Abstract
Background
Neurologic outcomes in patients with multiple sclerosis (MS) and related disorders (MSRD) following COVID-19 is not well understood. The objective of this study was to investigate neurologic outcomes in patients with MSRD post-COVID-19.
Methods
This was a retrospective medical records review study of adult patients with MSRD and COVID-19 infection at the Brigham MS Center. Neurologic worsening post-COVID-19 was defined as having a relapse, pseudorelapse, new brain MRI activity, worsening of preexisting MSRD symptoms, or development of other long-term neurologic symptoms.
Results
111 patients, 85 (76.6%) females, with a mean [SD] age of 49.3 [12.2] years and median [range] EDSS of 2.5 [0, 8.5] were identified. 41 patients (36.9%) had neurologic worsening post-COVID-19. Of those, 19 (46.3%) had pseudorelapses, 2 (4.8%) had relapses, and 24 (58.5%) patients reported worsening of preexisting MSRD symptoms, or other new long-term neurologic symptoms. Neurologic worsening was associated with hospitalized (moderate or severe) COVID-19 (p = 0.001), treatment for COVID-19 (p = 0.006), and incomplete COVID-19 recovery (p = 0.0267) but not with age, sex, MS type, race, disease duration, EDSS, vitamin D use, or disease modifying therapy use.
Conclusions
COVID-19 severity and lack of complete systemic recovery were associated with new or worsening neurologic symptoms in 36.9% of MSRD patients.
Keywords: Multiple sclerosis, Clinical outcomes, COVID-19
Abbreviations: CDC, Centers for Disease Control and Prevention; CIS, Clinically isolated syndrome; CLIMB, Comprehensive Longitudinal Investigation of multiple sclerosis database at Brigham and Women's hospital; COPD, chronic obstructive pulmonary disease; DMT, disease modifying therapy; EDSS, expanded disability status scale; Gd+, gadolinium; ICU, intensive care unit; MOGAD, myelin oligodendrocyte glycoprotein associated disease; MRI, magnetic resonance imaging; MSRD, multiple sclerosis and related disorders; NLRP3, nod-like receptor pyrin domain containing protein; NMOSD, neuromyelitis optica spectrum disorder; PCR, polymerase chain reaction; PPMS, primary progressive MS; RRMS, relapsing remitting MS; SARs-CoV-19 infection, COVID-19; sNfL, serum neurofilament light chain; SPMS, secondary progressive MS; TNF, tumor necrosis factor; WHO, World Health Organization
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARs-CoV-2) is a coronavirus responsible for causing coronavirus disease 2019 (COVID-19) pandemic. Infection with COVID-19 can be associated with various neurologic symptoms, which have been well described in the literature including headache, anosmia and ageusia (Romero-Sánchez et al., 2020; Divani et al., 2020; Aghagoli et al., 2021). The exact manner in which the virus affects the brain is unclear but proposed mechanisms include systemic inflammatory responses which lead to blood-brain barrier breakdown, as well as possible hypoxic ischemic injury and neuronal damage (Mukerji and Solomon, 2021; Guasp et al., 2022).
Studies from the United Kingdom (Garjani et al., 2021) and Poland (Czarnowska et al., 2021) on multiple sclerosis (MS) patients with COVID-19 have demonstrated that many patients report an MS exacerbation or worsening of pre-existing symptoms after infection. COVID-19 vaccines are now widely available and are designed to trigger an immune response to the COVID-19 spike protein. Though there are case reports (Khayat-Khoei et al., 2022) of the vaccines causing central nervous system demyelination, larger studies in MS patients show that they are safe and do not appear to increase risk of MS relapse after vaccination (Kelly et al., 2021; Kalincik, 2015).
We hope to better understand the impact of COVID-19 on patients with MS and related disorders (MSRD) and risk factors for neurologic worsening. In our study we retrospectively collected and examined a series of neurologic outcomes in patients with MSRD who developed COVID-19 infection with the goal to analyze the risk factors for developing new or worsening neurologic symptoms after COVID-19 infection.
2. Methods
We included adult patients (ages 18 or older) with MSRD who were followed at the Brigham MS center with a confirmed COVID-19 infection defined as a positive reverse transcriptase polymerase chain reaction (PCR) (Corman et al., 2020) on nasal or pharyngeal swab test or a positive serological test (Lee et al., 2020) between March 9, 2020 and April 1, 2021. Patients were identified if they directly contacted their neurologist due to a positive test, or via a query for a COVID-19 lab test in our electronic medical record system. Demographic data, MSRD type, duration, Expanded Disability Status Scale (EDSS), and disease modifying therapy (DMT) use, as well as information about neurologic symptoms after COVID-19, and Magnetic Resonance Imaging (MRI) data were queried from the medical record and the Brigham MS center's Comprehensive Longitudinal Investigation of Multiple Sclerosis database at Brigham and Women's Hospital (CLIMB).
For COVID-19 data, infection date, severity, hospitalization, treatment, and outcome were reviewed. COVID-19 severity was divided into 4 categories: asymptomatic, mild, moderate, and severe. Mild disease was defined as illness managed at home, moderate disease was defined if patients requiring hospitalization but not intensive care unit (ICU) level care, and severe disease was defined if patients required ICU level care. Though these definitions for disease severity differ from World Health Organization definitions, we chose these definitions to simplify classification in our patient population (WHO R and D, 2022). Similar definitions of COVID-19 outcomes were used early on in the pandemic by the Centers for Disease Control and Prevention (Severe Outcomes, 2020). If information about COVID-19 was not included in the medical record this was coded as “unknown.” If a patient with no or mild symptoms were at home, and treatment information was not specifically documented, they were coded as “untreated.”
COVID-19 recovery was defined as follows: complete recovery, recovery with mild sequelae, recovery with significant sequelae, or death. Mild sequalae included cough or shortness of breath at follow up, significant sequelae included persistent oxygen requirement at follow up. If information about COVID-19 recovery was not included in the medical record this was coded as “unknown.”
Neurologic symptoms post-COVID-19 were defined as having a relapse, pseudorelapse, worsening of preexisting MSRD symptoms, development of other neurologic symptoms, or new gadolinium enhancing or T2 lesions on brain MRI. Relapse was defined as new neurologic symptoms or worsening of old neurologic symptoms lasting for greater than 24 hours in the absence of fever or infection (Thompson et al., 2018). Pseudorelapse was defined as reemergence of prior neurologic symptoms in the setting of active COVID-19 infection occurring within 4 weeks of symptom onset of COVID-19, and improving after resolution of acute infection (Rodríguez de Antonio et al., 2021). Long term neurologic symptoms or post-COVID-19 syndrome was classified as non-localizing neurologic symptoms not otherwise consistent with MSRD relapse or MSRD disease worsening that persisted for at least 3 months post-COVID-19 infection (Soriano et al., 2022). If fatigue and cognitive impairment were part of a patient's pre-existing MSRD syndrome and worsened post-COVID-19 infection, this was coded as worsening of a preexisting MSRD symptom.
We compared patients with and without neurologic symptoms post-COVID-19 on a set of demographic and disease variables. For continuous variables age and disease duration, we used a two-sample t-test. For the ordinal variable EDSS, we used a Wilcoxon rank sum test. For dichotomous variables including disease type, DMT type, race, treatment for COVID-19, vitamin D use, COVID-19 severity, and COVID-19 recovery, we used a chi-squared test. In all analyses, we used p < 0.05 to indicate statistical significance. Patients who passed away or had an unknown value for either of the variables were excluded from these analyses. Statistical analyses were performed in the statistical package R (www.r-project.org).
3. Results
3.1. Patient demographics and neurologic disease characteristics
111 patients met the study inclusion criteria. 109 patients had COVID-19 confirmed by PCR. Two patients did not have PCR testing but had COVID-19 diagnosed by having a consistent clinical syndrome with subsequent nucleocapsid antibody positivity. If a patient had multiple positive PCR tests, the PCR date associated with worse disease severity was used (2 patients). If the exact date for a positive PCR test was unknown, it was coded as the 15th of the respective month (10 patients).
Table 1 lists demographic cohort characteristics. The average EDSS was median [range] 2.5 [0, 8.5] and 54 (48.6%) were on anti-CD20 therapies. For the patients on anti-CD20 therapies, the mean [SD] days between last infusion and positive COVID-19 PCR was 141.4 [99.1] days, and the mean [SD] CD19 count was 1.28% [2.64]. 88 (79.2%) were taking vitamin D. No patients in this cohort were fully vaccinated at the time of COVID-19 infection as defined by the Center for Disease Control's definition, which is 2 weeks after the second vaccine dose in a 2-dose series or 2 weeks after a single-dose vaccine (When You've Been Fully Vaccinated, 2022). The average [SD] neurology follow-up interval after COVID-19 infection was 17.1 [13.6] weeks.
Table 1.
Baseline demographics and neurological disease characteristics.
| Demographics | Disease characteristics | DMT use | |||
|---|---|---|---|---|---|
| Age, mean [SD], y | 49.3 [12.2] | Disease duration, mean [SD], y | 13.1 [9.6] | Anti-CD20, n (%) | 54 (48.6) |
| Sex, n (% female) | 85 (76.6) | EDSS, median [range] | 2.5 [0, 8.5] | Natalizumab, n (%) | 3 (2.7) |
| Race, n (% white) | 80 (72.1) | Disease type | Fingolimod, n (%) | 9 (8.1) | |
| Hypertension, n (%) | 23 (20.7) | RRMS, n (%) | 72 (64.9) | Dimethyl fumarate, n (%) | 6 (5.4) |
| Diabetes, n (%) | 23 (20.7) | SPMS, n (%) | 21 (18.9) | Teriflunomide, n (%) | 4 (3.6) |
| Asthma or COPD, n (%) | 7 (6.3) | PPMS, n (%) | 8 (7.2) | Glatiramer, n (%) | 10 (9) |
| Former or current cigarette use, n (%) | 11 (9.9) | CIS, n (%) | 2 (1.8) | Interferon beta, n (%) | 2 (1.8) |
| Related disorders, n (%) | 8 (7.2) | Other therapies, n (%)⁎⁎ | 3 (2.7) | ||
| NMOSD | 4 | None | 20 (18) | ||
| MOGAD | 1 | ||||
| Other* | 3 | ||||
Definitions: CIS = clinically isolated syndrome, COPD = chronic obstructive pulmonary disease, MOGAD = myelin oligodendrocyte glycoprotein associated disease, NMOSD = neuromyelitis optica spectrum disorder, PPMS = primary progressive MS, RRMS = relapsing remitting MS, SPMS = secondary progressive MS.
Legend: Table 1 shows demographic data and neurological disease characteristics of our cohort.
Other related disorders included 1 patient with Neuro Behcet's, 1 patient with tumor necrosis factor (TNF) exposure-related demyelination, and 1 patient with a single demyelinating brainstem lesion.
Other therapies: Intravenous immune globulin and anti-CD20 therapy combination (1), chronic monthly high-dose steroid infusions (1), glatiramer and chronic monthly high-dose steroid infusions combination (1).
3.2. COVID-19 severity, recovery, and treatment
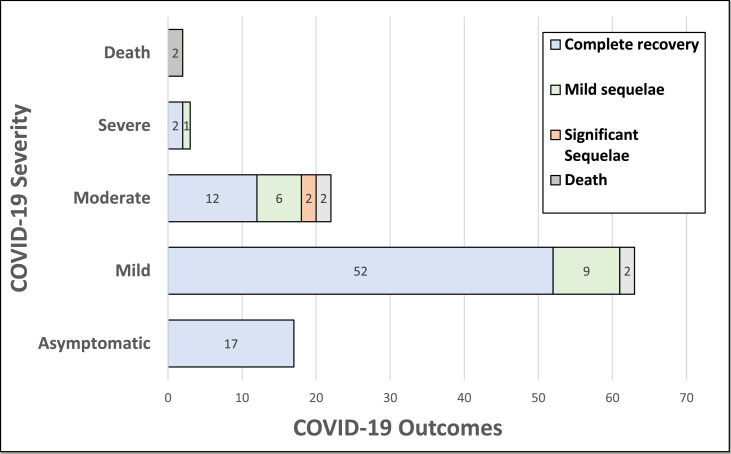
Most patients, 80 (72%), were asymptomatic or had mild COVID-19, and 85 (76.5%) recovered completely after infection (Fig. 1 ). The most common presenting symptom in our cohort was fever, which occurred in 37 patients (33.3%), followed by cough in 30 (27%) and shortness of breath in 27 (24.3%). 28 (25.2%) patients received treatment for COVID-19, including supplemental oxygen, steroids, remdesivir, monoclonal antibody treatment, tocilizumab, convalescent serum or a combination of these treatments. Two patients with pre-morbid EDSS 8.5 and on B-cell depleting therapies, died from COVID-19.
Fig. 1.
COVID-19 severity and recovery: COVID-19 outcomes are indicated by colored sections on the bar graph and are grouped in rows by COVID-19 severity (y-axis). The x-axis represents numbers of patients. Not shown are 4 patients who had unknown COVID-19 severity.
3.3. Neurologic outcomes
41 patients (36.9%) had neurologic worsening post-COVID-19. Of those with neurologic worsening, 2 (4.8%) had relapses requiring high dose intravenous solumedrol treatment, 19 (46.3%) had pseudorelapses, 19 (46.3%) reported worsening of preexisting MSRD symptoms, and 7 (17.1%) reported new long-term neurologic symptoms not meeting the definition of relapse at the last follow up visit (Table 2 ). Of the two patients who had relapses post-COVID-19, both had RRMS and treated with ocrelizumab. More information about these patients and patients who had pseudorelapses is available in supplementary Tables 1 and 2. Of the 19 patients with MSRD symptoms worsening post-COVID-19, 12 had worsening of baseline fatigue, 4 had worsening motor symptoms, 2 had worsening sensory symptoms, 2 had worsening of pre-existing cognitive impairment, 2 had worsening dizziness, 1 had worsening spasticity, and 1 had worsening of baseline nystagmus. Of the 7 patients who developed long term neurologic symptoms not related to their MSRD, 4 had anosmia, 1 had headaches, 1 had severe fatigue, and 1 had worsening of baseline sensory ganglionopathy.
Table 2.
Neurologic outcomes in MSRD patients post-COVID-19 (n = 111 patients).
| N (%) | |
|---|---|
| No neurologic worsening | 62/111 (55.8) |
| Unknown | 6/111 (5.4) |
| Neurologic worsening | 41/111 (36.9) |
| ▒Relapse | ▒2/41 (4.9) |
| ▒Pseudorelapse | ▒19/41 (46.3) |
| ▒Worsening of preexisting MSRD symptoms | ▒19/41 (46.3) |
| ▒Other long term neurologic non MSRD symptoms | ▒7/41 (17.1) |
| ▒MRI changes | ▒5/41 (12.2) |
Legend: Table 2 shows neurologic outcomes in MSRD patients post-COVID-19. The two patients who passed away are not included in this table.
One patient in this cohort suffered from an asymptomatic cerebro-vascular event during or after COVID-19 illness, incidentally discovered on routine MRI screening.
3.4. Imaging
There were 55 patients who had brain MRIs post-COVID-19. 1 patient had an incidentally discovered occipital lobe stroke. 1 patient had a new T2 and gadolinium (Gd+) enhancing lesion, 2 patients had new T2 lesions without enhancement, and 2 patients had asymptomatic Gd+ enhancing lesions. The mean [SD] number of days between MRI and infection was 144.6 [107.8]. Of the patients with MRI activity, 3 had MRI changes alone without neurologic symptoms, 1 had a pseudorelapse and 1 had worsening of MS-related fatigue.
3.5. Additional analyses
Neurologic worsening was associated with moderate or severe COVID-19 (p = 0.001), treatment for COVID-19 (p = 0.006), and incomplete COVID-19 recovery (p = 0.027) but not with age, sex, disease type, race, disease duration, EDSS, vitamin D use, or type or presence of disease modifying therapy (p > 0.05) (Table 3 ).
Table 3.
Association between subject characteristics and neurologic worsening post- COVID-19.
| Neurologic worsening post- COVID-19 | No neurologic worsening post- COVID-19 | p-value | |
|---|---|---|---|
| N | 41 | 62 | |
| Age, years (SD) | 48.2 (11.9) | 49.2 (12.3) | 0.701 |
| Female; n (%) | 32 (78.0) | 47 (75.8) | 0.980 |
| Disease type (n) | 0.166 | ||
| RRMS | 24 | 44 | |
| CIS | 2 | 0 | |
| SPMS | 7 | 13 | |
| PPMS | 4 | 2 | |
| Related disorders | 4 | 3 | |
| DMT type (n) | 0.071 | ||
| None | 11 | 8 | |
| Teriflunomide | 1 | 3 | |
| Fingolimod | 0 | 9 | |
| Anti-CD20 | 23 | 27 | |
| Glatiramer | 2 | 7 | |
| Interferon-beta | 0 | 1 | |
| Dimethyl fumarate | 2 | 3 | |
| Natalizumab | 0 | 3 | |
| Other | 2 | 1 | |
| Disease duration, years (SD) | 12.5 (10.7) | 12.5 (8.7) | 0.969 |
| EDSS median (range) | 2.5 (0, 8.5) | 2 (0, 8.5) | 0.978 |
| Non-white; n (%) | 13 (31.7) | 16 (25.8) | 0.669 |
| Treatment for COVID-19; n (%)⁎ | 17 (45.0) | 9 (15.5) | 0.006 |
| Vitamin D use; n (%)⁎⁎ | 29 (74.4) | 54 (87.1) | 0.173 |
| Moderate/severe COVID-19; n (%)⁎⁎ | 18 (43.9) | 7 (11.7) | 0.001 |
| Recovered from COVID-19; n (%)⁎⁎⁎ | 27 (71.1) | 56 (90.3) | 0.027 |
Legend: For age and disease duration, the reported p-value is from a two-sample t-test. For EDSS, the reported p-value is from a Wilcoxon rank sum test. For the remaining variables, the reported p-value is from a chi-squared test.
: Five patients were missing COVID-19 treatment.
: Two patients were missing vitamin D use and COVID-19 severity.
: Three patients were missing recovered from COVID-19.
4. Discussion
This study adds to the body of literature that focuses on neurologic outcomes post-COVID-19 in MSRD patients. In our cohort, worsening neurologic symptoms in MSRD patients were identified in 36.9%. This was largely due to pseudorelapses or worsening of preexisting MSRD symptoms. The death rate in our cohort was 1.8%, and the hospitalization rate was 22.5%. These rates are similar to other groups reporting on COVID-19 outcomes in MSRD populations, although the death rate is lower than previously reported (Klineova et al., 2021; Louapre et al., 2020; Sormani et al., 2021; Salter et al., 2021).
Several studies have described COVID-19 outcomes in patients with multiple sclerosis and related disorders (MSRD), and risk factors for the development of severe COVID-19, which include age, EDSS and comorbidities such as obesity (Klineova et al., 2021; Louapre et al., 2020; Sormani et al., 2021). Other studies from international registries have shown that certain DMTs, specifically B-cell depleting therapies may be associated with increased risk of severe COVID-19 (Sormani et al., 2021; Simpson-Yap et al., 2021). This has led some providers to safely extend the dosing interval for patients on anti-CD20 therapies (Rolfes et al., 2021).
Long-COVID is generally defined as delayed recovery after an acute infection with COVID-19 and is characterized by persistence of symptoms or onset of new symptoms for longer than would be expected from the acute illness alone (Alwan and Johnson, 2021; Datta et al., 2020). The fact that some patients have persistent symptoms following recovery from acute COVID-19 infection is not surprising as post-infectious sequelae have been reported following infection with other coronaviruses (Moldofsky and Patcai, 2011). Possible mechanisms for long-COVID include direct viral invasion of the central nervous system (Edén et al., 2021), cytokine storm and pro-inflammatory state (Zhang et al., 2020), blood-brain barrier breakdown, and immune mediated neuro-toxicity (Gupta and Weaver, 2021). Other mechanisms include neuronal injury as evidenced by studies showing elevated serum neurofilament light chain (sNFfL) in patients with COVID-19 compared to ICU patients without COVID-19 (Sutter et al., 2021).
Severity of COVID-19 infection has also been reported to correlate with the prevalence of the long-COVID syndrome in non-MSRD patients, with up to 80% in hospitalized patients and ∼ 5% of non-hospitalized patients suffering from these complications.. (Cabrera Martimbianco et al., 2021; Carfì et al., 2020; Carvalho-Schneider et al., 2021; Huang et al., 2021; Jacobs et al., 2020) Neurologic symptoms post-COVID-19 are common and data from a meta-analysis show that older age and COVID-19 severity were risk factors (Misra et al., 2021). A study on all neurologic manifestations in patients hospitalized for COVID-19 in Wuhan, China found that 36% had neurologic symptoms (either central nervous system, peripheral nervous system, or skeletal muscle injuries). Similar to our study, severe COVID-19 infection was associated with a higher rate of neurologic manifestations, with 45.5% of those with severe infection developing neurologic symptoms versus 30.2% of those with non-severe infection (Mao et al., 2020). However, prolonged ICU admissions have been independently associated with neurologic complications, and it might be difficult to separate these two variables from the available data thus far (Romero-Sánchez et al., 2020; Abenza-Abildúa et al., 2020).
In our study, COVID-19 severity and lack of complete systemic recovery were associated with neurologic worsening. Treatment for COVID-19 was also associated with neurologic worsening but we suspect this is a variable reflecting COVID-19 severity, due to the higher rate of hospitalizations and treatment in patients with more severe COVID-19. Our study supports earlier observations about worsening of MS symptoms after COVID-19 infection (Garjani et al., 2021; Michelena et al., 2022). Neurologic worsening post-COVID-19 specifically in MS patients has been associated with a higher pre-COVID-19 EDSS, longer disease duration, and lack of DMT use (Garjani et al., 2021). A study from the United Kingdom on post-acute sequelae of COVID-19 in MS patients found that preexisting neurologic impairment and mental health disease also increased the risk of developing long term symptoms post-COVID-19 (Garjani et al., 2021). Microglial activation either from oxidative stress or from activation of nod-like receptor pyrin domain containing protein (NLRP3) inflammasome are potential mechanisms for neurologic worsening specifically in MS patients with COVID-19 and both have also been linked to the onset and progression of MS (Soares et al., 2019; Gogoleva et al., 2018).
Two of 111 patients in our cohort had on-study relapses post-COVID-19. There is conflicting data regarding increased risk of relapses post-COVID-19. A 44-patient study found an increased risk of relapse after COVID-19 infection (Barzegar et al., 2021). However, larger studies including a New York University study found that only 2 of 474 MSRD patients developed disease relapses post-COVID-19 (Klineova et al., 2021). An Iranian study compared MS patients with and without relapses during COVID-19 and found no difference in relapse rate 6 months post infection. There was also no difference in relapse rate between hospitalized and non-hospitalized patients but investigators acknowledge sample size limitation to detect possible significance (Etemadifar et al., 2021). The blunting of expansion of autoreactive T cells from COVID-19-associated lymphopenia has been proposed as a possible hypothesis for the lack of increase in relapses in MSRD patients post-COVID-19 (Liu et al., 2020). Larger prospective studies are needed to further understand the impact of the virus on relapse rate.
Over the past 18 months, various COVID-19 severity scales have been proposed (WHO R and D, 2022; Severe Outcomes, 2020) Our study cohort spans the timeframe between the early pandemic and the more recent COVID-19 cases. Initially, the Centers for Disease Control and Prevention (CDC) stratified COVID-19 patients by those requiring hospitalization, ICU level care and death. The World Health Organization (WHO) separately proposed a comprehensive 8-point severity rating scale; however, the level of detail required to stratify patients into those groups was not available to us due to the nature of retrospective data collection and insufficient early pandemic documentation. Therefore, we chose to adopt COVID-19 severity definitions as proposed by the CDC for the purposes of this study. We also used our own recovery scale, as there was no validated recovery scale available the time of this study.
Further, we were specifically interested in the data on hospital-administered medications and correlations with neurologic outcomes. In the early times of the pandemic, there were not uniformly consistent or recommended outpatient treatments for COVID-19 infection. Thus, for non-hospitalized patients, we assumed no therapy was administered unless it was specifically documented that a patient received a monoclonal antibody or steroids as an outpatient. Therefore, our study does not account for any home remedies, vitamins or supplements that may have been used, and this is another study limitation.
In addition, our study is limited by small sample size, and the retrospective data collection. Specifically, there was limited power to detect a difference in neurologic outcomes based on DMT type. In addition, the pathophysiological mechanism underlying new relapse, pseudorelapse, and long-term post-COVID-19 neurological symptoms might be different. A separate analysis of the risk factors for each category of neurological worsening should be undertaken in studies of larger sample sizes. Our study sample did not have sufficient power for a more granular analysis, and we acknowledge this as a limitation of the study. Our study is also limited because data was obtained via chart review, and not by direct interview. This might have resulted in underreporting of neurologic symptoms as the patients would be less likely to seek medical care for milder issues. Information about the prior history of post-infectious relapses or pseudorelapses preceding COVID-19 pandemic was not available for our cohort, and this is a study limitation. However, with these considerations, we believe that ∼37% is likely an accurate representation of the fraction of MSRD patients experiencing broadly defined, more serious neurologic worsening during and after COVID-19 infection.
In our study, COVID-19 severity and lack of complete systemic recovery was associated with new or worsening neurologic symptoms. This has important clinical and public health implications for our patients especially as anti-SARS-CoV-2 monoclonal antibodies and other therapeutics that significantly decrease the risk of severe illness and hospitalization are increasingly available for early treatment and post-exposure prophylaxis.
Disclosures
SC, JZ, CS, LS, and KG report no disclosures. BH has received research support from Analysis Group, Celgene (Bristol-Myers Squibb), Verily Life Sciences, Merck-Serono, Novartis and Genzyme. TK has served on scientific advisory boards for Biogen, Novartis, Genzyme-Sanofi, Bristol Myers Squibb, and Genentech.TC has received compensation for consulting from Biogen, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme. She has received research support from the National Institutes of Health, National MS Society, US Department of Defense, Sumaira Foundation, Brainstorm Cell Therapeutics, EMD Serono, I-Mab Biopharma, Mallinckrodt ARD, Novartis Pharmaceuticals, Octave Bioscience, Roche Genentech, and Tiziana Life Sciences. MH served as a consultant for Roche/Genentech, Novartis, Biogen, Jansen pharmaceuticals, and Genzyme and has received research support from Biogen, Roche, Genzyme.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Sarah E. Conway: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft. Brian C. Healy: Formal analysis, Writing – original draft, Visualization. Jonathan Zurawski: Writing – original draft. Christopher Severson: Writing – original draft. Tamara Kaplan: Writing – original draft. Lynn Stazzone: Writing – original draft. Kristin Galetta: Writing – original draft. Tanuja Chitnis: Writing – original draft. Maria K. Houtchens: Conceptualization, Methodology, Investigation, Writing – original draft.
Acknowledgments
We would like to acknowledge Mariann Polgar-Turcsanyi for her assistance in data acquisition.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103946.
Appendix. Supplementary materials
References
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1e70. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani A.A., Andalib S., Biller J., Di Napoli M., Moghimi N., Rubinos C.A., et al. Central nervous system manifestations associated with COVID-19. Curr. Neurol. Neurosci. Rep. 2020;20(12):60. doi: 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghagoli G., Gallo Marin B., Katchur N.J., Chaves-Sell F., Asaad W.F., Murphy S.A. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit. Care. 2021;34(3):1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S.S., Solomon I.H. What can we learn from brain autopsies in COVID-19? Neurosci. Lett. 2021;742 doi: 10.1016/j.neulet.2020.135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasp M., Muñoz-Sánchez G., Martínez-Hernández E., Santana D., Carbayo Á., Naranjo L., et al. CSF biomarkers in COVID-19 associated encephalopathy and encephalitis predict long-term outcome. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.866153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnowska A., Kapica-Topczewska K., Zajkowska O., Adamczyk-Sowa M., Kubicka-Bączyk K., Niedziela N., et al. Symptoms after COVID-19 infection in individuals with multiple sclerosis in Poland. J. Clin. Med. 2021;10(22) doi: 10.3390/jcm10225225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat-Khoei M., Bhattacharyya S., Katz J., Harrison D., Tauhid S., Bruso P., et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J. Neurol. 2022;269(3):1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H., Sokola B., Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021;356 doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management. A systematic review. Neuroepidemiology. 2015;44(4):199–214. doi: 10.1159/000382130. [DOI] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Lin R.T.P., Renia L., Ng L.F.P. Serological Approaches for COVID-19: epidemiologic perspective on surveillance and control. Front. Immunol. 2020;11:879. doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severe Outcomes Among patients with coronavirus disease 2019 (COVID-19) - United States. MMWR Morb. Mortal. Wkly. Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. February 12-March 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Antonio L.A., García Castañón I., Aguilar-Amat Prior M.J., Puertas I., González Suárez I., Oreja Guevara C. Non-inflammatory causes of emergency consultation in patients with multiple sclerosis. Neurologia. 2021;36(6):403–411. doi: 10.1016/j.nrleng.2018.02.005. (Engl Ed) [DOI] [PubMed] [Google Scholar]
- Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22(4):e102–e1e7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- When You've Been Fully Vaccinated. 2022.
- Klineova S., Harel A., Straus Farber R., DeAngelis T., Zhang Y., Hentz R., et al. Outcomes of COVID-19 infection in multiple sclerosis and related conditions: one-year pandemic experience of the multicenter New York COVID-19 neuroimmunology consortium (NYCNIC) Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699–708. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e1e85. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes L., Pawlitzki M., Pfeuffer S., Nelke C., Lux A., Pul R., et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2021;8(5) doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan N.A., Johnson L. Defining long COVID: going back to the start. Med. 2021;2(5):501–504. doi: 10.1016/j.medj.2021.03.003. (N Y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.D., Talwar A., Lee J.T. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324(22):2251–2252. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.M., et al. CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology. 2021;96(2):e294–e300. doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- WHO R. Blueprint novel coronavirus COVID-19 therapeutic trial synopsis. https://cdn.who.int/media/docs/default-source/blue-print/covid-19-therapeutic-trial-synopsis.pdf?sfvrsn=44b83344_1&download=true
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Gupta M., Weaver D.F. COVID-19 as a trigger of brain autoimmunity. ACS Chem. Neurosci. 2021;12(14):2558–2561. doi: 10.1021/acschemneuro.1c00403. [DOI] [PubMed] [Google Scholar]
- Sutter R., Hert L., De Marchis G.M., Twerenbold R., Kappos L., Naegelin Y., et al. Serum neurofilament light chain levels in the intensive care unit: comparison between severely Ill patients with and without coronavirus disease 2019. Ann. Neurol. 2021;89(3):610–616. doi: 10.1002/ana.26004. [DOI] [PubMed] [Google Scholar]
- Cabrera Martimbianco A.L., Pacheco R.L., Bagattini Â.M., Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int. J. Clin. Pract. 2021;75(10):e14357. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L.G., Gourna Paleoudis E., Lesky-Di Bari D., Nyirenda T., Friedman T., Gupta A., et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S., Kolappa K., Prasad M., Radhakrishnan D., Thakur K.T., Solomon T., et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. 2021;97(23):e2269–e2e81. doi: 10.1212/WNL.0000000000012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza-Abildúa M.J., Ramírez-Prieto M.T., Moreno-Zabaleta R., Arenas-Valls N., Salvador-Maya M.A., Algarra-Lucas C., et al. Neurological complications in critical patients with COVID-19. Neurologia. 2020;35(9):621–627. doi: 10.1016/j.nrleng.2020.07.012. (Engl Ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena G., Casas M., Eizaguirre M.B., Pita M.C., Cohen L., Alonso R., et al. ¿ Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103368. [DOI] [PubMed] [Google Scholar]
- Garjani A., Middleton R.M., Hunter R., Tuite-Dalton K.A., Coles A., Dobson R., et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.L., Oliveira E.M., Pontillo A. Variants in NLRP3 and NLRC4 inflammasome associate with susceptibility and severity of multiple sclerosis. Mult. Scler. Relat. Disord. 2019;29:26–34. doi: 10.1016/j.msard.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Garjani A., Middleton R.M., Nicholas R., Evangelou N. Recovery from COVID-19 in multiple sclerosis: a prospective and longitudinal cohort study of the United Kingdom multiple sclerosis register. Neurol. Neuroimmunol. Neuroinflamm. 2021;9(1) doi: 10.1212/NXI.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoleva V.S., Atretkhany K.N., Drutskaya M.S., Mufazalov I.A., Kruglov A.A., Nedospasov S.A. Cytokines as mediators of neuroinflammation in experimental autoimmune encephalomyelitis. Biochemistry. 2018;83(9):1089–1103. doi: 10.1134/S0006297918090110. (Mosc) [DOI] [PubMed] [Google Scholar]
- Barzegar M., Vaheb S., Mirmosayyeb O., Afshari-Safavi A., Nehzat N., Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Sedaghat N., Aghababaee A., Kargaran P.K., Maracy M.R., Ganjalikhani-Hakemi M., et al. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult. Scler. Relat. Disord. 2021;51 doi: 10.1016/j.msard.2021.102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Long W., Tu M., Chen S., Huang Y., Wang S., et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.