Abstract
KCNK3 mutations identified in sleep apnea probands affect TASK-1 X-gate function. These changes lead to an increase in potassium current and open probability, as well as impaired sensitivity to G-protein-coupled receptor inhibitors.
An Article published in this issue of Nature Genetics by Sörmann et al.1 yields insights into the role of two-pore-domain potassium channels (K2P) in the rare disease developmental delay with sleep apnea (DDSA). This study stems from a recent powerful integration of health-related and exome data from 31,058 parent–offspring trios in individuals with developmental disorders2. In the study, KCNK3 was identified among 28 genes with excessive mutational burden not previously associated with developmental disorders. KCNK3 encodes the K2P TASK-1 channel that is expressed in central and peripheral chemoreceptors, lung and heart tissue and pulmonary smooth muscle, and is involved in the control of breathing and implicated in sleep apnea3–7 — a highly prevalent disease associated with cardiovascular and metabolic disease8,9. The follow-up analyses by Sörmann et al.1 in nine probands with shared sleep apnea phenotypes, developmental impairments, hypotonia and structural malformations revealed associations between six key missense mutations in two clusters of the TASK-1 protein. Such analyses highlight the utility of large-scale genomic datasets to identify potential functional variants that can be tested in controlled experimental settings, which are pivotal components of precision and personalized medicine.
Sörmann et al.1 assessed the functional consequences of KCNK3 mutations by cloning TASK-1 receptors in Xenopus oocytes and revealed a gain-of-function whole-cell current effect for non-wild-type variants identified in the probands (Fig. 1). The authors1 further revealed domain-specific associations with sleep apnea severity among probands and, in each case, these gain-of-function effects are notably distinct from previously characterized loss-of-function KCNK3 variants associated with pulmonary arterial hypertension10.
Fig. 1 |. Mutations observed in sleep apnea probands show impaired TASK-1 channel function, as evaluated in X. oocytes.
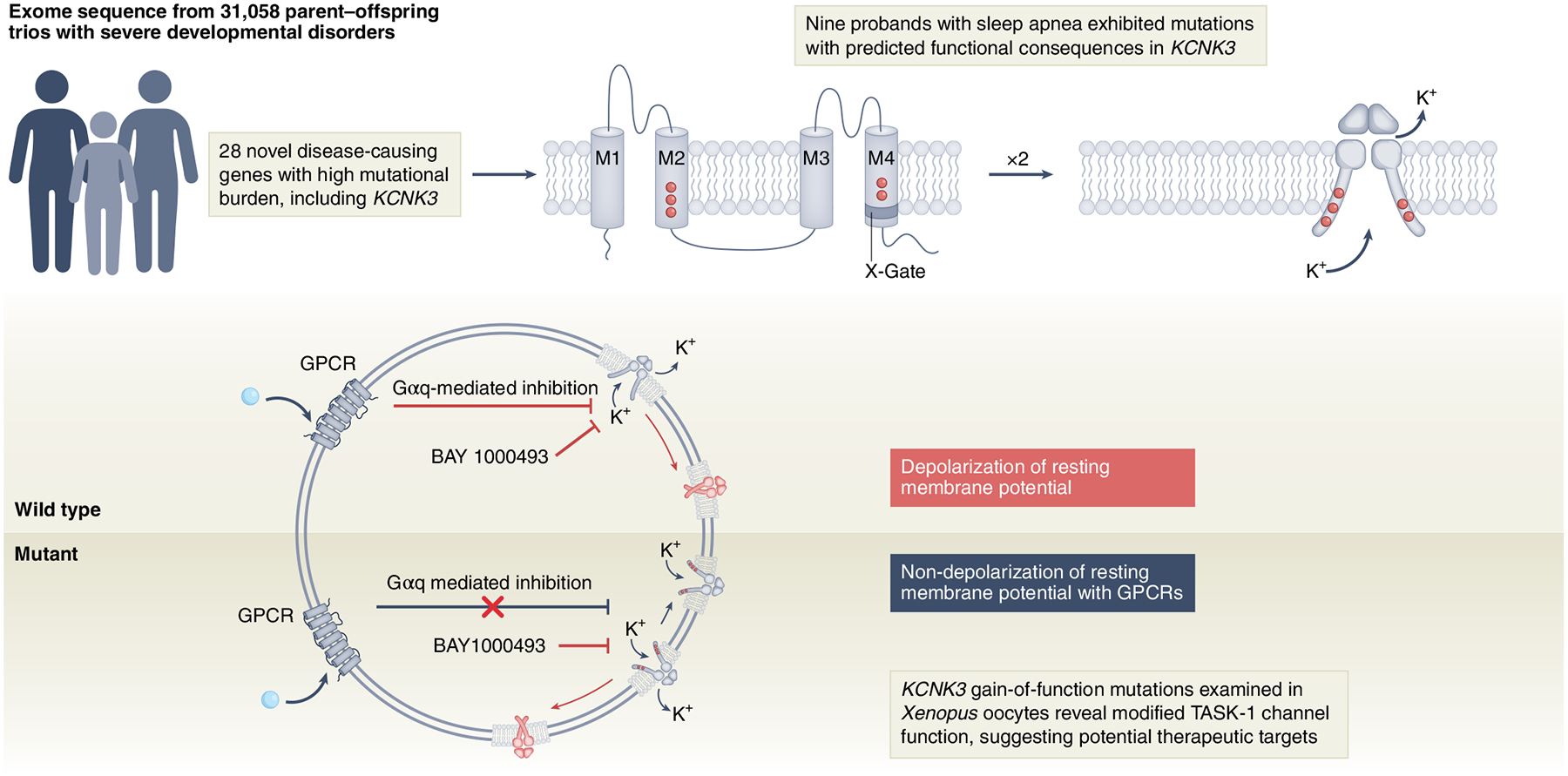
Mutations in KCNK3 results in reduced open probability (PO) and deficient activation through GPCRs in TASK-1 channels, disrupting the proper functioning of the TASK-1 channel X-gate. BAY1000493 denotes a potent TASK-1 inhibitor compound. M1–M4, transmembrane helix units; Gαq, protein G alpha q subunit.
The authors’ effective use of structural three-dimensional analysis revealed specific clustering of mutations in the X-gate area of TASK channels, which are crucial for the opening and closing function that establishes the resting membrane, potential controlling K+ ions conductance1. Typically, sustained stimulus or activation of second messenger pathways initiated by G-protein-coupled receptors (GPCRs) can induce a decrease in the open probability (PO) of the channel, reducing the conductance of K+ and favoring cell excitability. The reported mutations destabilize the proper closed state of the X-gate in the TASK-1 channel and, as the authors elegantly describe, the mutations associated with the novel DDSA condition increases TASK-1 PO as a consequence of the X-gate dysfunction1.
Activation of muscarinic GPCRs with carbachol or adenosine triphosphate (for P2Y receptors) in oocytes expressing wild-type TASK-1 receptors decreased K+ currents owing to the closed state of the channel, but GPCR-induced activation has no effect in the TASK-1 mutants. Therefore, mutations observed in DDSA TASK-1 channels render them insensitive to activation triggered by GPCR pathways (Fig. 1).
As noted by Sörmann et al.1, the mechanisms underlying sleep apnea are complex and not well understood. Although the authors did not investigate the cell-specific contributions of DDSA-associated mutations to sleep apnea, they carefully characterized specific functional changes in TASK-1 receptor mutations observed in humans with severe DDSA. This provides a step forward in the understanding of potential mechanisms for sleep apnea presentation, including contributions of dysfunctional TASK-1 channels. Additional studies that aim to delineate specific contributions to central and obstructive sleep apnea based on this work will be a promising direction for future research.
Given that sleep apnea is thought to affect up to one billion people worldwide, and the fact that existing therapies are imperfect, there is considerable interest in new therapeutic approaches for this disease. Personalized medicine is now being approached whereby underlying mechanisms can be individually targeted in an effort to ameliorate sleep apnea. The concept of loop gain (ventilatory control instability) has been applied to sleep apnea to define the propensity for breathing instability. Individuals with sleep apnea often have high loop gain, which describes a propensity for periodic breathing. Drugs that target the TASK-1 channels would be predicted to affect control of breathing but may need to be approached in a careful manner. Efforts to increase chemosensitivity may increase loop gain and could worsen sleep apnea in a subset of individuals. On the other hand, in patients with low loop gain or blunted chemosensitivity, the agents that enhance chemosensitivity could improve breathing pattern. Thus, careful physiological considerations would need to be applied in the design of sophisticated studies for the new findings to yield therapeutic progress. Nonetheless, we applaud the authors on important new findings and bringing the field a step closer to the pharmacological management of sleep apnea.
Footnotes
Competing interests
A.M. reports income related to medical education from Zoll, Livanova, Eli Lilly and Jazz. ResMed provided a philanthropic donation to University of California, San Diego. T.S.S. and E.A.M. declare no competing interests.
References
- 1.Sörmann J et al. Nat. Genet 10.1038/s41588-022-01185-x (2022). [DOI] [PMC free article] [PubMed]
- 2.Kaplanis J et al. Nature 586, 757–762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg EA et al. Pflugers Archiv. 467, 907–916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapp S et al. J. Neurosci 28, 8844–8850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckler KJ Pflugers Archiv. 467, 1013–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiper AK et al. Pflugers Archiv. Eur. J. Physiol 467, 1081–1090 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Wang J et al. Resp. Physiol. Neurobiol 161, 23–28 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Benjafield AV et al. Lancet Respir. Med 7, 687–698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan AS, McSharry DG & Malhotra A Lancet 383, 736–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L et al. N. Engl. J. Med 369, 351–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


