OBJECTIVES:
Respiratory failure is a lethal complication of COVID-19 that has remained resistant to drug therapy. Vasoactive intestinal peptide (VIP) is shown in nonclinical studies to upregulate surfactant production, inhibit cytokine synthesis, prevent cytopathy, and block replication of the severe acute respiratory syndrome coronavirus 2 virus in pulmonary cells. The study aims to determine whether Aviptadil (synthetic VIP) can improve survival and recovery in patients with COVID-19 respiratory failure compared with placebo and demonstrate biological effects in such patients.
DESIGN:
A multicenter, placebo-controlled trial.
SETTING:
Ten U.S. hospitals: six tertiary-care hospitals and four community hospitals.
PATIENTS:
A total of 196 patients with COVID-19 respiratory failure.
INTERVENTIONS:
Participants were randomized 2:1 to receive 3 days of IV Aviptadil or placebo.
MEASUREMENTS AND MAIN RESULTS:
The primary end point (alive and free from respiratory failure at day 60) did not reach statistical significance (odds ratio [OR], 1.6; 95% CI, 0.86–3.11) for patients treated with Aviptadil when controlling for baseline ventilation status as prespecified in the protocol. There was, however, a statistically significant two-fold odds of improved survival (OR, 2.0; 95% CI, 1.1–3.9) at 60 days (p = 0.035). There was significant improvement in respiratory distress ratio and reduced interleukin 6 cytokine release (p = 0.02) by day 3.
Subgroup analysis identified a statistically significant likelihood of achieving primary end point among those treated with high-flow nasal oxygen at baseline (p = 0.039). Subjects on mechanical ventilation also experienced a 10-fold increased odds of survival with drug versus placebo (p = 0.031).
CONCLUSIONS:
The primary end point did not reach statistical significance, indicating that there was no difference between Aviptadil versus placebo. However, Aviptadil improves the likelihood of survival from respiratory failure at day 60 in critical COVID-19 across all sites of care. Given the absence of drug-related serious adverse events and acceptable safety profile, we believe the benefit versus risk for the use of Aviptadil is favorable for patient treatment.
Keywords: acute lung injury, acute respiratory distress syndrome, alveolar type II, coronavirus, COVID-19, severe acute respiratory syndrome coronavirus 2, surfactant, vasoactive intestinal peptide
Aviptadil, a synthetic form of vasoactive intestinal peptide (VIP), has been granted U.S. Food and Drug Administration (FDA) Fast Track Designation for treating critical COVID-19 with respiratory failure. Respiratory Failure in COVID-19 is caused by selective binding of the severe acute respiratory syndrome coronavirus (SARS-CoV-2) to the alveolar type 2 (AT2) cell in the pulmonary epithelium via the angiotensin-converting enzyme 2 receptor (1). VIP is concentrated in the lung, where it binds to the VIP receptor 1 of the AT2 cell (2). As a result, VIP protects the lung against a broad array of experimental caustic, immune, and infectious injuries (3–8) by upregulating surfactant production (7, 9, 10), blocking apoptosis, and blocking cytokine effects. In addition, VIP inhibits replication of SARS-CoV-2 in human pulmonary-derived Calu-3 cells and inhibits virus-induced cytopathy of those cells (11). IV VIP has shown promise in a phase 1 trial for treating sepsis-related acute respiratory distress syndrome (ARDS) (12). The administration of VIP by inhalation has shown promising results in treating sarcoid (13), pulmonary hypertension (14, 15), and checkpoint-inhibitor pneumonitis (16). An open-label VIP trial showed enhanced recovery and survival among critically ill patients with respiratory failure in COVID-19 (17, 18).
We report the results of a phase 2b/3 multicenter trial (NCT04311697) designed to test whether IV Aviptadil administered to critically ill patients with respiratory failure in COVID-19 is associated with an increased likelihood of recovery from respiratory failure and/or survival at 60 days posttreatment and to determine whether there is evidence of biological effect as measured by changes in oxygenation and cytokine levels.
Trial Design: Participants, Methods, and Blinding
The study was conducted as a phase 2b/3 clinical trial under FDA Investigational New Drug Application 149,152 with fast track designation. Human subjects’ protection was overseen and approved by Advarra Institutional Review Board (IRB) (approval Pro000431143), IRBs of the study sites, and an independent data monitoring committee (DMC), operating under an FDA-reviewed charter.
Eligible participants were 18 years or older and had positive polymerase chain reaction critical COVID-19 by FDA resource-based definition, which requires treatment with high-flow nasal cannula (HFNC) greater than 20 L, noninvasive ventilation (NIV), or mechanical ventilation (MV) (19). Exclusion criteria are shown in Supplementary Table 1 (http://links.lww.com/CCM/H192). The study was performed in six tertiary-care hospitals and four regional (community) hospitals in the United States. Participants were enrolled between May and December 2020. Tertiary-care hospitals were defined as having a 24-hour presence of board-certified critical care physicians, fellows, and respiratory therapists in the ICU. Community hospitals had a lower level of staffing. The study follows Consolidated Standards of Reporting Trials (CONSORT) rules for reporting clinical trials (20).
The FDA initially approved the study as a 144 patient 28-day trial based on the ARDS experience. In December 2020, the sponsor submitted a modified protocol and statistical analysis plan to FDA in consultation with the IRB and DMC extending the trial to 60 days of observation and a sample size of 198 subjects. FDA similarly identified a need for longer periods of observation in treatments for COVID-19 respiratory failure and published revised guidance to the industry before unblinding the day 60 end point of this trial (20).
The original study protocol identified survival as the primary study end point and recovery from respiratory failure as the key secondary end point. In February 2021, at the suggestion of the DMC, these end points were reversed, and the design was changed to a primary end point was “alive at day 60 and free of respiratory failure,” ascertained as being alive, discharged from acute care, and no longer requiring more than 2 L of low-flow oxygen. The key secondary end point was survival at 60-day posttreatment, as ascertained by study-site personnel. Respiratory distress ratio (RDR; Pao2:Fio2 [P:F]) and levels of inflammatory cytokines were included as end points to detect biological response to treatment. The severity of illness was assessed via the National Institute of Allergy and Infectious Diseases (NIAID) 8-point ordinal scale where 1 = death and 8 = not hospitalized with no symptoms (Supplementary Table 2, http://links.lww.com/CCM/H193).
High mortality at community hospitals was identified from blinded data based on adverse event reports reported to the DMC and the FDA. A design change was implemented before unblinding to add a covariate for the site of care (tertiary versus community hospital). Results are reported for primary and key secondary end points both with and without the site of care design change.
The study intervention was an administration of either three 12-hour IV infusions of Aviptadil at graduating doses of 50, 100, and 150 pmol/kg/hr or a normal saline placebo on 3 successive days (12). The investigational product was prepared according to Good Manufacturing Practices, with manufacturing records submitted to the FDA before patient treatment. An unblinded statistician prepared interim analyses for assessment of safety and futility by the Data and Safety Monitoring Board, which reviewed this material only in closed sessions.
Randomization, Power Calculation, and Statistical Methods
Participants were randomized 2:1 Aviptadil versus placebo via a random seed-validated interactive voice response system in block sizes of four, visible only to the unblinded research pharmacist who prepared all IV infusions. Aviptadil and placebo were presented identically, labeled only by patient and date. Study personnel, patients, families, and sponsors were blinded to treatment assignment. All procedures concerning blinding, statistical analysis, and study conduct were reviewed in type A meetings with the FDA.
The initial power calculation required a sample size of 144 participants to achieve 80% study power (α = 0.05, β = 0.2), based on an assumption of 30% survival in patients with COVID-19 respiratory failure in the April 2020 time frame. Following enrollment of 102 participants, DMC reviewed reports of clinical trials for other agents, together with evidence of improved survival associated with preferential use of HFNC versus MV, proning of patients, use of steroids, and other improvements in care. DMC suggested an increase in sample size with adjustment of type 1 error (21). At 196 subjects, the study was projected to have 81% power to detect a 0.52 hazard ratio (HR; i.e., a 22% absolute increase in the percent achieving respiratory failure resolution) at a two-sided 5% type 1 error.
All analysis was by modified intention to treat (mITT), defined as randomized subjects minus those deemed ineligible or withdrew consent before treatment, as was agreed with FDA and is customary in critical care trials. Per the original protocol, binary end points were assessed by logistic regression, controlling for baseline NIAID score, use of remdesivir, baseline RDR, and body mass index (BMI). However, before the completion of data collection, the blinded analysis noted higher overall survival among those treated initially with HFNC than those treated with MV or NIV. Therefore, the statistical analysis plan was modified before unblinding to include baseline ventilation instead of baseline NIAID score to analyze the primary end point and survival.
Mixed-model repeated measure (MMRM) was used to analyze mean differences in daily NIAID score, RDR (P:F ratio), and cytokine levels (SAS/STAT 9.4 User’s Guide, SAS Institute Inc., Cary, NC). The F statistic correlates change in interleukin 6 (IL-6) levels with primary and secondary end points. Subgroup analyses were preplanned based on baseline ventilation status and use of concurrent medications.
RESULTS
Baseline Characteristics and Treatment
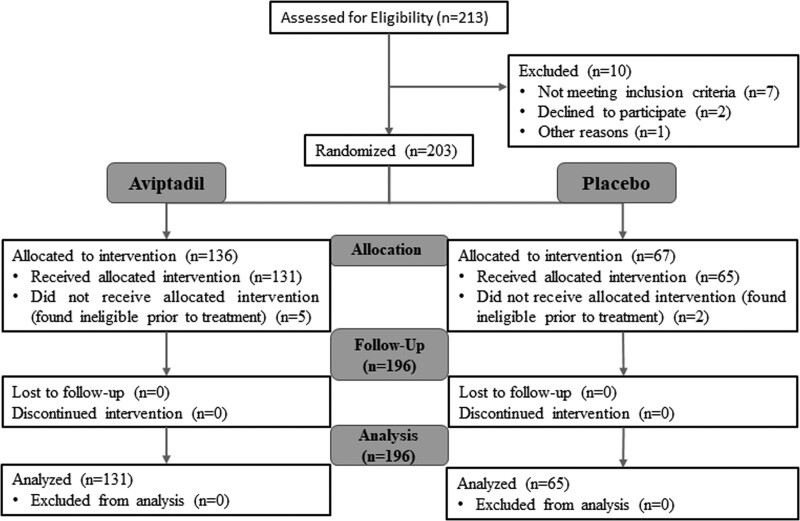
Participants were enrolled between May 15, 2020, and December 24, 2020. Of 213 participants screened, 203 were deemed eligible and consented to be randomized. Of those, five were considered ineligible before treatment and 2 withdrew consent before treatment (Fig. 1).
Figure 1.
Consolidated Standards of Reporting Trials flow diagram.
More severe disease (such as those on MV) was documented at baseline in the Aviptadil-treated group (27.5% vs 16.9%) with no other significant differences between the groups (Table 1), emphasizing the need to control for baseline ventilation status in measuring the primary and secondary end points. In addition, 91% of participants assigned to the Aviptadil arm received all three infusions at the targeted dose, with 19 (6.9%) requiring dose adjustments primarily for diarrhea. All placebo-treated participants received all three infusions of placebo. Ten patients refused repeated phlebotomy after several days but consented to ongoing noninvasive data collection. There were no losses to follow-up through day 60. For patients discharged alive from the hospital before days 28 and 60, patient status was assessed via telephone call initiated by study site personnel. Background standard of care was not protocoled, and study site physicians made all decisions regarding ventilation, use of concomitant medications, and other aspects of care. Background standard of care included remdesivir, tocilizumab, high-dose steroids, and proning (Table 1). No differences between aviptadil-treated and placebo-treated groups were seen with regard to any of these baseline characteristics.
TABLE 1.
Baseline Characteristics: Treated Participants
| Aviptadil (n = 131) | Placebo (n = 65) | |
|---|---|---|
| Age, yr, mean (range) | 60.1 (31–88) | 62.7 (35–85) |
| Age: <65 yr, n (%) | 82 (62.6) | 36 (55.4) |
| Sex: male, n (%) | 85 (64.9) | 50 (76.9) |
| Race: Caucasian, n (%) | 110 (84.0) | 57 (87.7) |
| Ethnicity: Hispanic or Latino, n (%) | 66 (50.4) | 39 (60.0) |
| BMI, mean (range) | 33.3 (20.1–60.3) | 32.1 (22.5–60.3) |
| NIAID Score: 2 (invasive mechanical ventilation), n (%) | 36 (27.5) | 11 (16.9) |
| Previous anti-viral therapy, n (%) | 91 (69.5) | 45 (69.2) |
| On remdesivir, n (%) | 86 (65.6) | 41 (63.1) |
| On steroids, n (%) | 69 (52.7) | 35 (53.8) |
| On tocilizumab, n (%) | 21 (16.0) | 11 (16.9) |
| On anticoagulants (anti-platelet, Heparins, warfarin, and factor Xa inhibitors), n (%) | 35 (26.7) | 18 (27.7) |
| Number (%) of patients who were pronated, n (%) | 7 (5.3) | 2 (3.1) |
| Tertiary site—mechanical ventilation, n (%) | 38 (29.0) | 8 (12.3) |
| Regional site—mechanical ventilation, n (%) | 17 (13.0) | 6 (9.2) |
| Tertiary site—HFNC, n (%) | 56 (42.7) | 42 (64.6) |
| Regional site—HFNC, n (%) | 20 (15.3) | 9 (13.8) |
| Pao2/Fio2 ratio, mean (range) | 107.7 (42–453) | 104.9 (51–318) |
| RDR ratio: <150 mm Hg, n (%) | 106 (80.9) | 55 (84.6) |
BMI = body mass index, HFNC = high-flow nasal cannula, NIAID = National Institute of Allergy and Infectious Diseases, RDR = respiratory distress ratio.
Primary End Point: Alive and Free of Respiratory Failure at Day 60
As shown in the CONSORT diagram (Fig. 1), there were no treatment misclassifications or losses to follow-up. Primary and secondary end points were analyzed in all 196 treated participants, 131 Aviptadil and 65 placebo-treated (the mITT population) (Table 2). A numerical (63% vs 50%) but not statistically significant increased odds of achieving the primary end point at day 60 was identified (odds ratio [OR], 1.48; 95% CI, 0.78–2.8), regardless of baseline ventilation status (Supplementary Table 3, http://links.lww.com/CCM/H194). The primary end point (alive and free from respiratory failure at day 60) did not reach statistical significance for patients treated with Aviptadil when controlling for baseline ventilation status as prespecified in the protocol, indicating that there was no difference between Aviptadil versus placebo.
TABLE 2.
Outcomes of All Patients at 28 and 60 d
| Treatment | Baseline Ventilation | Outcomes NIAID | Day 28, n (%) | Day 60, n (%) |
|---|---|---|---|---|
| Aviptadil | Ventilation (n = 55)a | Deceasedb | 25 (45.5) | 28 (50.9) |
| Alive but on respiratory supportc | 18 (32.7) | 7 (12.7) | ||
| Alive + free of respiratory supportd | 12 (21.8) | 20 (36.4) | ||
| HFNC (n = 76)e | Deceasedb | 16 (21.1) | 18 (23.7) | |
| Alive but on respiratory supportc | 9 (11.8) | 6 (7.9) | ||
| Alive + free of respiratory supportd | 51 (67.1) | 52 (68.4) | ||
| Total (n = 131) | Deceasedb | 41 (31.3) | 46 (35.1) | |
| Alive but on respiratory supportc | 27 (20.6) | 13 (9.9) | ||
| Alive + free of respiratory supportd | 63 (48.1) | 72 (55.0) | ||
| Placebo | Ventilation (n = 14)a | Deceasedb | 9 (64.3) | 11 (78.6) |
| Alive but on respiratory supportc | 3 (21.4) | 0 | ||
| Alive + free of respiratory supportd | 2 (14.3) | 3 (21.4) | ||
| HFNC (n = 51)e | Deceasedb | 9 (17.6) | 19 (37.3) | |
| Alive but on respiratory supportc | 16 (31.4) | 1 (2.0) | ||
| Alive + free of respiratory supportd | 26 (51.0) | 31 (60.8) | ||
| Total (n = 65) | Deceasedb | 18 (27.7) | 30 (46.2) | |
| Alive but on respiratory supportc | 19 (29.2) | 1 (1.5) | ||
| Alive + free of respiratory supportd | 28 (43.1) | 34 (52.3) |
HFNC = high-flow nasal cannula, NIAID = National Institute of Allergy and Infectious Diseases.
NIAID2.
NIAID 1.
NIAID 2/3/4.
NIAID 5/6/7/8.
NIAID3.
The use of remdesivir, P:F ratio, and BMI did not make statistically significant contributions to the regression and was dropped to conserve degrees of freedom. Using the Cox proportional hazards model with similar statistical significance at 28 and 60 days, primary and secondary end points were also examined by time series analysis.
Key Secondary End Point: Survival Through Day 60
A statistically significant increased odds of survival at day 60 was identified across all patients and hospitals (OR, 2.0; 95% CI, 1.05–3.87; p = 0.035) when controlling for baseline ventilation status as prespecified in the protocol. The increased odds of survival at day 60 on the drug was especially pronounced in the subgroup of those who scored NIAID 2 (MV), with a 10-fold increased odds of survival for those on the drug (OR, 10.4; 95% CI, 1.23–87.2; p = 0.031).
In addition, there was a statistically significant HR (HR, 0.59; 95% CI, 0.37–0.96; p = 0.032) through 60 days, controlling for baseline ventilation status (Supplementary Table 3, http://links.lww.com/CCM/H194).
Effect of Baseline Remdesivir Use
The effect on the primary end point could not be analyzed in regression equations because of treatment site imbalances in remdesivir use. Prior treatment with remdesivir was prespecified as a covariate and a significant Aviptadil effect (OR, 2.5; p = 0.019) when baseline remdesivir use was included as a covariate (Supplementary Table 3, http://links.lww.com/CCM/H194). Accordingly, a subgroup analysis of patients who developed respiratory failure despite treatment with remdesivir (n = 127) was performed (Supplementary Table 4, http://links.lww.com/CCM/H195). A significant advantage favoring Aviptadil was identified for the primary end point (OR, 3.1; p = 0.028) and day 60 Survival (OR, 4.15; p = 0.006). This finding should be considered hypothesis-generating.
Intermediate Clinical End Point: Respiratory Distress Ratio (Prespecified)
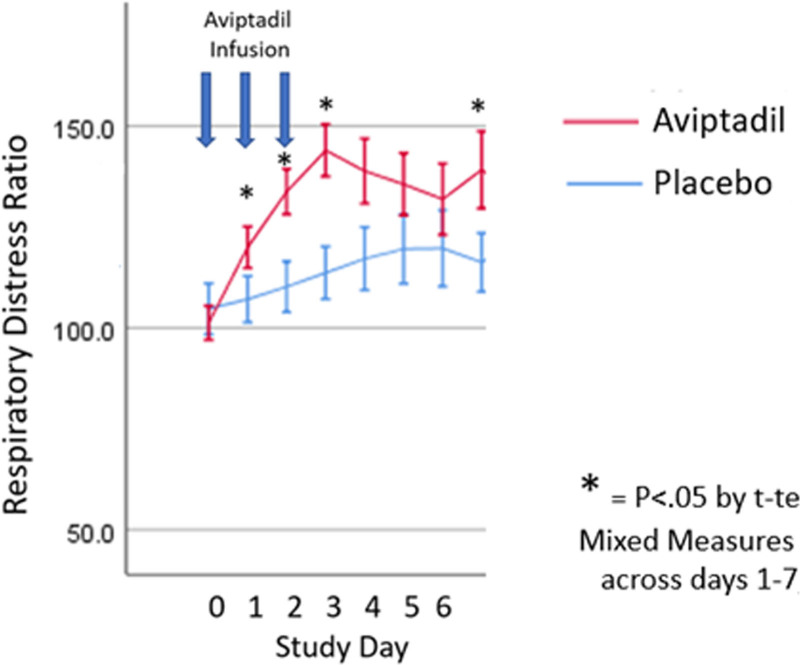
RDR was defined as the ratio of P:F. Although FDA guidance makes clear that end points for drug approval depend on measures of how patients “feel, function, and survive,” RDR may be an early indicator of the biological effect of Aviptadil on pulmonary function (8). Across all patients and hospitals, mean RDR was comparable at baseline with statistically significant advantages identified for Aviptadil, noted across the first 7 days following treatment with Aviptadil or placebo (Fig. 2). The difference is statistically significant by MMRM: F = 9.725; p = 0.02). Differences between Aviptadil and placebo were somewhat larger in the HFNC subgroup (day 2: 124 vs 93, day 3: 142 vs 105; p = 0.01) but not greater in tertiary versus community hospitals, suggesting a comparable biological effect of the drug in all settings. The higher mean RDR seen in Aviptadil versus placebo-treated participants was highly predictive of achieving the primary end point on MMRM (F = 16.0; p < 0.001).
Figure 2.
Respiratory distress ratio data results.
Biomarker End Point: Cytokine IL-6 Levels (Prespecified)
Serum levels of IL-6 and tumor necrosis factor α were targeted for collection at baseline and daily through day 7 in the first 144 patients. Analysis was performed on the first-morning laboratory sample and successfully collected in 134 through day 3 (92 Aviptadil/42 placebo), 123 participants on day 5 (83/40), and 111 participants through day 7 (75/36). Intraday changes in IL-6 were not significant.
Subjects treated with placebo demonstrated a five-fold greater rise in mean day 7 IL-6 level than those treated with Aviptadil (p < 0.05). In addition, MMRM regression demonstrated Aviptadil to be associated with preventing IL-6 rise across days 3–7 relative to placebo (F = 4.93; p = 0.024), with significant independent effects on days 3 (F = 9.78; p = 0.002) and 7 (F = 4.09; p = 0.046).
Effect of IL-6 Change on Primary End Point and Day 60 Survival (Exploratory)
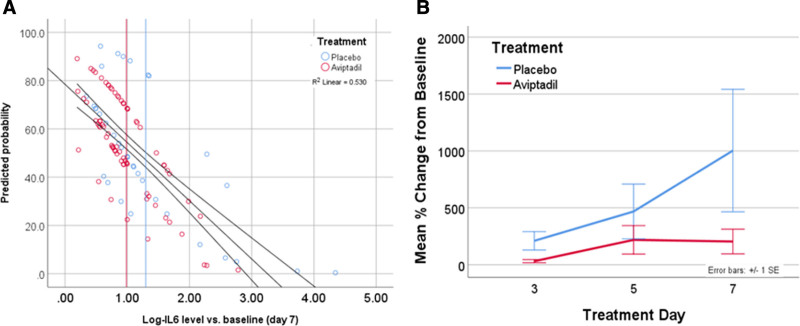
Among the subgroup of subjects for whom biomarker data were collected, the probability of achieving day 60 primary success was inversely related to the change in IL-6 level from baseline to day 7 (Fig. 3A). Day 7 IL-6 level strongly predicted achieving primary end point and survival at day 60 (–2 Log-Likelihood 136.3 vs 261.3, χ²=125, df = 1; p < 0.0001) and was collinear with treatment type (p = 0.95). Type of hospital was not significantly associated with this biological effect. Treatment with Aviptadil versus placebo was similarly predictive of outcome in a main-effects only model (p < 0.001) but was no longer significant once IL-6 was included in the model (p = 0.3).
Figure 3.
Percent change cytokine interleukin 6 (IL-6) and log-IL-6 level versus baseline. A, Predicted probability of primary outcome versus change in Log IL-6 level from baseline to day 7. Increase in IL-6 at day 7 predicted >50% of the variance in treatment outcome (R2 = 0.53). Treatment with Aviptadil is less likely to be associated with a day 7 increase in IL-6 and is associated with higher probability of primary end point (P < 0.001). B, Percent change from pretreatment in group cytokine IL-6 level. A significant difference is seen overall on mixed-model repeated measure between days 3 and 7 favoring Aviptadil (P = 0.024) with independent significance on days 3 (P = 0.002) and 7 (P = 0.06).
Post Hoc Analysis: Effect of Site of Care
When a covariate was included for care at tertiary-care hospitals versus community hospitals on a post hoc basis, a significant treatment site interaction was noted, with substantially higher mortality rates seen at community hospitals. With the inclusion of this site of care variable, statistical significance was seen for both the primary (p = 0.023) and secondary (p = 0.0009) end points, using the Wilks Comprehensive Global Hypothesis Test (Supplementary Table 5, http://links.lww.com/CCM/H196; and Supplementary Table 6, http://links.lww.com/CCM/H197). However, this difference must be considered hypothesis-generating.
Adverse Events
Treatment-emergent adverse event (TEAE) prevalence for each system organ class was ascertained (Supplementary Table 7, http://links.lww.com/CCM/H198). There were no significant differences between treatments overall or for any individual system organ class except for mild-to-moderate diarrhea (a known side effect of exposure to VIP), which occurred more frequently among Aviptadil-treated participants (32.8% vs 1.5%; p < 0.0001). Hypotension was common in both drug- and placebo-treated participants and managed with pressors per ICU protocol. There was comparable prevalence in the Aviptadil-treated group (26.0% vs 21.5%; p = 0.6), which was not statistically significant, and no treatment discontinuations were associated with hypotension. No unanticipated drug-related serious adverse events (SAEs), including mortality, were recorded.
DISCUSSION
In this multicenter, randomized, placebo-controlled trial among critically ill patients with COVID-19 and respiratory failure, we did not reach statistical significance (OR, 1.6; 95% CI, 0.86–3.11) on the primary end point of being alive and free of respiratory failure at 60 days. There was a two-fold, statistically significant increased odds of day 60 survival (the key secondary end point) (p = 0.035). In those most severely ill at baseline, the odds of mortality on placebo versus drug increased to 10-fold (p = 0.031), with the results confirmed in the subgroup on remdesivir at baseline; thus, Aviptadil efficacy did not depend upon remdesivir administration.
The difference in statistical significance between primary and secondary end points was a function of 10 subjects treated with Aviptadil who were alive but remained in rehabilitation centers with ongoing ventilator requirements at day 60. These patients received a tracheostomy and continued to require ventilatory support due to respiratory musculature loss rather than primary respiratory failure. However, per protocol, these patients were not classified as recovered from respiratory failure. The near-immediate improvement in RDR seen in the Aviptadil-treated patients, together with the substantial differences in the first week following treatment with Aviptadil vs placebo, suggests a biological effect in the first few days of treatment that is associated with long-term survival and recovery. This potential biological effect was seen across all patients and sites of care. In addition, Aviptadil treatment was associated with a reduction in the likelihood of increased cytokine IL-6 levels, often referred to as “Cytokine Storm” (22). This biomarker effect was highly predictive of survival and primary end point success (Fig. 3B). Despite the biological impact of Aviptadil in preventing cytokine elevation, many other hospital care components contribute to the ultimate survival and recovery of patients with COVID-19. Given the absence of drug-related SAEs and a suggestion (Fig. 2) that the short-term effect on RDR (P:F ratio) may narrow after cessation of infusion with Aviptadil, a longer duration of treatment should be considered in future trials.
The findings of this trial, at least among those patients treated at tertiary-care hospitals, are comparable with those reported in an open-label trial of highly comorbid patients with COVID-19 respiratory failure (SAS/STAT 9.4 User’s Guide, SAS Institute Inc.). Those treated with Aviptadil in addition to standard of care were more likely to be alive and free of respiratory failure (the primary prespecified end point) than those treated with standard of care alone (p = 0.02). The open-label trial similarly reported increased survival, reduction in IL-6, and strikingly similar differences in RDR between Aviptadil- and standard of care–treated groups.
Our findings regarding IL-6 contributions to COVID-related death are consistent with an increasing body of data demonstrating the correlation between IL-6 levels and outcome (18). Tocilizumab and other IL-6 receptor antagonists block the downstream effects of IL-6 elevation. However, monoclonal antibodies do not modify the inflammatory processes leading to elevations in IL-6 (23). In the current study, Aviptadil treatment appeared to prevent elevation of IL-6 levels early in the course of treatment, and this effect was associated with statistically significant increased survival.
No toxicity or lethal dose of Aviptadil has been observed, although VIP is well known to cause diarrhea and flushing. In addition, the safety profile of Aviptadil has been well-demonstrated in previous trials (13). Although hypotension can be seen with administration of Aviptadil, this TEAE was seen in 26% of Aviptadil-treated participants versus 21.5% of placebo-treated participants and did not cause cessation of therapy or any SAEs.
Use of remdesivir at baseline was not a statistically significant covariate for either primary or secondary end point. However, a statistically significant effect of Aviptadil was seen on both primary and secondary end points in the subgroup (n = 127) of patients who were enrolled having received remdesivir.
The study was underpowered for demonstrating the primary end point because of unexpected high mortality seen in several regional study sites during the December 2020 Delta variant surge in the pandemic with resulting ICU overcapacity and losses of medical staff to the effects of the public health emergency. In each category of baseline ventilation status (HFNC/NIV vs MV), numerical advantages are seen in both survival and recovery from respiratory failure in aviptadil-treated versus placebo-treated subjects. However, the study power does not allow for hypothesis tests within the baseline categories.
Another limitation was the randomization imbalance in baseline ventilation status as determined by the NIAID score. The interaction seen between the site of care and treatment outcome may be a function of the more advanced resources available in tertiary-care medical centers or may be an artifact of the November 2020 to February 2021 surge in COVID prevalence that affected the community hospitals included in this study. Before enrolling patients in the multicenter trial, each community hospital participated in an FDA-sanctioned open-label Expanded Access Protocol (NCT 04453839) and demonstrated competence in managing the investigational therapy and good clinical practice results. However, the COVID surge challenged these sites with 200% higher ICU demand resulting in makeshift patient beds erected in parking lots and other non-traditional sites of care, a scenario that has demonstrated that as ICU capacity reaches 100%, mortality in treatment of critical Covid-19 doubles (24).
CONCLUSIONS
We did not find any statistically significant difference between Aviptadil versus placebo for the primary end point (recovery from respiratory failure at 60 d). Aviptadil treatment is associated with clinically meaningful improved survival from respiratory failure at day 60 in COVID-19 across all care sites. Failure to reach significance on the primary end point was associated with Aviptadil-treated patients who were alive but still undergoing respiratory rehabilitation at day 60, suggesting that a longer observation period is required for studies of the most critically ill patients with COVID-19. We believe that the findings of this trial concerning ventilated patients are generalizable to all sites. The evidence of improved lung oxygenation and reduced cytokine-storm in the first week of therapy suggests that the biological effect of Aviptadil was seen across all baseline severities and hospital types.
Supplementary Material
Footnotes
*See also p. 1662.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by NRx Pharmaceuticals, Wilmington, DE. The sponsor participated in the design and conduct of the study, data collection, site monitoring, and data interpretation. Data analysis and interpretation were performed by David A Schoenfeld, PhD, in consultation with Lavin Consulting LLC, Framingham, MA. Clinical trial funding was provided by NRx and Relief Therapeutics, AG (Geneva, Switzerland).
Drs. Youssef, Lee, Lenhardt, Park, Fernandez, and Jayaweera are employed by institutions that received research support funding from NRx Pharmaceuticals. Drs. Morganroth and Lavin are consultants to NRx Pharmaceuticals. Drs. Youssef, Lee, Lenhardt, Morganroth, and Javitt disclosed the off-label product use of Vasoactive intestinal Peptide Aviptadil. Dr. Lavin’s institution received funding from NRx Pharmaceuticals. Drs. Lavin, Schoenfeld, and Javitt received funding from NRx Pharmaceuticals. Dr. Schoenfeld received funding from Immunity Pharma; he disclosed that he has consulted with external entities unrelated to this work. Drs. Schoenfeld and Javitt disclosed work for hire. Drs. Lenhardt’s, Morganroth’s, and Jayaweera’s institutions received funding from NeuroRX. Dr. Lenhardt received funding from Merck, CSL Behring, and Trevena. Dr. Fernandez received funding from NRx Consulting. Dr. Javitt disclosed that he is an employee and shareholder of NRx Pharmaceuticals and that his spouse and children have beneficial ownership in the stock of NRx Pharmaceuticals. Dr. Jayaweera’s institution received funding from Gilead Pharmaceuticals, VIIV, and Janssen; he received support for article research from the National Institutes of Health.
REFERENCES
- 1.Mason R: Pathogenesis of COVID-19 from a cell biologic perspective. Eur Respir J 2020; 55:2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Said SI, Mutt V: Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970; 225:863–864 [DOI] [PubMed] [Google Scholar]
- 3.Said SI: Vasoactive intestinal peptide in the lung. Ann N Y Acad Sci. 1988; 527:450–464 [DOI] [PubMed] [Google Scholar]
- 4.Said SI: VIP as a modulator of lung inflammation and airway constriction. Am Rev Respir Dis. 1991; 143:S22–S24 [DOI] [PubMed] [Google Scholar]
- 5.Said SI, Dickman KG: Pathways of inflammation and cell death in the lung: Modulation by vasoactive intestinal peptide. Regul Pept. 2000; 93:21–29 [DOI] [PubMed] [Google Scholar]
- 6.Javitt J, Youssef JG: VIP: A COVID-19 Therapeutic That Blocks Coronavirus Replication. 2020. Available at SRN: https://ssrn.com/abstract=3670129. August 23, 2022
- 7.Li L, Luo ZQ, Zhou FW, et al. : Effect of vasoactive intestinal peptide on pulmonary surfactants phospholipid synthesis in lung explants. Acta Pharmacol Sin. 2004; 25:1652–1658 [PubMed] [Google Scholar]
- 8.Quartuccio L, Sonaglia A, Pecori D, et al. : Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: A possible indication for deeper targeting of IL-6. J Med Virol. 2020; 92:2852–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, She H, Yue SJ, et al. : Role of c-fos gene in vasoactive intestinal peptide promoted synthesis of pulmonary surfactant phospholipids. Regul Pept. 2007; 140:117–124 [DOI] [PubMed] [Google Scholar]
- 10.Li L, Hua S, Yue S, et al. Vasoactive intestinal polypeptide induces surfactant protein A expression in ATII cells through activation of PKC/c-Fos pathway. Peptides. 2010; 31:2016–2051 [DOI] [PubMed] [Google Scholar]
- 11.Temerozo JR, Sacramenta Q, Fintelman-Rodriques N, et al. : The neuropeptides VIP and PACAP inhibit SARS-CoV-2 replication in monocytes and lung epithelial cells, decrease the production of proinflammatory cytokines, and VIP levels are associated with survival in severe Covid-19 participants. bioRxiv 2020.07.25.220806 [Google Scholar]
- 12.Jihad GY, Sami S, George Y, et al. : Treatment of acute respiratory distress syndrome with vasoactive intestinal peptide. J Infect Dis Treat. 2021; 7 [Google Scholar]
- 13.Prasse A, Zissel G, Lützen N, et al. : Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010; 182:540–548 [DOI] [PubMed] [Google Scholar]
- 14.Petkov V, Mosgoeller W, Ziesche R, et al. : Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2003; 111:1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leuchte HH, Baezner C, Baumgartner RA, et al. : Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008; 32:1289–1294 [DOI] [PubMed] [Google Scholar]
- 16.Frye BC, Meiss F, von Bubnoff D, et al. : Vasoactive intestinal peptide in checkpoint inhibitor-induced pneumonitis. N Engl J Med. 2020; 382:2573–2574 [DOI] [PubMed] [Google Scholar]
- 17.Beshay S, Youssef JG, Zahiruddin F, et al. Rapid clinical recovery from critical COVID-19 pneumonia with vasoactive intestinal peptide treatment. J Heart Lung Transplant. 2021; 40(4 Supp): S501 [Google Scholar]
- 18.Youssef JG, Javitt JC, Lavin P, et al. VIP in the treatment of critical COVID-19 with respiratory failure in patients with severe comorbidity: A prospective externally controlled. J Infect Dis Treat. 2021; 7 [Google Scholar]
- 19.U.S. Food and Drug Administration: COVID-19: Developing Drugs and Biological Products for Treatment or Prevention Guidance for Industry. 2021. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention. Accessed August 23, 2022
- 20.Zwarenstein M, Treweek S, Gagnier JJ, et al. ; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group: Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ. 2008; 337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui L, Hung HM, Wang SJ: Modification of sample size in group sequential clinical trials. Biometrics. 1999; 55:853–857 [DOI] [PubMed] [Google Scholar]
- 22.Fajgenbaum DC, June CH: Cytokine storm. N Engl J Med. 2020; 383:2255–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorham J, Moreau A, Corazza F, et al. : Interleukine-6 in critically ill COVID-19 patients: A retrospective analysis. PLoS One. 2020; 15:e0244628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of intensive care unit patient load and demand with mortality rates in US department of veterans affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.