Abstract
Acute pancreatitis (AP) is a disease characterized by an acute inflammatory phase followed by a convalescent phase. Diabetes mellitus (DM) was historically felt to be a transient phenomenon related to acute inflammation; however, it is increasingly recognized as an important late and chronic complication. There are several challenges that have prevented precisely determining the incidence rate of DM following AP and understanding the underlying mechanisms. The Diabetes RElated to Acute Pancreatitis and its Mechanisms (DREAM) Study is a prospective cohort study designed to address these and other knowledge gaps to provide the evidence needed to screen for, prevent, and treat DM following AP. In the following article, we summarize literature regarding the epidemiology of DM following AP, and provide the rationale and an overview of the DREAM study.
Keywords: pancreatogenic diabetes, type 3c diabetes, beta cell, insulin, autoantibody, epidemiology
BACKGROUND AND RATIONALE
Acute pancreatitis (AP) is an inflammatory disease of the pancreas that accounts for more than 300,000 hospitalizations and health care costs exceeding $2 billion annually in the United States.1,2 The incidence of AP is increasing in both North America and Europe.3 Complications can develop during hospitalization or subsequently during follow-up, and include diabetes mellitus (DM), exocrine pancreatic insufficiency, complications from walled-off pancreatic necrosis, and recurrent episodes of AP.4–6 Hyperglycemia was historically considered a transient phenomenon of AP during hospitalization; however, meta-analyses have reported that approximately one-quarter of AP patients will develop DM within three years of discharge, suggesting it is a relatively common long term complication.7,8 The suboptimal rigor of previous research has made it challenging to precisely define the incidence rate, accurately identify risk factors, and understand the underlying mechanisms of this problem.
Defining an accurate incidence rate requires prospective ascertainment of DM status over a sustained period of time, preferably with testing methodology that is both highly sensitive and specific. Passive or retrospective determination of the development of DM is likely to underestimate the true incidence, and extended follow-up periods are necessary to understand the cumulative risk. Understanding the risk, both in the short and long term, is necessary to guide patient education and surveillance recommendations and to identify those at highest risk who may benefit from preventative strategies. Prospective data collection helps to minimize various forms of bias, including the ascertainment of DM status. One recent prospective study of 152 AP subjects without pre-existing DM reported a cumulative incidence of DM of 7% at 1 year and 11% at 2 years, as well as the composite endpoint of DM or pre-DM in 43% at 2 years.9 This likely represents the most precise risk estimate to date. However, this study included few patients with moderate or severe disease. Various risk factors have been identified in prior studies, including the development of severe AP (particularly with pancreatic necrosis), exocrine pancreatic insufficiency, and a non-biliary etiology, but simultaneous assessment of comprehensive risk factors, including canonical risk factors for type 2 DM (T2D) has not been previously performed.8,10 Considering these limitations, screening for DM is not currently recommended in routine clinical practice following AP discharge.
In addition to estimating the risk of DM following AP, there are a number of additional knowledge gaps. Importantly, the pathophysiology is incompletely understood. The development of DM in patients with mild, interstitial AP suggests the role of factors other than mere reduction of islet cell mass from physical destruction of the pancreatic parenchyma due to necrosis. A number of potential mechanisms of disease have been proposed, including localized inflammation leading to insulin resistance and beta-cell autoimmunity; however, they are primarily based on inferential associations from clinical observations in AP and/or other forms of pancreatogenic DM.10,11 In light of these gaps, it is not currently possible to provide tailored recommendations for prevention or treatment of DM following AP.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) supported Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC) was formed in 2020, and has developed and launched the Diabetes RElated to Acute pancreatitis and its Mechanisms (DREAM) study to address these knowledge gaps and related scientific objectives.12 The current article describes the design of the DREAM study and, along with other articles in this issue, outlines a series of primary and ancillary studies to permit a spectrum of metabolic, immunologic, imaging, and other analyses to better understand DM following AP.
STUDY DESIGN
Synopsis
The DREAM study is a multicenter, observational cohort study prospectively enrolling adults with a recent episode of AP to investigate the incidence, etiology, and pathophysiology of DM following AP. Participants will undergo prospective characterization of glycemic status, islet function, and beta cell autoimmunity, as well as serial collection of clinical data, participant-completed questionnaires, imaging studies, and biological specimens to address a host of scientific objectives.
Primary Objective
The primary objective of the DREAM study is to define the cumulative incidence and clinical characteristics associated with the development of DM after one or more episode of AP. Therefore, patients with a recent diagnosis of AP and without pre-existing DM will undergo longitudinal laboratory assessment of glycemic status after AP resolution. This will be assessed using a combination of fasting glucose, glycated hemoglobin (HbA1c), and 2-hour postprandial glucose (from an oral glucose tolerance test [OGTT]).
Secondary Objectives
In addition to characterizing the incidence of DM following AP, we are keen to investigate the various potential mechanisms contributing to this complication. Dynamic metabolic testing (including OGTT, mixed meal tolerance tests (MMTT), and frequently sample intravenous glucose tolerance tests (FSIGTT)) and longitudinal collection of additional data, including beta cell autoantibodies and biosamples, from participants will provide a rich dataset to simultaneously pursue a number of objectives (Table 1), including to:
TABLE 1.
Selected Outcome Measures for the Diabetes Related to Acute Pancreatitis and Its Mechanisms (DREAM) Study
| Category | Selected Outcome Measure(s) |
|---|---|
| Diabetes risk |
|
| Metabolic alterations |
|
| Immunological |
|
| Imaging |
|
| Acute Pancreatitis |
|
| Patient-reported Outcomes |
|
| Biorepository |
|
Comprehensively characterize islet cell function and endocrine alterations after AP and their relationship with the development of DM13
Investigate the immunologic mechanisms of DM after AP, including the contribution of beta-cell autoimmunity14
Examine the utility of morphological changes seen with magnetic resonance imaging (MRI) and computed tomography (CT) as a biomarker for the development of DM after AP15
Eligibility Criteria
Adults (ages 18–75 at the time of enrollment) with a diagnosis of AP within the preceding 90 days and agreeable to longitudinal assessments will be eligible for participation. The study will recruit patients with no preference to sex or race. For the purposes of this study, AP will be diagnosed according to the revised Atlanta criteria, which requires the presence of two of the following criteria: upper abdominal pain, elevation of serum amylase and/or lipase to ≥3 times the upper limit of normal, and/or features of AP on cross-sectional imaging.16 Since patients undergoing an endoscopic retrograde cholangiopancreatography (ERCP) may frequently experience post-procedural pain and elevated pancreatic enzymes, these patients must be hospitalized for >48 hours to satisfy the study definition for AP. Patients with a prior history of AP are eligible for participation.
There are a number of exclusion criteria that are intended to define the target study population, eliminate unacceptable sources of bias or confounding, and to protect the safety of participants (Table 2). In brief, the criteria include the presence of any of the following prior to or at the time of enrollment: calcific chronic pancreatitis, direct endoscopic or percutaneous necrosectomy or drainage of necrotic collection(s), pancreatic surgery, pancreatic tumors, use of a medication with high diabetogenic potential, pregnancy (at the time of enrollment), or any severe systemic illness that will confound outcome assessments of DM and immunological outcomes or pose additional risk for harms. Patients with autoimmune pancreatitis (diagnosed according to the International Consensus Diagnostic Criteria) are eligible for participation if they otherwise meet the eligibility criteria, including the study definition of AP.17
TABLE 2.
Exclusion Criteria for the Diabetes Related to Acute Pancreatitis and Its Mechanisms (DREAM) Study
|
AIDS indicates acquired immunodeficiency syndrome; CP, chronic pancreatitis; CT, computed tomography; eGFR, estimated glomerular filtration rate; ERCP, endoscopic retrograde cholangiopancreatography; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; PI, principal investigator
Study Assessments
The planned assessments are comprehensive, including collection of clinical data, patient-reported outcomes, detailed metabolic testing, cross-sectional imaging, and biospecimens for future analyses (Table 3). All participants will undergo screening for pre-existing DM at enrollment. While the primary study population involves patients without a previous clinical diagnosis of DM, we will also enroll a smaller number of patients with pre-existing DM to complete baseline assessments and serve as disease controls. Standardized procedures will be used to collect participant (eg, age, sex, race/ethnicity), clinical, laboratory, and imaging data, as well as biospecimens at baseline and during longitudinal follow-up for additional planned studies to investigate the mechanisms of DM after AP. The collection method of all biosamples is prescribed in a study-specific biospecimen standard operating procedure to ensure consistency across the study sites.18
TABLE 3.
Schedule of Activities for the Diabetes Related to Acute Pancreatitis and Its Mechanisms (DREAM) Study
| Assessment/Procedure | 0 mo Enrollment | +3 mo (Metabolic Visit) | +6 mo | +12 mo | 18 mo | 24–60 mo (Annual) | New Diagnosis of DM |
|---|---|---|---|---|---|---|---|
| Research visit (Time window) | X (0–90 d after AP) | X (−2 to +6 wk) | X or  * (−6 wk to +3 mo) * (−6 wk to +3 mo) |
X (−3 to +3 mo) | X or  (−3 to +3 mo) (−3 to +3 mo) |
X (±6 mo) | |
| General | |||||||
| Demographics and medical history | X | ||||||
| Interim medical history | X | X | X | X | X | X | |
| Height, weight, waist circumference | X | X | X | X | X | ||
| Questionnaires | |||||||
| Quality of life, EPI GI Symptom tracker | X | X | X | X | |||
| Food frequency questionnaire | X | X | X | ||||
| Labs | |||||||
| FBG, HbA1c, autoantibodies | X | X | X | X | X | X | |
| CBC with diff, creatinine, FE-1 | X | X | X | X | |||
| Metabolic testing | |||||||
| OGTT | X | ||||||
| Serial OGTT arm | X | X | X (MMTT) | ||||
| Serial MMTT arm† | X† | X | X | X | |||
| Serial FSIGTT arm† | X† | X | X (MMTT) | ||||
| Imaging | |||||||
| Research MRI/MRCP‡ | X | X | X3 | X | |||
| Biorepository | |||||||
| Blood§ | X | X | X | X | X | ||
| Stool|| | X | X | X |
The reference time point zero represents the date a participant first met the diagnostic criteria for their qualifying AP.
 symbol indicates this visit could be conducted by telephone;
symbol indicates this visit could be conducted by telephone;
Serial MMTT and FSIGTT will be performed in a subset of participants. These tests will be offered to participants without DM at 3-mo visit;
Research MRI/MRCP will be performed in a subset of participants through 36 mo;
Blood will be collected for serum, plasma, peripheral blood mononuclear cells, blood cell stabilizers (whole blood added to SmartTube Proteomic Stabilizer), Tempus tubes (RNAseq), and DNA (from a single blood collection);
Stool samples will be collected using methods that will allow microbiome and metabolic analyses.
diff indicates differential; EPI, exocrine pancreatic insufficiency; FBG, fasting blood glucose; FE-1, fecal elastase-1; GI, gastrointestinal; OGTT, oral glucose tolerance test; MMTT, mixed meal tolerance test; FSIGTT, frequently sampled intravenous glucose tolerance test.
Baseline assessments will include history, exam, CT or MRI imaging, and laboratory assessments. Demographics, concomitant medications, and family history will be collected. Details related to AP, including the etiology, clinical course, and severity grade of the qualifying episode will be recorded. Questionnaires will be administered to assess quality of life (using PROMIS Global Health and PROMIS-29), dietary intake patterns (using Vioscreen Food Frequency Questionnaire [FFQ]), and symptoms of exocrine pancreatic dysfunction (using Exocrine Pancreatic Insufficiency GI Symptom Tracker). Computed tomography and MRI scans performed for clinical care will be reviewed using standardized forms to assess the pancreas and severity of disease by a dedicated site radiologist, and scans will be uploaded to a central imaging repository. Lastly, blood and stool samples will be obtained for the study biorepository.
Participants without DM at baseline (ie, at risk for DM) will undergo longitudinal assessments. At the first follow-up visit (approximately 3 months after the diagnosis of the qualifying AP episode) all participants will undergo assessment of DM status and beta cell function with an OGTT (Fig. 1). Subsequently, participants will be followed over time with serial assessment of their glycemic status using fasting glucose and HbA1c at specified intervals. The majority of participants will also undergo serial OGTT to enhance the sensitivity to diagnose DM and pre-DM status, and estimate changes in beta cell function, as further described in this issue.13 Alternatively, smaller groups of participants without pre-existing DM may undergo either MMTTs or FSIGTTs to obtain further metabolic insights.13 Information related to interval changes in participants’ medical history (eg, recurrence of AP, change in medications) and patient-reported outcomes (eg, quality of life, symptoms of exocrine pancreatic dysfunction) will be collected. Interval cross-sectional imaging studies will be reviewed by site radiologists. Additionally, a subset of participants without DM at 3 months will also be invited to undergo serial cross-sectional imaging with MRI using a standardized research imaging protocol to assess for imaging predictors of DM following AP.15 Lastly, participants who develop DM during follow-up will be invited for a one-time visit for additional collection of questionnaire data, MMTT, research MRI, and biospecimens.
FIGURE 1.
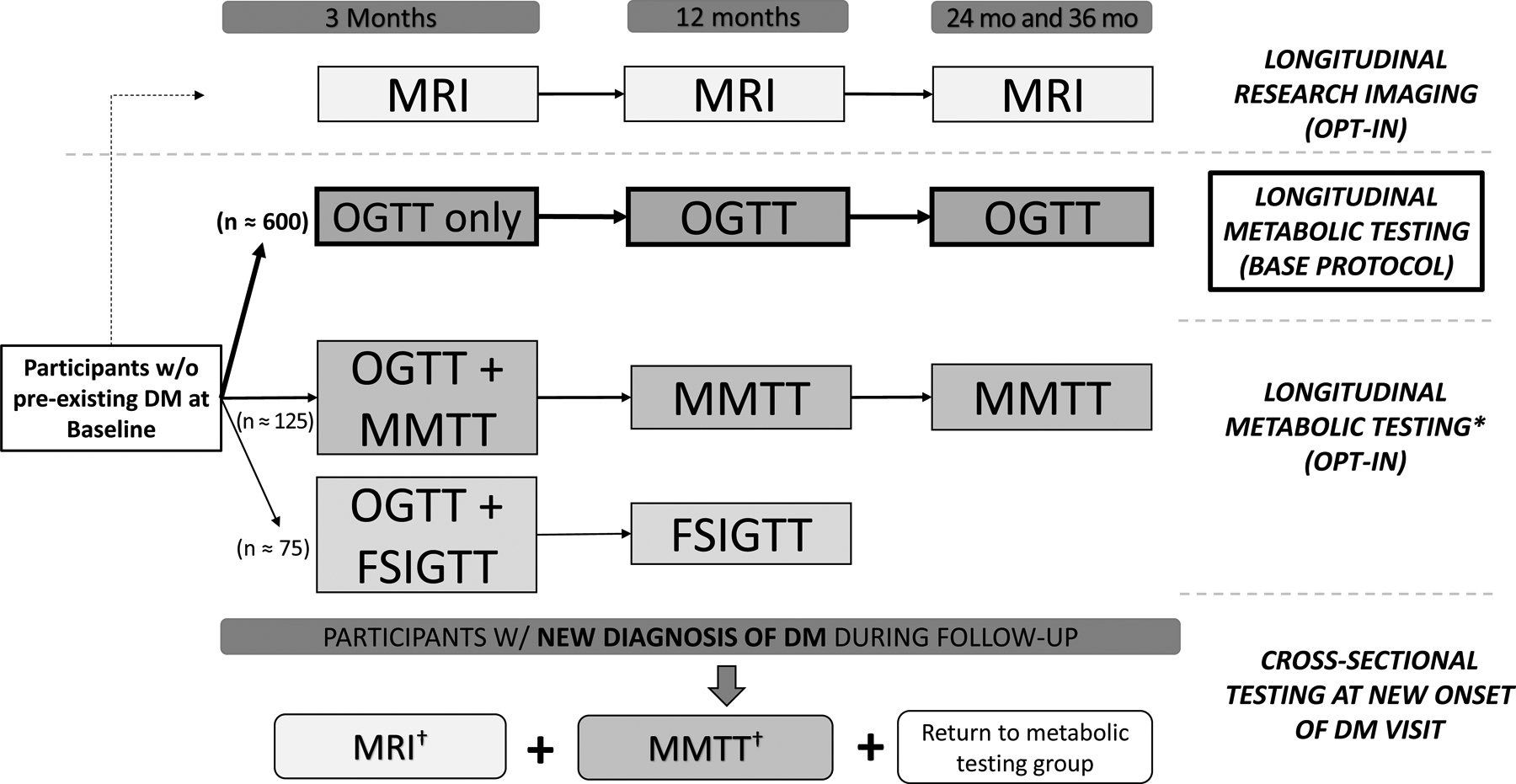
Overview of metabolic testing and imaging groups for the Diabetes Related to Acute Pancreatitis and its Mechanisms (DREAM) Study. Participation in the longitudinal metabolic groups is mutually exclusive, whereas participation in the longitudinal research magnetic resonance imaging (MRI) can occur irrespective of the metabolic testing group. *For participants without DM at 3 months. †One-time MMTT and MRI are performed if participant is not previously enrolled in MRI and MMTT groups, respectively. w/o, without.
OUTCOMES
The primary endpoint of the DREAM study is the incidence of DM following the qualifying episode of AP defined as the combination of both concurrent newly diagnosed DM (ie, initially diagnosed at the time of the qualifying AP episode) and new-onset DM (ie, diagnosed during longitudinal follow-up).19 The chronology of DM in relation to the qualifying episode of AP and definition for both DM and pre-diabetes for the DREAM study are described in Table 4. DM status will be serially assessed using the combination of fasting glucose and HbA1c, according to the American Diabetes Association diagnostic criteria.19 Furthermore, to identify individuals with lack of concordance of tests for diagnosing DM, we will also measure a 2-hour postprandial glucose level (during OGTT) as a sensitive method of diagnosis for participants in the serial OGTT arm. When participants partially satisfy diagnostic criteria for DM, they will be asked to repeat a fasting plasma glucose and HbA1c during the same time window. We will also assess the development of either diabetes or prediabetes as a secondary endpoint. Lastly, the frequency, distribution, and temporal emergence of islet autoantibodies will be evaluated in relation to DM, as described elsewhere14.
TABLE 4.
Chronology of Diabetes (DM) in Relation to the Qualifying Episode of AP and Definition for DM and Prediabetes for the Diabetes Related to Acute Pancreatitis and Its Mechanisms (DREAM) Study
| Chronology of DM | |
| Pre-existing DM | Assigned at baseline for participants with DM present prior to the onset of the qualifying episode of AP |
| Unclassified | Assigned at baseline for the situation where a participant without pre-existing DM has at least one abnormal parameter of DM during the qualifying AP episode, but cannot be further classified until follow-up testing is completed |
| Concurrent newly diagnosed DM | DM in which an abnormal parameter of DM is identified during the qualifying AP episode, but DM criteria are not confirmed until the 3-month visit |
| Transient hyperglycemia | Situation in which an abnormal parameter of DM is identified during the AP episode, but DM criteria are not met at the 3-month visit. |
| New-onset DM after AP | DM was not present during qualifying episode of AP, but is diagnosed at the 3-month visit or during subsequent follow-up |
| Definitions for DM and prediabetes | |
| DM diagnosis | Any of the three following criteria are met during follow-up:
|
| Prediabetes | Abnormal values on two or more of the following tests at the same visit:
|
Parameters of DM include: fasting blood glucose ≥126 mg/dl, 2-hour blood glucose ≥200 mg/dl during a 75-g oral glucose tolerance test, HbA1c level ≥6.5%, and the presence of classic symptoms of DM (polyuria, polydipsia, polyphagia, weakness, weight loss) with a random blood glucose ≥200 mg/dl (does not require a second abnormal parameter
While the primary objective is to evaluate the incidence and mechanisms of DM, the study design affords the opportunity to investigate other aspects of the natural history of AP. Specifically, we will evaluate the readmission rate, incidence of exocrine pancreatic dysfunction (serially measured with a combination of symptoms and fecal elastase-1 levels), changes in quality of life, healthcare utilization, and the recurrence of AP and/or progression to chronic pancreatitis. Additional secondary endpoints related to metabolic and immunological mechanisms of diabetes as well as imaging findings are discussed elsewhere.13–15
STATISTICAL CONSIDERATIONS
We will apply a discrete time-to-event regression analysis with interval censoring because we anticipate the occurrence of DM for most participants will be detected at the scheduled visits (eg, 3, 6, 12, 18, 24, and 36 months). We will enroll up to 1000 participants (including up to 800 without pre-existing DM who will undergo longitudinal follow-up and up to 200 with pre-existing DM who will only undergo baseline assessment) into the DREAM study. We are estimating cumulative dropout rates of 5%, 10%, 15%, 20%, 25%, and 30% at 6, 12, 18, 24, 30, and 36 months of follow-up, respectively. Assuming cumulative DM incidence rates of 4.5%, 9%, 12%, 15%, 18%, and 21% at 6, 12, 18, 24, 30, and 36 months of follow-up, respectively, the 95% confidence interval for a sample size of 800 participants without pre-existing DM will have limits of ± 4% at 24 months based on a simulation study with 1000 Monte Carlo samples; confidence intervals for different enrollment sizes are depicted in Supplemental Figure 1. Analyses for all primary and secondary outcomes will be repeated using a broader definition of new-onset dysglycemia, which includes the primary statistical endpoint of DM as well as pre-DM.
We will also investigate relevant clinical factors (eg, AP severity, AP etiology, baseline body mass index, etc.) as to whether they significantly affect the discrete-time hazard function by including them in the regression model. We will perform secondary analyses using: 1) the discrete time-to-events hazards regression model, and 2) nested case-control designs with a 1:4 allocation ratio of cases to controls. Such nested case-control study designs with 800 participants (allowing for 20% loss to follow-up at 24 months) will yield 80% statistical power with a two-sided 0.05 significance level test to detect an odds ratio of 1.80 for a binary risk factor that has 50% prevalence in the controls; alternate power calculations for different enrollment sizes are shown in Supplemental Figure 2.
STUDY EXECUTION
The DREAM study includes the 10 clinical centers (with at least 3 additional satellite sites) of the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC) from throughout the United States. Investigators have formed a combination of working groups and committees that worked collaboratively in an iterative manner to develop the DREAM study protocol and execution plans, as previously introduced.12 In addition to these teams, the Data Coordinating Center provides central administrative, regulatory, and statistical support for T1DAPC, including DREAM.20 In accordance with NIH policies, the study is approved through a single institutional review board, and reviewed on a biannual basis by an NIH-appointed Data and Safety Monitoring Board (DSMB).
DISCUSSION
There are multiple knowledge gaps in the understanding of AP and its complications. In the DREAM study, we will directly address important clinical questions related to the development of DM following AP and its underlying mechanisms, including beta-cell autoimmunity. In addition to the lack of robust data on the incidence of diabetes following AP, there is limiting understanding of the phenotype and pathophysiology of the disease, which we aim to investigate. Important questions include the factors associated with and the frequency at which islet cell autoimmunity occurs following exocrine pancreas injury and whether the kinetics of immune response and resolution relate to subsequent clinical phenotype. Further, the natural history of beta cell function in those who do and do not develop diabetes is unknown. Detailed metabolic testing will provide refined estimates of the incidence of DM and characterize the type and trajectory of metabolic and endocrine alterations, including beta cell function and alterations in pancreatic and incretin hormone responses. Serial assessments of clinical, immunological, cross-sectional imaging, and other data elements will be combined with paired biological specimens to create a comprehensive dataset that can be used to assess additional questions related to the natural history of AP and other disease-related complications.
One of the guiding principles in the design of the DREAM study has been to create a rigorous protocol that addresses limitations of previous research. Importantly, prospective assessment of DM status is critical to minimize biased estimates of case ascertainment. In addition to fasting glucose and HbA1c values, we are implementing the use of serial OGTT to provide a sensitive estimate of DM. By performing systematic screening, we will increase the accuracy of identifying incident DM and its timing in relation to the qualifying AP episode. Similarly, the prospective and systematic collection of comprehensive data elements will allow investigation of the potential influence of covariates, including, but not limited to: etiology of AP, clinical severity of AP, imaging severity of AP, beta-cell autoantibodies, exocrine pancreatic dysfunction, changes in pancreas volume, and alterations in the intestinal microbiome. The assessment of patient-related outcomes (eg, quality of life) has not been attempted in a study of this scale in the United States, and will help identify patient-centric outcomes warranting additional investigation. Lastly, the large sample size will allow us to conduct robust subgroup and sensitivity analyses to understand the various interactions between patient and disease-related factors.
Proactive measures have been undertaken to protect the scientific rigor and enhance the feasibility of the DREAM study, utilizing the interdisciplinary team of investigators from the disciplines of gastroenterology, endocrinology, immunology, and biostatistics. First, considering the critical role of biospecimen analyses for the planned translational studies, we convened a Biospecimen Committee to develop study-specific biospecimen standard operating procedures.18 This guidance is intended to minimize heterogeneity across the clinical centers due to confounding related to variations in biosample collection, processing, storage, and/or shipping. Next, the Protocol Committee developed a series of study-specific Manual of Procedures to provide explicit guidance for completion of study assessments, including case report forms, for the participating sites. This manual will be updated on an as needed basis to address common questions or recurring situations where clarifying information is needed. We sought guidance from T1DAPC investigators and the NIH-representatives participating in recent and ongoing studies in pancreatitis to refine, and when possible harmonize, study definitions, data elements, case report forms, and research methodology.21–25 Lastly, we have convened a Recruitment and Retention Committee concurrently with study planning to provide guidance and feedback regarding implications of decisions on participant burden.26 There is not a precedent in the United States for a prospective cohort study in AP of this scale, so we will prospectively monitor recruitment and retention rates, completion of study assessments, and collaboratively discuss any needs to alter the sample size, number, and/or frequency of study assessments to maintain feasibility. Final decisions regarding modifications to the study protocol will be made by the T1DAPC Steering Committee and approved by the NIDDK, DSMB, and single institutional review board before implementation.
In summary, the DREAM study is a prospective cohort study of patients with one or more episode of AP at risk for developing DM. The primary goal of the DREAM study is to investigate the incidence and mechanisms of DM following AP. Study results will generate an evidence base that will provide clinicians with further guidance regarding the surveillance of DM following AP, and generate the data needed to further investigate tailored preventative and treatment strategies to improve the lives of our patients.
Supplementary Material
Supplemental Figure 1. The 95% confidence intervals for estimating the incidence rate of DM in the Diabetes Related to Acute Pancreatitis and its Mechanisms (DREAM) Study across different sample size estimates according to a discrete time-to-event analysis.
Supplemental Figure 2. Sample sizes of enrollment in the Diabetes Related to Acute Pancreatitis and its Mechanisms (DREAM) Study needed to identify odds ratios for incident diabetes with 80% statistical power using a nested case-control study design with 1:4 allocation ratio.
Grant Support:
Research reported in this publication was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC) under award numbers U01 DK127367 (GT, MB), U01 DK127377 (AEP, FGST, DY), U01 DK127378 (CY), U01 DK127382 (JJE, EF, TT), U01 DK127384 (AMD, VC, JLM, MW), U01 DK127388 (PAH, GIP, DLC, KMD, PJL), U01 DK127392 (CF), U01 DK127395 (WJP), U01 DK127400 (EA, VA, RRK, VS, AZ), U01 DK127403 (JB, MK, SJP, SSV), and U01 DK127404 (CG, RK, CS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest/disclosures:
The following authors disclose potential conflicts of interest: E.A. is on the advisory board for Nestle; M.D.B. receives research support from Viacyte and Dexcom, advisory board for Insulet; K.M.D. receives research support from Sanofi, Viacyte, Abbott, and Dexcom; consulting activities with Eli Lilly, Boehringer Ingelheim, Elsevier; honoraria from UptoDate, Elsevier, Medscape, Academy for Continued Healthcare Learning; C.E.F. receives research support from Abbvie; consulting activities with Nestle; G.I.P. receives research support from Abbvie; consulting activities with Nestle; C.S. is on the advisory board for Vertex Pharmaceuticals; F.G.S.T. receives research support from Dompé Pharmaceuticals; consulting activities with Sanofi, Eli Lilly, and AstraZeneca. The remaining authors declare no conflicts of interest.
Abbreviations:
- AP
acute pancreatitis
- CP
chronic pancreatitis
- DCC
Data Coordinating Center
- DM
diabetes mellitus
- DREAM
Diabetes RElated to Acute pancreatitis and its Mechanisms
- DSMB
Data and Safety Monitoring Board
- FSIGTT
frequently sampled intravenous glucose tolerance testing
- OGTT
oral glucose tolerance testing
- MMTT
mixed meal tolerance testing
- T1DAPC
Type 1 Diabetes in Acute Pancreatitis Consortium
- T2D
type 2 diabetes mellitus
REFERENCES
- 1.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019;156:254–272 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302–307. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology. 2022;162:122–134. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096–103. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Lee E, Papachristou GI, et al. A population-based evaluation of readmissions after first hospitalization for acute pancreatitis. Pancreas. 2014;43:630–637. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738–746. [DOI] [PubMed] [Google Scholar]
- 7.Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818–831. [DOI] [PubMed] [Google Scholar]
- 8.Zhi M, Zhu X, Lugea A, et al. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: A systematic review and meta-analysis. Front Physiol. 2019;10:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharmal SH, Cho J, Alarcon Ramos GC, et al. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol. 2020;55:775–788. [DOI] [PubMed] [Google Scholar]
- 10.Hart PA, Bradley D, Conwell DL, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav D, Whitcomb DC, Tang G, et al. Autoimmunity may explain diabetes in a subset of patients with recurrent acute and chronic pancreatitis: A pilot study. Clin Gastroenterol Hepatol. 2021. Nov 16. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Serrano J, Laughlin M, Bellin MD, et al. Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC): From concept to reality. Pancreas. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dungan KM, Hart PA, Andersen DK, et al. Assessing the pathophysiology of hyperglycemia in the Diabetes RElated to Acute pancreatitis and its Mechanisms (DREAM) study: From the Type 1 Diabetes in Acute Pancreatitis Consortium (T!DAPC). Pancreas. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casu A, Grippo PJ, Wasserfall C, et al. Evaluating the immunopathogenesis of diabetes following acute pancreatitis in the Ddiabetes RElated to Acute pancreatitis and its Mechanisms (DREAM) study: From the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Pancreas. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terkes T, Chinchilli VM, Bagci U, et al. Design and rationale for the use of magnetic resonance imaging biomarkers to predict diabetes following acute pancreatitis in the Diabetes RElated to Acute pancreatitis and its Mechanisms (DREAM) study: From the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Pancreas. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis−-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 17.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. [DOI] [PubMed] [Google Scholar]
- 18.Wasserfall C, Dyer AM, Speake C, et al. Standard operating procedures for biospecimen collection, processing, and storage: From the Type 1 Diabetes in Acute Pancreatitis Consortium. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. [DOI] [PubMed] [Google Scholar]
- 20.Dyer AM, Baab K, Merchlinski A, et al. Rationale and development of a data coordinating center to support the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papachristou GI, Machicado JD, Stevens T, et al. Acute pancreatitis patient registry to examine novel therapies in clinical experience (APPRENTICE): an international, multicenter consortium for the study of acute pancreatitis. Ann Gastroenterol. 2017;30:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart PA, Andersen DK, Mather KJ, et al. Evaluation of a mixed meal test for Diagnosis and characterization of pancrEaTogEniC diabeTes secondary to pancreatic cancer and chronic pancreatitis: Rationale and methodology for the DETECT study from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav D, Park WG, Fogel EL, et al. PROspective evaluation of Chronic pancreatitis for EpidEmiologic and translational stuDies: Rationale and study design for PROCEED from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coté GA, Durkalski-Mauldin VL, Serrano J, et al. SpHincterotomy for Acute Recurrent Pancreatitis randomized trial: Rationale, methodology, and potential implications. Pancreas. 2019;48:1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paragomi P, Phillips AE, Machicado JD, et al. Post-Acute Pancreatitis Pancreatic Exocrine Insufficiency: Rationale and methodology of a prospective, observational, multicenter cohort study. Pancreas. 2021;50:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yazici C, Dyer AM, Conwell DL, et al. Recruitment and Retention Strategies for the Diabetes RElated to Acute Pancreatitis and Its Mechanisms (DREAM) Study: From the Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Pancreas. 2022;51:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The 95% confidence intervals for estimating the incidence rate of DM in the Diabetes Related to Acute Pancreatitis and its Mechanisms (DREAM) Study across different sample size estimates according to a discrete time-to-event analysis.
Supplemental Figure 2. Sample sizes of enrollment in the Diabetes Related to Acute Pancreatitis and its Mechanisms (DREAM) Study needed to identify odds ratios for incident diabetes with 80% statistical power using a nested case-control study design with 1:4 allocation ratio.


