Abstract
Background
Data on medium-term outcomes in indivduals with myocarditis after mRNA COVID-19 vaccination are scarce. We aimed to assess clinical outcomes and quality of life at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults.
Methods
In this follow-up surveillance study, we conducted surveys in US individuals aged 12–29 years with myocarditis after mRNA COVID-19 vaccination, for whom a report had been filed to the Vaccine Adverse Event Reporting System between Jan 12 and Nov 5, 2021. A two-component survey was administered, one component to patients (or parents or guardians) and one component to health-care providers, to assess patient outcomes at least 90 days since myocarditis onset. Data collected were recovery status, cardiac testing, and functional status, and EuroQol health-related quality-of-life measures (dichotomised as no problems or any problems), and a weighted quality-of-life measure, ranging from 0 to 1 (full health). The EuroQol results were compared with published results in US populations (aged 18–24 years) from before and early on in the COVID-19 pandemic.
Findings
Between Aug 24, 2021, and Jan 12, 2022, we collected data for 519 (62%) of 836 eligible patients who were at least 90 days post-myocarditis onset: 126 patients via patient survey only, 162 patients via health-care provider survey only, and 231 patients via both surveys. Median patient age was 17 years (IQR 15–22); 457 (88%) patients were male and 61 (12%) were female. 320 (81%) of 393 patients with a health-care provider assessment were considered recovered from myocarditis by their health-care provider, although at the last health-care provider follow-up, 104 (26%) of 393 patients were prescribed daily medication related to myocarditis. Of 249 individuals who completed the quality-of-life portion of the patient survey, four (2%) reported problems with self-care, 13 (5%) with mobility, 49 (20%) with performing usual activities, 74 (30%) with pain, and 114 (46%) with depression. Mean weighted quality-of-life measure (0·91 [SD 0·13]) was similar to a pre-pandemic US population value (0·92 [0·13]) and significantly higher than an early pandemic US population value (0·75 [0·28]; p<0·0001). Most patients had improvements in cardiac diagnostic marker and testing data at follow-up, including normal or back-to-baseline troponin concentrations (181 [91%] of 200 patients with available data), echocardiograms (262 [94%] of 279 patients), electrocardiograms (240 [77%] of 311 patients), exercise stress testing (94 [90%] of 104 patients), and ambulatory rhythm monitoring (86 [90%] of 96 patients). An abnormality was noted among 81 (54%) of 151 patients with follow-up cardiac MRI; however, evidence of myocarditis suggested by the presence of both late gadolinium enhancement and oedema on cardiac MRI was uncommon (20 [13%] of 151 patients). At follow-up, most patients were cleared for all physical activity (268 [68%] of 393 patients).
Interpretation
After at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination, most individuals in our cohort were considered recovered by health-care providers, and quality of life measures were comparable to those in pre-pandemic and early pandemic populations of a similar age. These findings might not be generalisable given the small sample size and further follow-up is needed for the subset of patients with atypical test results or not considered recovered.
Funding
US Centers for Disease Control and Prevention.
Introduction
Evidence from the USA and multiple international vaccine safety monitoring systems support a small but increased risk of myocarditis after mRNA COVID-19 vaccination.1 In 2021, data from the Vaccine Adverse Event Reporting System (VAERS) indicated that in US individuals aged 12 years or older, approximately 4·8 cases of myocarditis per million doses of mRNA COVID-19 vaccines administered were reported, with the highest reporting rates in those aged 12–29 years.2 Despite the higher than expected occurrence of myocarditis after COVID-19 vaccination, the benefits of mRNA COVID-19 vaccines have been shown to outweigh the risk of myocarditis.2, 3
Research in context.
Evidence before this study
In December, 2020, the US Food and Drug Administration (FDA) issued emergency use authorisations (EUAs) for the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine and the Moderna COVID-19 (mRNA-1273) vaccine. In May, 2021, FDA expanded the EUA for the BNT162b2 vaccine to include adolescents aged 12–15 years. By July, 2022, more than 200 million people in the USA had received two doses of a COVID-19 mRNA vaccine and more than 1500 cases of myocarditis with onset after mRNA COVID-19 vaccination were reported to the Vaccine Adverse Events Reporting System (VAERS). We searched PubMed for articles published up to April 30, 2022, using the keywords “mRNA vaccine” and “myocarditis”, without any language restrictions. Systematic reviews published in 2022 included more than 5299 individuals with myocarditis after mRNA vaccination and suggested the risk was highest in adolescents and young males after a second vaccine dose. Findings from these systematic reviews suggest that most cases of myocarditis after mRNA COVID-19 vaccination have resolution of symptoms at or soon after discharge from a short hospital stay. However, data on medium-term prognoses for adolescents and young adults diagnosed with myocarditis after mRNA COVID-19 vaccination are scarce.
Added value of this study
To our knowledge, this is the largest evaluation of outcomes among patients diagnosed with myocarditis after mRNA COVID-19 vaccination, with follow-up at least 90 days since onset. We collected data from both patients (or their parents or guardians) and health-care providers, and evaluated a comprehensive range of outcomes, including follow-up cardiac biomarkers, cardiac magnetic resonance imaging, echocardiograms, troponin levels, and electrocardiograms. We found that 320 (81%) of 393 patients with a health-care provider assessment were considered recovered from myocarditis, and quality of life measures were similar to pre-pandemic or early pandemic measurements. No single diagnostic test or clinical feature appeared to be associated with recovered status.
Implications of all the available evidence
Myocarditis after mRNA COVID-19 vaccination is rare, but potentially serious. To better understand possible longer term sequalae of myocarditis, continued follow-up is important, particularly for the patients not recovered by at least 90 days since symptom onset. Vaccination remains the most effective way of preventing morbidity and mortality from COVID-19.
Cardiac assessment of patients diagnosed with myocarditis after mRNA COVID-19 vaccination often shows increased cardiac biomarkers (eg, troponin concentrations) and atypical cardiac imaging (eg, echocardiograms), which are similar findings to those shown for viral or acute myocarditis.4, 5 Viral myocarditis unrelated to mRNA COVID-19 vaccination can lead to heart failure, cardiac transplantation, or death.6 Conversely, case descriptions suggest that clinical outcomes following a diagnosis of myocarditis after mRNA COVID-19 vaccination are more favourable than those associated with viral myocarditis, with resolution of symptoms often described at or soon after discharge from a short hospital stay for myocarditis after mRNA COVID-19 vaccination.5, 7, 8, 9 However, data on follow-up prognoses for adolescents and young adults diagnosed with myocarditis after mRNA COVID-19 vaccination are scarce.10, 11, 12 To conduct surveillance, the US Centers for Disease Control and Prevention (CDC) developed a working myocarditis case definition with a team of subspecialists that has been used in several studies.2, 5, 9, 13
In August, 2021, the CDC began follow-up of myocarditis cases to describe medium-term outcomes in the age group with the highest risk of myocarditis after mRNA COVID-19 vaccination diagnosis (ie, individuals aged 12–29 years). We report findings of clinical outcomes and quality of life at least 90 days since the onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults aged 12–29 years.
Methods
Study design and population
In this follow-up surveillance study, we included US patients who were aged 12–29 years at the time of mRNA COVID-19 vaccination and for whom the time to myocarditis symptom onset was more than 90 days since vaccination and a VAERS report was filed between Jan 12, to Nov 5, 2021. VAERS is a national passive surveillance system coadministered by the CDC and the US Food and Drug Administration (FDA).14 Any vaccine recipients, health-care providers, and vaccine providers can submit a report to VAERS. Under emergency use authorisations, vaccination providers are subject to mandatory reporting requirements for certain adverse events after COVID-19 vaccination, including hospitalisation.15, 16 The CDC encourages both vaccination providers and recipients report any clinically significant adverse event, regardless of the plausibility of the vaccine causing the event. Signs and symptoms of adverse events are coded using the Medical Dictionary for Regulatory Activities.
Physicians at the CDC reviewed all identified VAERS reports and available medical records to determine if the case met CDC case definition criteria2 for confirmed or probable myocarditis or myopericarditis (henceforth referred to as myocarditis; appendix 1 p 6).2
This activity was determined to meet the requirements of public health surveillance as defined in Title 45 of the Code of Federal Regulations, part 46.102(l)(2) and no institutional review board approval was needed. Verbal consent was obtained from either adult patients or parents or guardians of minor patients.
Procedures
From Aug 24, 2021, to Jan 12, 2022, we administered a two-component telephone survey to assess patient outcomes at least 90 days since the onset of myocarditis symptoms after mRNA COVID-19 vaccination (further details on the implementation of the survey are specified in appendix 1 [pp 2–3]). The first component administered to adult patients or to the parents or guardians of minor patients ascertained quality of life, previous medical history, need for ongoing medication for myocarditis, and presence of clinical symptoms in the 2 weeks before the date of the survey, including chest pain, shortness of breath, fatigue, and palpitations, hospitalisations, and days of school or work missed in the 2 weeks before the survey.
To assess patient quality of life and overall health after myocarditis diagnosis, we administered the EuroQol 5-dimension, 5-level (EQ-5D-5L) questionnaire that characterises health across five dimensions: mobility, self-care, pain or discomfort, perform usual activities, and anxiety or depression.17, 18 The EQ-5D-5L instrument has been validated for use in people aged 12–29 years19 and was used in this surveillance activity to describe the overall wellbeing of the patient group, not as an indicator of myocarditis recovery. For the quality of life questionnaire, five levels of response, from no problems to extreme problems, were dichotomised as no problems (severity level 1) or any problems (severity levels 2–5).20 Weighted analysis converted patient responses to a numerical scale ranging from 0 (equivalent to death) to 1 (full health; appendix 1 p 7).21 Overall health was self-rated by patients using the EuroQol visual analog scale (EQ-VAS), with scores ranging from 0 to 100 (100 representing the best possible health and 0 representing the worst possible health). We compared the patients' EQ-5D-5L survey responses with published EQ-5D-5L survey results of 18–24-year-old US population respondents before and during the COVID-19 pandemic.22, 23
The second component of the two-part survey was a survey of health-care providers who provided care to eligible patients for this study with myocarditis after mRNA COVID-19 vaccination, which ascertained patient cardiac health and functional status (appendix 2 pp 1–19). Follow-up assessments of cardiac health after the initial myocarditis diagnosis or hospitalisation for myocarditis after COVID-19 vaccination included findings from electrocardiograms, echocardiograms, cardiac MRIs, troponin concentrations, exercise stress testing, and ambulatory rhythm monitoring (appendix 1 pp 1–2). Assessments of functional status included ongoing treatment or health-care provider-recommended restrictions on physical activity.
In both parts of the survey, health-care providers and patients were asked about any previous SARS-CoV-2 infection in the patient before the diagnosis of myocarditis, as determined by a positive laboratory-confirmed test; however, we did not ask about the severity of infection. To assess myocarditis recovery, health-care providers were asked the following: based on your clinical assessment and any testing information, please describe the patient's cardiac recovery status as of the date of your last visit or consultation (compared with the time of initial myocarditis diagnosis; appendix 2 p 4). Survey options were fully recovered, probably fully recovered, improved but not fully recovered, same cardiac status as at the initial diagnosis (ie, no worse or no better), worse cardiac status, unsure, or declined to answer. For this evaluation, patients determined to be fully or probably fully recovered by the health-care provider were designated recovered and patients deemed to be improved but not fully recovered or with the same cardiac status at initial diagnosis were designated not recovered. CDC's Clinical Immunization Safety Assessment (CISA) Project provided technical input for survey development.24
Statistical analysis
To assess non-response bias, demographic and clinical characteristics of survey respondents were compared with characteristics of non-respondents (eg, those who were unreachable), including age, sex (male or female), self-reported race and ethnicity, geographical census region, and findings from initial echocardiograms (appendix 1 p 8). Additionally, we compared VAERS reporter type (health-care provider or patient), geographical census region, age, sex, initial echocardiogram findings, and race and ethnicity among survey respondents and non-respondents.
Descriptive analyses were conducted to determine frequencies, percentages, means, and SDs to characterise cases of myocarditis after mRNA COVID-19 vaccination by age, sex, time elapsed since symptom onset, quality of life measures, and clinical outcome. All cases meeting the CDC definition for myocarditis were included, regardless of whether a suspected alternative cause was identified. For assessment of recovery status, we conducted a sensitivity analysis limited to individuals with symptom onset at least 7 days after vaccination and without a suspected alternative cause identified by their health-care provider (appendix 1 p 4). Missing values were not imputed. To assess statistical significance of comparisons, we used the χ2 test for categorical variables, Fisher's exact test for variables with small sample sizes (n<5), and the Student's t-test or the Mann–Whitney U test for continuous variables. All analyses were performed with R software (version 4.1.1). p<0·05 was considered to be statistically significant.
Role of the funding source
The funder led data collection, data analysis, data interpretation, writing, and submission of the manuscript.
Results
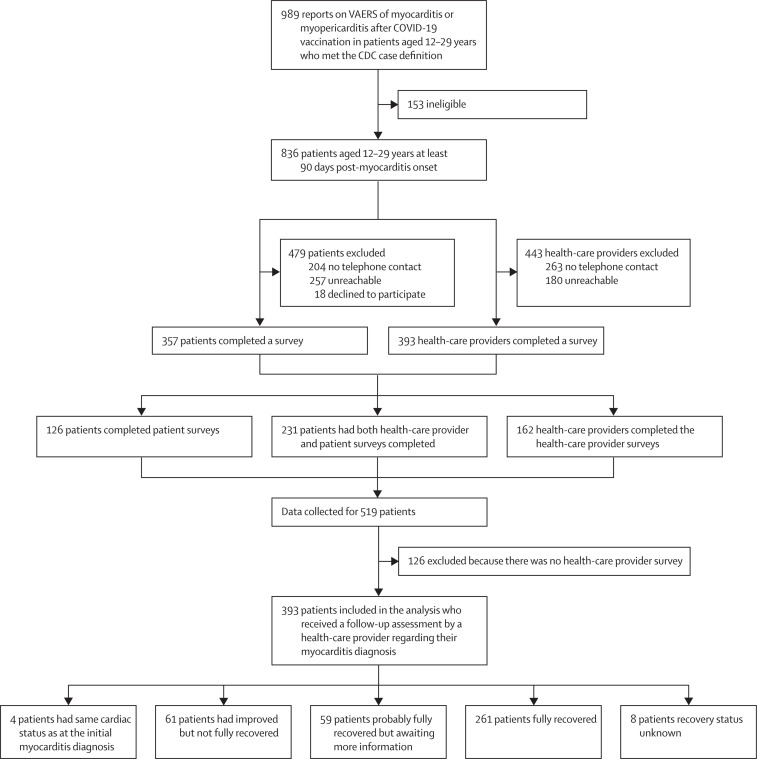
Between Jan 12 and Nov 5, 2021, 989 cases of myocarditis after mRNA COVID-19 vaccination in patients aged 12–29 years were reported to VAERS and met the CDC's case definition for myocarditis. Of these, 836 (85%) patients were at least 90 days post-myocarditis onset (figure 1 ). Of the 836 patients, 204 (24%) patients had no telephone number available for contact and 257 (31%) patients were unreachable. Of the remaining 375 patients, 357 (95%) patients consented to the survey and 18 (5%) patients declined. Between Aug 24, 2021, and Jan 12, 2022, we contacted and collected data for 519 (62%) of the 836 eligible patients: 126 patients via patient survey only, 162 patients via health-care provider survey only, and 231 patients via both the patient and health-care provider survey (figure 1). Median interval from myocarditis onset to survey completion was 143 days (IQR 131–162) for patients and 191 days (170–216) for health-care providers. We found no significant differences in VAERS reporter type (health-care provider or patient), geographical census region, age, sex, initial echocardiogram findings, or race or ethnicity in patients surveyed compared with patients who were not surveyed (appendix 1 p 8). In a subset of patients with abnormal echocardiograms, the abnormality identified was a left ventricular ejection fraction (LVEF) of less than 50%. Of the 100 survey respondents with LVEF values recorded at their initial diagnosis, 33 (33%) had LVEF values less than 50%, which was not statistically different from the results in non-respondents (27 [42%] of 65 non-respondents; χ2=1·24, p=0·265).
Figure 1.
Survey participation of patients with myocarditis after mRNA COVID-19 vaccination reported to VAERS at least 90 days since symptom onset
CDC=US Centers for Disease Control and Prevention. VAERS=Vaccine Adverse Event Reporting System.
For 393 (47%) of 836 patients, health-care providers were contacted; 241 (61%) of 393 were cardiologists. At the time of the survey, health-care providers considered 320 (81%) of 393 patients to be recovered: 261 (66%) patients were considered fully recovered and 59 (15%) patients were considered to be probably recovered but awaiting further information. An additional 61 (16%) patients had improved and four patients had the same cardiac status as at the initial myocarditis diagnosis; these 65 patients were categorised as not fully recovered (figure 1). The cumulative proportion of patients considered recovered in the time (weeks) since the last health-care provider encounter is shown in appendix 1 (p 15). The median time from myocarditis symptom onset to the last health-care provider encounter for patients who were considered probably fully recovered or fully recovered was 92 days (IQR 43–133), and for patients who were considered fully recovered the median time was 84 days (36–135).
Most patients were male (457 [88%] of 519 patients) and White non-Hispanic (274 [53%]), and the median age of all patients was 17 years (IQR 15–22; table 1 ). 98 (19%) of 519 patients were Hispanic of any race. There was no notable difference between recovered individuals compared with individuals who were not recovered across any ethnic or racial groups. Overall, patients considered to be recovered and not recovered from myocarditis were similar with respect to age (median age 17 years [IQR 15–21] for patients considered recovered vs 17 years [15–21] for those considered not recovered) and sex (290 [91%] male individuals who were considered recovered vs 56 [86%] of male individuals who were considered not to be recovered, and 30 [9%] female individuals who were considered recovered vs 9 [14%] of female individuals who were not considered recovered). The median time from illness onset to health-care provider interview for the 320 (81%) of 393 individuals who were considered recovered was 189 days (IQR 167–214), and for the 61 (16%) of 393 patients who were considered improved but not fully recovered the median time was 195 days (179–195).
Table 1.
Demographic characteristics and symptoms of patients by provider-reported recovery status from myocarditis after mRNA COVID-19 vaccination
| Patients fully or probably fully recovered (n=320) | Patients not recovered (n=65) | All patients (n=519) | p value | ||
|---|---|---|---|---|---|
| Median age, years (IQR) | 17 (15–21) | 17 (15–21) | 17 (15–22) | .. | |
| Age group, years | |||||
| 12–14 | 58 (18%) | 9 (14%) | 92 (18%) | 0·84 | |
| 15–19 | 160 (50%) | 35 (54%) | 245 (47%) | .. | |
| 20–24 | 69 (22%) | 15 (23%) | 120 (23%) | .. | |
| 25–29 | 33 (10%) | 6 (%9) | 62 (12%) | .. | |
| Sex | |||||
| Male | 290 (91%) | 56 (86%) | 457 (88%) | 0·39 | |
| Female | 30 (9%) | 9 (14%) | 61 (12) | .. | |
| Unknown | 0 | 0 | 1 (<1%) | .. | |
| Race, ethnicity | |||||
| White, non-Hispanic | 182 (57%) | 32 (49%) | 274 (53%) | 0·32 | |
| Asian, non-Hispanic | 16 (5%) | 1 (2%) | 25 (5%) | 0·33 | |
| Black, non-Hispanic | 10 (3%) | 2 (3%) | 16 (3%) | 0·71 | |
| Other race, non-Hispanic | 11 (3%) | 0 | 12 (2%) | 0·22 | |
| Multiple races, non-Hispanic | 10 (3%) | 1 (2%) | 12 (2%) | 0·69 | |
| American Indian or Alaskan native, non-Hispanic | 1 (<1%) | 0 | 1 (<1%) | .. | |
| Hispanic | 53 (17%) | 14 (22%) | 98 (19%) | 0·33 | |
| Unknown | 37 (12%) | 13 (20%) | 81 (16%) | .. | |
| Previous SARS-CoV-2 infection* | 28 (9%) | 4 (6%) | 48 (9%) | 0·61 | |
| Received two COVID-19 vaccine doses | 278 (87%) | 58 (89%) | 448 (86%) | 0·75 | |
| Underlying medical condition | |||||
| At least one condition, excluding obesity | 63 (20%) | 16 (25%) | 99 (19%) | 0·46 | |
| Asthma† | 29 (9%) | 4 (6%) | 41 (8%) | 0·60 | |
| Autoimmune disease | 10 (3%) | 1 (2%) | 13 (3%) | 0·69 | |
| Arrhythmia | 9 (3%) | 1 (2%) | 16 (3%) | 0·86 | |
| Congenital heart disease | 8 (2%) | 2 (3%) | 10 (2%) | 0·68 | |
| Genetic or chromosomal | 7 (2%) | 8 (12%) | 15 (3%) | 0·0005 | |
| Previous heart failure | 1 (<1%) | 1 (2%) | 2 (<1%) | 0·31 | |
| Kawasaki disease | 1 (<1%) | 0 | 2 (<1%) | .. | |
| Myocarditis | 4 (1%) | 1 (2%) | 7 (1%) | .. | |
| Type 1 diabetes | 1 (<1%) | 1 (2%) | 3 (1%) | 0·31 | |
| BMI-based obesity‡ | 80/291 (27%) | 16/63 (25%) | 99/359 (28%) | 0·86 | |
| Patient-reported symptoms in the patient survey | n=195§ | n=28§ | n=357 | .. | |
| At least one symptom | 94 (48%) | 18 (64%) | 178 (50%) | 0·16 | |
| Chest pain or discomfort | 55 (28%) | 13 (46%) | 113 (32%) | 0·082 | |
| Chest pain or discomfort while resting | 45 (23%) | 11 (39%) | 92 (26%) | 0·011 | |
| Fatigue | 40 (21%) | 12 (43%) | 89 (25%) | 0·018 | |
| Fatigue while resting | 28 (14%) | 10 (36%) | 63 (18%) | 0·012 | |
| Shortness of breath | 38 (19%) | 9 (32%) | 80 (22%) | 0·28 | |
| Shortness of breath while resting | 15 (8%) | 4 (14%) | 38 (11%) | 0·42 | |
| Heart palpitations | 36 (18%) | 6 (21%) | 77 (22%) | 0·71 | |
| Heart palpitations while resting | 28 (14%) | 5 (18%) | 59 (17%) | 0·84 | |
Data are n (%) unless specified otherwise. Data are based on the completion of 357 patient surveys, 393 provider surveys, and 231 linked surveys, resulting in 519 patients for which data were collected. Health-care provider determination of patient myocarditis recovery was provided for 393 patients, of whom 320 were considered fully or probably fully recovered and 65 were not considered recovered (and eight patients had an undetermined recovery status; figure 1). Based on the last patient encounter, health-care providers reported that 62 (16%) of 393 patients had at least one symptom that might occur with myocarditis.
Previous SARS-CoV-2 infection before the diagnosis of myocarditis, as determined by a positive laboratory-confirmed test; the interval from a positive SARS-CoV-2 test result to mRNA COVID-19 vaccination was a median of 139 days (IQR 92–198; n=15 with a date provided).
Asthma, for which prescription medicine within the past 2 years was needed; if asthma was only with exercise, it was not recorded.
BMI was calculated using measurements obtained at the earliest follow-up visit: the formula weight (pounds) / [height (inches)]2 × 703. The denominators reflect the number of individuals with data available to calculate BMI.
All patients who self-reported symptoms in the patient survey and had a provider-reported recovery status.
In the 2 weeks before the survey date, 178 (50%) of 357 patients reported having at least one symptom that might occur with myocarditis (chest pain or discomfort, fatigue, shortness of breath, or palpitations). Patients who were not considered recovered from myocarditis more frequently reported fatigue than did patients who were considered recovered (12 [43%] vs 40 [21%]; p=0·018; table 1). By contrast, based on the last patient encounter, health-care providers reported that 62 (16%) of 393 patients at least one symptom that might occur with myocarditis (table 1).
Of 357 patients surveyed, 267 (75%) were enrolled in school or in paid employment; 43 (16%) of whom reported missing school or workdays in the 2 weeks before the survey date. Of those 43 patients, 15 (35%) believed it was associated with myocarditis.
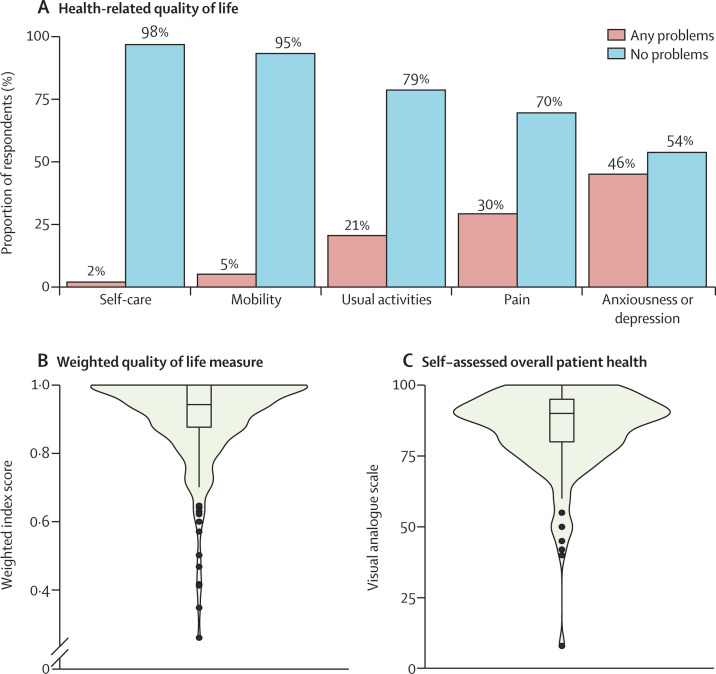
Of 357 patients surveyed, 249 (71%) consented to completing both the EQ-5D-5L and EQ-VAS components of the patient survey. Of 249 patients, four (2%) reported problems with self-care, 13 (5%) with mobility, 49 (21%) with performing usual activities, 74 (30%) with pain, and 114 (46%) with anxiousness or depression (figure 2A ). Overall, patients reported having good health, reflected by the high median weighted index score (0·94; IQR 0·88–1·00) and median overall health status (EQ-VAS) score (90; 80–95; figure 2B, C).
Figure 2.
Self-assessment of health-related quality of life among patients with myocarditis after mRNA COVID-19 vaccination
(A) Bar plot of health-related quality of life among patients. Patients were administered the EuroQol 5-dimension 5-severity level questionnaire; for analysis, the five health-related dimensions were dichotomised into the frequency of problems (severity levels 2–5) and no problems (level 1). (B) Violin plot of weighted quality of life measure (using value weights in appendix 1 p 7) converted from each patient health profile from (A) to an index score between 1 (perfect health) and 0 (equivalent to death). (C) Violin plot of patient self-assessed overall health on a scale from 0 to 100 (with 100 representing best possible health and 0 representing the worst possible health). The denominator for the EuroQol questionnaire was 249 respondents. In the violin plots (B, C), the limits of the boxes denote IQR and the horizontal line denotes median values. Whisker endpoints are equal to the maximum and minimum values below or above the median plus or minus 1·5 times the IQR. The width of the outer shape around the box plots indicates the probability density of values or responses with a given result.
The mean EQ-5D-5L weighted utility score in our group (0·91 [SD 0·13]) was significantly higher than that for US respondents aged 18–24 years who completed a EQ-5D-5L questionnaire during the pandemic, as reported by Hay and colleagues22 (0·75 [0·28]; p<0·0001). Mean EQ-5D-5L weighted utility scores from the pre-pandemic timepoint among US respondents aged 18–24 years have also been reported by Jiang and colleagues, from face-to-face surveys and online surveys.23 The weighted score from our survey was not significantly different to that obtained in the face-to-face surveys (0·92 [0·13]), but our score was significantly higher than that from the online surveys (0·84 [0·18]; p<0·0001).
Most patients were admitted to hospital after an initial diagnosis of myocarditis (484 [93%] of 519 patients). Of these 484 patients, 393 (81%) patients had information on level of care, according to the health-care provider surveys; 99 (25%) of these 393 patients were treated in an intensive care unit and one (<1%) patient required extracorporeal membrane oxygenation (table 2 ). To our knowledge, no deaths occurred during follow-up among the patients eligible for the survey. Six (2%) of 357 patients who self-reported re-admission to hospital had a hospital admission because of an adverse event after myocarditis treatment (n=3; adverse reactions to intravenous immune globulin) or had any cardiac abnormality identified (n=3; appendix 1 p 6); all patients were discharged within 1 week.
Table 2.
Level of care, testing, and treatment by recovery status among patients with myocarditis after mRNA COVID-19 vaccination
| Patients fully or probably fully recovered (n=320) | Patients not recovered (n=65) | Patient recovery unknown (n=8) | p value | ||
|---|---|---|---|---|---|
| Highest level of care | |||||
| Hospitalised with no intensive care | 210 (66%) | 40 (62%) | 4 (50%) | 0·66 | |
| Hospitalised with intensive care | 85 (27%) | 12 (18%) | 2 (25%) | 0·22 | |
| Not hospitalised, managed as outpatient | 14 (4%) | 9 (14%) | 2 (25%) | 0·0074 | |
| Intensive care with ECMO | 0 | 1 (2%) | 0 | .. | |
| Unknown* | 11 (3%) | 3 (5%) | 0 | .. | |
| Patient restrictions on physical activity | |||||
| At time of initial myocarditis hospitalisation or diagnosis | 267 (83%) | 53 (82%) | 6 (75%) | 0·84 | |
| At time of last health-care provider follow-up | 91 (28%) | 31 (48%) | 3 (38%) | 0·0038 | |
| Cleared for physical activity and date of clearance known | 160/267 (60%) | 16/53 (30%) | 2/6 (33%) | 0·026 | |
| Median days from myocarditis onset to physical activity clearance (IQR) | 104 (63–135) | 114 (73–156) | 80 | 0·12 | |
| Patient cardiac MRI | |||||
| At time of initial myocarditis hospitalisation or diagnosis | 137 (43%) | 32 (49%) | 0 | 0·56 | |
| At time of healthcare provider follow-up | 114 (36%) | 36 (55%) | 1 (13%) | 0·0023 | |
| Patient echocardiogram | |||||
| At time of initial myocarditis hospitalisation or diagnosis | 257 (80%) | 55 (85%) | 7 (88%) | 0·53 | |
| At time of health care provider follow-up | 230 (72%) | 51 (78%) | 3 (38%) | 0·35 | |
| Patient troponin | |||||
| At time of initial myocarditis hospitalisation or diagnosis | 318 (99%) | 65 (100%) | 7 (88%) | 0·76 | |
| At time of healthcare provider follow-up | 166 (52%) | 33 (51%) | 1 (13%) | 0·86 | |
| Patient electrocardiogram | |||||
| At time of initial myocarditis hospitalisation or diagnosis | 210 (66%) | 34 (52%) | 6 (75%) | 0·059 | |
| At time of health-care provider follow-up | 251 (78%) | 55 (85%) | 5 (63%) | 0·34 | |
| Patient exercise stress test | |||||
| At time of health-care provider follow-up | 91 (28%) | 16 (25%) | 2 (25%) | 0·63 | |
| Patient ambulatory rhythm monitoring | |||||
| At time of health-care provider follow-up | 86 (27%) | 18 (28%) | 1 (13%) | 0·89 | |
| Prescribed medication at last provider follow-up | 68 (21%) | 33 (51%) | 3 (38%) | <0·0001 | |
| Daily medication types prescribed† | |||||
| Colchicine | 31 (10%) | 17 (26%) | 0 | 0·0005 | |
| β-blocker | 29 (9%) | 12 (18%) | 1 (13%) | 0·043 | |
| Non-steroidal anti-inflammatory | 21 (7%) | 9 (14%) | 1 (13%) | 0·081 | |
| Aspirin | 9 (3%) | 5 (8%) | 1 (13%) | 0·069 | |
| Angiotensin-converting enzyme inhibitor | 8 (3%) | 6 (9%) | 0 | 0·018 | |
| Diuretic | 3 (1%) | 3 (5%) | 0 | 0·063 | |
| Corticosteroid | 1 (<1%) | 3 (5%) | 0 | 0·016 | |
| Angiotensin II receptor blocker | 2 (1%) | 7 (11%) | 0 | <0·0001 | |
| Other medication | 3 (1%) | 2 (3%) | 1 (13%) | 0·19 | |
Data are n (%) unless otherwise specified. Data are based on the completion of 393 health-care provider surveys. Health-care provider determination of patient myocarditis recovery was provided for 393 patients, of whom 320 were considered fully or probably fully recovered, 65 were not considered recovered, and the health-care provider was unsure of the recovery status in eight patients, as shown in figure 1. Follow-up cardiac testing was performed, although the result of the test was not available for troponin concentration in three patients, echocardiogram in five patients, cardiac MRI in seven patients, exercise stress testing in five patients, and ambulatory rhythm monitoring in nine patients.
Some data were unknown because not all health-care providers who were surveyed knew the level of care the patient received as not all cared for the patient while they were in the hospital.
The denominator is based on patients who, as of their last health-care provider encounter, were recommended to use daily medication. ECMO=extracorporeal membrane oxygenation.
At follow-up, fewer patients had restrictions on physical activity than at initial diagnosis, and 34 (52%) of 65 individuals with restrictions on physical activity at the time of follow-up who were not considered recovered were cleared for all physical activity; 31 (48%) individuals still had restrictions (table 2). Median interval from myocarditis onset to approval for all physical activity was 98 days (IQR 57–134; table 2).
104 (26%) of 393 patients were prescribed daily medications related to myocarditis at the last health-care provider encounter (table 2). Patients who were not considered recovered from myocarditis were more frequently prescribed daily medication than were patients who were considered to be recovered. The most prescribed medications, as of the last health-care provider follow-up, were colchicine, β-blockers, and non-steroidal anti-inflammatory drugs (table 2).
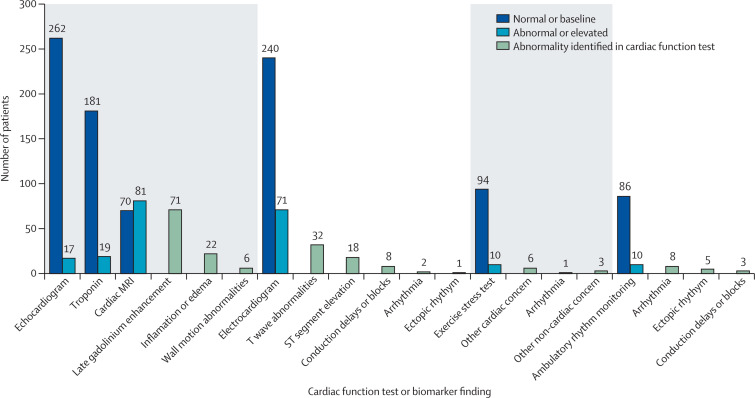
At follow-up, most patients had improvements in diagnostic marker and imaging data, including normal or back-to-baseline troponin concentrations, echocardiograms, exercise stress testing, ambulatory rhythm monitoring, and electrocardiograms (figure 3 ). In the ten patients with abnormal ambulatory rhythm monitoring results, we found eight (80%) had atrial, supraventricular, or ventricular arrhythmia, three (30%) had a conduction delay or block, and five (50%) had frequent atrial or ventricular ectopy. Of these 10 patients, three (30%) had evidence of late gadolinium enhancement on follow-up cardiac MRI; of the three with evidence of late gadolinium enhancement, two (67%) had evidence of an atrial, supraventricular, or ventricular arrhythmia. Among the 151 patients who had cardiac MRIs during outpatient follow-up, 81 (54%) patients had one or more abnormalities. Abnormal cardiac MRI findings included the presence of late gadolinium enhancement (71 [47%] patients), inflammation or oedema (22 [15%] patients), or wall motion abnormalities (six [4%] patients; figure 3, appendix 1 p 9). Evidence of ongoing myocarditis, defined by both late gadolinium enhancement and oedema using modified Lake Louise criteria,25 was uncommon (20 [13%] of 151 patients; appendix 1 p 9). Median interval from symptom onset to evidence of ongoing myocarditis was 26 days (IQR, 9–94) and from symptom onset to evidence of late gadolinium enhancement was 109 days (58–163; appendix 1 p 10). Of the 67 patients with late gadolinium enhancement or evidence of ongoing myocarditis, additional follow-up testing indicated abnormal echocardiograms in five (7%) patients, abnormal troponin concentrations in five (7%) patients, and abnormal electrocardiograms in 14 (21%) patients (appendix 1 p 13).
Figure 3.
Follow-up functional status, biomarker testing, and cardiac imaging in patients at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination
Cardiac biomarker testing or imaging findings are from the health-care provider surveys completed for 393 patients. Not all patients received diagnostic testing or imaging and the denominator for each follow-up test is equal to the sum of the normal and abnormal findings; the type of abnormalities identified are not mutually exclusive.
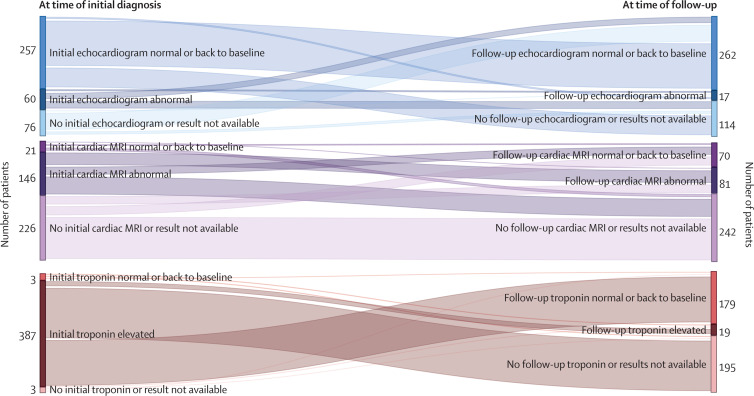
In the subset of patients with abnormal findings at the time of myocarditis diagnosis, abnormal diagnostic markers or abnormal imaging were also observed at follow-up in seven (12%) of 60 with initial abnormal echocardiograms, 19 (5%) of 387 with initial elevated troponin levels, and 47 (32%) of 146 with initial abnormal cardiac MRIs (figure 4 ). There was substantial heterogeneity in cardiac biomarkers, imaging, and patient functional status between patients considered recovered or not recovered from myocarditis (appendix 1 p 16). All cardiac test results (ie, echocardiogram, electrocardiogram, cardiac MRI, and troponin) were available for follow-up review in only 199 (62%) of 320 patients considered recovered, 44 (68%) of 65 considered not recovered, and three (38%) of eight with an unknown recovery status.
Figure 4.
Changes in cardiac biomarker and imaging from the initial encounter and the health-care provider follow-up
Testing, including echocardiograms, cardiac MRIs, and troponin concentrations, performed at the time of initial myocarditis diagnosis and at follow-up are not necessarily matched because each patient had testing (or not) at the discretion of the treating health-care providers.
Discussion
We ascertained outcomes at least 90 days since onset of myocarditis among 519 patients aged 12–29 years who received an mRNA COVID-19 vaccination and met the CDC case definition for myocarditis. Most (81%) patients for whom a follow-up health-care provider survey was completed were considered recovered from myocarditis, and most self-reported overall good health on the EQ-5D-5L. Readmissions to hospital were uncommon, and no deaths were identified during the follow-up period. Myocarditis after mRNA COVID-19 vaccination is rare yet potentially serious, and although most patients were considered recovered by health-care providers at least 90 days since onset, nearly half of patients continued to self-report symptoms, including chest pain, and a quarter were prescribed daily cardiac medications. These findings suggest that continued follow-up and assessment of myocarditis after mRNA COVID-19 vaccination is needed to more fully understand recovery after vaccine-associated myocarditis.
From a clinical standpoint, our findings suggest that myocarditis after mRNA COVID-19 vaccination could have a more favourable prognosis than myocarditis after viral infection, based on data available from the pre-COVID-19 period. In a published study of outcomes in children within 90 days of viral or acute myocarditis onset in the USA, 119 (23%) of 514 individuals required extracorporeal membrane oxygenation or a ventricular-assisted device and 58 (11%) of 514 individuals required cardiac transplant or died.6 In a recent nationwide study in Denmark of adults, 90-day all-cause mortality among those with acute myocarditis was 4·9%.26 A longer term follow-up study of acute myocarditis among older adults (median age 34 years [IQR 24–42]) in Italy observed cardiac mortality or heart transplant rates at 1 year and 5 years of 3·0% and 4·1%, respectively, although complicated cases had rates of adverse cardiac outcomes that were several times higher.27 In contrast with these studies, we found that only four (1%) patients had the same cardiac status as at the initial myocarditis diagnosis (ie, did not improve but did not worsen), whereas more than 95% (381 of 393 patients) showed improvement or recovery. Consistent with our findings, a recent report comparing classic myocarditis to COVID-19 vaccine-related myocarditis in individuals aged younger than 21 years observed similar clinical presentations and found COVID-19 vaccine-related myocarditis had better outcomes and a more rapid cardiac recovery.28
Published data for health-related quality of life in the USA among individuals aged 18–24 years, before the COVID-19 pandemic, showed that 45 (42%) of 107 individuals reported anxiety or depression and 35 (33%) of 107 individuals reported pain or discomfort.23 More recent quality of life measure data from Hay and colleagues22 among US respondents during the early stages of the COVID-19 pandemic showed that 1653 (60·2%) of 2746 individuals reported anxiety or depression.22 Consistent with these observations, we found that patients with myocarditis after mRNA COVID-19 vaccination reported similar or better quality of life measures than the general US population, with fewer patients with myocarditis reporting anxiety or depression than did individuals during the pandemic (46% [114/249] vs 60·2% [1653/2746]). However, absence of age-specific data in the previous analyses22, 23 precluded any further statistical comparisons in this study.
Despite clinical improvements and normalisation of most diagnostic test results, as noted by health-care providers, half of patients (178/357) surveyed continued to report at least one symptom potentially associated with myocarditis after COVID-19 vaccination. One possible explanation for the persistence of symptoms is that approximately 50% of patients reported depression or anxiety, conditions that can manifest as symptoms associated with myocarditis, such as chest pain or palpitations.29
The meaning of the cardiac MRI findings among the subset of patients who received cardiac imaging is unclear. Evidence of ongoing myocarditis on follow-up cardiac MRIs based on modified Lake Louise criteria25 was uncommon. However, consistent with the few published case series of myocarditis after mRNA COVID-19 vaccination, we observed that nearly half of patients (71/151) with follow-up cardiac MRIs had residual late gadolinium enhancement, suggestive of myocardial scarring.10, 11, 12, 25 We did not note the degree of late gadolinium enhancement identified during follow-up, but a recent study that assessed serial cardiac MRIs in patients younger than 19 years with myocarditis after COVID-19 vaccination and persistent late gadolinium enhancement showed improvement over time.30 In a small subset of patients, initial cardiac imaging at diagnosis was normal but follow-up imaging was abnormal. It is possible that clinical findings in these patients continued to evolve after diagnosis. Another possibility is that the initial and follow-up imaging results were evaluated by different health-care providers, who had varying interpretations.
In previous studies during the pre-COVID era, cardiac scarring related to myocarditis on follow-up MRI was not uncommon, yet its clinical significance has remained controversial.10, 31, 32, 33 Although late gadolinium enhancement during the acute episode of myocarditis has been shown in children and adults to be a possible indication of future adverse cardiac events, including arrythmias, extracorporeal membrane oxygenation, transplantation, and death,31, 34, 35, 36 the importance of late gadolinium enhancement noted on follow-up cardiac MRIs in patients with viral myocarditis is unclear.31 Indeed, guidelines regarding clearance of athletes for competitive sports after myocarditis acknowledge the unclear role of cardiac MRI in the follow-up of such patients.37
Our follow-up evaluation is subject to several limitations. First, and most importantly, the absence of clear clinical practice guidelines for the outpatient follow-up of myocarditis meant that comparing clinical course among patients was challenging, as no standard level of care was provided. Therefore, some data pertinent to understanding potential residual symptoms and disease were unavailable. We found substantial heterogeneity in the initial evaluation and follow-up of patients, particularly in the cardiac diagnostic imaging received. Current guidelines recommend restricting patients with myocarditis (eg, athletes) from competitive sports for 3–6 months,38 although we noted some variability among health-care providers in clearing patients for a return to all physical activity. There are no standard criteria for myocarditis recovery, and we did not identify any clinical feature or diagnostic test results associated with recovery status in the patients we evaluated. Forthcoming expert guidelines regarding the follow-up management and testing of patients with myocarditis could help standardise care in the future.
A second limitation is the passive (or spontaneous) nature of VAERS reporting. Some US cases of myocarditis associated with mRNA COVID-19 vaccination will not have been reported; however, it is unclear how cases reported or not reported initially to VAERS could differ. Selection bias is a possible limitation in any survey activity. Third, although 519 (62%) of the 836 eligible patients with myocarditis who filed a report to VAERS were included in this follow-up evaluation, 275 (33%) declined to participate or were unreachable. Reassuringly, we found no significant differences in the age, sex, race, or census region of respondents compared with non-respondents, although our findings might not be generalisable to all US individuals aged 12–29 years who develop myocarditis after mRNA COVID-19 vaccination due to the small sample size. Fourth, we relied on health-care provider reports for all diagnostic data results. Unlike prospective studies, we did not have access to central interpretation of tests (eg, electrocardiograms, echocardiograms, and cardiac MRIs). Although this limitation probably introduces some variability into the findings, it also reflects real-world practice and data appeared not to be missing at random. A fifth limitation is the absence of a control group for the analysis of patient symptoms. Control groups are important for contextualising symptoms. For example, in a study of long COVID among children and adolescents (aged <21 years) in the USA, Rao and colleagues39 found that 41·9% of patients with a history of COVID-19 reported at least one symptom of post-acute sequelae of SARS-CoV-2 infection, as did 38·2% of a control group without a history of COVID-19. Although no pre-myocarditis measures were available for our group of patients with myocarditis, we found that quality of life measures among those with COVID-19 vaccine-associated myocarditis at follow-up were similar to or better than those of contemporary populations studied before or early in the pandemic.22, 23 Finally, given limitations on the ability to determine causes of myocarditis other than mRNA vaccination, we included all cases in our analyses.
In summary, after at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination, 81% of patients were considered recovered by their health-care provider. At the time of follow-up, these patients reported quality of life measures similar to pre-pandemic reports among individuals of similar ages in the USA. 50% of patients reported at least one symptom at follow-up. Among a subset of 151 patients who had follow-up cardiac MRI results, 54% had an abnormal finding. The CDC is conducting additional follow-up on patients who were not considered recovered at least 12 months since symptom onset, to better understand their longer term outcomes.
Data sharing
Patient data from this public health investigation are not available to be shared publicly. Limited, deidentified VAERS data are publicly available online.
This online publication has been corrected. The corrected version first appeared at thelancet.com/child-adolescent on November 16, 2022 and further corrections were made on November 30, 2022
Declaration of interests
MEO reports a grant from the US National Institutes of Health (NIH). BC reports a Clinical and Translational Science grant from NIH and participation on the data and safety advisory board for Astellas. EBW reports a grant from Moderna, Pfizer, Sequiris, and NIH, and participation on the data and safety advisory board for Vaxcyte and Iliad Biotechnologies.
Acknowledgments
Acknowledgments
We thank all the patients, parents, guardians, and health-care providers who participated in the surveys. We also thank the state and local health departments that assisted in data collection. We thank all health-care providers who made reports to VAERS and who were involved in the care of the patients described in this investigation. We acknowledge the technical contributions of Dr Michael J Smith. The US Centers for Disease Control and Prevention (CDC) provided financial support for the CDC authors' salaries and project materials. This work was also supported by the CDC CISA Project contracts 200–2012–50430–0005 to Vanderbilt University Medical Center, 200–2012–53663–0011 to Duke University, 200–2012–53661–0008 to Cincinnati Children's Hospital Medical Center, and 200–2012–53709–0007 to Boston Medical Center. Other authors received salary support from their institutions. The findings and conclusions in this Article are those of the authors and do not necessarily represent the official position of the CDC. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Contributors
MEO, KRB, MJC, MMC, JS, JRS, SSM, JMD, CBC, EBW, DKS, and TTS were responsible for project conceptualisation. IK, JW, PM, and JS were responsible for data curation and data analysis. KRB, MMC, MG, KS, BR, ALV, SSM, AA, AR-C, SN, SSM, DKS, TTS, and SVB provided project administration, supervision, and resources. IK, SVB, MEO, DKS, and TTS wrote the original draft. All authors edited the final version. IK and JW had access to all the data and had final responsibility for the decision to submit for publication.
Contributor Information
Myocarditis Outcomes After mRNA COVID-19 Vaccination Investigators and the CDC COVID-19 Response Team:
Paula Campbell, Chidera Anugwom, Colenda Arvelo Jefferson, Kimberly Badger, Nastocia Bafford, Chandra Barnes, Stephanie Boles, Emory Collins, Mitesh Desai, Theresa Dulski, Barbara Dyleski, Kathryn Edwards, Melanie Feyereisen, Stephanie Gonsahn, Tchernavia Gregory, Jyothi Gunta, Kara Jacobs Slifka, Charlotte Kabore, Bryan K. Kapella, Susan Karol, Kalah Kennebrew, Nancy Kluisza, Sean Lang, Labretta Lanier Gholston, Marcella Law, Jennifer Lehman, Jacek M. Mazurek, Henraya McGruder, Kiara McNamara, Maria-Luisa Moore, Pedro Moro, John F. Moroney, Oidda Museru, Cassandra Nale, Andi Neiman, Kim Newsome, Erika Odom, Brooke Pantazides, Suchita Patel, Agam Rao, Laura Reynolds, Sonya Robinson, Frederick L. Ruberg, Tammy Schaeffer, Dipesh Solanky, Laurence Sperling, Toscha Stanley, Regina Sullivan, Allan Taylor, Kimberly Thomas, Shayle Thompson, Jigsa Tola, Cuc H. Tran, Steven Wiersma, and Kimberly Works
Supplementary Materials
References
- 1.WHO COVID-19 subcommittee of the World Health Organization Global Advisory Committee on Vaccine Safety: updated guidance regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. 2021. https://www.who.int/news/item/09-07-2021-gacvs-guidance-myocarditis-pericarditis-covid-19-mrna-vaccines
- 2.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January, 2021–January, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December, 2020 to August, 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:622–627. doi: 10.1161/CIRCOUTCOMES.112.965749. [DOI] [PubMed] [Google Scholar]
- 7.Sinagra G, Anzini M, Pereira NL, et al. Myocarditis in clinical practice. Mayo Clin Proc. 2016;91:1256–1266. doi: 10.1016/j.mayocp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145:345–356. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 10.Amir G, Rotstein A, Razon Y, et al. CMR imaging 6 months after myocarditis associated with the BNT162b2 mRNA COVID-19 vaccine. Pediatr Cardiol. 2022 doi: 10.1007/s00246-022-02878-0. published online March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fronza M, Thavendiranathan P, Karur GR, et al. Cardiac MRI and clinical follow-up in COVID-19 vaccine-associated myocarditis. Radiology. 2022 doi: 10.1148/radiol.220802. published online May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148 doi: 10.1542/peds.2021-053427. [DOI] [PubMed] [Google Scholar]
- 13.Goddard K, Lewis N, Fireman B, et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022 doi: 10.1016/j.vaccine.2022.07.007. published online July 12. 10.1016/j.vaccine.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Fact Sheet for Healthcare Providers Administering Vaccine (vaccination providers): Emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) 2021. https://www.fda.gov/media/159307/download/
- 16.US Food and Drug Administration (FDA) Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency use authorization (EUA) of Pfizer-Biontech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) 2021. (https://www.fda.gov/media/144413/download/)
- 17.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22:1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. 2017;15:127–137. doi: 10.1007/s40258-017-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devlin N, Parkin D, Janssen B. Springer Nature; Cham: 2020. Methods for analysing and reporting EQ-5D data. [PubMed] [Google Scholar]
- 21.Pickard AS, Law EH, Jiang R, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value Health. 2019;22:931–941. doi: 10.1016/j.jval.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Hay JW, Gong CL, Jiao X, et al. A US population health survey on the impact of COVID-19 using the EQ-5D-5L. J Gen Intern Med. 2021;36:1292–1301. doi: 10.1007/s11606-021-06674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang R, Janssen MFB, Pickard AS. US population norms for the EQ-5D-5L and comparison of norms from face-to-face and online samples. Qual Life Res. 2021;30:803–816. doi: 10.1007/s11136-020-02650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Clinical Immunization Safety Assessment (CISA) Project. 2020. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html./
- 25.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 26.Kragholm KH, Lindgren FL, Zaremba T, et al. Mortality and ventricular arrhythmia after acute myocarditis: a nationwide registry-based follow-up study. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammirati E, Cipriani M, Moro C, et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 28.Patel T, Kelleman M, West Z, et al. Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipsitz JD, Masia-Warner C, Apfel H, et al. Anxiety and depressive symptoms and anxiety sensitivity in youngsters with noncardiac chest pain and benign heart murmurs. J Pediatr Psychol. 2004;29:607–612. doi: 10.1093/jpepsy/jsh062. [DOI] [PubMed] [Google Scholar]
- 30.Hadley SM, Prakash A, Baker AL, et al. Follow-up cardiac magnetic resonance in children with vaccine-associated myocarditis. Eur J Pediatr. 2022;181:2879–2883. doi: 10.1007/s00431-022-04482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grün S, Schumm J, Greulich S, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 33.Dubey S, Agarwal A, Nguyen S, Adebo D. Persistence of late gadolinium enhancement on follow-up CMR imaging in children with acute myocarditis. Pediatr Cardiol. 2020;41:1777–1782. doi: 10.1007/s00246-020-02445-5. [DOI] [PubMed] [Google Scholar]
- 34.Aquaro GD, Perfetti M, Camastra G, et al. Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 35.Lota AS, Tsao A, Owen R, et al. Prognostic significance of nonischemic myocardial fibrosis in patients with normal LV volumes and ejection-fraction. JACC Cardiovasc Imaging. 2021;14:2353–2365. doi: 10.1016/j.jcmg.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gräni C, Eichhorn C, Bière L, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2362–2371. doi: 10.1016/j.jacc.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Bonow RO, Nishimura RA, Thompson PD, Udelson JE. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 5: valvular heart disease: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e292–e297. doi: 10.1161/CIR.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 39.Rao S, Lee GM, Razzaghi H, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022 doi: 10.1001/jamapediatrics.2022.2800. published online Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient data from this public health investigation are not available to be shared publicly. Limited, deidentified VAERS data are publicly available online.
This online publication has been corrected. The corrected version first appeared at thelancet.com/child-adolescent on November 16, 2022 and further corrections were made on November 30, 2022