Abstract
The α-aminoadipate pathway for lysine biosynthesis is present only in fungi. The α-aminoadipate reductase (AAR) of this pathway catalyzes the conversion of α-aminoadipic acid to α-aminoadipic-δ-semialdehyde by a complex mechanism involving two gene products, Lys2p and Lys5p. The LYS2 and LYS5 genes encode, respectively, a 155-kDa inactive AAR and a 30-kDa phosphopantetheinyl transferase (PPTase) which transfers a phosphopantetheinyl group from coenzyme A (CoA) to Lys2p for the activation of Lys2p and AAR activity. In the present investigation, we have confirmed the posttranslational activation of the 150-kDa Lys2p of Candida albicans, a pathogenic yeast, in the presence of CoA and C. albicans lys2 mutant (CLD2) extract as a source of PPTase (Lys5p). The recombinant Lys2p or CLD2 mutant extract exhibited no AAR activity with or without CoA. However, the recombinant 150-kDa Lys2p, when incubated with CLD2 extract and CoA, exhibited significant AAR activity compared to that of wild-type C. albicans CAI4 extract. The PPTase in the CLD2 extract was required only for the activation of Lys2p and not for AAR reaction. Site-directed mutational analysis of G882 and S884 of the Lys2p activation domain (LGGHSI) revealed no AAR activity, indicating that these two amino acids are essential for the activation. Replacement of other amino acid residues in the domain resulted in partial or full AAR activity. These results demonstrate the posttranslational activation and the requirement of specific amino acid residues in the activation domain of the AAR of C. albicans.
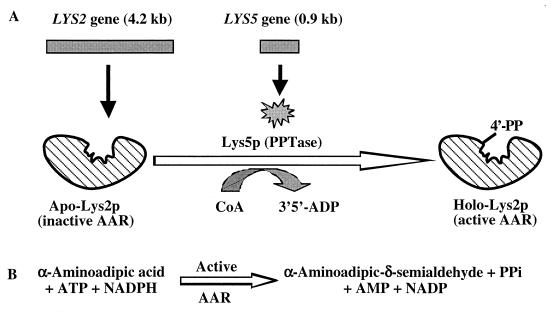
Two distinct pathways exist in nature for the biosynthesis of the amino acid lysine. The diaminopimelic acid pathway is present in bacteria, lower fungi, and plants (12, 35, 36). Candida albicans and other higher fungi exclusively use the α-aminoadipic acid pathway (3, 10, 33, 36). A modified α-aminoadipic acid pathway has been identified recently in thermophilic bacteria such as Thermus thermophilus (24). The α-aminoadipic acid pathway of yeast and other higher fungi has eight enzyme-catalyzed steps which convert α-ketoglutarate and acetyl coenzyme A (acetyl-CoA) to lysine (4, 6, 10, 33). α-Aminoadipic acid is a key intermediate which serves as a common precursor for the biosynthesis of lysine and β-lactam antibiotics such as penicillin (3, 14, 22). The complex α-aminoadipate reductase (AAR) reaction requires α-aminoadipic acid, ATP, and NADPH to produce α-aminoadipate-δ-semialdehyde (Fig. 1) (10, 18, 29, 32).
FIG. 1.
Involvement of two distinct genes (LYS2 and LYS5) and enzymes in the complex AAR reaction for the biosynthesis of lysine in C. albicans. (A) The LYS2 gene produces an apo-Lys2p (inactive AAR). The LYS5 gene produces Lys5p, which serves as a PPTase and catalyzes the transfer of 4′-phosphopantetheine (4′-PP) from CoA to the serine 884 of the activation domain of Lys2p. The resulting holo-Lys2p serves as the active AAR. (B) Posttranslationally activated AAR catalyzes the conversion of α-aminoadipic acid to α-aminoadipic-δ-semialdehyde in the presence of ATP and NADPH.
Two unlinked genes, LYS2 and LYS5, of Saccharomyces cerevisiae are required for the complex AAR reaction (5, 31). Specific functions of the LYS2 and LYS5 genes and their encoded proteins (Lys2p and Lys5p) have not been investigated in detail in any pathogenic fungus. C. albicans, a dimorphic and diploid yeast, is a major opportunistic fungal pathogen of immunocompromised hosts, such as AIDS, cancer, and transplant patients (7, 13, 26, 27). There is an urgent need to gain knowledge of novel metabolic processes of pathogenic fungi in order to develop rapid and sensitive detection methods as well as specific targets for antifungal drugs. The exclusive nature of the α-aminoadipate pathway makes the genes and enzymes of this pathway important targets for detection probes and antifungal drugs (2, 7, 16).
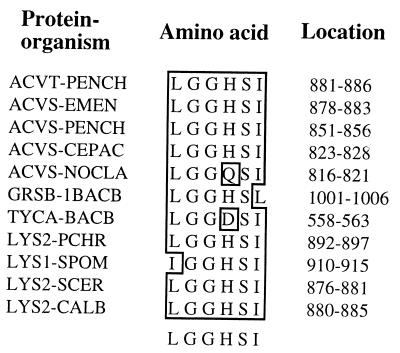
The presence of the α-aminoadipate pathway has been determined in several pathogenic fungi (16). The open reading frame of the LYS2 gene consists of 4,176 nucleotides (nt) encoding 1,392 amino acid residues in S. cerevisiae (1, 25), 4,173 nt encoding 1,391 amino acid residues in C. albicans (21, 34), 4,330 nt encoding 1,409 amino acid residues in Penicillium chrysogenum (11), and 4,245 nt encoding 1,415 amino acid residues in Schizosaccharomyces pombe (8). These genes and the encoded approximately 150-kDa proteins exhibit more than 60% identity at the nucleotide level and 55% identity at the amino acid level. Additionally, there exist several highly conserved core sequences and functional domains in the Lys2p which correspond to those in the nonribosomal peptide synthetases such as α-aminoadipyl-l-cysteinyl-d-valine synthetase for penicillin biosynthesis (8, 11, 18, 34). Computer analysis also revealed the presence of a highly conserved phosphopantetheinylation domain (LGGHSI, amino acid residues 880 to 885) in Lys2p (34). The LYS5 gene (816 nt encoding 272 amino acid residues) of S. cerevisiae does not contain any of the functional domains for the catalytic activity of AAR (23). The phosphopantetheinylation domain plays an important role for the posttranslational activation of several enzymes, including polyketide synthase, nonribosomal peptide synthase, and siderophore synthase (17, 19, 20, 28). However, such posttranslational activation of an amino acid biosynthetic enzyme is highly unusual. Ehmann et al. (15) demonstrated that the LYS2 gene of S. cerevisiae encodes an apo-Lys2p (inactive AAR), which in the presence of CoA and the LYS5 gene-encoded phosphopantetheinyl transferase (PPTase) is activated as the holo-Lys2p (active AAR) (Fig. 1A). The PPTase transfers the 4′-phosphopantetheinyl group from CoA to the serine 880 residue of the posttranslational phosphopantetheinylation (activation) domain of Lys2p for AAR activity (Fig. 1B). We hypothesize that the obligatory requirement for the posttranslational activation of AAR is widespread in organisms, including pathogenic fungi which employ the α-aminoadipic acid pathway for the biosynthesis of lysine. We report here for the first time heterologous expression in Escherichia coli and characterization of the recombinant C. albicans Lys2p, posttranslational activation of apo-Lys2p by CoA-mediated phosphopantetheinylation to the holo-Lys2p (active AAR), and the site-directed mutational analysis of the amino acid residues in the activation domain of Lys2p.
MATERIALS AND METHODS
Organisms and media.
The organisms and plasmids used in this study are listed in Table 1. C. albicans was grown at 30°C in yeast extract-peptone-dextrose (YEPD) medium (2% peptone, 2% dextrose, 1% yeast extract). Escherichia coli was grown at 37°C in Luria-Bertani (LB) medium containing ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml) when appropriate.
TABLE 1.
Organisms and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | F′/endA1 hsdR17(rK−mK−) supE44 thi-1 recA1 gyrA(Nalr) relA1 D(lacZYA-argF)U169 deoR [F80dlacD(lacZ)M15] | ATCC |
| E. coli BL21 (DE3) pLysS | F ompT hsdSB(rB−rB) dcm gal (DE3) pLsS Cmr | Promega |
| C. albicans CAI4 | LYS2, Δura3::imm434/Δura3::imm434 | 7 |
| C. albicans CLD2 | Δlys2::hisG/Δlys2::hisG::ura3::hisG; Δura3::imm434/Δura3::imm434 | 7 |
| Plasmids | ||
| pRSETA | f1 ori Ampr pUC ori | Invitrogen |
| pBS-CaLYS2 | LYS2 Ampr | This study |
| pCaLYS2SE1 | LYS2 Ampr | This study |
| pCaLYS2SE1 mutant strainsa | lys2 mutant 2; Ampr | This study |
See Table 3.
Molecular biology techniques.
Plasmid isolation, restriction analysis, DNA ligations, PCR amplification, sequencing, and E. coli transformations were done as described by Sambrook et al. (30) or according to manufacturers' instructions.
Construction of recombinant pCaLYS2SE1.
The C. albicans LYS2 open reading frame was amplified from pBS-CaLYS2 using the primers 5′-ATTCCGGATCCACTGACTTTTGGTTGAATTA-3′ and 5′-CCCTTCGAATTTTGGCATCTGAACCTCGTG-3′. The primers introduced BamHI and BstBI restriction sites (underlined) into the amplified product. The amplified product was digested with BamHI and BstBI and then ligated into the similarly digested and purified pRSETA expression vector (Invitrogen, Carlsbad, Calif.) to generate the C. albicans LYS2 recombinant plasmid pCaLYS2SE1. This plasmid was used for the expression, posttranslational (in vitro) activation, and site-directed mutational analysis of Lys2p.
Expression and characterization of Lys2p.
E. coli BL21(DE3) pLysS transformants with pCaLYS2SE1 were grown in 500 ml of LB medium to an optical density at 600 nm of 0.6 and were induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 28°C. The cells were harvested and resuspended in sonication buffer (20 mM NaH2PO4, 500 mM NaCl, pH 8.0) and were sonicated with a HeatSystems-Ultrasonics W-225 sonicator. The supernatant was passed through a ProBond resin column (Invitrogen) and washed sequentially with sonication buffer, washing buffer (20 mM NaH2PO4, 500 mM NaCl, 10% glycerol, pH 6.0), washing buffer with 10 mM imidazole, and washing buffer with 50 mM imidazole. Lys2p was eluted off the column with washing buffer with 200 mM imidazole and was further concentrated with a Centricon YM-10 filter (Millipore, Bedford, Mass.). Protein concentration was determined with the Bio-Rad protein assay. Total cell lysate and the purified protein were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) (30) and were used in the activation and AAR assay.
Site-directed mutagenesis.
Point mutations for each of the conserved amino acid residues in the activation domain of C. albicans Lys2p (Fig. 2) were generated using the QuickChange mutagenesis kit (Stratagene, La Jolla, Calif.) as per manufacturer's instructions. The amino acid changes (9) were made in Lys2p using appropriate PCR primers (unmutagenized control primer 5′-CTTCGATTTAGGAGGTCACTCTATTTTGGGTACCAGAATATTTAC-3′; mutant primers provided upon request). The mutated plasmid was transformed into E. coli XLI Blue Supercompetent cells, and the mutation was verified by sequence determination using an ABI Prism 310 (Applied Biosystems, Foster City, Calif.). Control and mutant plasmids were transformed into E. coli BL21(DE3) pLysS cells and induced with IPTG. Lysate and purified proteins were analyzed by SDS-PAGE and were used for the coupled activation and AAR assay.
FIG. 2.
The highly conserved core sequence of amino acid residues (LGGHSI) in the phosphopantetheinylation (activation) domain of the antibiotic synthetases and Lys2p. Abbreviations for organisms: PENCH, P. chrysogenum; EMEN, Emericella nidulans; CEPAC, Cephalosporium acremonium; NOCLA, Nocardia lactamdurans; 1BACB, Bacillus brevis; BACB, Bacillus brevis; PCHR, P. chrysogenum; SPOM, S. pombe; SCER, S. cerevisiae; CALB, C. albicans.
In vitro activation and AAR assay.
The activation of Lys2p and its AAR activity were measured using a previously described assay (16, 34). The AAR reaction mixture contained 12.5 mM dl-α-aminoadipate, 15 mM ATP, 10 mM MgCl2, 1 mM reduced glutathione, 0.625 mM β-NADPH, and 250 mM Tris, pH 8.0. One milligram of C. albicans CAI4 cell extract or 50 to 100 μg of recombinant Lys2p was added to the reaction mixture. Reactions lacking α-aminoadipate were used as negative controls. A reaction containing wild-type C. albicans CAI4 cell extract was used as a positive control. For the coupled activation and AAR assay, recombinant Lys2p, C. albicans CLD2 extract, and 200 μM CoA were mixed and then added to the AAR reaction mixture listed above without any other protein addition and incubated at 30°C for 1 h, and the reaction product was determined at A460.
RESULTS
Expression and characterization of the C. albicans Lys2p.
On SDS-PAGE, E. coli BL21(pCaLYS2SE1) lysate and His tag column-purified proteins showed a 150-kDa protein band which was not present in E. coli BL21(pRSETA) lysate (data not shown). The other visible protein bands were common between E. coli BL21(pRSETA) and E. coli BL21(pCaLYS2SE1) lysates and purified proteins.
Posttranslational activation of the C. albicans Lys2p.
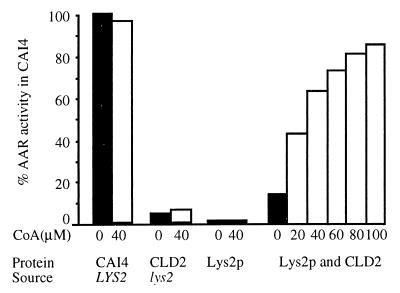
Cell extract from C. albicans wild-type strain CAI4 exhibited full (A460, 2.2; 100%) AAR activity. The recombinant Lys2p exhibited no AAR activity (Fig. 3). However, in the coupled activation and AAR assay (recombinant Lys2p, CLD2 extract, and CoA mixed with the AAR reagents), significant AAR activity was observed in the presence of 20 μM CoA, and the activity increased close to 90% of C. albicans CAI4 with increasing amounts of CoA (Fig. 3). The activity was also dependent on the concentration of Lys2p. The C. albicans CLD2 is a double gene disrupted lys2 deletion mutant (7), which serves as the source of the LYS5-encoded PPTase for the activation of Lys2p. The CLD2 extract or recombinant Lys2p individually showed no significant AAR activity even in the presence of 40 μM CoA. The difference of AAR activity in the CAI4 extract, with or without CoA, was not considered significant. Acetyl-CoA, benzoyl-CoA, and phenylacetyl-CoA each gave high AAR activity when substituted for CoA (results not shown). These results demonstrate that the cloned C. albicans LYS2 gene produces an inactive AAR which, in the presence of CoA as the phosphopantetheine donor and CLD2 extract as the source of the LYS5 encoded PPTase, becomes active AAR for the catalysis of the complex AAR reaction in vitro. C. albicans Lys2p also exhibited significant AAR activity when CLD2 extract was replaced by S. cerevisiae SR36 lys2 mutant extract as the source of a heterologous PPTase (result not shown). This observation confirms our published in vivo result that S. cerevisiae SR36 lys2 mutant, when transformed with pCaLYS2, exhibits a high level of AAR activity (34). It is important to note that the Lys2p expressed in E. coli BL21 cells was not activated by the E. coli PPTase in the presence of intracellular CoA. These observations suggest that the C. albicans Lys2p is specifically activated by C. albicans and S. cerevisiae extracts but not by E. coli PPTases.
FIG. 3.
Posttranslational (in vitro) activation of the C. albicans Lys2p and AAR activity. AAR activity (A460 as the measure of α-aminoadipic-δ-semialdehyde formation) in the C. albicans CAI4 (wild-type) extract without CoA addition was used as 100%. C. albicans CLD2 (lys2 double knockout mutant) extract served as the source of Lys5p (PPTase), pCaLYS2SE1-expressed purified protein was used as Lys2p, and the effect of increasing concentrations of CoA on the activation of Lys2p (Lys2p and CLD2 extract incubated with CoA and AAR reagents) was shown. CLD2 extract and Lys2p with or without CoA served as negative controls.
Requirement of Lys5p only for the activation of Lys2p.
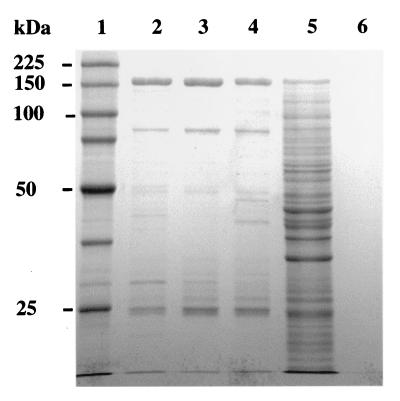
An experiment was designed to determine whether Lys5p is required only for the activation of Lys2p or for both activation and the AAR reaction (Fig. 1). Purified Lys2p (500 μg) was incubated for activation in the presence of 500 μM CoA and 1 mg of C. albicans CLD2 extract as the source of Lys5p. After 1 h of incubation, Lys2p was repurified using a ProBond resin column, examined by SDS-PAGE, and used in the AAR assay. Repurified activated Lys2p exhibited the predicted 150-kDa protein band upon SDS-PAGE. This band also was present following repurification of Lys2p alone and in the activation reaction mixture prior to repurification but not present in the CLD2 extract (Fig. 4). This reaction mixture had many protein bands from the CLD2 extract. Column-purified CLD2 extract showed no protein band which could form a complex with Lys2p. Activated and repurified Lys2p also exhibited full AAR activity, without further addition of CLD2 extract or CoA, compared to CAI4 extract as the positive control (Table 2). The addition of CLD2 extract or CoA showed no significantly higher AAR activity, and inactivated Lys2p showed no AAR activity. The results of the SDS-PAGE clearly demonstrate that no aggregate was formed between Lys2p and Lys5p from the CLD2 extract during the activation and that Lys5p in the CLD2 extract is required only for the activation of Lys2p and not for the catalytic activity of AAR.
FIG. 4.
E. coli BL21-expressed activated and repurified C. albicans Lys2p on SDS-PAGE. Lanes shown contain ProBond column-purified Lys2p (lane 2), column-repurified Lys2p (lane 3), purified Lys2p following activation and repurification (lane 4), purified Lys2p in the activation mixture before repurification (lane 5), ProBond column- purified CLD2 extract (lane 6), and molecular markers (lane 1).
TABLE 2.
Requirement of Lys5p (PPTase) only for activation of Lys2p and not for AAR reaction
| Source of Lys2p | Addition of PPTase (CLD2 lys2 extract) | Addition of CoA | AAR activitya (A460) |
|---|---|---|---|
| CAI4, LYS2 | − | − | 2.24 |
| Purified Lys2p | − | − | 0.00 |
| Purified Lys2p | − | + | 0.00 |
| Purified Lys2p | + | + | 2.15 |
| Repurified Lys2p | − | − | 0.00 |
| Repurified Lys2p | + | + | 2.15 |
| Activated repurified Lys2p | − | − | 1.90 |
| Activated repurified Lys2p | − | + | 1.86 |
| Activated repurified Lys2p | + | − | 2.20 |
| Activated repurified Lys2p | + | + | 2.15 |
Activity is expressed as units of absorbance at 460 nm (A460) per 1 mg of CAI4 extract or 100 μg of recombinant Lys2p.
Site-directed mutational analysis of the amino acid residues in the activation domain of C. albicans Lys2p.
Prior to the mutagenesis experiments, the nucleotide sequence of the activation domain region was determined from pCaLYS2SE1 and C. albicans genomic DNA. The conserved amino acid residues in the posttranslational activation domain were found to be LGGHSI and not LGSHSI as reported previously (34). Substitutions of each amino acid residue in the highly conserved activation domain (Fig. 2) were generated by changing a single nucleotide for each amino acid in the recombinant plasmid. Each mutant recombinant Lys2p showed a 150-kDa band on SDS-PAGE (results not shown). In the coupled activation and AAR assay, two different mutations changing serine 884 to alanine and to phenylalanine exhibited no AAR activity (Table 3). Similarly, two different changes of glycine 882 to serine and to arginine resulted in little or no AAR activity. Changing histidine 883 to arginine showed very little AAR activity; however, the change of histidine to glutamine showed significant activity. The change of glycine 881 to glutamic acid and the change of isoleucine 885 to leucine resulted in less than 50% AAR activity. The substitution of leucine 880 to valine and phenylalanine as well as changes in the flanking amino acid residues, aspartic acid 879 to asparagine and leucine 886 to valine, also showed no significant effect on AAR activity. The mutagenic procedure had no effect on pCaLYS2SE1, which was used as a control.
TABLE 3.
Site-directed mutational analysis of the amino acid residues in phosphopantetheinylation domain (LGGHSI residues 880 to 885) of C. albicans Lys2p and AAR activity
| Amino acid location | Amino acid (nucleotide) | Mutated amino acida (nucleotide) | AAR activityb in purified protein
|
|
|---|---|---|---|---|
| A460 | % Wild type | |||
| pCaLYS2SE1 | Wild type | None | 1.87 | 100 |
| 879 | D (GAT) | N (AAT) | 1.81 | 97 |
| 880 | L (TTA) | V (GTA) | 1.63 | 87 |
| F (TTT) | 2.07 | 111 | ||
| 881 | G (GGA) | A (GCA) | 2.12 | 113 |
| E (GAA) | 0.69 | 37 | ||
| 882 | G (GGT) | S (AGT) | 0.17 | 9 |
| R (CGT) | 0.00 | 0 | ||
| 883 | H (CAC) | R (CGC) | 0.08 | 4 |
| Q (CAG) | 1.61 | 86 | ||
| 884 | S (TCT) | A (GCT) | 0.00 | 0 |
| F (TTT) | 0.00 | 0 | ||
| 885 | I (ATT) | L (CTT) | 0.76 | 41 |
| M (ATG) | 2.14 | 114 | ||
| 886 | L (TTG) | V (GTG) | 2.11 | 113 |
Mutated nucleotides are underlined.
Activity is expressed as units of absorbance at 460 nm (A460) per 100 μg of recombinant Lys2p.
DISCUSSION
α-Aminoadipic acid serves as a common precursor for the biosynthesis of the secondary metabolite penicillin and the primary metabolite lysine. The first committed reaction catalyzed by AAR in the second half of the pathway for the biosynthesis of lysine is a highly complex one (Fig. 1). The LYS2 gene, mapped on chromosome II, and the LYS5 gene, mapped on chromosome VII, of S. cerevisiae are required for the AAR reaction. Mutants in either of these two genes lack AAR activity (31). Two genes (lys1+ and lys7+) also have been identified for this reaction in S. pombe (37). A homology search has revealed the presence of several highly conserved functional domains in Lys2p (substrate α-aminoadipate binding domain, ATP binding domain, dehydrogenation domain, and novel adenylation domains) required for the catalytic activity of AAR (18, 34). None of these catalytic domains are present in the LYS5-encoded protein of S. cerevisiae.
The specific function of the phosphopantetheinylation domain, LGGHSI (residues 880 to 885), in Lys2p (34) and the LYS5 gene in the AAR reaction is the focus of the present investigation. The phosphopantetheinylation reaction is catalyzed by a phosphopantetheinyl transferase which transfers a 4′-phosphopantetheine group from CoA to a specific serine residue of the posttranslational activation domain (15, 20). Such posttranslational activation by phosphopantetheinylation is highly unusual for an amino acid biosynthesis enzyme like the AAR. The results presented in this report clearly demonstrate that the recombinant 150-kDa Lys2p of C. albicans is completely inactive for the catalysis of the AAR reaction. This inactive Lys2p is activated in vitro in the presence of CoA as the source of the phosphopantetheine group and C. albicans CLD2 extract as the source of the LYS5-encoded PPTase (activation reaction) (Fig. 1A). The fact that CLD2 (7) extract activated the recombinant Lys2p is strong evidence for the existence and requirement of a LYS5-encoded PPTase in C. albicans. Our results also show that the PPTase in the CLD2 extract is required for the activation of Lys2p and not for its AAR activity. In the wild-type C. albicans or S. cerevisiae, Lys2p is phosphopantetheinylated in vivo by the LYS5-encoded PPTase in the presence of intracellular CoA. The lys5 mutant of S. cerevisiae and the equifunctional lys7 mutant of S. pombe do not exhibit any AAR activity. Since the LYS2 gene and its equifunctional lys1+ gene encode the inactive AAR, the lys5 mutant and the lys7 mutant lack the PPTase for the activation of AAR. The lack of AAR activity in these mutants also indicates that there is no other PPTase in yeast for the activation of Lys2p and that the PPTase is highly specific for the posttranslational activation of Lys2p.
The site-directed mutational analysis of the amino acid residues in the highly conserved phosphopantetheinylation domain of the C. albicans Lys2p demonstrates that the serine 884 residue is an absolute requirement for the activation of Lys2p. The glycine 882 residue is also very important for the activation of Lys2p compared to other amino acids in this domain (Table 3). It is important to note that the replacement of serine 562 in the activation domain of the phenylalanine-activating tyrocidine synthase (TycA) to alanine and glycine reduced the activity to 30% of the wild-type level (17). These authors did not report results of mutational analysis of other amino acid residues in the activation domain. Since the replacement of serine 884 in the Lys2p to alanine and phenylalanine reduced the AAR activity to 0% of the wild-type level, the function of the serine residue in the activation of AAR is highly specific and most significant. The results reported here represent for the first time complete mutational analysis of all amino acid residues in the activation domain of any Lys2p.
C. albicans is an important opportunistic fungal pathogen. LYS2 is a large gene with multiple functional domains. The LYS2 and LYS5 gene functions are unique in fungi compared to animals and humans. Therefore, the α-aminoadipic acid pathway could be considered as a model for investigation in other pathogenic fungi and to target its unique genes and enzymes for molecular (PCR) detection of fungal pathogens and antifungal drugs (2, 3, 34).
ACKNOWLEDGMENTS
We thank L. A. Actis, G. R. Janssen, K. Suvarna, and Sean O'Donnell for technical advice and Sondra Karipides for the synthesis of PCR primers.
This research was supported by Public Health Service grant IR15GM55912-0–1A1 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Barnes D A, Thornes J. Genetic manipulation of Saccharomyces cerevisiae by use of the LYS2 gene. Mol Cell Biol. 1986;6:2828–2838. doi: 10.1128/mcb.6.8.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, J. K., R. C. Garrad, P. L. Skatrud, and R. B. Peery. July 1999. Methods and reagents for detecting fungal pathogens in a biological sample. U.S. patent 5,919,617.
- 3.Bhattacharjee J K. Evolution of α-aminoadipate pathway for the synthesis of lysine in fungi. In: Mortlock R P, editor. Evolution of metabolic function. Boca Raton, Fla: CRC Press; 1992. pp. 47–80. [Google Scholar]
- 4.Bhattacharjee J K. α-Aminoadipate pathway for the synthesis of lysine in lower eukaryotes. Crit Rev Microbiol. 1985;17:131–151. doi: 10.3109/10408418509104427. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee J K, Sinha A K. Relationship among the genes, enzymes, and intermediates of the biosynthetic pathway of lysine in Saccharomyces. Mol Gen Genet. 1972;115:26–30. doi: 10.1007/BF00272214. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee J K, Strassman M. Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant. J Biol Chem. 1967;242:2542–2546. [PubMed] [Google Scholar]
- 7.Bhattacherjee V, Bhattacharjee J K. Characterization of a double gene disruption in the LYS2 locus of the pathogenic yeast, Candida albicans. Med Mycol. 1999;37:411–417. doi: 10.1046/j.1365-280x.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacherjee V, Bhattacharjee J K. Nucleotide sequence of the Schizosaccharomyces pombe lys1+ gene and similarities of the lys1+ protein to peptide antibiotic synthetases. Yeast. 1998;14:479–484. doi: 10.1002/(SICI)1097-0061(19980330)14:5<479::AID-YEA236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Bordo D, Argos P. Suggestions for “safe” residue substitutions in site-directed mutagenesis. J Mol Biol. 1991;217:721–729. doi: 10.1016/0022-2836(91)90528-e. [DOI] [PubMed] [Google Scholar]
- 10.Broquist H P. Lysine biosynthesis in yeast. Methods Enzymol. 1971;17:112–129. [Google Scholar]
- 11.Casqueiro J, Gutierrez S, Bonuelos O, Fierro F, Velasco J, Martin J F. Characterization of the lys2 gene of Penicillium chrysogenum encoding α-aminoadipic acid reductase. Mol Gen Genet. 1998;259:549–556. doi: 10.1007/s004380050847. [DOI] [PubMed] [Google Scholar]
- 12.Davis B D. Biosynthetic interrelations of diaminopimelic acid, lysine and threonine in mutants of E. coli. Nature. 1952;169:534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- 13.DeBacker M D, Magee P T, Pla J. Recent developments in molecular genetics of Candida albicans. Annu Rev Microbiol. 2000;54:463–498. doi: 10.1146/annurev.micro.54.1.463. [DOI] [PubMed] [Google Scholar]
- 14.Demain A L, Masurekar P S. Lysine inhibition of the in vivo homocitrate synthase in Penicillium chrysogenum. J Gen Microbiol. 1974;82:143–151. doi: 10.1099/00221287-82-1-143. [DOI] [PubMed] [Google Scholar]
- 15.Ehmann D E, Gehring A M, Walsh C T. Lysine biosynthesis in Saccharomyces cerevisiae: mechanism of α-aminoadipate reductase (Lys2) involves posttranslational phosphopantetheinylation by Lys5. Biochemistry. 1999;38:6171–6177. doi: 10.1021/bi9829940. [DOI] [PubMed] [Google Scholar]
- 16.Garrad R, Bhattacharjee J K. Lysine biosynthesis in selected pathogenic fungi: characterization of lysine auxotrophs and the cloned LYS1 gene of Candida albicans. J Bacteriol. 1992;174:7379–7384. doi: 10.1128/jb.174.22.7379-7384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gocht M, Marahiel M A. Analysis of core sequences in the d-Phe activating domain of the multifunctional peptide synthetase TycA by site-directed mutagenesis. J Bacteriol. 1994;176:2654–2662. doi: 10.1128/jb.176.9.2654-2662.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hijarrubia M J, Aparicio J F, Casqueiro J, Martin J F. Characterization of the lys2 gene of Acremonium chrysogenum encoding a functional α-aminoadipate activating and reducing enzyme. Mol Gen Genet. 2001;264:755–762. doi: 10.1007/s004380000364. [DOI] [PubMed] [Google Scholar]
- 19.Kleinkauf H M, Von Dohren H. A nonribosomal system of peptide biosynthesis. Eur J Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Lambalot R, Gehring A, Flugel R S, Zuber P, LaCelle M, Marahiel M A, Reid R, Khosla C, Walsh C T. A new enzyme superfamily—the phosphopantetheinyl transferases. Chem Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 21.Magee B B, Koltein Y, Gorman J A, Magee P T. Assignment of the cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988;8:4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin J F, Gutierrez S, Demain A L. β-Lactams. In: Anke T, editor. Fungal biotechnology. Weinheim, Germany: Chapman and Hall; 1997. pp. 91–127. [Google Scholar]
- 23.Miller K G, Bhattacharjee J K. The LYS5 gene of S. cerevisiae. Gene. 1996;171:167–168. doi: 10.1016/0378-1119(96)00105-9. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki J, Kobashi N, Nishiyama M, Yamane H. Functional and evolutionary relationship between arginine biosynthesis and prokaryotic lysine biosynthesis through α-aminoadipate. J Bacteriol. 2001;183:5067–5073. doi: 10.1128/JB.183.17.5067-5073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris M E, Jinks-Robertson S. Nucleotide sequence of the LYS2 gene of S. cerevisiae, homology to Bacillus brevis tyrocidine synthase I. Gene. 1991;98:141–145. doi: 10.1016/0378-1119(91)90117-t. [DOI] [PubMed] [Google Scholar]
- 26.Perfect J R. Molecular targets for new antifungal drugs. Can J Bot. 1995;73(Suppl. 1):S1187–S1191. [Google Scholar]
- 27.Pfaller M A, Messer S A, Hollis R J, Jones R N, Doera G V, Brandt M E, Hajjeh R A. Trends in species distribution and susceptibility to fluconazole among bloodstream isolates of Candida species in the United States. Diagn Microbiol Infect Dis. 1999;33:217–222. doi: 10.1016/s0732-8893(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 28.Quadri L E N, Weinreb P H, Lei M, Nakano M M, Zuber P, Walsh C T. Characterization of sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry. 1998;37:1983–1993. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 29.Sagisaka S, Shimura K. Studies in lysine biosynthesis. IV. Mechanism of activation and reduction of α-aminoadipic acid (AAA) J Biochem. 1962;52:155–159. doi: 10.1093/oxfordjournals.jbchem.a127590. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Sinha A K, Bhattacharjee J K. Control of a lysine biosynthetic step by two unlinked genes of Saccharomyces. Biochem Biophys Res Commun. 1970;39:1205–1210. doi: 10.1016/0006-291x(70)90689-3. [DOI] [PubMed] [Google Scholar]
- 32.Sinha A K, Bhattacharjee J K. Lysine biosynthesis in Saccharomyces, conversion of α-aminoadipate into α-aminoadipic δ-semialdehyde. Biochem J. 1971;125:743–749. doi: 10.1042/bj1250743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strassman M, Weinhouse S. Biosynthetic pathways. III. The biosynthesis of lysine in T. utilis. J Am Chem Soc. 1953;75:1680–1684. [Google Scholar]
- 34.Suvarna K, Seah L, Bhattacherjee V, Bhattacharjee J K. Molecular analysis of the LYS2 gene: homology to antibiotic synthetases and the regulation of the α-aminoadipate reductase. Curr Genet. 1998;33:268–275. doi: 10.1007/s002940050336. [DOI] [PubMed] [Google Scholar]
- 35.Umbarger H E. Biosynthesis of the branched-chain amino acids. In: Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 352–367. [Google Scholar]
- 36.Vogel H J. Distribution of lysine pathways among fungi: evolutionary implications. Am Nat. 1960;98:446–455. [Google Scholar]
- 37.Ye Z H, Bhattacharjee J K. Lysine biosynthesis pathway and biochemical blocks of lysine auxotrophs of S. pombe. J Bacteriol. 1988;170:5968–5970. doi: 10.1128/jb.170.12.5968-5970.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]