NAc neurons are shown in gray, and the isolation-activated NAc neurons are shown in red. The cyan-eGRASP signals (in cyan circles) are the synapses that come from isolation-nonactivated DRNTH neurons. The yellow-eGRASP signals (in yellow circles) are the synapses that come from isolation-activated DRNTH neurons. (E) Representative images of dual-eGRASP in isolation-activated and isolation-nonactivated NAcsh dendrite with cyan-eGRASP and yellow-eGRASP puncta in both sexes. Scale bars, 5 μm. (F) In male mice, isolation-activated DRNTH neurons have higher synaptic density with isolation-activated NAcsh neurons compared to isolation-nonactivated NAcsh neurons. Each data point represents a dendrite (n = 37 for NAc nonactivated dendrites, n = 40 for NAc-activated dendrites, from six mice; Mann-Whitney two-tailed test; ***P < 0.0001). (G) In female mice, isolation did not change spine density between the activated populations (n = 35 for NAc nonactivated dendrites, n = 33 for NAc-activated dendrites, from six mice; Mann-Whitney two-tailed test; P = 0.0949). (H) Representative image of dual-eGRASP signals on dendritic shaft (white arrow) and spine head/neck (blue arrow) in isolation-activated NAcsh dendrites. The percentage of cyan-eGRASP and yellow-eGRASP on dendritic shaft and spine head/neck, in isolated males and female. Scale bar, 2 μm.
To express pre-eGRASP only in DRNTH neurons in a cre-dependent manner, we designed a pre-eGRASP construct combined with a double-floxed inverted open reading frame (DIO) sequence (Fig. 5B). Yellow pre-eGRASP expression was driven by the TRE3G promoter controlled by c-fos promoter–driven rtTA3G in activated cells, and cyan pre-eGRASP was constitutively expressed by the EF1α promoter. Post-eGRASP was expressed bilaterally in NAcsh, with the TRE3G promoter–driven myristoylated mScarlet-I in activated cells and myristoylated iRFP670 constitutively expressed in this neuronal population distinct from c-fos activity. Myristoylated iRFP670 was driven by the hSyn promoter with calcium/calmodulin-dependent protein kinase II (CaMKIIα) promoter–driven flippase (FLPO) (54). We injected the virus cocktails into the DRN and NAcsh of TH::cre mice and delivered doxycycline at the onset of isolation (Fig. 5C). After socially isolated for 24 hours, the mice were put back to their home cage with cagemates for an extra day to obtain a higher expression of dual-eGRASP (35, 53). Because the working time point of doxycycline is ~8 hours after injection (55), the activated synapses cannot be captured when the mice return to their home cage. We confirmed that the increased sociability following 24 hours of social isolation still remained after a further 24-hour isolation or a further 24-hour group-housing (fig. S5, A to C). Through this approach, we specifically labeled the isolation-activated DRNTH synapses as yellow-eGRASP and activity-independent synapses as cyan-eGRASP, on isolation-activated and isolation-nonactivated NAcsh neurons, respectively (Fig. 5, D and E). We validated the expression specificity under the Fos-rtTA system, such that the yellow-eGRASP and myristoylated mScarlet-I were expressed in a doxycycline-dependent manner (fig. S5D). Because iRFP670 is expressed regardless of fos expression, we analyzed nonactivated neurons that only expressed iRFP670 and without mScarlet-I expression. We performed a quantitative analysis of dual-eGRASP by reconstructing three-dimensional (3D) models using the IMARIS program (fig. S5E).
In isolated male mice, isolation-activated DRNTH neurons showed a significantly greater synaptic density with the isolation-activated NAcsh neurons compared with the nonisolation-activated NAcsh neurons (Fig. 5F). To determine whether the increased synaptic connection in males was specific to the isolation experience, we performed the same experiment with the group-housed male mice. The synaptic density did not show any differences, regardless of their activity (fig. S5F). This suggests that the higher connections between the activated ensembles were isolation specific. In isolated females, isolation-activated DRNTH neurons had a similar synaptic density with isolation-activated and nonisolation-activated NAcsh neurons (Fig. 5G). The ratio of eGRASP signals on dendritic shaft or spine head/neck did not differ between cyan-eGRASP and yellow-eGRASP in both sexes, suggesting that the activated synapses are evenly distributed on dendritic shaft and spine head/neck (Fig. 5H). Together, the male-specific increase in synaptic density extends our previous sex-dependent results and implies the sexual dimorphic involvement of activated synaptic ensembles in DRNTH-NAcsh.
Isolation-activated neurons in the NAcsh are D1R expressing
Isolation-activated NAcsh neurons received higher synaptic connection from isolation-activated DRNTH neurons. To further elucidate the characteristics of isolation-activated neuronal population in NAcsh, we examined whether this activated population exhibits unique electrophysiological properties (Fig. 6A). Only the isolation-activated NAcsh neurons in male mice, but not in female mice, showed higher excitability (Fig. 6B), in line with our previous observations in DRNTH (Fig. 2C). Because D1R- and D2R-expressing neurons are the two main cell types in NAc (30), we then wondered which cell types are mainly activated following isolation. AAV2/1-Fos-rtTA with AAV2/1-TRE3G-mCherryNuc was injected bilaterally into the NAcsh of D1R-eGFP and D2R-eGFP transgenic mice lines, respectively (Fig. 6C). Among the isolation-activated NAcsh neurons, we found that a significantly greater proportion of D1R-expressing neurons were labeled compared with D2R-expressing neurons (Fig. 6D).
Fig. 6. D1R-expressing neurons of NAcsh are required for sociability following isolation.
(A) Labeling of isolation-activated NAcsh neurons. Scale bar, 100 μm. (B) In isolated male mice, the neuronal excitability (delivery of a 50-pA current pulse) is significantly higher in the mEmeraldNuc+ neurons (mEmeraldNuc−, n = 17; 1.000 ± 0.4287; mEmeraldNuc+, n = 17; 5.412 ± 1.018; unpaired t test; ***P = 0.0004). In isolated female mice, mEmeraldNuc+ and mEmeraldNuc− neurons show comparable excitability (mEmeraldNuc−, n = 18; 1.000 ± 0.4918; mEmeraldNuc+, n = 16; 1.813 ± 0.7595; unpaired t test; P = 0.3656). Each group from three mice. Scale bar, 50 mV, 100 ms. (C) Experimental scheme with D1R-eGFP and D2R-eGFP mice. (D) Labeling of isolation-activated NAcsh neurons. Scale bars, 50 μm. D1R-expressing neurons show higher activation ratio (mCherryNuc+ eGFP+/mCherryNuc+) than D2R-expressing neurons (D1, n = 36 from four mice; 13.03 ± 1.449; D2, n = 17 from two mice; 7.543 ± 2.126; unpaired t test; *P = 0.0373). (E and G) Experimental scheme of chemogenetically inhibiting D1R-expressing neurons (E) and D2R-expressing neurons (G) during three-chamber test. (F) Inhibition of D1R-expressing neurons abolish isolation-induced social interaction (n = 6; paired t test; **P = 0.0054). (H) Isolation-induced sociability remains intact, although D2R-expressing neurons are inhibited during the three-chamber test (n = 7; paired t test; P = 0.6365). (I and K) Experimental scheme of bilateral intracranial infusion of D1R antagonist (I) and D2R antagonist (K) into the NAcsh of isolated male mice. (J) Isolation-induced sociability is significantly attenuated after infusing D1R antagonist (Saline, n = 8; 86.03 ± 3.214; antagonist, n = 9; 73.79 ± 4.218; unpaired t test; *P = 0.0389). (L) Isolation-induced sociability is not affected by D2R antagonist infusion (Saline, n = 7; 83.52 ± 3.849; antagonist, n = 12; 80.33 ± 2.794; unpaired t test; P = 0.5055).
Inhibiting D1R-expressing neurons in the NAcsh attenuates isolation-induced sociability
To define the involvement of D1R- and D2R-expressing neurons in isolation-induced sociability, we inhibited each cell type during social interaction. First, AAV2/1-Ef1a-DIO-hM4Di-mCherry was bilaterally injected into the NAcsh of D1::cre or D2::cre mice to block the response to both glutamatergic and dopaminergic inputs (Fig. 6, E and G). Isolation-induced sociability disappeared when D1R-expressing neurons were inhibited during social interaction (Fig. 6F). In contrast, sociability remained intact in CNO-injected D2::cre mice (Fig. 6H). Inhibition of D1R- and D2R-expressing neurons did not change the mobility of the mice (fig. S6, A and B), indicating that D1R-expressing neurons are required for the expression of isolation-induced sociability. By infusing dopamine receptor antagonist intracranially into the NAcsh, we further examined whether the expression of social behavior following 24-hour isolation required the dopamine release. We bilaterally infused D1R antagonist (SCH 23390) or D2R antagonist (eticlopride) into the NAcsh 10 min before the three-chamber test (Fig. 6, I and K). The increased sociability following 24-hour isolation was abolished by D1R antagonist but not by D2R antagonist (Fig. 6, J and L). Infusions had no effect on the locomotion (fig. S6, C and D). Twenty-four hours of social isolation had no detectable effect on the protein level of D1Rs and D2Rs in NAcsh (fig. S6, E and F). These results indicate that dopamine signaling through D1Rs of NAcsh is critical for the expression of isolation-induced sociability.
DISCUSSION
By combining IEG-based tagging methods with the dopaminergic neurons and circuits, we have identified a neuronal mechanism underlying sex-specific isolation-induced sociability. The underlying mechanism uses synaptic-level alterations in activated ensembles in DRNTH-NAcsh circuit. Dopaminergic neurons in the DRN become activated upon social interaction following 24 hours of isolation (6). We found that only male mice showed an isolation-induced enhancement in sociability, which is similar to the observed sex differences in humans (9). We speculated that neural changes during the 24-hour isolation period drove the behavioral and circuit-level changes. A subpopulation of DRNTH neurons showed a robust increase in endogenous activity during the first 2 hours of isolation. This increased activity may serve as a trigger to activate the circuit and subsequent downstream regions. When silencing DRNTH during the 24-hour isolation period, we did not detect an increase in sociability. Moreover, isolation-activated ensembles in DRNTH and NAcsh showed higher neuronal excitability, higher reactivation ratio, and higher synaptic connectivity in between. These characteristics reflect more than solely activated neurons, as activated neurons under the group-housing condition did not show these features. Together, we conclude that activated neurons during the isolation period may represent a memory of social isolation experience. The male-specific increase in synaptic density between activated neuronal ensembles, coupled with a larger dopamine release into the NAcsh, provides additional mechanistic and functional insight into how circuits and synapses give rise to sexual dimorphic behaviors.
Behavioral stimuli, including social interactions, can induce changes in spine morphology (56). However, the synaptic dynamics at the afferent synapses in dopaminergic neurons, especially in activated synapses, are not well understood. Using our dual-eGRASP technique, we focused on the synaptic connections between the activated populations in DRNTH-NAcsh circuit to reveal the association between social interactions and resulting synaptic changes. Previously, a combination of dual-eGRASP with memory-encoding excitatory neurons in the hippocampus and amygdala revealed that synaptic potentiation between the activated ensembles represents memory (35, 53). Our study presented here expands this mechanism to neuromodulatory neurons and provides insight into the synaptic changes underlying a pivotal form of social behavior. Unique features of isolation-activated DRNTH neurons include increased synapse-specific involvement with activated neuronal population, implying that isolation experiences can elicit persistent changes in DRNTH neurons. The question remains whether this visualized synaptic strengthening is presynaptically or/and postsynaptically driven. We posit that both presynaptic and postsynaptic mechanisms are involved. Isolated male mice showed higher GRABDA signals, reflecting the increased presynaptic dopamine release. Increased dual-eGRASP spine density in isolated male mice may occur by a postsynaptic mechanism. These observations appear to be isolation specific and sexual dimorphic, such that group-housing experience and female mice did not show the differences between activated and nonactivated neurons. Our results provide an initial indication of the underlying synaptic mechanisms in sexual dimorphism. Because 2 weeks of social isolation permanently changed the sociability of males (57), our study prompts further examination of the persistent synaptic changes in DRNTH-NAcsh.
The complex functions of dopamine have not coalesced around a single function. Several studies indicate that dopaminergic neurons corelease glutamate (6, 58, 59) and heterogeneity among DRNTH exists (20, 60). Our observations further imply that the subpopulation of DRNTH neurons, which corelease dopamine/glutamate into the NAcsh, contribute to the visualized synaptic strengthening. Because dopaminergic neurons can synapse on the neck of dendritic spines and form a triadic circuit with glutamatergic (nondopaminergic) synapses (61, 62), the increased synaptic density between activated ensembles in DRNTH-NAcsh may occur by multiple scenarios: (i) The very same activated synapses receive other glutamatergic inputs or (ii) presynaptic DRN dopaminergic release acts on the axonal arbors of dopamine/glutamate coreleasing neurons itself. In either case, the synaptic strengthening appears to specifically occur in activated NAcsh neurons, as nonactivated NAcsh neurons did not show increased synaptic features. We visualized evenly distributed activated contacts on the synaptic head/neck and dendritic shaft, further suggesting that additional glutamatergic input may be less important.
Multiple inputs to the NAc, which is subdivided into central core and surrounding shell (63), regulate social behaviors by acting on integrating accumbal microcircuits modulated primarily by gamma-aminobutyric acid (GABA)ergic, glutamatergic, and dopaminergic signaling. The neuronal projections of the core remain within the basal ganglia and serve to evaluate reward-related actions. In contrast, the shell is interconnected with regions outside the basal ganglia to drive motivation and reward-related processes (64, 65). We observed that DRNTH neurons only project to the shell of NAc. This is in contrast to VTATH neurons, which project to both NAc shell and core (66). We suggest that the anatomical projections from DRNTH to the NAcsh drive motivated responding to increase social interactions after isolation. We posit that this circuit exclusively uses D1R-expressing neurons in NAcsh, as our inhibition experiments revealed the functional involvement of D1R, but not D2R in isolation-induced sociability although the activation of D1R-expressing neurons can affect D2R-expressing neurons (67). Our results are consistent with reports that the activity of D1R-expressing neurons in the NAc is important for modulating social behavior (25, 68).
A challenging question is how the brain can generate sexually dimorphic social behaviors. The behavioral effect of 24-hour social isolation in both sexes are markedly different, as isolated males show higher sociability levels, while isolated females show higher anxiety levels (8, 69). Modulation of DRNTH-NAcsh circuit only affected isolation-induced sociability, not isolation-induced anxiety in females, suggesting the distinct circuits to yield different sexual dimorphisms.
Impairment in social interaction is one trait of autism spectrum disorders (ASDs), as social isolation and loneliness can occur in patients with ASD (70, 71). Moreover, ASD shows a 4:1 gender distribution of males:females, although the treatment of sexual dimorphism in patients with ASD remains very broad (72, 73). Although complex interactions may exist between neural circuits that govern social behavior, increased sociability following social isolation and our proposed underlying mechanism associated with sexual dimorphic DRNTH-NAcsh circuit likely provide additional characteristics of ASD pathophysiology. We speculate that our study may inform further treatments to enhance social participation in both genders to reduce the prevalence of loneliness.
In conclusion, our findings reveal the underlying neural mechanism of sexual dimorphism in social isolation that bridges the synaptic, cellular, and circuit levels to behavior. We propose that modulating this circuit may treat gender-specific deficiencies in sociability found in ASD.
MATERIALS AND METHODS
Animals
All mice used in this study were maintained in a C57BL/6J background. Depending on the experiments, male or/and female mice were used with information mentioned in the figures or figure legends. Animals were housed with food and water ad libitum on a 12-hour light-dark cycle and were housed in four to six animals per cage before being group-housed or socially isolated. TH::cre (JAX008601) mice were crossed with Ai14 reporter mice (JAX007914) to visualize dopamine neurons in a cre-dependent manner (74). D1-eGFP (MMRRC000297) and D2-eGFP (MMRRC000230), D1::cre (MMRRC036916) and D2::cre (MMRRC032108) mice were used to visualize and target D1R- and D2R-expressing neurons in NAcsh (75, 76). The number of animals used in each experiment is noted in the figure legends. All mice were 8 to 18 weeks old during experiments. Eight- to 12-week-old mice underwent stereotaxic surgery, and all experiments were completed within 6 weeks. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University.
Stereotaxic surgery
Mice (8 to 12 weeks) were anesthetized with a ketamine/xylazine solution and positioned on a stereotaxic apparatus (Stoelting Co.). The virus mixture was injected into target regions through a 32-gauge needle with a Hamilton syringe at a rate of 0.1 μl/min, and the total injection volume per site was 0.3 to 0.5 μl. The needle tip was positioned 0.05 mm below the target coordinate right before the injection for 2 min. After the injection was completed, the needle remained in place for an additional 7 min and was withdrawn slowly. The stereotaxic coordinates for each target site were as follows: NAc (AP: +1.5; ML: ±0.65; DV: −4.55), VTA (AP: −2.9; ML: ±0.7; DV: −4.65), and DRN (AP: −4.2; ML: 0; DV: −3.2). For bilateral cannula implantations, cannula (custom and Newdoon) were implanted 0.5 mm above NAcsh and held in place with dental cement.
IEG-based tagging system
An activity-dependent tagging system was used as previously reported (53). Fos-rtTA and TRE3G promoter–based Tet-on labeling strategy was used (35, 48, 77). When rtTA proteins are expressed in fos-expressing neurons, it will bind to TRE3G promoter under doxycycline-presenting conditions. The working time point of doxycycline is ~8 hours after injection; thus, events longer than 24 hours cannot be captured (55). Subsequently, DNA constructs downstream of TRE3G promoter are induced.
Chemogenetic manipulations
The designer drug CNO (10 mg/kg for inhibition, 3 mg/kg for activation, i.p.; Hellobio) was administered 40 min before the behavioral tasks. CNO (5 μM) was used for ex vivo patch-clamp recording, and the effect was measured 10 min after CNO perfusion.
Optogenetic manipulations
Optical activation (23) was achieved by delivering 473-nm, 20-Hz train of eight pulses of 5-ms pulse width (6). Optical inhibition delivered constant 593 nm. AAV1-Ef1a-DIO-eYFP served as the control for both activation and inhibition experiments. Behavior tasks were performed after 4 weeks of viral expression.
Electrophysiology
Adult mice of both sexes were anesthetized with isoflurane, and their brains were quickly dissected. N-methyl-d-glucamine (NMDG) solution (93 mM NMDG, 2.5 mM KCl, 1.2 mM NaH2PO4, 30 mM NaHCO3, 20 mM Hepes, 25 mM glucose, 5 mM sodium ascorbate, 2 mM thiourea, 3 mM sodium pyruvate, 10 mM MgSO4, and 0.5 mM CaCl2) was used during brain slicing and recovery (35). Transverse slices (250 μm for DRN and 300 μm for NAc) were prepared in an ice-cold NMDG solution with a vibratome and then recovered in a 32°C NMDG solution for 6 to 7 min. After at least 1 hour of recovery in artificial cerebrospinal fluid (ACSF; 124 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4, 25 mM NaHCO3, 10 mM glucose, 2 mM CaCl2, and 2 mM MgSO4), the slice was placed in the recording chamber perfused with 32°C ACSF. To obtain reliable recordings, patched cells were allowed to stabilize for at least 3 min, and resting membrane potential was then recorded with no injected current. Only cells with a change in access resistance <20% were included in the analysis. The recording pipettes (2 to 4 megohm) were filled with an internal solution containing 145 mM K-gluconate, 5 mM NaCl, 10 mM Hepes, 1 mM MgCl2, 0.2 mM EGTA, 2 mM MgATP, and 0.1 mM Na3GTP (280 to 300 mOsm, adjusted to pH 7.2 with KOH). For combination with optogenetics, neurons that exhibited an average current (over four sweeps) of ≥10 pA recorded at −70 mV were defined as connections.
Behavior tasks
Three-chamber test
During the habituation session, mice were allowed to freely move in the apparatus for 10 min. After the habituation, an unfamiliar juvenile mouse (stranger) was placed under one cup and an object was placed under the other cup (78). Then, the test mice were recorded for the preference to the sex-matched unfamiliar juvenile mouse for 10 min, which is the test session. The time of sniffing to each cup (stranger and object) was analyzed. Time spent sniffing the stranger was divided by the total time of sniffing both stranger and object, which was regarded as sociability. Ethovision software generated the heatmaps and analyzed the velocity and total distance moved.
Juvenile interaction test
During the first 1 min, test mice were placed in a cage with new bedding for habituation. Then, sex-matched juvenile stranger mice were introduced into the cage for 5 min. Every task used new bedding. Observers counted the interaction time, which included nose-to-nose sniffing and nose-to-anogenital sniffing.
Open-field test
Mice were gently placed in the center and left to explore the chamber for 10 min. After every task, the chamber was cleaned with 75% ethanol and water. The total distance moved and the time spent in the center were analyzed using the Ethovision software (79).
Auditory fear conditioning
Auditory fear conditioning occurred in two different contexts (context A and context B) to minimize the influence of contextual associations. Context A was a square chamber with steel grid floor (Coulbourn Instruments; H10-11 M-TC), and context B was a rectangular plastic box with striped walls and a hardwood laboratory bedding (Beta Chip). For fear conditioning, mice were placed in context A and could explore the context for 150 s, followed by three exposures to auditory tone CS (30 s), each of which coterminated with 2 s, 0.75-mA footshock US, with a 30-s intertrial interval. After conditioning, mice were immediately placed in their home cages. One day after conditioning, mice were placed into a previously unexperienced context B and exposed to the auditory tone to measure the freezing behavior. Freezing behavior was recorded and scored using a video-based FreezeFrame fear-conditioning system.
Brain clearing
SeeDB2 protocol (80) was used for brain clearing. The brain sample was perfused with 4°C chilled 1× phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA) in 1× PBS. Perfused brains were fixed in 4% PFA for 12 hours. Samples were sliced by a vibratome into 1-mm thickness for clearing. All SeeDB2 incubation procedures were performed at room temperature.
Sample preparation and confocal imaging for dual-eGRASP
All viruses used for dual-eGRASP were confirmed in prior reports (35, 53). AAV2/1-CamKII-FLPO and AAV2/1-hSyn-FRT (flippase recognition target site)–Floxed-myriRFP670V5-P2A-post-eGRASP were newly constructed in this paper. Perfused brains were fixed with 4% PFA in PBS overnight at 4°C and dehydrated in 30% sucrose in PBS for 2 days at 4°C. Brains were sliced into 50-μm sections for dual-eGRASP analysis. Sections were mounted in Vectashield mounting medium (H-1000, Vector Laboratories). For dual-eGRASP analysis, NAc dendrites were imaged in Z-stack using a Leica SP8 confocal microscope with a 63× objective and distilled water immersion.
Image analysis for dual-eGRASP
Processing the confocal image and 3D reconstruction of dendrites were performed using IMARIS (Bitplane, Zurich, Switzerland) software. Before analysis, all image samples were blinded to exclude any bias. Each mScarlet-I–positive or iRFP670-positive dendrite was manually marked as a filament, while hiding other fluorescent signals. iRFP670-only dendrites were chosen for nonactivated ones. Each cyan- or yellow-eGRASP signal was marked as a cyan- or yellow-sphere through IMARIS automatic detection. Cyan- and yellow-eGRASP puncta on dendrites were manually counted, and overlapped cyan- and yellow-eGRASP signals were considered yellow signals.
Every confocal image had all four colors for normalization (iRFP-only–expressing and RFP-expressing dendrites, as well as cyan- and yellow-eGRASP signals). The spine density data were exported in blind to the experimenter. Then, the raw data for each dendrite were paired with the GRASP information (cyan, yellow, or no GRASP). Dendrites without any cyan-eGRASP were excluded for a more precise analysis. For normalization within one confocal image, the raw value of yellow density on every iRFP dendrite was averaged. Then, the raw value of each yellow density on iRFP dendrite and yellow density on mScarlet dendrite were divided by the calculated average. This normalized value was used for further statistical analysis.
Immunohistochemistry
Brains were postfixed in 4% PFA at 4°C overnight and transferred to 30% sucrose for 2 days at 4°C. Brains were embedded in optimal cutting temperature mounting medium, and 40-μm sections were cut using a cryostat (Leica). For immunohistochemistry, primary antibodies rabbit anti-TH (AB152; Millipore), chicken anti-TH (ab76442; Abcam), rat anti-DAT (MAB369, Sigma-Aldrich) and rabbit anti–c-fos (sc-52; Santa Cruz Biotechnology, 226003; SySy) were diluted to 1:300 to 1:1000 in blocking solution and incubated for 24 hours at 4°C. Secondary antibodies in the blocking solution were incubated for 2 hours at room temperature, and sections were mounted with Vectashield. Images were viewed under Zeiss LSM 700 and Leica SP8 confocal microscopes.
Ovariectomy
Juvenile female mice (5 to 7 weeks of age) were ovariectomized under subcutaneous injections of ketamine. The fallopian tubes were traced, both ovaries were removed, and the skin was sutured. For the control group, the cagemates of ovariectomized mice were selected and subjected to sham surgery. After 3 to 4 weeks of recovery in the home cage, the ovariectomized and sham groups underwent the behavioral tasks.
Western blot
Following 24 hours after group-housing or social isolation, the brains were rapidly removed. The NAcsh was dissected under a dissecting microscope and immediately frozen with liquid nitrogen. Samples were homogenized in radioimmunoprecipitation assay buffer containing 50 mM TriCl at pH 7.6; 150 mM NaCl; 1 mM EDTA; 1 mM dithiothreitol; 1% NP-40; 0.1% SDS; 0.5% sodium deoxycholate. The protein concentration was quantified using a Pierce BCA protein assay kit (Thermo Fisher Scientific) following the manufacturer’s protocol. Protein samples were electrophoresed on a 4 to 12% gradient gel (Invitrogen, NW04125BOX) and transferred to a nitrocellulose blotting membrane (GE Healthcare, 10600003). The membranes were blocked with 5% skim milk and incubated with primary antibodies: D1R (1:500; AB1765P, Merck Millipore), D2R (1:500; AB5084P, Merck Millipore), and pan-cadherin (1:5000; sc-59876, Santa Cruz Biotechnology) overnight at 4°C. Blots were rinsed out three times in 1X Tris-Buffered Saline, 0.1% Tween® 20 (TBST) and exposed to secondary antibody at room temperature for 2 hours: goat anti-mouse immunoglobulin G (IgG; 1:1000; Q-11071MP, Invitrogen) or goat anti-rabbit IgG (1:1000; A0545, Sigma-Aldrich). Bands were visualized using a Chemiluminescent horseradish peroxidase substrate kit (Millipore Corporation, Billerica, MA 91821, USA) and ChemiDoc XRS+ (721BR02655, Bio-Rad). Signals were detected and quantified using ChemiDoc MP device (Bio-Rad).
Intracranial drug delivery
Twenty-six–gauge guide cannulas were implanted bilaterally into the NAc (AP: +1.4; ML: ±1.5; DV: −4.0) at a 14° angle. After recovery, the mice were injected bilaterally with 0.3 μl of either 5 mM SCH23390 (Tocris) or 1 mM eticlopride (Sigma-Aldrich) or saline vehicle in the NAcsh. The 30-gauge needle was injected 0.2 mm lower than the guide cannula. After the volumes were injected, it was left in place for 3 min to allow diffusion. Three-chamber tests were started 10 min after infusion.
Fiber photometry
We used TH::cre mice to detect dopamine release from DRNTH in NAcsh. The dopamine sensor AAV2/1-hSyn-GRABDA (Addgene 113050) was injected into the NAcsh (AP: +1.5; ML: 0.65, DV: −4.5) with optic fiber (400-μm core diameter, AP: +1.5; ML: 0.65; DV: −4.4), and AAV2/1-Ef1a-DIO-Chronos-mCherry [further modified from a previous paper (35)] were injected into the DRN (AP: −4.2; ML: 0; DV: −3.2) with optic fiber (200-μm core diameter, AP: −4.2; ML: 0; DV: −2.8). For combination with social interaction following group-housed and socially isolated mice, wild-type mice were injected with AAV2/1-hSyn-GRABDA and AAV2/1-CamKII-TagRFP-T. Fibers were implanted in NAcsh (400-μm core diameter, AP: +1.5; ML: 0.65; DV: −4.4). All experiments were performed at least 1.5 weeks after surgery for recovery.
For the optogenetic fiber photometry experiment, we placed the test mouse into a rectangular acrylic cage for a habituation period of least 5 min. After habituation, we recorded GRABDA responses in NAcsh. During measurements, we delivered blue light (10 mW) into the DRN at 30-s intervals for 1.5 s to activate DRNTH neurons.
For fiber photometry combined with behavior, 24-hour group-housed or isolated mice were placed in rectangular acrylic cage and habituated over 5 min to stabilize before measuring the baseline dopamine signals in NAcsh. The secondary virus (AAV2/1-CamKII-TagRFP-T) assessed motion artifacts before the GRABDA recordings (whether the cannula had not shifted and was well connected to the sleeve while the animal was freely moving). After this habituation period, we recorded GRABDA signals for 60 s to measure the dopamine response at baseline. After baseline recording, we introduced sex-matched juvenile mouse into the cage and measured the dopamine signals for 60 s at the onset of social interaction. Then, we withdrew the juvenile mouse and recorded an additional 60 s of dopamine responses.
Fiber photometry analysis was performed as previously published (81). Fluorescence signals were measured by the Doric studio (ver 5.3.3.14) program. To measure the dopamine dynamics in NAcsh, the moment immediately before the test mouse interacted with a juvenile mouse was denoted as 0 s. Next, we calculated (F − F0)/F0 (ΔF/F)%. We considered F0 as the baseline fluorescence signal averaged over a 3-s time window between −4 and −1 s before the social interaction starts. We analyzed and plotted the dopamine dynamics for the first 30 s after the social interaction started.
Quantification and statistical analysis
All behavioral data were scored by a trained observer blind to experimental conditions or scored using an automated system (Ethovision). Then, data were processed and analyzed using Excel and GraphPad Prism 8. Statistical analyses were conducted using unpaired t tests, paired t test, two-way analysis of variance (ANOVA), and Mann-Whitney two-tailed test when appropriate. Unless specified otherwise, all data are shown as mean values ± SEM with the n value for each dataset. Statistically significant effects are reported in the figure legend. The significance threshold was held at 0.05, two-tailed (not significant, ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). No exclusion of any data was performed.
Acknowledgments
We thank K. Tye and G. Matthews for critical comments and suggestions on an earlier version of the manuscript.
Funding: This work was supported by the National Honor Scientist Program (NRF-2012R1A3A1050385) of Korea.
Author contributions: J.E.C., D.I.C., and B.-K.K. contributed to the study design and wrote the paper. J.E.C. designed and conducted all the animal behavior and electrophysiology experiments. D.I.C. conducted fiber photometry, subcloning, virus preparation, image analysis, and stereotaxic surgery. J.L., Jooyoung Kim, M.J.K., I.H., H.J., Y.S., Ji-il Kim, T.K., N.-K.Y., and S.-H.L. assisted with virus preparation, stereotaxic surgery, immunohistochemistry, and data analysis. H.K.C., J.W.K., and J.-H.K. contributed to the interpretation of the data and revising the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S6
Other Supplementary Material for this manuscript includes the following:
Data file S1
REFERENCES AND NOTES
- 1.Baumeister R. F., Leary M. R., The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 117, 497–529 (1995). [PubMed] [Google Scholar]
- 2.Van Loo P. L. P., de Groot A. C., Van Zutphen B. F. M., Baumans V., Do male mice prefer or avoid each other’s company? influence of hierarchy, kinship, and familiarity. J. Appl. Anim. Welf. Sci. 4, 91–103 (2001). [Google Scholar]
- 3.Thoenig R. H., Solitary confinement-punishment within the letter of the law, or psychological torture. Wisconsin Law Rev. , 223–311 (1972). [Google Scholar]
- 4.Cacioppo J. T., Hughes M. E., Waite L. J., Hawkley L. C., Thisted R. A., Loneliness as a specific risk factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychol. Aging 21, 140–151 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Fone K. C. F., Porkess M. V., Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 32, 1087–1102 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Matthews G. A., Nieh E. H., Vander Weele C. M., Halbert S. A., Pradhan R. V., Yosafat A. S., Glober G. F., Izadmehr E. M., Thomas R. E., Lacy G. D., Wildes C. P., Ungless M. A., Tye K. M., Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomova L., Wang K. L., Thompson T., Matthews G. A., Takahashi A., Tye K. M., Saxe R., Acute social isolation evokes midbrain craving responses similar to hunger. Nat. Neurosci. 23, 1597–1605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senst L., Baimoukhametova D., Sterley T.-L., Bains J. S., Sexually dimorphic neuronal responses to social isolation. eLife 5, e18726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandervoort D., Social isolation and gender. Curr. Psychol. 19, 229–236 (2000). [Google Scholar]
- 10.Ruigrok A. N. V., Salimi-Khorshidi G., Lai M.-C., Baron-Cohen S., Lombardo M. V., Tait R. J., Suckling J., A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bálint S., Czobor P., Komlósi S., Mészáros A., Simon V., Bitter I., Attention deficit hyperactivity disorder (ADHD): Gender-and age-related differences in neurocognition. J. Psychol. Med. 39, 1337–1345 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Wooten G., Currie L., Bovbjerg V., Lee J., Patrie J., Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borland J. M., Aiani L. M., Norvelle A., Grantham K. N., O’Laughlin K., Terranova J. I., Frantz K. J., Elliott Albers H., Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology 44, 785–792 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brancato A., Bregman D., Ahn H. F., Pfau M. L., Menard C., Cannizzaro C., Russo S. J., Hodes G. E., Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350, 180–189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asher M., Aderka I. M., Gender differences in social anxiety disorder. J. Clin. Psychol. 74, 1730–1741 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Greenberg G. D., Laman-Maharg A., Campi K. L., Voigt H., Orr V. N., Schaal L., Trainor B. C., Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front. Behav. Neurosci. 7, 223–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver D. K., Intson K., Sargin D., Power S. K., Nabb J. M., Ramsey A. J., Lambe E. K., Chronic social isolation exerts opposing sex-specific consequences on serotonin neuronal excitability and behaviour. Neuropharmacology 168, 108015 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Stratford T. R., Wirtshafter D., Ascending dopaminergic projections from the dorsal raphe nucleus in the rat. Brain Res. 511, 173–176 (1990). [DOI] [PubMed] [Google Scholar]
- 19.Trulson M. E., Cannon M. S., Raese J. D., Identification of dopamine-containing cell bodies in the dorsal and median raphe nuclei of the rat brain using tyrosine hydroxylase immunochemistry. Brain Res. Bull. 15, 229–234 (1985). [DOI] [PubMed] [Google Scholar]
- 20.Huang K. W., Ochandarena N. E., Philson A. C., Hyun M., Birnbaum J. E., Cicconet M., Sabatini B. L., Molecular and anatomical organization of the dorsal raphe nucleus. eLife 8, e46464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vander Weele C. M., Siciliano C. A., Matthews G. A., Namburi P., Izadmehr E. M., Espinel I. C., Nieh E. H., Schut E. H. S., Padilla-Coreano N., Burgos-Robles A., Chang C.-J., Kimchi E. Y., Beyeler A., Wichmann R., Wildes C. P., Tye K. M., Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witten I. B., Steinberg E. E., Lee S. Y., Davidson T. J., Zalocusky K. A., Brodsky M., Yizhar O., Cho S. L., Gong S., Ramakrishnan C., Stuber G. D., Tye K. M., Janak P. H., Deisseroth K., Recombinase-driver rat lines: Tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai H.-C., Zhang F., Adamantidis A., Stuber G. D., Bonci A., de Lecea L., Deisseroth K., Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bariselli S., Hörnberg H., Prévost-Solié C., Musardo S., Hatstatt-Burklé L., Scheiffele P., Bellone C., Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat. Commun. 9, 3173 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunaydin L. A., Grosenick L., Finkelstein J. C., Kauvar I. V., Fenno L. E., Adhikari A., Lammel S., Mirzabekov J. J., Airan R. D., Zalocusky K. A., Tye K. M., Anikeeva P., Malenka R. C., Deisseroth K., Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin R., Liang J., Wang R., Yan T., Zhou Y., Liu Y., Feng Q., Sun F., Li Y., Li A., Gong H., Luo M., The raphe dopamine system controls the expression of incentive memory. Neuron 106, 498–514.e8 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Cho J. R., Treweek J. B., Robinson J. E., Xiao C., Bremner L. R., Greenbaum A., Gradinaru V., Dorsal raphe dopamine neurons modulate arousal and promote wakefulness by salient stimuli. Neuron 94, 1205–1219.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Groessl F., Munsch T., Meis S., Griessner J., Kaczanowska J., Pliota P., Kargl D., Badurek S., Kraitsy K., Rassoulpour A., Zuber J., Lessmann V., Haubensak W., Dorsal tegmental dopamine neurons gate associative learning of fear. Nat. Neurosci. 21, 952–962 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salgado S., Kaplitt M. G., The nucleus accumbens: A comprehensive review. Stereotact. Funct. Neurosurg. 93, 75–93 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Gerfen C. R., Surmeier D. J., Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith R. J., Lobo M. K., Spencer S., Kalivas P. W., Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr. Opin. Neurobiol. 23, 546–552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolen G., Darvishzadeh A., Huang K. W., Malenka R. C., Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace D. L., Han M.-H., Graham D. L., Green T. A., Vialou V., Iñiguez S. D., Cao J.-L., Kirk A., Chakravarty S., Kumar A., Krishnan V., Neve R. L., Cooper D. C., Bolaños C. A., Barrot M., McClung C. A., Nestler E. J., CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 12, 200–209 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Josselyn S. A., Tonegawa S., Memory engrams: Recalling the past and imagining the future. Science 367, eaaw4325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J.-H., Sim S.-E., Kim J.-I., Choi D. I., Oh J., Ye S., Lee J., Kim T. H., Ko H.-G., Lim C.-S., Kaang B.-K., Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Ryan T. J., Roy D. S., Pignatelli M., Arons A., Tonegawa S., Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Ramirez S., Pang P. T., Puryear C. B., Govindarajan A., Deisseroth K., Tonegawa S., Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koya E., Golden S. A., Harvey B. K., Guez-Barber D. H., Berkow A., Simmons D. E., Bossert J. M., Nair S. G., Uejima J. L., Marin M. T., Mitchell T. B., Farquhar D., Ghosh S. C., Mattson B. J., Hope B. T., Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci. 12, 1069–1073 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz F. C., Babin K. R., Leao R. M., Goldart E. M., Bossert J. M., Shaham Y., Hope B. T., Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J. Neurosci. 34, 7437–7446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wall N. R., Neumann P. A., Beier K. T., Mokhtari A. K., Luo L., Malenka R. C., Complementary genetic targeting and monosynaptic input mapping reveal recruitment and refinement of distributed corticostriatal ensembles by cocaine. Neuron 104, 916–930.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillies G. E., McArthur S., Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 62, 155–198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riecher-Rössler A., Häfner H., Stumbaum M., Maurer K., Schmidt R., Can estradiol modulate schizophrenic symptomatology? Schizophr. Bull. 20, 203–214 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Hille B., Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 22, 283–294 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLaren D. A. A., Browne R. W., Shaw J. K., Radhakrishnan S. K., Khare P., España R. A., Clark S. D., Clozapine N-oxide administration produces behavioral effects in long–Evans rats: Implications for designing DREADD experiments. eNeuro 3, ENEURO.0219-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S., Kramer E. E., Mercaldo V., Rashid A. J., Insel N., Frankland P. W., Josselyn S. A., Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 41, 2987–2993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Haasteren G., Li S., Ryser S., Schlegel W., Essential contribution of intron sequences to Ca2+-dependent activation of c-fos transcription in pituitary cells. Neuroendocrinology 72, 368–378 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Loew R., Heinz N., Hampf M., Bujard H., Gossen M., Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol. 10, 81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reijmers L. G., Perkins B. L., Matsuo N., Mayford M., Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Zhou X., Vink M., Klaver B., Berkhout B., Das A. T., Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 13, 1382–1390 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto K., McBride W. J., Regulation of nucleus accumbens dopamine release by the dorsal raphe nucleus in the rat. Neurochem. Res. 17, 401–407 (1992). [DOI] [PubMed] [Google Scholar]
- 51.De Deurwaerdère P., Stinus L., Spampinato U., Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: Role of 5-HT3 receptors. J. Neurosci. 18, 6528–6538 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y., Zhu H., Liu Z., Chen X., Su X., Ma C., Tian Z., Huang B., Yan E., Liu X., Ma L., A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat. Neurosci. 22, 1986–1999 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Choi D. I., Kim J., Lee H., Kim J.-i., Sung Y., Choi J. E., Venkat S. J., Park P., Jung H., Kaang B.-K., Synaptic correlates of associative fear memory in the lateral amygdala. Neuron 109, 2717–2726.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Jung H., Kim S.-W., Kim M., Hong J., Yu D., Kim J. H., Lee Y., Kim S., Woo D., Shin H.-S., Park B. O., Heo W. D., Noninvasive optical activation of Flp recombinase for genetic manipulation in deep mouse brain regions. Nat. Commun. 10, 314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucchetti J., Fracasso C., Balducci C., Passoni A., Forloni G., Salmona M., Gobbi M., Plasma and brain concentrations of doxycycline after single and repeated doses in wild-type and APP23 mice. J. Pharmacol. Exp. Ther. 368, 32–40 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Pignatelli M., Bonci A., Role of dopamine neurons in reward and aversion: A synaptic plasticity perspective. Neuron 86, 1145–1157 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Soares-Cunha C., Coimbra B., David-Pereira A., Borges S., Pinto L., Costa P., Sousa N., Rodrigues A. J., Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 7, 11829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuber G. D., Hnasko T. S., Britt J. P., Edwards R. H., Bonci A., Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J. Neurosci. 30, 8229–8233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tecuapetla F., Patel J. C., Xenias H., English D., Tadros I., Shah F., Berlin J., Deisseroth K., Rice M. E., Tepper J. M., Koos T., Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J. Neurosci. 30, 7105–7110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dougalis A. G., Matthews G. A. C., Bishop M. W., Brischoux F., Kobayashi K., Ungless M. A., Functional properties of dopamine neurons and co-expression of vasoactive intestinal polypeptide in the dorsal raphe nucleus and ventro-lateral periaqueductal grey. Eur. J. Neurosci. 36, 3322–3332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broussard J. I., Co-transmission of dopamine and glutamate. J. Gen. Physiol. 139, 93–96 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tritsch N. X., Sabatini B. L., Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voorn P., Gerfen C. R., Groenewegen H. J., Compartmental organization of the ventral striatum of the rat: Immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J. Comp. Neurol. 289, 189–201 (1989). [DOI] [PubMed] [Google Scholar]
- 64.Heimer L., Alheid G. F., de Olmos J. S., Groenewegen H. J., Haber S. N., Harlan R. E., Zahm D. S., The accumbens: Beyond the core-shell dichotomy. J. Neuropsychiatry Clin. Neurosci. 9, 354–381 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Sesack S. R., Grace A. A., Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology 35, 27–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heymann G., Jo Y. S., Reichard K. L., McFarland N., Chavkin C., Palmiter R. D., Soden M. E., Zweifel L. S., Synergy of distinct dopamine projection populations in behavioral reinforcement. Neuron 105, 909–920.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Francis T. C., Yano H., Demarest T. G., Shen H., Bonci A., High-frequency activation of nucleus accumbens D1-MSNs drives excitatory potentiation on D2-MSNs. Neuron 103, 432–444.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okuyama T., Kitamura T., Roy D. S., Itohara S., Tonegawa S., Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann I. D., Landgraf R., Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659 (2012). [DOI] [PubMed] [Google Scholar]
- 70.C. Kasari, L. Sterling, Loneliness and social isolation in children with autism spectrum disorders, in The Handbook of Solitude: Psychological Perspectives on Social Isolation, Social Withdrawal, and Being Alone, R. J. Coplan, J. C. Bowker, Eds. (Wiley Blackwell, 2014), pp. 409–426. [Google Scholar]
- 71.Orsmond G. I., Shattuck P. T., Cooper B. P., Sterzing P. R., Anderson K. A., Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord. 43, 2710–2719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitjans M., Begemann M., Ju A., Dere E., Wüstefeld L., Hofer S., Hassouna I., Balkenhol J., Oliveira B., van der Auwera S., Tammer R., Hammerschmidt K., Völzke H., Homuth G., Cecconi F., Chowdhury K., Grabe H., Frahm J., Boretius S., Dandekar T., Ehrenreich H., Sexual dimorphism of AMBRA1-related autistic features in human and mouse. Transl. Psychiatry 7, e1247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werling D. M., Parikshak N. N., Geschwind D. H., Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun. 7, 10717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindeberg J., Usoskin D., Bengtsson H., Gustafsson A., Kylberg A., Söderström S., Ebendal T., Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Lee J. H., Ribeiro E. A., Kim J., Ko B., Kronman H., Jeong Y. H., Kim J. K., Janak P. H., Nestler E. J., Koo J. W., Kim J. H., Dopaminergic regulation of nucleus accumbens cholinergic interneurons demarcates susceptibility to cocaine addiction. Biol. Psychiatry 88, 746–757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong S., Zheng C., Doughty M. L., Losos K., Didkovsky N., Schambra U. B., Nowak N. J., Joyner A., Leblanc G., Hatten M. E., Heintz N., A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Hasan M. T., Althammer F., da Gouveia M. S., Goyon S., Eliava M., Lefevre A., Kerspern D., Schimmer J., Raftogianni A., Wahis J., Knobloch-Bollmann H. S., Tang Y., Liu X., Jain A., Chavant V., Goumon Y., Weislogel J.-M., Hurlemann R., Herpertz S. C., Pitzer C., Darbon P., Dogbevia G. K., Bertocchi I., Larkum M. E., Sprengel R., Bading H., Charlet A., Grinevich V., A fear memory engram and its plasticity in the hypothalamic oxytocin system. Neuron 103, 133–146.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Lim C.-S., Kim M. J., Choi J. E., Islam M. A., Lee Y.-K., Xiong Y., Shim K.-W., Yang J.-E., Lee R. U., Lee J., Park P., Kwak J.-H., Seo H., Kim C. H., Lee J.-H., Lee Y.-S., Hwang S.-K., Lee K., Lee J.-A., Kaang B.-K., Dysfunction of NMDA receptors in neuronal models of an autism spectrum disorder patient with a DSCAM mutation and in Dscam-knockout mice. Mol. Psychiatry 26, 7538–7549 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park J., Lim C.-S., Seo H., Park C.-A., Zhuo M., Kaang B.-K., Lee K., Pain perception in acute model mice of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol. Pain 11, 28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ke M. T., Nakai Y., Fujimoto S., Takayama R., Yoshida S., Kitajima T. S., Sato M., Imai T., Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent. Cell Rep. 14, 2718–2732 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Sun F., Zhou J., Dai B., Qian T., Zeng J., Li X., Zhuo Y., Zhang Y., Wang Y., Qian C., Tan K., Feng J., Dong H., Lin D., Cui G., Li Y., Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S6
Data file S1