Background:
Evidence is emerging that preterm birth (PTB, birth before 37 completed weeks of gestation), a risk factor for neonatal mortality and future morbidity, may be induced by maternal nitrate () exposure from drinking water. The objective of this study is to assess the association between maternal exposure to nitrate and the risk of PTB in a nationwide study of liveborn singletons.
Methods:
We estimated maternal nitrate exposure from household tap water for 1,055,584 births in Denmark to Danish-born parents during 1991–2015 by linkage of individual home address(es) with nitrate concentrations from a national monitoring database. Nitrate exposure during pregnancy was modeled using four categories and continuously. Logistic models adjusted for sex, birth year, birth order, urbanicity, and maternal age, smoking, education, income, and employment, with generalized estimating equations were used to account for sibling clusters.
Results:
A total of 1,009,189 births were included, comprising 51,747 PTB. An increase in the risk of PTB was seen across categories of exposure (P < 0.001) with an odds ratio (OR) in the uppermost category (>25 mg/L nitrate) of 1.05 (95% confidence interval [CI] = 1.00, 1.10). Evidence of an exposure–response relationship was observed in models using continuous nitrate (OR = 1.01 [95% CI = 1.00, 1.03] per 10 mg/L nitrate). In sensitivity analyses, results were robust to the addition of variables for short inter-pregnancy interval (<1 year between births), maternal pre-pregnancy body mass index, paternal socioeconomic status and age, season of birth, and inclusion of post-term births. Results were virtually unchanged when the analysis was restricted to women exposed to less than the current European Union standard of 50 mg/L.
Conclusion:
We observed an increasing risk of PTB with increases in nitrate in household tap water. These findings add to a growing body of evidence of adverse effects from nitrate in drinking water at levels below current regulatory levels.
Keywords: Children, Drinking water, Environment, Epidemiology, Gestational age, Infant, Nitrate, Preterm birth
What this study adds
Nitrate is one of the most common water contaminants in the world. Only three previous epidemiologic studies have examined whether maternal exposure to nitrate in drinking water increases the risk of preterm births, and all reported some evidence of an association. Our study with over 1 million births and high-quality data on nitrate exposure during pregnancy, adds substantially to the evidence that nitrate in drinking water increases the risk of preterm births. Notably exposures in our study were low and an increased risk was evident among women who were exposed to nitrate concentrations below current regulatory standards.
Introduction
Globally, 15 million children are born preterm (i.e., before 37 weeks of gestation) each year. Complications from preterm birth (PTB) result in 1.1 million or 35% of all neonatal deaths; the single largest cause of neonatal death in high- and middle-income countries and second only to pneumonia in low-income countries.1
Health outcomes stemming from PTB are costly to society, with the frequency, severity, and cost of complications increasing as the length of gestation decreases.2 Disability occurs in 60% of survivors born at 26 weeks and in 30% of those born at 31 weeks.3 Complications include respiratory distress, chronic lung disease, neonatal sepsis, and neonatal and childhood mortality.4 Impaired neurodevelopmental function5, learning impairment, visual disorders,6 and a higher risk of social and behavioral problems are also seen in those born preterm.4
Only three epidemiologic studies of nitrate in drinking water and PTB have previously been conducted, all of which were in the United States. These US studies all provide some evidence for an association between nitrate in drinking water and PTB7–9; however, two were based on ecologic estimates of nitrate exposure and all of them were based on birth in areas with high nitrate concentrations and possible pesticide exposure. Maternal consumption of nitrosatable drugs along with dietary intake of nitrite during pregnancy has been associated with increased risk of PTB in a survey-based study of liveborn infants in the US National Birth Defects Prevention Study.10
Nitrate is one of the world’s most common drinking water contaminants.11 Nitrate pollution of drinking water supplies is of particular concern in agricultural countries, such as Denmark, that use nitrogen fertilizers and have intensive animal production.12–14 While nitrate concentrations in Danish public water supplies are typically below the European Union regulatory level (50 mg/L ), household tap water nitrate at this sub-regulatory level has been associated with birth defects15, reduced birth weight and other markers of fetal growth restriction16 in similarly designed nationwide studies in Denmark.
Our study leverages extensive nitrate measures in drinking water samples collected across Denmark, detailed information on individual household water supply, and largely complete residential and birth registries for the entire Danish population over the span of more than two decades. Furthermore, the study population has access to free health and prenatal care, reducing the possibility of confounding by this important factor.8,17,18 The aim of this analysis is to examine the associations between maternal exposure to nitrate in drinking water during pregnancy and the risk of PTB in a large population exposed to relatively low levels of nitrate.
Methods
Study design
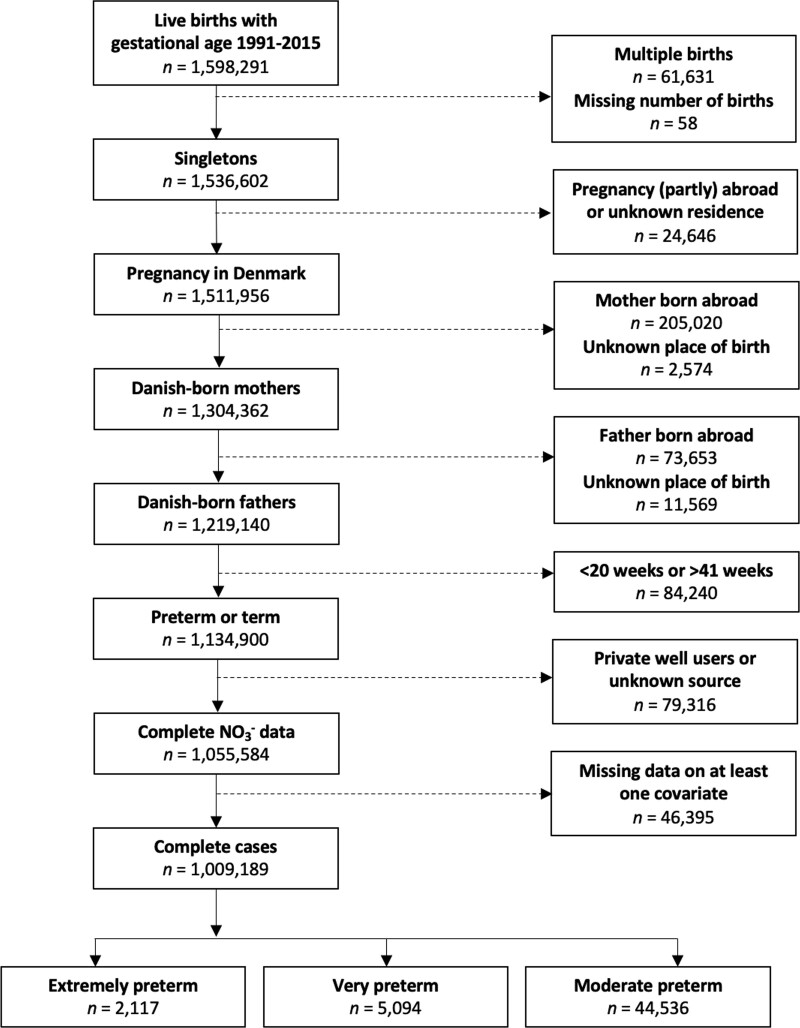
We linked data on gestational age, sex, and smoking from the Danish Medical Birth Registry (DNBR)19 with estimates of household levels of nitrate in drinking water from the Danish national monitoring geodatabase Jupiter.20 Data were linked using the unique personal identification number assigned to each liveborn resident in Denmark.21 We restricted our population to liveborn singletons born from 1 January 1991 to 31 December 2015 who had a gestational age of ≥140 and ≤293 days and who had Danish born parents, and mothers who had residence in Denmark throughout the index pregnancies (Figure 1).
Figure 1.
Flow diagram of restriction of the cohort to the main analysis population. Main model covariates included calendar year, sex, gravidity, urbanicity, and maternal age, smoking, education, income, and employment status.
Exposure assessment
We estimated annual average nitrate levels at the waterworks-level using the monitoring database Jupiter, for all Danish water supply areas. The water supply dataset is described in detail in Schullehner.21 Nitrate was quantified via high-performance liquid chromatography by certified Danish laboratories. As analytic tests improved over the two decades this cohort spans, the limit of detection decreased from typically 1 to ≤0.3 mg/L or even lower. Analyses of water samples at the exit of treatment plants and from the home faucets they serve have demonstrated nearly identical nitrate levels (R2: 0.98).22
Residential address histories for each mother–child dyad were obtained from the Danish Civil Registrations System,23 which were linked to the annual average nitrate levels for the water system(s) supplying water to their home address during the pregnancy. For women who moved during pregnancy, we computed time-weighted average exposures, weighted by the number of days living at the respective residences.
We computed annual averages on water supply area level, as we have earlier shown that seasonality is negligible.24 This is due to water production exclusively being based on groundwater, where seasonal variations are attenuated, and trends tend to change gradually. An individual’s exposure level could therefore change when the person changed residence, or when the pregnancy covered two calendar years. Given the stability of nitrate concentrations in drinking water,24 if a nitrate measurement was available within a 3-year time window, we imputed missing years at a residence by linear interpolation. We did not consider using trimester specific estimates of exposure since the correlations between these measures and pregnancy averages was extremely high (R = 0.93–0.98).
We restricted analyses to individuals with complete nitrate exposure information during pregnancy (Figure 1). Missingness could arise if a mother moved to a residence without a linkable measurement during the pregnancy, or if the pregnancy spans into a calendar year without a nitrate estimate.
Households supplied by private wells (3% of the Danish population) were identified based on their proximity to a private well in Jupiter. Those supplied by well water were excluded due to incomplete exposure data, with estimates for only 53% of the private well users.24
Outcome definition
As recommended by Quinn et al,25 PTB (140–258 days or 20–36 weeks of gestation) was subdivided into extremely preterm (EPTB; 140–195 days or 20–27 weeks), very preterm (VPTB; 196–223 days or 28–31 weeks), and moderate preterm (MPTB; 224–258 days or 32–36 weeks of gestation). Term births were defined as 259–293 days (or 37–41 weeks) of gestation. To assign the date of conception, we used the date of birth and the gestational age registered in the DMBR, which reflects the period between the date of birth and the date of last menstrual period corrected by two ultrasounds, if ultrasound data were available and different from the estimated gestational period (ultrasound measurements were largely regularly used since 2004 in Denmark).
Covariates
Data on potential confounders were obtained from the Integrated Database for Longitudinal Labour Market Research and the Danish Medical Birth Registry. Continuous covariates were modeled as restricted cubic splines with four knots that were defined by Stata using Harrell’s recommended percentiles.26 Covariates included in the main analyses were sex (male/female), year of birth (spline), gravidity (1, 2, or ≥3), urbanicity (five categories), and maternal age at birth (spline), maternal smoking during pregnancy (yes/no), and markers of socioeconomic status (SES) including maternal income normalized for inflation using the Consumer Price Index (The World Bank 2019) (spline), maternal educational attainment (less than high school, high school, higher), and maternal employment status (employed, unemployed, not in the workforce). All SES variables (i.e., income, education, and employment) were as recorded 2 years before birth. For children born in the period before 1997 smoking was recorded at the first pregnancy healthcare visit with no specifications as to the timing. For children born from 1997 onward smoking is during pregnancy.
Statistical analyses
Multivariable logistic regression models were fitted for the risk of PTB, MPTB, VTPB, and ETPB, using generalized estimating equations (GEE) to account for the non-independence of siblings with the same mother. We excluded extremely preterm cases in the analysis of VPTB, and to exclude EPTB and very preterm births (VPTB) in the analysis of moderate preterm. This was done so that the referent group would not include more severe forms of preterm. No exclusions were made in the analyses for PTB or for EPTB since PTB is defined as including all births that were less than 259 days, and EPTB was the most extreme outcome used.
Pregnancy average nitrate concentrations were modeled as categorical or continuous variables. Three cut points for the categorical analysis were defined a priori based on the distribution of exposure in the population and their usefulness for assessing current regulatory standards. The referent category was defined as any weighted average ≤2 mg/L while the highest category included those with weighted averages >25 mg/L , which is half the European Union regulatory limit and chosen due to the relatively low concentrations within our data.
Effect modification by mother’s and father’s income and education, maternal smoking with nitrate exposure was examined by computing a likelihood ratio test comparing the main model with the model containing the interaction terms. GEE was not used in this analysis because it does not permit the estimation of a likelihood ratio test.
We performed a series of sensitivity analyses to test the robustness of our findings. In the first sensitivity analysis, we included a binary variable for short interpregnancy interval (<1 year for those with more than one birth), dropping those with an interval of ≤36 days as implausible (n = 56). Short interpregnancy interval has been shown to be strongly correlated with PTB, but necessitates at least two births to the same woman in the study period, reducing the dataset by 440,806 births.
In the second sensitivity analysis, we adjusted for pre-pregnancy maternal body mass index (BMI; restricted cubic splines with four knots defined by Stata)—variables which was only available from 2003 onward (a reduction of 555,626 births).
The third sensitivity analysis examined additional adjustment by the covariates not considered a priori confounders: season of birth (four categories), paternal age, income, education, and employment status 2 years before birth.
Due to differences in definitions of full-term across studies, a fourth sensitivity analysis included post-term births up to 44 weeks (n = 74,067) in the full-term definition.
Finally, a fifth sensitivity analysis was performed in which children with mothers who had pregnancy average exposures greater than the EU and WHO standards of 50 mg/L were excluded to determine whether adverse effects occur below the current allowable limits. The US EPA MCL of 10 mg/L as NO3-N is nearly equivalent to the WHO limit of 50 mg/L as NO3 (multiply 50 × 0.2258=11.3 mg/L).
All statistical analyses were conducted using Stata (StataCorp. 2019. Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX).
Results
Main analyses
We included a total of 1,055,584 births in the study (Figure 1). The median nitrate exposure in the cohort, averaged over the entire pregnancy, was 1.9 (inter quartile range [IQR]: 1.0–3.4) mg/L , and 3.6% experienced drinking water with nitrate contamination >25 mg/L . Approximately 5% of the births were PTB (Table 1) and the distribution of relevant covariates differed between PTB-cases and non-cases (all P < 0.001; Table S1; http://links.lww.com/EE/A198), as did the distribution of covariates between the four nitrate exposure categories (Table 1), where all X2 tests were highly significant (P < 0.001) except for sex (P = 0.04). There were moderately strong correlations (R = 0.42–0.49) between maternal education, income, and age. This correlation resulted in the program dropping maternal education variables from the models due to multicollinearity.
Table 1.
Characteristics of the study population by average nitrate concentration (mg/L ) in home drinking water during pregnancy.
| Estimated mean nitrate (mg/L NO3–) in household drinking water | ||||
|---|---|---|---|---|
| Characteristic | ≤2 | >2–5 | >5–25 | >25 |
| Total, n (%)a | 529,172 (52) | 318,135 (32) | 124,602 (12) | 37,280 (4) |
| Case status, n (%) | ||||
| Preterm (140–258 days) | 26,616 (51.4) | 16,547 (32.0) | 6,579 (12.7) | 2,005 (3.9) |
| Full term (259–293 days) | 502,556 (52.5) | 301,588 (31.5) | 118,023 (12.3) | 35,275 (3.7) |
| Gestational age (days), n (%) | ||||
| Q1 (≤273 days) | 163,537 (51) | 101,451 (32) | 42,122 (13) | 12,141 (4) |
| Q2 (274–280 days) | 146,194 (52) | 88,684 (32) | 35,756 (13) | 10,173 (4) |
| Q3 (281–287 days) | 147,762 (53) | 87,624 (31) | 33,070 (12) | 10,245 (4) |
| Q4 (288–293 days) | 71,679 (55) | 40,376 (31) | 13,654 (10) | 4,721 (4) |
| Sex, n (%) | ||||
| Female | 258,603 (53) | 154,775 (31) | 60,738 (12) | 17,998 (4) |
| Male | 270,569 (52) | 163,360 (32) | 63,864 (12) | 19,282 (4) |
| Birth order, n (%) | ||||
| 1 | 227,350 (52) | 146,330 (33) | 51,060 (12) | 16,066 (4) |
| 2 | 207,786 (53) | 122,971 (31) | 49,621 (13) | 14,274 (4) |
| ≥3 | 94,036 (54) | 48,834 (28) | 23,921 (14) | 6,940 (4) |
| Short interpregnancy interval, n (%) | ||||
| No | 1,144 (51) | 680 (30) | 355 (16) | 85 (4) |
| Yes | 300,678 (53) | 171,125 (30) | 73,187 (13) | 21,129 (4) |
| Missing (i.e., only child in this period) | 227,350 (52) | 146,330 (33) | 51,060 (12) | 16,066 (4) |
| Urbanicity, n (%) | ||||
| Capital | 55,352 (39) | 83,871 (58) | 4,385 (3) | 18 (0) |
| Suburb of the capital | 52,095 (41) | 58,310 (46) | 17,178 (13) | 163 (0) |
| Provincial cityb | 86,212 (68) | 10,880 (9) | 14,677 (11) | 15,944 (12) |
| Provincial townc | 150,204 (52) | 91,239 (32) | 40,736 (14) | 6,398 (2) |
| Rural areasd | 185,309 (58) | 73,835 (23) | 47,626 (15) | 14,757 (5) |
| Year of birth, n (%) | ||||
| Q1 (1991–1996) | 117,479 (47) | 77,511 (31) | 45,397 (18) | 11,078 (4) |
| Q2 (1997–2002) | 129,740 (53) | 74,448 (31) | 30,621 (13) | 8,974 (4) |
| Q3 (2003–2008) | 133,675 (54) | 79,816 (32) | 26,467 (11) | 8,831 (4) |
| Q4 (2009–2015) | 148,278 (56) | 86,360 (33) | 22,117 (8) | 8,397 (3) |
| Season of birth, n (%) | ||||
| January–March | 126,971 (52) | 77,154 (32) | 31,084 (13) | 9,189 (4) |
| April–June | 133,726 (52) | 80,246 (31) | 31,909 (13) | 9,382 (4) |
| July–September | 141,982 (52) | 85,831 (32) | 32,989 (12) | 9,859 (4) |
| October–December | 126,493 (53) | 74,904 (31) | 28,620 (12) | 8,850 (4) |
| Maternal age (years), n (%) | ||||
| <25 | 65,613 (48) | 43,750 (32) | 20,418 (15) | 5,541 (4) |
| 25–29 | 192,586 (53) | 111,068 (30) | 47,825 (13) | 15,087 (4) |
| 30–34 | 187,860 (54) | 110,196 (32) | 39,819 (11) | 11,949 (3) |
| ≥35 | 83,113 (53) | 53,121 (34) | 16,540 (11) | 4,703 (3) |
| Maternal smokinge, n (%) | ||||
| No | 426,069 (54) | 249,304 (31) | 92,204 (12) | 28,852 (4) |
| Yes | 103,103 (48) | 68,831 (32) | 32,398 (15) | 8,428 (4) |
| Maternal BMI, n (%) | ||||
| <18.5 | 10,032 (55) | 6,153 (34) | 1,543 (8) | 616 (3) |
| 18.5–24.9 | 157,553 (56) | 93,070 (33) | 23,844 (8) | 9,328 (3) |
| 25–29.9 | 52,754 (56) | 28,471 (30) | 9,861 (10) | 3,327 (4) |
| ≥30 | 32,085 (56) | 16,351 (29) | 6,491 (11) | 2,084 (4) |
| Missingf | 276,748 (50) | 174,090 (31) | 82,863 (15) | 21,925 (4) |
| Maternal educationg, n (%) | ||||
| Primary school | 111,898 (49) | 73,156 (32) | 34,718 (15) | 9,406 (4) |
| High school | 247,303 (53) | 141,880 (30) | 60,735 (13) | 17,958 (4) |
| Higher education | 169,971 (54) | 103,099 (33) | 29,149 (9) | 9,916 (3) |
| Maternal employment statusg, n (%) | ||||
| Employed | 431,525 (53) | 258,286 (32) | 98,825 (12) | 29,387 (4) |
| Unemployed | 28,828 (48) | 18,710 (31) | 9,620 (16) | 2,868 (5) |
| Not seeking work | 68,819 (52) | 41,139 (31) | 16,157 (12) | 5,025 (4) |
| Maternal incomeh, n (%) | ||||
| Q1 | 128,705 (52) | 78,541 (32) | 31,045 (13) | 9,452 (4) |
| Q2 | 132,575 (53) | 71,866 (29) | 36,213 (14) | 11,060 (4) |
| Q3 | 134,520 (53) | 75,804 (30) | 33,217 (13) | 9,761 (4) |
| Q4 | 133,372 (52) | 91,924 (36) | 24,127 (9) | 7,007 (3) |
| Missing | 128,705 (52) | 78,541 (32) | 31,045 (13) | 9,452 (4) |
| Paternal age (years), n (%) | ||||
| <25 | 32,318 (48) | 22,662 (33) | 10,196 (15) | 2,662 (4) |
| 25–29 | 142,498 (52) | 84,008 (31) | 36,884 (13) | 11,422 (4) |
| 30–34 | 198,549 (53) | 114,862 (31) | 44,513 (12) | 13,649 (4) |
| ≥35 | 155,807 (53) | 96,603 (33) | 33,009 (11) | 9,547 (3) |
| Paternal educationg, n (%) | ||||
| Primary school | 107,349 (49) | 69,249 (32) | 32,089 (15) | 8,712 (4) |
| High school | 272,371 (53) | 155,999 (30) | 67,969 (13) | 20,005 (4) |
| Higher education | 144,258 (55) | 88,909 (34) | 23,115 (9) | 8,245 (3) |
| Missing | 5,194 (48) | 3,978 (36) | 1,429 (13) | 318 (3) |
| Paternal employment statusg, n (%) | ||||
| Employed | 477,356 (53) | 282,767 (31) | 111,471 (12) | 33,211 (4) |
| Unemployed | 18,294 (46) | 13,328 (34) | 6,038 (15) | 1,712 (4) |
| Not seeking work | 31,959 (52) | 20,749 (34) | 6,780 (11) | 2,302 (4) |
| Missing | 1,563 (49) | 1,291 (40) | 313 (10) | 55 (2) |
| Paternal incomeh, n (%) | ||||
| Q1 | 126,569 (51) | 81,466 (33) | 30,459 (12) | 9,635 (4) |
| Q2 | 131,468 (52) | 73,180 (29) | 35,687 (14) | 10,738 (4) |
| Q3 | 135,287 (53) | 76,857 (30) | 32,226 (13) | 9,421 (4) |
| Q4 | 135,570 (53) | 86,422 (34) | 26,188 (10) | 7,481 (3) |
| Missing | 278 (52) | 210 (39) | 42 (8) | 5 (1) |
All X2 tests for difference between strata were significant at P < 0.001 except for sex (P = 0.04).
aThe study population: full-term singleton live births in Denmark from 1 January 1991 to 31 December 2015 to Danish-born parents where the mother resided in Denmark for the full pregnancy and who had a nitrate estimate for each day of pregnancy, and with non-missing covariates in the base model.
bMunicipalities having a town with >100,000 inhabitants.
cMunicipalities having a town with between 10,000 and 100,000 inhabitants.
dMunicipalities in Denmark where the largest town has <10,000 inhabitants.
eFor children born in the period before 1997 smoking was recorded at the first visit with the midwife with no specifications as to the timing. For children born from 1997 onward smoking is during pregnancy.
fAvailable from 2003 onward only.
gAs reported 2 years before birth.
hAs reported 2 years before birth and standardized to 2013 values.
Preterm birth
The risk of PTB increased monotonically across the four exposure categories compared with the lowest (≤2 mg/L ) in the adjusted models (Table 2). An adjusted odds ratio (OR) of 1.05 (95% CI = 1.00, 1.10) was estimated for those with levels >25 mg/L compared with the referent category. Based on the continuous model, was associated with a significant (P < 0.04) increase in the risk of preterm births (OR for a 10 mg/L increase in : 1.01; 95% CI = 1.00, 1.03; Table 2; Figure 2). There was no evidence of effect modification by maternal (P = 0.50) or paternal (P = 0.66) education or by maternal (P = 0.31) or by paternal (P = 0.65) income or by maternal smoking (P = 0.44).
Table 2.
Adjusted odds of preterm birth and preterm birth subcategories given pregnancy concentrations of nitrate in drinking water.
| Mean pregnancy nitrate exposure (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Category of birth | ≤2 | >2–5 | >5–25 | >25 | Trend | Continuous (per 10 mg/L) |
| All preterm (140–258 days) | ||||||
| Total (N) | 529,172 | 318,135 | 124,602 | 37,280 | 1,009,189 | 1,009,189 |
| Cases (n) | 26,616 | 16,547 | 6,579 | 2,005 | 51,747 | 51,747 |
| OR (95% CI) | Ref (1) | 1.03 (1.01, 1.06) | 1.04 (1.01, 1.07) | 1.05 (1.00, 1.10) | 1.01 (1.00, 1.03) | |
| P value | 0.002 | 0.01 | 0.06 | <0.001 | 0.04 | |
| Extremely preterm (140–195 days) | ||||||
| Total (N) | 529,172 | 318,135 | 124,602 | 37,280 | 1,009,189 | 1,009,189 |
| Cases (n) | 1,094 | 690 | 263 | 70 | 2,117 | 2,117 |
| OR (95% CI) | Ref (1) | 1.03 (0.93, 1.14) | 1.06 (0.93, 1.22) | 0.93 (0.72, 1.19) | 1.01 (0.94, 1.07) | |
| P value | 0.52 | 0.39 | 0.55 | 0.70 | 0.86 | |
| Very preterm (196–223 days) | ||||||
| Total (N) | 528,078 | 317,445 | 124,339 | 37,210 | 1,007,072 | 1,007,072 |
| Cases (n) | 2,642 | 1,581 | 670 | 201 | 5,094 | 5,094 |
| OR (95% CI) | Ref (1) | 1.01 (0.94, 1.08) | 1.05 (0.96, 1.15) | 1.03 (0.89, 1.19) | 1.01 (0.97, 1.05) | |
| P value | 0.84 | 0.25 | 0.73 | 0.34 | 0.72 | |
| Moderate preterm (224–258 days) | ||||||
| Total (N) | 525,436 | 315,864 | 123,669 | 37,009 | 1,001,978 | 1,001,978 |
| Cases (n) | 22,880 | 14,276 | 5,646 | 1,734 | 44,536 | 44,536 |
| OR (95% CI) | Ref (1) | 1.04 (1.01, 1.06) | 1.04 (1.01, 1.07) | 1.06 (1.01, 1.12) | 1.02 (1.00, 1.03) | |
| P value | 0.001 | 0.01 | 0.03 | <0.001 | 0.03 | |
Models were fitted using logistic regression with generalized estimating equations to control for the non-independence of births from the same mother and were controlled for calendar year, sex, gravidity, urbanicity, and maternal age, smoking, education, income, and employment status.
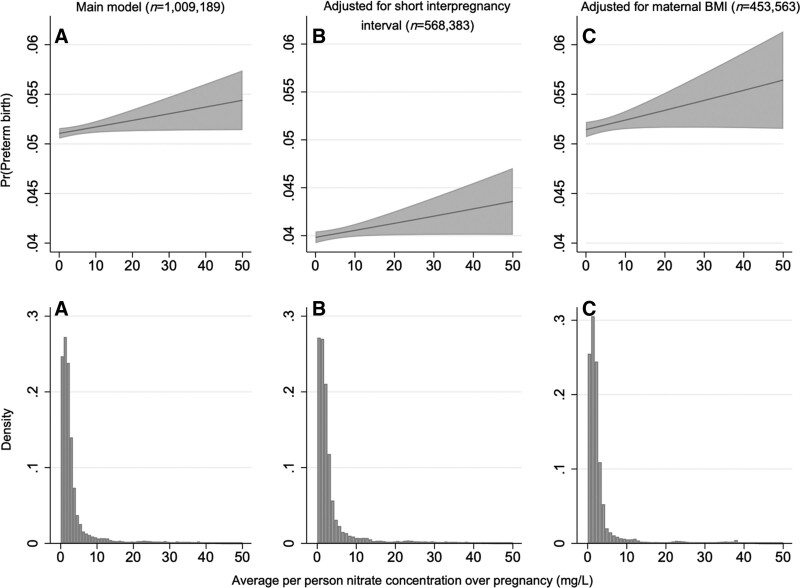
Figure 2.
Distribution of the pregnancy average nitrate exposure, truncated to those with ≤50 mg/L average nitrate exposure, and the corresponding probability of preterm birth in (A) the main model, (B) main model with further adjustment for short interpregnancy interval, and (C) main model with further adjustment for maternal pre-pregnancy body mass index. Main model covariates included calendar year, sex, birth order urbanicity, and maternal age, smoking, education, income, and employment status.
Subcategories of preterm birth
Within the cohort, there were 2,117 cases of extremely PTB, 5,094 cases of very PTB, and 44,536 cases of moderate PTB. The risk of moderate PTB was the only category with evidence of an exposure–response relationship (Table 2). A monotonic increase in moderate PTB risk was observed across categories of exposure (P < 0.001) and we estimated an OR: 1.02 (95% CI = 1.00, 1.03) with every 10 mg/L increase in based on the continuous exposure model.
Overall, our results were robust in our sensitivity analyses. Our findings did not change meaningfully when controlling for short interpregnancy interval (Table S2; http://links.lww.com/EE/A198; Figure 2), maternal pre-pregnancy BMI (Table S3, http://links.lww.com/EE/A198; Figure 2), season or paternal SES (Table S4, http://links.lww.com/EE/A198). No change in effect estimates was observed when post-term births were included in the analysis (Table S5, http://links.lww.com/EE/A198). Finally, dropping children whose mothers had pregnancy average exposures greater than the current EU standard (50 mg/L) had nearly no effect on the results (Table S6, http://links.lww.com/EE/A198).
Discussion
In this study, spanning 24 years and with more than 1 million births, we examined the association between maternal exposure to nitrate from household drinking water and PTB at the individual level for the entire country of Denmark. We observed the strongest evidence of an elevated risk of PTB among those born moderately preterm (224–258 days or 32–37 weeks gestation). Changes from main model results in sensitivity analyses were generally minor and appeared to be due to changes in the sample and not confounding (Tables S2–S5, http://links.lww.com/EE/A198). Notably, we observed an increased risk below current allowable limits (EU: 50 mg/L US: 44 mg/L ) suggesting that this level is not protective for PTB (Table S6, http://links.lww.com/EE/A198).
Comparison with other studies
Our results are largely consistent with the three prior studies that have examined the relationship between nitrate in drinking water and PTB.7–9 All three studies reported evidence of an increased risk of PTB from maternal consumption of nitrate in drinking water. However there, are some differences worth noting. The strongest association with nitrate exposure in a study in the Midwestern US7 was for very preterm births (<32 weeks), and in one of the California studies9 the strongest association was with births between 20 and 31 weeks, which corresponds most closely to our definition of extremely preterm (20–27 weeks). In contrast, in our study, the risk was most pronounced among moderate preterm births (OR>25mg/L = 1.06 [1.01, 1.12]; Ptrend < 0.001) and we only observed a weak association with very PTB (OR>25mg/L = 1.03 [0.89, 1.19]; Ptrend = 0.34), and no evidence of an association with extremely preterm (OR>25mg/L = 0.93 [0.72, 1.19]; Ptrend = 0.55). These differences might be explained by higher exposures in the US studies which could result in more severe outcomes. The prior studies had a higher percentage (0.6%–1.8%) of exposures above the current EU and US standards than our study (<0.1%). It is also possible that mothers in the United States had higher exposures to other water contaminants (e.g., pesticides) than mothers in our study; however, we have no evidence of this. Finally, differences in levels of other risk factors (e.g., obesity) that might modify the risk could conceivably explain these differences.
Support from mechanistic studies
An association between nitrate in drinking water and PTB may be mediated by nitric oxide metabolites. Endogenous nitrosation of nitrate is a precursor to the formation of N-nitroso compounds, most of which are believed to be carcinogenic and teratogenic.27 Approximately 6%–7% percent of nitrate is converted to nitrite in the salivary glands, which can be converted to nitrous oxide, nitrous acid and other metabolites that promote the formation of N-nitroso compounds.28 Higher levels of nitric oxide metabolites have been found in the serum and urine of mothers delivering PTB compared to those delivering at full-term and controls.29 High levels of these reactive oxygen species are known to lead to cell cycle arrest, apoptosis, and senescence.30 Nitric oxide metabolites may also damage the collagen of the membrane that surrounds the fetus during gestation (i.e., the chorioamnion),31 which is associated with elevated risk of PTB.32 It has also been shown that nitrate consumed from public water supplies may increase the risk of congenital hypothyroidism33 and the consumption of nitrate-rich food may increase maternal thyroid autoantibodies34 and hypothyroidism.35 These conditions could lead to higher risk of PTB.34,36
Design limitations
Our study was unable to account for differences in individual dietary sources of nitrate and nitrite, or with vitamin C and other antioxidant supplementation which may impede the effect of nitrate,27,37 or with nitrosatable drug use that may interact with nitrate,38 or with the maternal oral microbiome, which contributes to transformation of nitrate in the body.27 We were also unable to adjust for pesticides and other compounds found in Danish drinking water which might be correlated with nitrate. However, tap water in Denmark originates from groundwater which is typically less contaminated than in places relying on surface water.
We were also unable to quantify the amount of water a woman consumed and assumed equivalent consumption for all pregnancies. Exposure misclassification due to consumption of bottled water is possible, but we do not consider this to be a large source of bias, as the use of pre-packaged bottled water in Denmark is minimal (19.4 L per capita per year).39 Exposure misclassification could also arise from water consumed from outside the home tap. It is also possible that a residence is supplied by an unregistered private well; private wells typically have higher nitrate concentrations than public waterworks. Misclassification of gestational age is also possible. Generalizability in our study is limited to those experiencing nitrate contamination of drinking water at levels largely below regulatory limits and to high-income nations with widespread access to prenatal care. We were unable to study more specific definitions of PTB and we encourage future studies to study the associations with spontaneous PTB where contractions begin before 37 weeks completed gestation and to exclude PTB by induction due to pregnancy or birth complications, such as preeclampsia, to have a more homogeneous definition of PTB.
Strengths
With more than 1 million births, our population-based study is well-powered, and we were able to make use of individual-level covariates, outcomes, and exposure: importantly accounting for nitrate concentrations at each maternal residence during pregnancy, not just at residence at the time of birth. Our nitrate data, based on measurements performed by certified laboratories, have been shown to be a reliable proxy for measurements taken in homes at the faucet.23 The validity of the Danish Medical Birth Registry with children born at hospital or at home in Denmark is also considered very high and includes information on maternal smoking.40 Further, appreciable confounding by other risk factors for PTB such as environmental pollutants, extreme temperature, physical demanding workload or SES such as substantial inequalities in income and access to health care, as in the United States41 are far less likely.
Conclusions
The findings from our study support the existing evidence of an increased risk of PTB with increasing nitrate concentrations. This adds to a growing body of evidence of an increase in adverse birth outcomes related to nitrate in drinking water. Although the effect sizes were relatively small, given the ubiquity of nitrate in drinking water and the severity of long-term consequences associated with being born preterm, our findings have large public health implications. Consistent with the prior studies, our findings suggest that current EU and US nitrate standards may be inadequate to protect children from PTB. It is of great concern that we are seeing these effects at exposures to such relatively low nitrate concentrations. One may expect much more severe changes in gestational age in developing countries and in private well users with higher exposures. Additional studies are needed that consider dietary intake of nitrate and nitrite and of anti-oxidants that may inhibit the formation of N-Nitroso compounds, the use of nitrosatable drugs that may promote the formation of these compounds, and under higher exposure conditions.
Supplementary Material
Footnotes
Published online 23 August 2022
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
This work was supported by the US National Institutes of Health (NIH)/National Institute for Environmental Health Sciences (NIEHS) grant R01 ES027823-01A1. This grant included funding for open access publication of this paper. This study was also supported by The Danish Big Data Centre for Environment and Health funded by the Novo Nordisk Foundation Challenge Programme (grant NNF17OC0027864).
Due to privacy concerns, the data from this study are not available to outside researchers unless they have approval from the Danish Data Protection Agency.
The authors declare that they have no conflicts of interest with regard to the content of this report.
References
- 1.World Health Organization [WHO], March of Dimes, PMNCH, Save the Children. Born Too Soon: The Global Action Report on Preterm Birth. Eds Howson CP, Kinney MV, Lawn JE. World Health Organization. Geneva. 2012. Available at: https://www.marchofdimes.org/materials/born-too-soon-the-global-action-report-on-preterm-.pdf. Accessed 19 June 2020. [Google Scholar]
- 2.Petrou S, Eddama O, Mangham L. A structured review of the recent literature on the economic consequences of preterm birth. Arch Dis Child Fetal Neonatal Ed. 2011;96:F225–F232. [DOI] [PubMed] [Google Scholar]
- 3.Koppe JGV-VP, Ilsen A. Long-term outcome. In: Kurjak A, ed. Textbook of Perinatal Medicine. Parthenon Publishing; 1998:1362–1374. [Google Scholar]
- 4.Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299:1429–1436. [DOI] [PubMed] [Google Scholar]
- 5.Pietz J, Peter J, Graf R, et al. Physical growth and neurodevelopmental outcome of nonhandicapped low-risk children born preterm. Early Hum Dev. 2004;79:131–143. [DOI] [PubMed] [Google Scholar]
- 6.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417–421. [DOI] [PubMed] [Google Scholar]
- 7.Stayner LT, Almberg K, Jones R, Graber J, Pedersen M, Turyk M. Atrazine and nitrate in drinking water and the risk of preterm delivery and low birth weight in four Midwestern states. Environ Res. 2017;152:294–303. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Woodruff TJ, Baer RJ, et al. Investigation of association between environmental and socioeconomic factors and preterm birth in California. Environ Int. 2018;121(Pt 2):1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherris AR, Baiocchi M, Fendorf S, Luby SP, Yang W, Shaw GM. Nitrate in drinking water during pregnancy and spontaneous preterm birth: a retrospective within-mother analysis in California. Environ Health Perspect. 2021;129:57001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuong AM, Shinde MU, Brender JD, et al. ; National Birth Defects Prevention Study Investigators. Prenatal exposure to nitrosatable drugs, dietary intake of nitrites, and preterm birth. Am J Epidemiol. 2016;183:634–642. [DOI] [PubMed] [Google Scholar]
- 11.Shukla S, Saxena A. Global status of nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures. In: Hussain CM, ed. Handbook of Environmental Materials Management. Springer International Publishing; 2018;869–888. [Google Scholar]
- 12.Hansen B, Thorling L, Schullehner J, Termansen M, Dalgaard T. Groundwater nitrate response to sustainable nitrogen management. Sci Rep. 2017;7:8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burow KR, Nolan BT, Rupert MG, Dubrovsky NM. Nitrate in groundwater of the United States, 1991-2003. Environ Sci Technol. 2010;44:4988–4997. [DOI] [PubMed] [Google Scholar]
- 14.Nolan BT, Ruddy BC, Hitt KJ, Helsel DR. Risk of nitrate in groundwaters of the United States a national perspective. Environ Sci Technol. 1997;31:2229–2236. [Google Scholar]
- 15.Stayner LT, Jensen AS, Schullehner J, et al. Nitrate in drinking water and risk of birth defects: findings from a cohort study of over one million births in Denmark. Lancet Reg Health Eur. 2022;14:100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffman VR, Jensen AS, Trabjerg BB, et al. Prenatal exposure to nitrate from drinking water and markers of fetal growth restriction: a population-based study of nearly one million danish-born children. Environ Health Perspect. 2021;129:27002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelhardt KA, Hisle-Gorman E, Gorman GH, Dobson NR. Lower preterm birth rates but persistent racial disparities in an open-access health care system. Mil Med. 2018;183:e570–e575. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen LB, Olsen J. The danish medical birth registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 20.Hansen M, Pjetursson B. Free, online Danish shallow geological data. Geological Survey Denmark Greenland Bulletin. 2011;23:53–56. [Google Scholar]
- 21.Schullehner J. Danish Water Supply Areas and their links to water production facilities: an open-access data set. GEUS Bulletin. 2022;49. doi: 10.34194/geusb.v49.8319. [Google Scholar]
- 22.Schullehner J, Stayner L, Hansen B. Nitrate, nitrite, and ammonium variability in drinking water distribution systems. Int J Environ Res Public Health. 2017;14:E276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen CB. The danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 24.Schullehner J, Hansen B. Nitrate exposure from drinking water in Denmark over the last 35 years. Environ Res Lett. 2014;9:095001. [Google Scholar]
- 25.Quinn JA, Munoz FM, Gonik B, et al. ; Brighton Collaboration Preterm Birth Working Group. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34:6047–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 27.Ward MH, Jones RR, Brender JD, et al. Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health. 2018;15:E1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenbrand G, Spiegelhalder B, Preussmann R. Nitrate and nitrite in saliva. Oncology. 1980;37:227–231. [DOI] [PubMed] [Google Scholar]
- 29.Chadha S, Jain V, Gupta I, Khullar M. Nitric oxide metabolite levels in preterm labor. J Obstet Gynaecol Res. 2007;33:710–717. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DD, Ridnour LA, Isenberg JS, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83:85–96. [DOI] [PubMed] [Google Scholar]
- 32.Wu HC, Shen CM, Wu YY, Yuh YS, Kua KE. Subclinical histologic chorioamnionitis and related clinical and laboratory parameters in preterm deliveries. Pediatr Neonatol. 2009;50:217–221. [DOI] [PubMed] [Google Scholar]
- 33.Mehrnejat N, Yazdanpanah H, Fadaei Nobari R, et al. Spatial analysis of neonatal congenital hypothyroidism and nitrate as an environmental pollutant in Isfahan Province during 2010-2013. Int J Prev Med. 2015;6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342:d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stagnaro-Green A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab. 2009;94:21–25. [DOI] [PubMed] [Google Scholar]
- 37.Super M, de Heese HV, MacKenzie D, Dempster WS, Du Plessis J, Ferreira JJ. An epidemiological study of well-water nitrates in a group of South West African/Namibian infants. Water Res. 1981;15:1265–1270. [Google Scholar]
- 38.Brender JD, Olive JM, Felkner M, Suarez L, Marckwardt W, Hendricks KA. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology. 2004;15:330–336. [DOI] [PubMed] [Google Scholar]
- 39.UNESDA (Union of European Soft Drinks Associations). Industry Volume Data: Denmark. Available at: https://www.unesda.eu/consumption/. Accessed 4 December 2019.
- 40.Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49:893–897. [DOI] [PubMed] [Google Scholar]
- 41.Schaider LA, Swetschinski L, Campbell C, Rudel RA. Environmental justice and drinking water quality: are there socioeconomic disparities in nitrate levels in U.S. drinking water? Environ Health. 2019;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.