Abstract
Background
Among antiretroviral therapy (ART)–treated people with human immunodeficiency virus (PWH), persistent systemic immune activation contributes to atherogenesis atherosclerotic, cardiovascular disease (CVD) events, and mortality. Factors associated with key immune activation indices have not previously been characterized among a global primary CVD prevention cohort of PWH.
Methods
Leveraging baseline Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) data, we evaluated factors associated with soluble CD14 (sCD14) and oxidized low-density lipoprotein (oxLDL).
Results
The primary analysis cohort included 4907 participants from 5 global-burden-of-disease regions (38% female, 48% Black, median age 50 years). In fully adjusted models for sCD14, female sex and White race (among those in high-income regions) were associated with higher sCD14 levels, while higher body mass index (BMI) and current use of nucleoside reverse transcriptase inhibitor + integrase strand transfer inhibitor ART were associated with lower sCD14 levels. In fully adjusted models for oxLDL, male sex, residence in high-income regions, White race (among those in high-income regions), and higher BMI were associated with higher oxLDL levels. In a subanalysis cohort of 1396 women with HIV, increased reproductive age was associated with higher sCD14 levels but not with higher oxLDL levels.
Conclusions
Factors associated with sCD14 and oxLDL, 2 key indices of immune-mediated CVD risk, differ. Future studies will elucidate ways in which medications (eg, statins) and behavioral modifications influence sCD14 and oxLDL and the extent to which dampening of these markers mediates CVD-protective effects.
Clinical Trials Registration
Keywords: HIV, cardiovascular disease risk, immune activation markers, women, reproductive aging
Among antiretroviral therapy–treated people with human immunodeficiency virus globally, factors associated with sCD14 and oxLDL, two key indices of immune-mediated cardiovascular disease (CVD) risk, differ. Future studies will elucidate ways in which medications/lifestyle influence these markers and the extent to which dampening these markers mediates CVD risk reduction.
Among antiretroviral therapy (ART)–treated people with human immunodeficiency virus (PWH), persistent systemic immune activation may contribute to atherogenesis, atherosclerotic cardiovascular disease (ASCVD) events, and mortality [1–3]. Although immune indices across pathways have been etiologically implicated, 2 indices in particular, rise to prominence. The first is soluble CD14 (sCD14), a nonspecific marker of systemic monocyte activation that is released into the circulatory system when myeloid cells detect and react to pathogen-associated ligands [4]. General population studies have shown that sCD14 levels predict ASCVD events [5]. Clinical studies among PWH have revealed that sCD14 levels relate to the degree of arterial inflammation/macrophage infiltration [6] and predict subclinical atherosclerosis progression [7], myocardial infarction [8], and mortality [9]. The second is oxidized low-density lipoprotein (oxLDL), an oxidized form of LDL cholesterol that may induce endothelial and smooth muscle cell dysfunction, activate innate and adaptive immune responses, and/or be engulfed by macrophages-turned foam cells, forming the fatty core of incipient atherosclerotic plaques [10]. A prospective population-based study showed an association between increased oxLDL levels and heightened risk of incident coronary heart disease [11]. Meanwhile, a small study among PWH illustrated that levels of oxLDL were associated with a greater volume of subclinical coronary atherosclerotic plaque, while reductions in oxLDL with statins were associated with concomitant reductions in plaque volume, independent of traditional CVD risk factors [12].
We set out to interrogate factors associated with sCD14 and oxLDL among a contemporary, global cohort of ART-treated PWH. The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) is the largest primary CVD prevention trial in the field. REPRIEVE will determine among ART-treated PWH whether therapy with pitavastatin calcium reduces major adverse cardiovascular events through lipid-lowering effects, antiinflammatory effects, or both [13, 14]. As part of REPRIEVE, 7770 PLWH with low to moderate traditional CVD risk were recruited from 2015 to 2019 through more than 100 clinical research sites spanning 5 continents.
Our primary study hypothesis was that among the whole group, assigned sex at birth (henceforth referred to as sex) and enrollment region (inferred to be region of residence) would influence levels of sCD14 and oxLDL. The former hypothesis was based on established sexual dimorphism in human immune responses [15–17], while the latter stemmed from documented regional differences in parameters relevant to systemic immune activation (eg, CVD risk factors [18], prevalence of non-AIDS infectious diseases [19], and types of ART prescribed to treat HIV [20]). Based on our previous findings from a small physiology study of US females living with HIV [21], we further hypothesized that ovarian aging may be associated with higher levels of either or both of these markers among cisgender female participants.
METHODS
Study Objectives
Our study objectives were as follows: to characterize levels of sCD14 and oxLDL by sex, age, and geographic region; to investigate participant characteristics related to sCD14 and oxLDL; and to determine relationships between reproductive (vs chronologic) age and levels of sCD14 and oxLDL among cisgender women with HIV (henceforth referred to as WWH).
Study Participants
Our primary analysis centered on 4907 PWH enrolled in REPRIEVE following integration of the REPRIEVE Women’s Objectives (protocol version 3, distributed 2 February 2016). A subanalysis focused on a subset of 1396 WWH with data sufficient for reproductive aging classification [22] (Supplementary Figure 1). Each REPRIEVE research site obtained institutional review board/ethics committee approval, as well as any other applicable regulatory entity approvals. Participants were provided with study information, including a discussion of risks and benefits, and were asked to sign the approved declaration of informed consent [13, 14].
Data Elements Assessed in REPRIEVE Trial Participants
Data on demographic parameters, traditional cardiometabolic risk parameters, and HIV-specific parameters were collected, as previously described [13]. Region of enrollment/residence was grouped according to the World Health Organization (WHO) global burden of disease (GBD) super region scheme [23]. Sex-specific thresholds outlined in WHO guidelines were applied to classify waist circumference (WC) as high or normal (≤102 cm for men and ≤88 cm for women) [24].
Quantification of sCD14 and oxLDL
Levels of sCD14 and oxLDL were evaluated with EDTA plasma specimens obtained at the study entry visit prior to initiation of study treatment. Plasma samples were stored and frozen at –80°C and subsequently thawed before testing in duplicate of 40 samples per batch (Lewis Katz School of Medicine at Temple University). Levels of sCD14 and oxLDL were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN; Mercodia Inc, Uppsala, Sweden, respectively). All ELISAs were performed with appropriate quality control samples.
Quantification of Anti-Müllerian Hormone Levels
Anti-Müllerian hormone (AMH) levels were evaluated via ELISA (picoAMH assay; Ansh Labs) from serum specimens obtained at study entry, as previously described [22].
Reproductive Aging Classification Scheme
WWH were categorized along an ordinal 3-group reproductive aging spectrum defined by time since last menstrual period and by AMH level as follows: premenopausal (group 1, menses within 12 months and AMH ≥20 pg/mL), premenopausal with reduced ovarian reserve (group 2, menses within 12 months and AMH <20 pg/mL), and postmenopausal (group 3, no menses within 12 months and AMH <20 pg/mL), as previously described [22].
Statistical Methods
Baseline characteristics were summarized for the primary analysis cohort (total and sex-stratified) and subanalysis cohort (total and stratified by reproductive age group). Spearman correlation was used to assess the relationship between sCD14 and oxLDL. Single and multivariable linear regression analyses were performed to evaluate associations between sCD14 and oxLDL (dependent variables) and select baseline characteristics. Covariates of interest were sex, age, and region for objective 1 and race, cigarette smoking status and substance use (current vs former or never), hypertension, WC, body mass index (BMI), nadir CD4 count, CD4 count, total duration of ART use, and ART regimen class (controlling for sex, age, and region) for objective 2. For objective 3 in the subanalysis cohort, the same covariates were used as for objective 2, substituting reproductive age group for chronologic age group. Given skewed biomarker distributions, modeling was performed on log-transformed values. Results were then backtransformed to geometric mean ratios (relative differences in geometric mean ratios [GMR]) with 95% confidence intervals. Type 3 Wald P values are provided for overall covariate effects.
For objectives 1 and 2, exploratory subgroup analyses by sex were performed to determine whether the overall effects were apparent across females and males. Interaction tests were included in the overall model if subgroup differences were apparent to formally evaluate for these sex differences.
Sensitivity analyses restricted to participants with undetectable HIV-1 RNA were performed; however, as these yielded analogous findings, only findings in the full cohort are shown. Analyses for oxLDL were repeated controlling for LDL cholesterol levels.
Inference was based on a nominal 0.05 significance level with no formal adjustment for multiple testing. Given high power to detect small differences, inference was focused on factors associated with effect sizes of 5% or greater (ie, GMR ≥1.05 or ≤0.95). All statistical analyses were performed using SAS (for the Linux platform) version 9.4.
RESULTS
Baseline Characteristics
Baseline characteristics of the primary analysis cohort (total and sex-stratified) and of the subanalysis cohort (total and stratified by reproductive age group) are presented in Table 1. The primary analysis cohort did not differ significantly from the main REPRIEVE cohort [14]. In the primary analysis cohort, median sCD14 levels were 1743 ng/mL (1468–2064) and median oxLDL levels were 53 U/L (42–68).
Table 1.
Participant Baseline Characteristics
| Cohort 1 | Cohort 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristica | Total (N = 4907) | Male (N = 3034) | Female (N = 1873) | Total (N = 1396) | LMP ≤12 Months With Measurable AMH (N = 469) | LMP ≤12 Months With Undetectable AMH (N = 215) | LMP >12 Months With Undetectable AMH (N = 712) | |
| Demographics and behavioral | ||||||||
| Age, years | Median (Q1, Q3) | 50 (45, 54) | 50 (45, 54) | 49 (44, 55) | 49 (45, 55) | 44 (41, 46) | 48 (45, 51) | 55 (51, 59) |
| Enrollment regionb | High Income | 2350 (48%) | 1802 (59%) | 548 (29%) | 343 (25%) | 96 (20%) | 55 (26%) | 192 (27%) |
| Latin America and Caribbean | 944 (19%) | 602 (20%) | 342 (18%) | 281 (20%) | 77 (16%) | 44 (20%) | 160 (22%) | |
| Southeast/East Asia | 412 (8%) | 158 (5%) | 254 (14%) | 206 (15%) | 104 (22%) | 35 (16%) | 67 (9%) | |
| South Asia | 242 (5%) | 179 (6%) | 63 (3%) | 55 (4%) | 30 (6%) | 10 (5%) | 15 (2%) | |
| Sub-Saharan Africa | 959 (20%) | 293 (10%) | 666 (36%) | 511 (37%) | 162 (35%) | 71 (33%) | 278 (39%) | |
| Racec | Black or African American | 2350 (48%) | 1144 (38%) | 1206 (64%) | 894 (64%) | 259 (55%) | 132 (61%) | 503 (71%) |
| White | 1533 (31%) | 1287 (42%) | 246 (13%) | 164 (12%) | 57 (12%) | 19 (9%) | 88 (12%) | |
| Asian | 687 (14%) | 368 (12%) | 319 (17%) | 262 (19%) | 135 (29%) | 45 (21%) | 82 (12%) | |
| Other | 337 (7%) | 235 (8%) | 102 (5%) | 76 (5%) | 18 (4%) | 19 (9%) | 39 (5%) | |
| Smoking status | Current | 1093 (22%) | 787 (26%) | 306 (16%) | 190 (14%) | 45 (10%) | 27 (13%) | 118 (17%) |
| Former | 1145 (23%) | 877 (29%) | 268 (14%) | 198 (14%) | 55 (12%) | 31 (14%) | 112 (16%) | |
| Never | 2668 (54%) | 1370 (45%) | 1298 (69%) | 1008 (72%) | 369 (79%) | 157 (73%) | 482 (68%) | |
| Substance used | Current | 83 (2%) | 67 (2%) | 16 (1%) | 11 (1%) | 3 (1%) | 2 (1%) | 6 (1%) |
| Former | 1343 (27%) | 1056 (35%) | 287 (15%) | 182 (13%) | 34 (7%) | 32 (15%) | 116 (16%) | |
| Never | 3479 (71%) | 1910 (63%) | 1569 (84%) | 1203 (86%) | 432 (92%) | 181 (84%) | 590 (83%) | |
| Cardiovascular and metabolic | ||||||||
| Hypertension | 1716 (35%) | 984 (32%) | 732 (39%) | 556 (40%) | 131 (28%) | 84 (39%) | 341 (48%) | |
| Body mass index, kg/m² | Median (Q1, Q3) | 26.0 (23.0, 29.8) | 25.4 (22.7, 28.6) | 27.3 (23.5, 32.2) | 27.0 (23.4, 31.4) | 26.4 (22.6, 31.1) | 28.1 (24.4, 32.6) | 27.2 (23.7, 31.4) |
| <18.5 | 174 (4%) | 111 (4%) | 63 (3%) | 49 (4%) | 19 (4%) | 8 (4%) | 22 (3%) | |
| 18.5–24.9 | 1924 (39%) | 1319 (43%) | 605 (32%) | 469 (34%) | 172 (37%) | 57 (27%) | 240 (34%) | |
| 25–29.9 | 1649 (34%) | 1085 (36%) | 564 (30%) | 435 (31%) | 142 (30%) | 70 (33%) | 223 (31%) | |
| 30+ | 1159 (24%) | 519 (17%) | 640 (34%) | 443 (32%) | 136 (29%) | 80 (37%) | 227 (32%) | |
| Waist circumference,e cm | Normal | 3080 (63%) | 2407 (80%) | 673 (36%) | 516 (37%) | 211 (45%) | 75 (35%) | 230 (33%) |
| High | 1773 (37%) | 593 (20%) | 1180 (64%) | 871 (63%) | 256 (55%) | 138 (65%) | 477 (67%) | |
| Low-density lipoprotein cholesterol, mg/dL | Median (Q1, Q3) | 107 (87, 129) | 106 (86, 127) | 109 (89, 131) | 109 (90, 131) | 102 (86, 123) | 108 (89, 132) | 114 (94, 134) |
| Estimated glomerular filtration rate by chronic kidney disease -EPI, mL/min per 1.73 mm² | Median (Q1, Q3) | 98 (82, 111) | 95 (80, 107) | 104 (88, 118) | 104 (89, 118) | 109 (96, 124) | 105 (88, 118) | 100 (85, 113) |
| HGB, g/dL | Median (Q1, Q3) | 14.0 (13.0, 15.1) | 14.7 (13.9, 15.6) | 12.9 (12.0, 13.7) | 12.9 (12.0, 13.7) | 12.6 (11.3,13.5) | 12.8 (11.9,13.6) | 13.1 (12.3,13.8) |
| HIV disease characteristics | ||||||||
| Time since HIV diagnosis, years | <5 | 493 (10%) | 320 (11%) | 173 (9%) | 131 (9%) | 44 (9%) | 22 (10%) | 65 (9%) |
| 5–10 | 1369 (28%) | 871 (29%) | 498 (27%) | 367 (26%) | 129 (28%) | 57 (27%) | 181 (25%) | |
| >10 | 3044 (62%) | 1843 (61%) | 1201 (64%) | 897 (64%) | 296 (63%) | 136 (63%) | 465 (65%) | |
| Nadir CD4 count, cells/mm³ | <200 | 2372 (48%) | 1455 (48%) | 917 (49%) | 692 (50%) | 207 (44%) | 116 (54%) | 369 (52%) |
| 200–349 | 1283 (26%) | 808 (27%) | 475 (25%) | 356 (26%) | 128 (27%) | 53 (25%) | 175 (25%) | |
| 350–499 | 605 (12%) | 388 (13%) | 217 (12%) | 163 (12%) | 68 (14%) | 18 (8%) | 77 (11%) | |
| 500+ | 506 (10%) | 289 (10%) | 217 (12%) | 153 (11%) | 57 (12%) | 22 (10%) | 74 (10%) | |
| Unknown | 141 (3%) | 94 (3%) | 47 (3%) | 32 (2%) | 9 (2%) | 6 (3%) | 17 (2%) | |
| History of AIDS-defining event | 1187 (24%) | 735 (24%) | 452 (24%) | 361 (26%) | 108 (23%) | 58 (27%) | 195 (27%) | |
| Total ART use, years | <5 | 1093 (22%) | 652 (21%) | 441 (24%) | 328 (23%) | 126 (27%) | 56 (26%) | 146 (21%) |
| 5–10 | 1444 (29%) | 921 (30%) | 523 (28%) | 389 (28%) | 138 (29%) | 59 (27%) | 192 (27%) | |
| 10+ | 2370 (48%) | 1461 (48%) | 909 (49%) | 679 (49%) | 205 (44%) | 100 (47%) | 374 (53%) | |
| ART regimen class | NRTI + INSTI | 1225 (25%) | 923 (30%) | 302 (16%) | 206 (15%) | 51 (11%) | 32 (15%) | 123 (17%) |
| NRTI + NNRTI | 2393 (49%) | 1283 (42%) | 1110 (59%) | 857 (61%) | 307 (65%) | 126 (59%) | 424 (60%) | |
| NRTI + Protease inhibitor | 891 (18%) | 537 (18%) | 354 (19%) | 269 (19%) | 94 (20%) | 45 (21%) | 130 (18%) | |
| NRTI-sparing | 101 (2%) | 67 (2%) | 34 (2%) | 25 (2%) | 7 (1%) | 4 (2%) | 14 (2%) | |
| Other NRTI-containing | 296 (6%) | 223 (7%) | 73 (4%) | 39 (3%) | 10 (2%) | 8 (4%) | 21 (3%) | |
| CD4 count,f cells/mm3 | Median (Q1, Q3) | 632 (464, 840) | 606 (433, 803) | 688 (499, 910) | 688 (497, 910) | 650 (496, 875) | 688 (483, 893) | 707 (511, 928) |
| 50–199 | 128 (3%) | 94 (3%) | 34 (2%) | 27 (2%) | 10 (2%) | 6 (3%) | 11 (2%) | |
| 200–349 | 486 (10%) | 346 (11%) | 140 (7%) | 108 (8%) | 40 (9%) | 20 (9%) | 48 (7%) | |
| 350–499 | 875 (18%) | 578 (19%) | 297 (16%) | 219 (16%) | 71 (15%) | 34 (16%) | 114 (16%) | |
| 500+ | 3418 (70%) | 2016 (66%) | 1402 (75%) | 1042 (75%) | 348 (74%) | 155 (72%) | 539 (76%) | |
| HIV-1 RNA, copies/mL | <LLQ | 3269 (87%) | 2155 (87%) | 1114 (88%) | 815 (88%) | 275 (88%) | 133 (89%) | 407 (88%) |
| LLQ < 400 | 394 (11%) | 276 (11%) | 118 (9%) | 74 (8%) | 25 (8%) | 12 (8%) | 37 (8%) | |
| 400+ | 80 (2%) | 40 (2%) | 40 (3%) | 34 (4%) | 11 (4%) | 4 (3%) | 19 (4%) | |
| Other comorbid conditions | ||||||||
| Chronic active hepatitis B virus | 127 (3%) | 97 (3%) | 30 (2%) | 21 (2%) | 8 (2%) | 4 (2%) | 9 (1%) | |
| Chronic active hepatitis C virus | 97 (2%) | 72 (2%) | 25 (1%) | 17 (1%) | 4 (1%) | 5 (2%) | 8 (1%) | |
| Inflammatory markers | ||||||||
| Soluble CD14, ng/mL | Median (Q1, Q3) | 1743 (1468, 2064) | 1690 (1432, 1998) | 1829 (1546, 2177) | 1835 (1555, 2194) | 1768 (1497, 2142) | 1821 (1507, 2209) | 1886 (1622, 2226) |
| Oxidized low-density lipoprotein cholesterol, U/L | Median (Q1,Q3) | 53 (42, 68) | 54 (43, 70) | 52 (41, 65) | 52 (41, 64) | 51 (40, 63) | 52 (40, 67) | 52 (42, 64) |
Abbreviations: AMH, anti-Müllerian hormone; ART, antiretroviral therapy; EPI, epidemiology collaboration; HGB, hemoglobin; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LLQ, lower limit of quantitation; LMP, last menstrual period; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor.
Frequency (%) for categorical measures, and median with lower and upper quartiles (Q1, Q3) for continuous measures. All statistics are calculated for participants with data collected. Missing data include smoking status (n = 1), substance use (n = 2), body mass index (n = 1), waist circumference (n = 54), low-density lipoprotein cholesterol (n = 42), estimated glomerular filtration rate by chronic kidney disease-EPI (n = 1), HGB (n = 18), time since HIV diagnosis (n = 1), HIV-1 RNA (n = 1164), sCD14 (n = 2), and oxidized low-density lipoprotein (n = 1).
Classified according to global burden of disease regions.
“Other” race includes participants self-identifying as native or indigenous to the enrollment region, more than 1 race (with no single race noted as predominant), or of unknown race.
Substance use includes use of cocaine, methamphetamine, and intravenous drugs.
Normal waist circumference is defined as ≤102 cm for men and ≤88 cm for women.
CD4 count obtained at entry.
Immune Biomarker Distributions
Distributions of sCD14 and oxLDL by key covariates are displayed in Supplementary Figure 2. Levels of sCD14 did not correlate with levels of oxLDL (r = 0.03).
Characterization of Levels of Systemic Immune Biomarkers by Sex, Age, and Region
sCD14
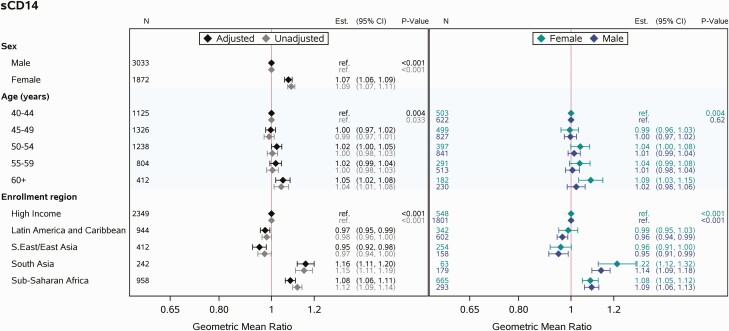
Unadjusted analyses suggested higher values of sCD14 among females, those in South Asia and sub-Saharan Africa, and older age groups. These trends all remained in the multivariable modeling. In both the unadjusted and adjusted modeling, higher age was associated with higher values of sCD14, but this effect was modest. Modeling stratified by sex suggested that the trend of higher values of sCD14 among older age groups was driven by females. However, after adjustment for region, the interaction between age and sex was not apparent (P = .27, data not shown; Figure 1).
Figure 1.
Multivariable linear regression analyses relating covariates of interest (sex, age, enrollment region) to sCD14. Abbreviations: CI, confidence interval; sCD14, soluble CD14.
oxLDL
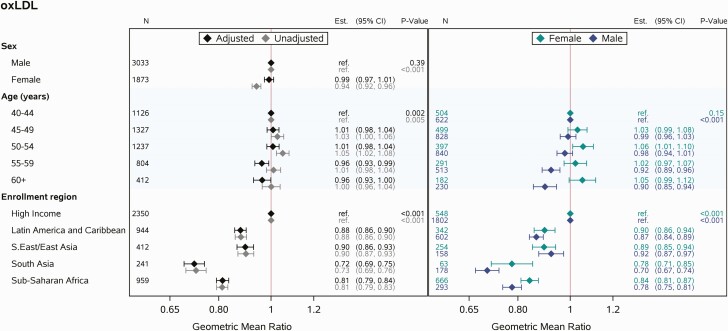
Unadjusted analyses suggested higher values of oxLDL among males and those residing in high-income regions; the lowest levels were apparent among those residing in South Asia and sub-Saharan Africa. After including sex, age, and region in the multivariable model, the trend of higher values of oxLDL among males was no longer apparent, while analogous trends across regions remained. Moreover, after adjustment for sex and region, a trend toward lower values of oxLDL among those in older age groups was observed. Multivariable modeling by sex suggested different effects of higher age on oxLDL among females vs males. Among females, oxLDL was on average higher among older age groups, while among males, oxLDL was on average lower among older age groups (interaction between age and sex adjusting for region, P = .0004; Figure 2).
Figure 2.
Multivariable linear regression analyses relating covariates of interest (sex, age, enrollment region) to oxLDL. Abbreviations: CI, confidence interval; oxLDL, oxidized low-density lipoprotein.
Multivariable Modeling for Levels of Systemic Immune Activation
sCD14
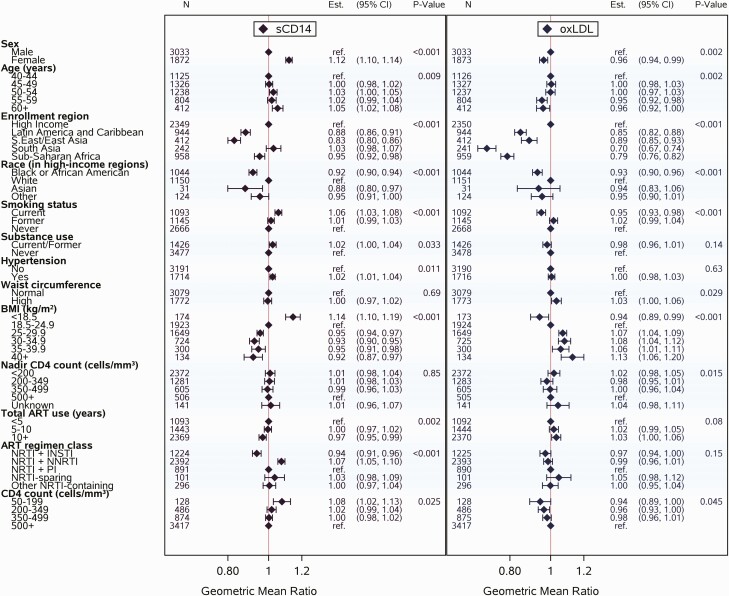
In the fully adjusted model for sCD14, similar effects of sex and age were apparent. Shifts in effect sizes were observed for region, and these shifts were noted to occur after adjustment for current ART regimen class. Other associated parameters included race (in high-income regions), cigarette smoking, BMI, entry CD4 count, and ART regimen class (Figure 3). Current cigarette smoking and entry CD4 count <200 cells/mm3 were associated with higher sCD14 levels, as was current use of nucleoside reverse transcriptase inhibitor plus nonnucleoside reverse transcriptase inhibitor (NRTI + NNRTI) ART. Meanwhile, sCD14 was lower among Black and Asian PWH relative to White PWH (in high-income regions), and levels tended to be lower among those with higher BMI and those currently using NRTI plus integrase strand transfer inhibitor (INSTI) ART. Of note, differences by ART regimen class remained consistent in sex-stratified analyses and in analyses stratifying PWH by BMI (data not shown). Statistically significant trends were seen for hypertension and total duration of ART use, though the effect sizes were small.
Figure 3.
Multivariable linear regression analyses relating covariates of interest (sex, age, enrollment region, race in high-income regions, cigarette smoking status, substance use, hypertension, waist circumference, BMI, nadir CD4, total ART use, ART regimen class, and entry CD4 count) to sCD14 and to oxLDL, respectively. Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; oxLDL, oxidized low-density lipoprotein; PI, protease inhibitor; sCD14, soluble CD14.
oxLDL
Similar effects of age and region were also apparent in the fully adjusted model for oxLDL. While statistically significant in the fully adjusted model, the estimated effect of sex was small. Other associated parameters included race (in high-income regions), cigarette smoking, and BMI (Figure 3). Higher BMI was associated with higher levels of oxLDL. Meanwhile, oxLDL was lower among Black and Asian PWH relative to White PWH (in high-income regions) and among PWH who were current cigarette smokers (vs former or never-smoking). Statistically significant trends were also observed for WC, nadir CD4 count, and entry CD4 count, though the effect sizes were small.
Overall, findings for both sCD14 and oxLDL remained consistent in sex-stratified analyses, and findings for oxLDL remained consistent when adjusting for levels of LDL cholesterol (data not shown).
Effects of Reproductive Age vs Chronologic Age on Levels of Systemic Immune Activation Among WWH
Fully adjusted models for sCD14 and oxLDL applied to a prespecified subset of WWH (see Methods section) yielded results consistent with the results provided above (Supplementary Figures 3 and 4). Among this group, when reproductive age category was substituted in place of chronologic age, being in reproductive age group 3 (no menses within 12 months and AMH <20 pg/mL) was associated with higher sCD14, but there was no effect of being in reproductive age group 2 (Supplementary Figure 3). In contrast, when substituted for chronologic age, reproductive age category was not associated with oxLDL (Supplementary Figure 4). In an effort to determine whether reproductive age might be associated with levels of sCD14 independent of the influence of chronologic age among WWH, distributions of sCD14 were presented by chronologic age strata across contiguous reproductive age groups. In comparable chronologic age groups, consistently higher levels of sCD14 were apparent among WWH in reproductive age group 3 vs groups 1 and 2 (Supplementary Figure 5).
DISCUSSION
In this analysis of factors associated with systemic immune activation indices in the largest global primary CVD prevention cohort of ART-treated PWH, our primary findings were as follows. First, levels of sCD14 and oxLDL were not correlated, in contrast to findings published in smaller cohorts [25]. Second, when levels of sCD14 and oxLDL were characterized by sex, age, and region, higher levels of sCD14 were associated with female sex and residence in South Asia or sub-Saharan Africa, while higher levels of oxLDL were associated with residence in high-income regions. Third, in fully adjusted multivariable models for sCD14, female sex, White race (among those in high-income regions), and current use of NRTI + NNRTI ART were associated with higher levels of sCD14, while higher BMI and current use of NRTI + INSTI ART regimens were associated with lower levels of sCD14. In fully adjusted models for oxLDL, male sex, residence in high-income regions, White race (among those in high-income regions), and higher BMI were associated with higher levels of oxLDL. Finally, among WWH, increased reproductive age (being in reproductive age group 3) was associated with higher levels of sCD14 but not with higher levels of oxLDL.
Our study revealed opposing effects of sex on levels of sCD14 and oxLDL. Female sex was associated with higher levels of sCD14 controlling for demographic, behavioral, cardiometabolic, and HIV-specific factors. This finding has a biologic basis, as females have been shown to have a more robust innate immune response to HIV infection than males [15]. Meanwhile, male sex was associated with higher levels of oxLDL in fully adjusted models. Notably, our finding held when controlling for LDL cholesterol levels, which may differ by sex among ART-treated PWH [26]. It is as yet unknown whether sex differences in oxLDL reflect sex differences in atherosclerotic plaque burden among PWH [27].
Among our global cohort, residence in high-income regions was associated with higher levels of oxLDL. While direct comparisons of coronary atherosclerotic plaque burden among PWH in high-income regions vs other GBD regions are lacking, synthesis of distinct studies using comparable techniques suggest such burden may be higher among PWH in high-income regions [28–31]. Whether regional differences in subclinical atherosclerotic plaque burden relate to regional differences in oxLDL remains to be determined. Meanwhile, more work is also required to better understand differences in sCD14 by region (influenced by ART).
Among PWH in our study, lower BMI was associated with higher levels of sCD14, while higher BMI and, to a lesser extent, increased WC were associated with higher levels of oxLDL. The latter positive association is anticipated, given that overweight/obesity and increased central adiposity are believed to contribute to a low-grade state of systemic immune activation. Indeed, general population studies have revealed relationships between increased WC and circulating levels of oxLDL [32]. By contrast, the former inverse association is consistent with previously published work [33] but less intuitive. Explanations for this inverse association remain incompletely understood but may potentially reflect increased intestinal epithelial permeability to enteric pathogens among PWH in a more catabolic or nutrient-deprived state. On balance, our findings suggest that a normal-weight state absent increased central adiposity may be least immunostimulatory for ART-treated PWH.
Among our cohort of ART-treated PWH, ART regimen class was associated with levels of sCD14. Specifically, levels of sCD14 were lower among PWH currently using NRTI + INSTI ART regimens compared with those using other regimens (NRTI + protease inhibitor [PI] and especially NRTI + NNRTI). Of interest, studies of newly initiated ART among ART-naive PWH have shown that an INSTI-based ART regimen tends to dampen select indices of systemic immune activation (eg, sCD14, LpPLA2) to a greater extent than an NNRTI-based ART regimen [34]. Furthermore, ART switch studies conducted specifically among females with HIV have shown that sCD14 levels decline in those who crossed over from PI- or NNRTI-based ART to INSTI-based ART [35].
One of the most intriguing and potentially clinically significant findings is the observation that among ART-treated WWH, more advanced reproductive age appeared to be associated with higher levels of sCD14. To arrive at this finding, our team synthesized menstrual history with laboratory data on the ovarian reserve marker AMH to categorize WWH along a reproductive aging spectrum, as previously described [22]. This categorization system helped us to avoid misclassifying as post-menopausal the large subset of aging WWH who have prolonged, albeit reversible, amenorrhea [36]. Our findings build on observations derived from smaller US cohorts of women, suggesting increases in sCD14 levels across the menopausal transition [37, 38]. Additional research is required to determine whether higher sCD14 levels among WWH with more advanced reproductive age relates to estrogen depletion or other associated changes.
Limitations of our study include the cross-sectional nature of the data and the modest effect sizes of many of the covariate-immune marker associations, which warrant cautious interpretation in context. Additional limitations are that REPRIEVE entry criteria may have influenced select findings, particularly the trends toward inverse associations between oxLDL and both age and current cigarette smoking. For example, participants with higher 10-year ASCVD risk scores, calculated using the pooled cohort equation, were only allowed to enter this primary CVD prevention trial in the setting of lower circulating LDL cholesterol levels [13, 14]. As age and cigarette smoking both increase the 10-year ASCVD risk score, there might have been a selection bias toward lower levels of circulating LDL cholesterol and, in turn, oxLDL among older PWH and among those who are active cigarette smokers.
Our observations regarding opposing effects of sex on levels of sCD14 and oxLDL, 2 key systemic immune activation markers related to CVD risk among ART-treated PWH, have potential clinical implications. Specifically, our findings highlight the perils of assuming that biologic sex exerts the same effect on different systemic immune pathways. Among PWH, sex-specific examinations of residual systemic immune activation profiles post-ART and how such profiles relate to risks of CVD and other comorbidities will be key to developing sex-specific disease prevention approaches. Furthermore, our finding of a relationship between more advanced reproductive age and heightened innate immune activation merits additional study to enhance our understanding of how menopause augments CVD risk among WWH [39, 40]. Initial work has suggested that statin therapy reduces levels of sCD14 and oxLDL both in the general population and among PWH [41, 42]. Future studies may elucidate ways in which medications such as statins and behavioral modifications (eg, healthy diet, exercise) influence sCD14 and oxLDL and the extent to which dampening of these markers mediates CVD-protective effects, with potential differential effects in females and males with HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, 2021; REPRIEVE Investigator Meeting, 2021; ACTG Women’s Health Collaborative Science Group Meeting, 2021.
Acknowledgments. The study investigators thank the study participants, site staff, and study-associated personnel for their ongoing contributions to the trial. Additionally, they thank the AIDS Clinical Trials Group (ACTG) for clinical site support; the ACTG specialists (Barbara Bastow, Laura Moran, and Jhoanna Roa) for regulatory support; the data management center, Frontier Science Foundation, for data support; and the Center for Biostatistics in AIDS Research for statistical support.
Disclaimer. The views expressed here are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute (NHLBI), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), or the US Department of Health and Human Services.
Financial support. This work was supported through NIH, National Institute of Heart, Lung, and Blood Institute grants U01HL123336 to the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) Clinical Coordinating Center and U01HL123339 to the REPRIEVE Data Coordinating Center, as well as through funding from ViiV Healthcare, Kowa Pharmaceuticals America, Inc, and Gilead Sciences. NIAID has supported this study through grants UM1 AI068636, which supports the ACTG Leadership and Operations Center, and UM1 AI106701, which supports the ACTG Laboratory. This work was also supported by NIH, NIAID grant R01AI123001 (to M. V. Z. and S. E. L.).
Contributor Information
Sara E Looby, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA; Yvonne L. Munn Center for Nursing Research, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Amy Kantor, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Tricia H Burdo, Department of Microbiology, Immunology, and Inflammation and Center for NeuroVirology and Gene Editing, Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, USA.
Judith S Currier, Division of Infectious Diseases, David Geffen School of Medicine, University of California–Los Angeles, Los Angeles, California, USA.
Carl J Fichtenbaum, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Edgar T Overton, Division of Infectious Diseases, University of Alabama at Birmingham School of Medicine, Birmingham, Alabama, USA.
Judith A Aberg, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Carlos D Malvestutto, Division of Infectious Diseases, Ohio State University Medical Center, Columbus, Ohio, USA.
Gerald S Bloomfield, Department of Medicine, Duke Global Health Institute and Duke Clinical Research Institute, Duke University, Durham, North Carolina, USA.
Kristine M Erlandson, Department of Medicine, Division of Infectious Disease, University of Colorado–nschutz Medical Campus, Aurora, Colorado, USA.
Michelle Cespedes, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Esper G Kallas, Departmento de Molestias Infecciosas e Parasitárias, University of Sao Paulo, Sao Paulo, Brazil.
Mar Masiá, Department of Infectious Diseases, Hospital General Universitario de Elche, Alicante, CIBER de Enfermedades Infecciosas, Instituto de Salud Carlos III, Spain.
Alice C Thornton, Division of Infectious Diseases, University of Kentucky College of Medicine, Lexington, Kentucky, USA.
Mandy D Smith, Department of Microbiology, Immunology, and Inflammation and Center for NeuroVirology and Gene Editing, Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, USA.
Jacqueline M Flynn, Department of Microbiology, Immunology, and Inflammation and Center for NeuroVirology and Gene Editing, Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, USA.
Emma M Kileel, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Evelynne Fulda, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Kathleen V Fitch, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Michael T Lu, Cardiovascular Imaging Research Center, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Pamela S Douglas, Duke University Research Institute, Duke University School of Medicine, Durham North Carolina, USA.
Steven K Grinspoon, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Heather J Ribaudo, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, Massachusetts, USA.
Markella V Zanni, Metabolism Unit, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, USA.
References
- 1. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW.. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsue PY, Deeks SG, Hunt PW.. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205:S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunt PW, Lee SA, Siedner MJ.. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shive CL, Jiang W, Anthony DD, Lederman MM.. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29:1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reiner AP, Lange EM, Jenny NS, et al. . Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013; 33:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zanni MV, Toribio M, Wilks MQ, et al. . Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis 2017; 215:1264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS.. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schnittman SB-EG, Shigenaga J, Ahn H, et al. . Sex modifies the association between inflammation and vascular events in treated HIV CROI. 2021;Abstract 98. [Google Scholar]
- 9. Sandler NG, Wand H, Roque A, et al. . Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhoads JP, Major AS.. How oxidized low-density lipoprotein activates inflammatory responses. Crit Rev Immunol 2018; 38:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koenig W, Karakas M, Zierer A, et al. . Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem 2011; 57:1196–200. [DOI] [PubMed] [Google Scholar]
- 12. Nou E, Lu MT, Looby SE, et al. . Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS 2016; 30:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grinspoon SK, Fitch KV, Overton ET, et al. . Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J 2019; 212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grinspoon SK, Douglas PS, Hoffmann U, Ribaudo HJ.. Leveraging a landmark trial of primary cardiovascular disease prevention in human immunodeficiency virus: introduction from the REPRIEVE coprincipal investigators. J Infect Dis 2020; 222:S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Addo MM, Altfeld M.. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209:S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gal-Oz ST, Maier B, Yoshida H, et al. . ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 2019; 10:4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roth GA, Mensah GA, Johnson CO, et al. . Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol 2020; 76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fichtenbaum CJ, Ribaudo HJ, Leon-Cruz J, et al. . Patterns of antiretroviral therapy use and immunologic profiles at enrollment in the REPRIEVE Trial. J Infect Dis 2020; 222:S8–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Looby SE, Fitch KV, Srinivasa S, et al. . Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS 2016; 30:383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanni MV, Currier JS, Kantor A, et al. . Correlates and timing of reproductive aging transitions in a global cohort of midlife women with human immunodeficiency virus: insights from the REPRIEVE Trial. J Infect Dis 2020; 222(Supplement_1):S20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390:1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Waist Circumference and Waist-Hip Ratio: A Report of a WHO Expert Consultation 2008. Available at: https://www.who.int/publications/i/item/9789241501491. Accessed 16 May 2011. . [Google Scholar]
- 25. Zidar DA, Juchnowski S, Ferrari B, et al. . Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 2015; 69:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frazier EL, Sutton MY, Tie Y, Fagan J, Fanfair RN.. Differences by sex in cardiovascular comorbid conditions among older adults (aged 50–64 or ≥65 years) receiving care for human immunodeficiency virus. Clin Infect Dis 2019; 69:2091–100. [DOI] [PubMed] [Google Scholar]
- 27. Foldyna B, Fourman LT, Lu MT, et al. . Sex differences in subclinical coronary atherosclerotic plaque among individuals with HIV on antiretroviral therapy. J Acquir Immune Defic Syndr 2018; 78:421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmann U, Lu MT, Foldyna B, et al. . Assessment of coronary artery disease with computed tomography angiography and inflammatory and immune activation biomarkers among adults with HIV eligible for primary cardiovascular prevention. JAMA Netw Open 2021; 4:e2114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bogorodskaya MM, Kityo CM, Nazzinda R, et al. . Sex modifies the association between HIV and coronary artery disease in Uganda. CROI 2021; Abstract 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lo J, Abbara S, Shturman L, et al. . Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010; 24:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Post WS, Budoff M, Kingsley L, et al. . Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinbrenner T, Schroder H, Escurriol V, et al. . Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr 2006; 83:30–5; quiz 181. [DOI] [PubMed] [Google Scholar]
- 33. Koethe JR, Dee K, Bian A, et al. . Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and nonobese HIV-infected adults on antiretroviral therapy. AIDS Res Hum Retroviruses 2013; 29:1019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hileman CO, Kinley B, Scharen-Guivel V, et al. . Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lake JE, McComsey GA, Hulgan T, et al. . Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV Med 2014; 15:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cejtin HE, Evans CT, Greenblatt R, et al. . Prolonged amenorrhea and resumption of menses in women with HIV. J Womens Health (Larchmt) 2018; 27:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters BA, Xue X, Sheira LA, et al. . Menopause is associated with immune activation in women with HIV. J Infect Dis 2022; 225:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA.. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 2020; 5:e134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raghavan A, Rimmelin DE, Fitch KV, Zanni MV.. Sex differences in select non-communicable HIV-associated comorbidities: exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep 2017; 14:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abelman RA, Mugo BM, Zanni MV.. Conceptualizing the risks of coronary heart disease and heart failure among people aging with HIV: sex-specific considerations. Curr Treat Options Cardiovasc Med 2019; 21:41. [DOI] [PubMed] [Google Scholar]
- 41. Jamialahmadi T, Baratzadeh F, Reiner Z, et al. . The effects of statin dose, lipophilicity, and combination of statins plus ezetimibe on circulating oxidized low-density lipoprotein levels: a systematic review and meta-analysis of randomized controlled trials. Mediators Inflamm 2021; 2021:9661752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toribio M, Fitch KV, Sanchez L, et al. . Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017; 31:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.