Abstract
We cloned the rpoN (ntrA, glnF) gene encoding the alternate sigma factor ς54 from the opportunistic multihost pathogen Pseudomonas aeruginosa strain PA14. A marker exchange protocol was used to construct the PA14 rpoN insertional mutation rpoN::Genr. PA14 rpoN::Genr synthesized reduced levels of pyocyanin and displayed a variety of phenotypes typical of rpoN mutants, including a lack of motility and the failure to grow on nitrate, glutamate, or histidine as the sole nitrogen source. Compared to wild-type PA14, rpoN::Genr was ca. 100-fold less virulent in a mouse thermal injury model and was significantly impaired in its ability to kill the nematode Caenorhabditis elegans. In an Arabidopsis thaliana leaf infectivity assay, although rpoN::Genr exhibited significantly reduced attachment to trichomes, stomata, and the epidermal cell surface, did not attach perpendicularly to or perforate mesophyll cell walls, and proliferated less rapidly in Arabidopsis leaves, it nevertheless elicited similar disease symptoms to wild-type P. aeruginosa PA14 at later stages of infection. rpoN::Genr was not impaired in virulence in a Galleria mellonella (greater wax moth) pathogenicity model. These data indicate that rpoN does not regulate the expression of any genes that encode virulence factors universally required for P. aeruginosa pathogenicity in diverse hosts.
In gram-negative bacteria, the alternate sigma factor ς54, working in concert with a transcriptional activator that belongs to the NtrC superfamily, activates a variety of genes that are regulated in response to external stimuli (1). For example, in various bacteria, ς54 is required for expression of the enzymatic pathways responsible for nitrogen utilization, dicarboxylate transport, xylene degradation, and hydrogen utilization (6, 32, 39, 41, 61). ς54 is also involved in the regulation of virulence-related factors in both plant and animal pathogens, including pilin, flagellin, and alginate synthesis in Pseudomonas aeruginosa (19, 58, 60); capsular expression in Klebsiella pneumoniae (3); and regulation of hrp gene expression and coronatine biosynthesis in Pseudomonas syringae (23, 24).
Our laboratory has developed a bacterial pathogenicity model that utilizes a clinical isolate of P. aeruginosa (strain UCBPP-PA14 [referred to here as PA14]) that elicits severe soft-rot-like symptoms and proliferates when infiltrated into Arabidopsis leaves (52), kills the larvae of the wax moth caterpillar Galleria mellonella (30), causes lethal sepsis in a mouse full-skin-thickness burn model (52), and kills the nematode Caenorhabditis elegans (40, 56, 57). Interestingly, there is significant overlap among the PA14 virulence factors required for pathogenesis in plants, nematodes, insects, and mice. For example, among 21 genes identified as being involved in pathogenesis by screening transposon-induced PA14 mutants in plants and nematodes, 18, 17, 19, and 21 of these genes were required for pathogenicity in Arabidopsis, nematodes, wax moths, and mice, respectively (30, 40, 53, 57).
In other studies, we showed that rpoN is a key virulence factor for the plant pathogen P. syringae (23, 24). Specifically, molecular and genetic analysis showed that the P. syringae rpoN gene is required for expression of the P. syringae hrp gene cluster, a block of contiguous genes, some of which encode components of a type III secretory system (2, 18, 26, 45, 46, 59).
Given the facts that RpoN activates the expression of a wide variety of environmentally regulated genes and is required for virulence in a variety of pathogens, we hypothesized that RpoN would play a central role in the evolution of P. aeruginosa's ability to be a pathogen of evolutionarily disparate hosts. In this study we describe the results of experiments that involved the construction of a P. aeruginosa PA14 rpoN mutant to study the role of ς54 in P. aeruginosa pathogenesis in a variety of plant and animal hosts. Surprisingly, we report that the P. aeruginosa rpoN gene is not a universal virulence factor required for multihost pathogenesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used and constructed in this study are listed in Table 1. Escherichia coli and P. aeruginosa strains were grown at 37°C in L broth, King's A (KA), King's B (KB) (33), or M9 minimal salts media. Nitrogen source utilization tests for PA14 rpoN mutants were performed in M9 salts minimal medium by replacing ammonium chloride with an alternative nitrogen source at 5 mM when required. Bacterial motility was tested on “swarm plates” (35). Pyocyanin assays (17) were carried out in KA broth containing 100 μM FeCl3 (17, 33). Pyoverdin was assayed on KB plates as described previously (57). C. elegans killing assays were carried out on NG agar (“slow killing” [56]) or PGS agar (“fast killing” [56]) as described elsewhere. Antibiotic concentrations for E. coli strains were as follows: streptomycin, 150 μg/ml; kanamycin, 25 μg/ml; tetracycline, 12 μg/ml; gentamicin, 5 to 10 μg/ml; and spectinomycin, 20 μg/ml. Antibiotic concentrations for P. aeruginosa were as follows: streptomycin, 200 μg/ml; kanamycin, 200 μg/ml; tetracycline, 75 μg/ml; gentamicin, 30 μg/ml; nalidixic acid, 50 μg/ml; rifampin, 100 μg/ml, and carbenicillin, 300 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype, phenotype, or rolea | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| PAK N1 | rpoN::Genr | S. Lory (28) |
| PA14 | Wild type | M. Schroth (52) |
| PA14 rpoN::Genr | Contains Genr cassette inserted into rpoN | This study |
| PA14(pSMC21) | PA14 expressing GFP | 56 |
| PA14 rpoN::Genr(pSMC21) | rpoN::Genr expressing GFP | This study |
| E. coli | ||
| DH5a | F−lacZΔM15 endA1 recA1 hsdR17 supE44 thi-1 gyrA relA1 λ−; host for cosmid library and other plasmids | Bethesda Research Laboratories (20) |
| MM294(pRK2013) | Donor of transfer functions for triparental crosses | 14 |
| Plasmids | ||
| pJSR1 | Apr, cosmid cloning vector | 52 |
| pBSK(+) | Apr, cloning vector | Stratagene, Inc. |
| pBR322 | Apr Tetr, cloning vector | New England Biolabs, Inc. |
| pKI11 | Source of P. aeruginosa PAK rpoN gene | S. Lory (28) |
| pPAR4 | 4-kb fragment containing PA14 rpoN gene in pBSK(+) | This study |
| pPAR4SR | 4-kb fragment containing PA14 rpoN gene in pJSR1 | This study |
| pRPONgent | PA14 rpoN::Genr in pBR322 | This study |
| pRPON10 | PA14 rpoN gene in pJSR1 | This study |
| pSMC21 | Derivative of plasmid pSMC2; carries the A. victoria GFP | G. A. O'Toole (7) |
Apr, ampicillin resistance; Tetr, tetracycline resistance.
Bacterial genetics.
pJSR1 derivatives were introduced into Pseudomonas strains via triparental matings with MM294/pRK2013 as the donor of transfer functions as described previously (14). Plasmid pSMC21 (7) containing the Aequorea victoria green fluorescent protein (GFP) was introduced into PA14 rpoN::Genr by electroporation.
Pyocyanin assays.
Pyocyanin was measured at 520 nm in acidic solution by using a modified version of a previously described method (17). Cultures were grown from a 100-fold dilution of a log-phase culture in KA broth modified with 100 μM FeCl3. After 20 h, 1 ml of the culture was extracted with 2 ml of chloroform and centrifuged for 5 min. The blue chloroform solution was transferred into a new tube containing 1 ml of 0.2 N HCl to extract pyocyanin into the acidic solution. The concentration was determined by measuring the optical density at 520 nm (OD520).
Plant material and growth of plants.
Arabidopsis ecotypes Llagostera (Ll-0) and Landsberg erecta (La-er) were obtained from the Arabidopsis Biological Resource Center, Columbus, Ohio. Arabidopsis plants were grown in Metro-Mix 2000 in either a climate-controlled greenhouse at 19°C under a 12-h light-dark cycle with supplemental fluorescent illumination or in a Percival AR-60L growth chamber at 20°C and 50% relative humidity.
Arabidopsis pathogenicity assays.
Six- to eight-week-old intact or detached Arabidopsis rosette leaves were used for pathogenicity assays. The pathogenicity of PA14 strains was tested by placing detached Ll-0 leaves on a 1.5% water agar surface with their petioles embedded into the agar and inoculating the leaves by growing lawns of PA14 or PA14 rpoN::Genr overnight on LB agar medium containing rifampin (PA14) and gentamicin (rpoN::Genr), cutting 3-mm-diameter agar cylinders from these plates, and placing the cylinders bacterial side down on one or both sides of the central vein in the top half of a leaf. The growth of PA14 or PA14 rpoN::Genr in Arabidopsis leaves was determined by infiltrating the leaves of intact La-er plants with 5 × 104 CFU/cm2 of leaf area as described previously (52). The growth of PA14 or PA14 rpoN::Genr in detached Arabidopsis leaves was determined by infiltrating Ll-0 leaves with a bacterial suspension in 10 mM MgSO4 at a density of 5 × 104 CFU/ml for 1 h under a slight vacuum at room temperature in the wells of a six-well microtiter plate. At days 0, 1, 2, 3, and 4, the titer of the bacteria in each of two punches in each of three leaves was determined as described previously (15).
Infection of G. mellonella larvae.
The 50% lethal dose (LD50) of PA14 strains in G. mellonella larvae was determined as described previously (30). In brief, overnight cultures grown in KB medium were diluted 1:100, allowed to grow until they reached an OD600 of 0.3 to 0.4, and resuspended in 10 mM MgSO4. After dilution to an OD600 of 0.1 with 10 mM MgSO4, serial 10-fold dilutions were made in 10 mM MgSO4 containing 1 mg of rifampin/ml and 10 mg of carbenicillin/ml. A 10-μl Hamilton syringe was used to inject 5-μl aliquots into the hindmost left proleg of fifth-instar G. mellonella larvae purchased from Van der Horst Wholesale, St. Mary's, Ohio. Groups of 10 larvae infected with the same dose of bacteria were placed in petri dishes and incubated at 25°C for 60 h. Larvae were scored as dead when they no longer moved upon shaking of the petri dish or poking with a pipette tip.
Mouse full-skin-thickness burn model.
Inbred AKR mice that had been subjected to a thermal burn injury were infected with PA14 strains as described previously (54). In brief, 6-week-old male mice were anesthetized by the injection of phenobarbital. The animals were then shaved, and a ventral skin fold was elevated. Two brass blocks preheated to 92 to 95°C were applied to the skin fold for 5 s to deliver a full-skin-thickness burn covering ca. 5% of the body surface area. The burn eschar was injected with 100 μl of bacterial suspension at a titer of 5 × 104 or 5 × 106 bacteria per ml. Bacteria for the inoculation were grown overnight in LB, diluted 1:100, allowed to grow until they reached an OD600 of 1.6 to 1.7, pelleted by centrifugation, and resuspended and diluted in 10 mM MgSO4. The mouse protocol was reviewed and approved by the Animal Care Committee of the Massachusetts General Hospital.
C. elegans killing assay.
The killing kinetics of C. elegans strain Bristol N2 by PA14 strains were determined on PGS agar (fast-killing assay) or NG agar (slow-killing assay) as described previously (56).
Nucleic acid manipulations.
Routine DNA manipulations such as DNA blots and plasmid DNA isolation were performed as described previously (4). Restriction enzymes, T4 DNA ligase, and calf intestine phosphatase were purchased from Boeringer Mannheim and New England BioLabs and used according to the manufacturers' specifications.
Cloning the P. aeruginosa PA14 rpoN gene and construction of a PA14 rpoN mutant.
A cosmid clone, pRPON10, containing a presumptive P. aeruginosa PA14 rpoN gene was identified in a pJSR1 cosmid library (52) by colony hybridization with P. aeruginosa strain PAK rpoN gene on plasmid pKI11 as a hybridization probe. The presumptive PA14 rpoN gene was mapped to a 4.0-kb XhoI-EcoRI fragment and was subcloned into pBSK(+) to produce pPAR4. DNA sequence analysis showed 95% identity out of 141 nucleotides sequenced between the PA14 rpoN gene and the P. aeruginosa PAO1 rpoN gene (reference 31 and data not shown).
A mutant derivative of the PA14 rpoN gene was constructed by inserting a DNA cassette conferring gentamicin resistance (Genr) into the ClaI site of the PA14 rpoN gene in pPAR4 to create rpoN::Genr and then excising a 5.5-kb XhoI-SpeI fragment containing rpoN::Genr and ligating it into the EcoRI site of pBR322 to create pRPONgent. pRPONgent (containing rpoN::Genr) was conjugated into PA14 via a triparental mating by using pRK2013 (14), and rpoN::Genr was marker exchanged into the PA14 genome by first selecting for gentamicin resistance and then screening for carbenicillin sensitivity.
Scanning electron and confocal microscopy.
To monitor the attachment of PA14 GFP-labeled strains to the epidermal cell walls of Ll-0 leaves and to Ll-0 trichomes by confocal laser microscopy, 4-mm-diameter leaf disks were immersed in bacterial suspensions at a density of ca. 109 cells/ml under weak vacuum to remove possible air bubbles from the surface of the leaf disks and make the leaf surface more accessible to bacteria. Subsequently, leaf disks were incubated for 24 h in the bacterial suspensions with shaking under normal pressure and room temperature. The leaf disks were examined with a confocal laser spectrophotometer (Leica TCS NT) by excitation at 488 nm and by monitoring the emission intensity at 511 nm. To monitor the development of PA14 strains in Ll-0 parenchyma cells by scanning electron microscopy, 4-mm-diameter leaf disks were cut from the central portion of a leaf from either side of the central vein with a cork borer and then immersed in a PA14 or PA14 rpoN::Genr suspension at an OD600 of 0.02 in 10 mM MgSO4 in the wells of a 24-well microtiter dish, followed by incubation at room temperature. Leaf disks were fixed at 1, 2, 3, and 4 days postinfection (dpi) in 4% paraformaldehyde, passed through an ethanol series (30, 50, 70, 96, and 100%), and freeze fractured in liquid nitrogen. The plant material was dried in a critical-point drying apparatus (Samdri-PVT-3B; Tousimis), mounted on stubs, coated with a 20- to 25-μm layer of gold-palladium in a Hummer II Sputter Coater (Technics), and studied by using an AMRAY 1000 scanning electron microscope.
RESULTS AND DISCUSSION
PA14 rpoN::Genr exhibits a variety of characteristic RpoN. phenotypes.
As described in Materials and Methods, an interspecies hybridization method was used to identify and clone the P. aeruginosa PA14 rpoN gene. The PA14 rpoN gene was partially sequenced and over this region showed 95% identity to both P. aeruginosa PAO1 and PAK rpoN sequences (data not shown). Also, as described in Materials and Methods, a PA14 rpoN mutant (rpoN::Genr) was constructed by inserting a DNA cassette conferring gentamicin resistance into the ClaI site of the PA14 rpoN gene and transferring the disrupted gene into the PA14 genome by homologous recombination. The Genr cassette is inserted within the N-terminal 33% of rpoN, before the highly conserved carboxy-terminal region (31, 41).
Unlike wild-type PA14, rpoN::Genr exhibited a variety of characteristic RpoN− phenotypes. Similar to a previously described P. aeruginosa PAK rpoN mutant (strain PAK N1) (58), PA14 rpoN::Genr was nonmotile and did not grow well on glutamate, histidine, or nitrate as the sole nitrogen source (Table 2). Electron microscopic examination showed that PA14 rpoN::Genr cells were nonflagellated (data not shown). In contrast to a previous report that P. aeruginosa PAK rpoN (strain N1) is a glutamine auxotroph (58), we found that both PAK rpoN (strain N1) and PA14 rpoN::Genr grew slowly in the absence of glutamine. Restricted nitrogen utilization, rather than glutamine auxotrophy, is also observed with rpoN mutants of P. syringae and P. putida (36).
TABLE 2.
Nitrogen utilization of P. aeruginosa strains
| Nitrogen source | Nitrogen utilization of strain:
|
||||
|---|---|---|---|---|---|
| PA14
|
PAK
|
||||
| Wild type (platesa) |
rpoN mutant
|
Wild type (platesa) | rpoN mutant (platesa) | ||
| Platesa | Liquidb | ||||
| Ammonia | 2 | 4 | + | 2 | 4 |
| Arginine | 2 | 3 | + | 2 | 3 |
| Glutamate | 2 | − | − | 2 | − |
| Glutamine | 2 | 2 | + | 2 | 2 |
| Histidine | 2 | − | − | 2 | − |
| Nitrate | 2 | − | − | 2 | − |
Measured as the number of days until the appearance of colonies. – indicates that only tiny colonies grew.
Growth (+) or absence of growth (−) in liquid culture. Growth experiments were conducted twice with identical results
To confirm that the RpoN− phenotypes of PA14 rpoN::Genr were due to the rpoN::Genr insertion, a 4.0-kb DNA fragment containing a wild-type copy of PA14 rpoN on plasmid pPAR4SR was introduced into the PA14 rpoN::Genr strain. This plasmid restored motility and wild-type growth on all of the nitrogen sources listed in Table 2. Although this complementation experiment did not rule out the possibility that the rpoN::Genr insertion has a polar effect on downstream genes, it appears likely that the RpoN− phenotypes of PA14 rpoN::Genr are primarily due to the disruption of the rpoN gene. This conclusion is based on the conservation of the genomic region surrounding rpoN in P. aeruginosa strain PAO1 in comparison to the analogous genomic regions in several bacterial species (29, 31, 42, 44, 50, 55). Upstream of the PAO1 rpoN gene is an open reading frame (ORF) with homology to the ATP-binding component of an ABC transporter (21). Downstream of rpoN in PAO1 are four ORFs (55). Homologous downstream ORFs are found in several species (29, 31, 42, 44, 50, 55). The second ORF is homologous to the enzyme IIA domains of several proteins of the bacterial sugar phosphotransferase system (PTS) (31). The fourth ORF is homologous to the gene for the HPr protein, which is also a component of the PTS (50). ORF1 and -3 encode peptides that have no homology to proteins of known function. These downstream ORFs appear to play a role in modifying RpoN activity, although the effects are relatively minor. Disruption of the second downstream ORF in P. aeruginosa strain PAK reduced the ability of PAK to grow without glutamine, but overexpression of ORF1 or ORF2 by the tac promoter had no measurable effect (31). Mutation of either the first or second downstream ORFs in K. pneumoniae increased expression of a number of rpoN-regulated genes (42). In Caulobacter crescentus and Rhizobium etli, mutations of the downstream ORFs had only a minor effect on one rpoN-regulated gene, fliK (29), or had no measurable effect (44), respectively.
PA14 rpoN::Genr synthesizes reduced levels of pyocyanin.
P. aeruginosa secretes a variety of compounds under nutrient-limiting conditions, including the yellow-green siderophore pyoverdin and the blue microbial toxin pyocyanin (10, 43). Pyoverdin may be a virulence factor in burn wound infections (5, 43), and pyocyanin has been implicated in pulmonary artery injury (9, 22). KA Fe3+ medium or swarm plates turned noticeably blue when PA14 was grown to stationary phase, whereas no blue color was observed after the growth of PA14 rpoN::Genr. Quantitative measurement showed that PA14 rpoN::Genr produced 56% ± 13% less pyocyanin than did the wild type and that pyocyanin production was restored to wild-type levels in PA14 rpoN::Genr(pPAR4SR). Pyoverdin production appeared to be unaffected in PA14 rpoN::Genr. PA14 colonies grown on KB plates express pyoverdin, which can be detected by fluorescence when the colonies are exposed to long-wavelength UV light. Visual inspection revealed no noticeable difference between wild-type and PA14 rpoN::Genr colonies.
PA14 rpoN::Genr exhibits reduced pathogenicity in mice.
As shown in Table 3, PA14 rpoN::Genr was significantly less pathogenic than wild-type PA14 in a mouse burn model. A high percentage of lethality was observed with PA14 at both a relatively high dose of 5 × 105 cells (100% lethality) and a relatively low dose of 5 × 103 cells (∼80% lethality). In contrast, only five of eight and one of seven animals died when inoculated with 5 × 105 and 5 × 103 PA14 rpoN::Genr, respectively. The use of two different doses allows a better estimation of the mutation's effects on virulence. In the case of rpoN, even the high dose was not as lethal as wild type at a 100-fold-lower dose, demonstrating a significant reduction in virulence. These burn model data are consistent with previous reports demonstrating that P. aeruginosa rpoN mutants or P. aeruginosa mutants which contain lesions in RpoN-regulated genes exhibit reduced colonization and virulence in a number of other model systems. rpoN mutants showed a reduced ability to colonize in a chronic murine intestinal mucosal model (48) or in human respiratory epithelial xenografts (11) and exhibited reduced virulence in a murine corneal scratch model (51) or in a murine model of acute pneumonia (12). One limitation of these studies, as well as of our own, however, is the fact that rpoN mutants are nonmotile, and it is not known to what extent the nonmotile phenotype of the rpoN mutants contributes to the loss of virulence. Nonmotile mutants of P. aeruginosa strains M2, PA01, and MT1200 exhibited significant reduction in virulence in a mouse burn model (16). The reduction was at the same level or greater than that seen with the PA14 rpoN mutant. Thus, the entire effect on virulence of the rpoN mutant in P. aeruginosa PA14 in the mouse burn model could be due to the loss of motility. RpoN is also an important virulence factor for virulence of Vibrio anguillarum in fish (47) and Vibrio cholerae in an infant mouse colonization model (13, 34). In the case of V. cholerae, it appears that unknown rpoN-regulated genes, in addition to the flagellum genes, are required for colonization.
TABLE 3.
Lethality of PA14 in a mouse burn model
| P. aeruginosa strain | Mortality ratio (no. of animals that died/total no. of animals [%]) at 10 days post burn
|
|
|---|---|---|
| Low dosea | High doseb | |
| PA14 | 7/7 (100) | 8/8 (100) |
| PA14 rpoN::Genr | 1/7 (14) | 5/8 (62) |
Six-week-old male AKR/J mice (Charles River Laboratories) were injected with 5 × 103 cells as described previously (54).
Injected with 5 × 105 cells.
PA14 rpoN::Genr exhibits reduced pathogenicity in Arabidopsis.
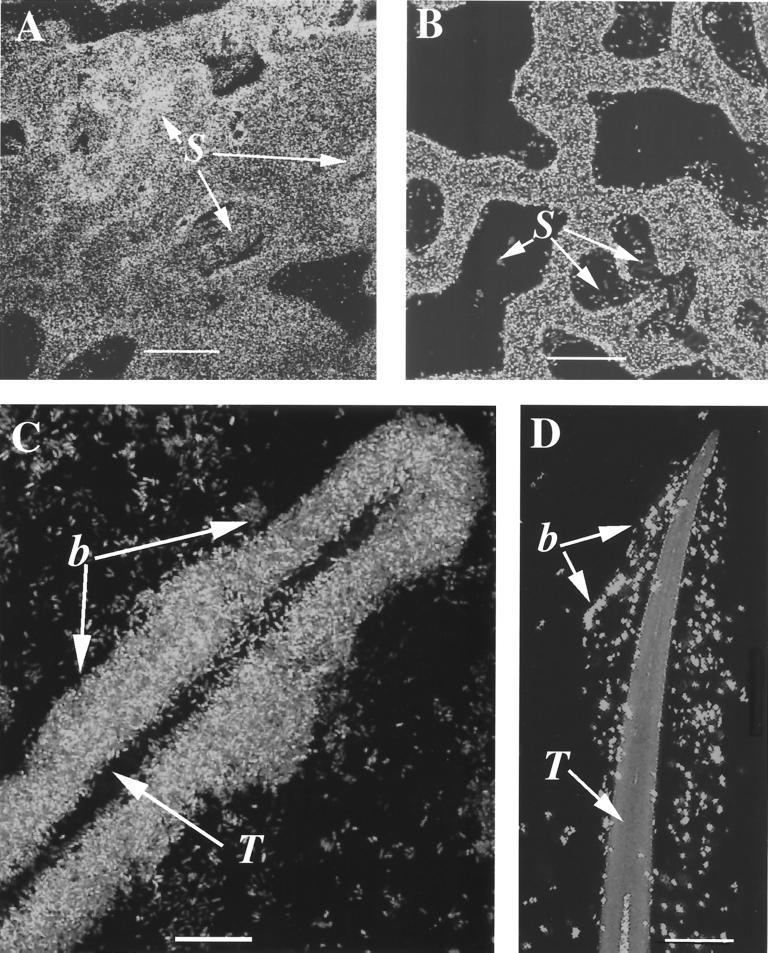
We have developed several assays to assess the pathogenicity of P. aeruginosa in Arabidopsis leaves, comparing attachment to plant leaf surfaces and monitoring growth rate and symptom development. We previously showed (49) that when Arabidopsis ecotype Ll-0 leaf disks are incubated in a dense bacterial suspension (i.e., ∼109 cells/ml) for 24 h, wild-type PA14 cells attached to the entire leaf epidermal cell surface, congregated above most of the stomata, and formed large, multilayered clusters on the trichomes (Fig. 1A and C). In contrast, as shown in Fig. 1B and D, PA14 rpoN::Genr cells primarily attached to the grooves formed at cell junctions, and very few of the mutant bacteria were located above the stomata or associated with trichomes.
FIG. 1.
P. aeruginosa adheres to leaf surfaces. Confocal scanning microscopy of PA14 or rpoN::Genr expressing GFP in association with Arabidopsis ecotype Ll-O. (A) PA14(pSCM21) adheres to the entire surface of the leaf, including the stomatal (S) openings. Bar, 20 μm. (B) PA14 rpoN::Genr(pSCM21) cells primarily attached to the grooves formed at cell junctions; very few of the mutant bacteria were located above the stomata (S). Bar, 20 μm. (C) PA14(pSCM21) (b) formed large multilayered clusters on trichomes (T). Bar, 10 μm. (D) PA14 rpoN-GFP::Genr(pSCM21) did not attach to the trichome (T) surface and concentrated loosely around them. Bar, 10 μm. Leaf disks were incubated with PA14/pSMC21 (A and C) or PA14 rpoN::Genr/pSMC21(B and D) in bacterial suspensions at a density of ∼109 CFU/ml under slight vacuum for 1 h, followed by incubation for 24 h in an orbital shaker at room temperature, and then examined by confocal laser microscopy as described in Materials and Methods.
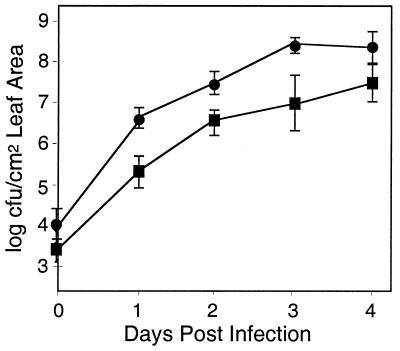
As shown in Fig. 2, PA14 rpoN::Genr showed an ∼10-fold decrease in its ability to proliferate in detached Arabidopsis Ll-0 leaves compared to PA14 after gentle vacuum infiltration of a relatively low inoculum of bacteria. Similar results were obtained when intact La-er Arabidopsis plants were hand infiltrated with a 1-ml syringe without a needle (data not shown). With either mode of infection, PA14 rpoN::Genr ultimately elicited severe soft-rot symptoms.
FIG. 2.
Growth of PA14 and PA14 rpoN::Genr in Arabidopsis leaves. Detached Ll-0 leaves were vacuum infiltrated with bacterial suspensions as described in Materials and Methods. The initial titers were ca. 5 × 104 CFU/cm2 leaf area. Bacterial titers in the infiltrated leaves were measured immediately after infiltration and at 1, 2, 3, and 4 days after infiltration as described in Materials and Methods. Each value represents the average of three replicates. Similar results were obtained when the leaves of intact 6-week-old La-er plants were infiltrated at a titer of 5 × 104 CFU/cm2 of leaf area and incubated as described in Materials and Methods (not shown). Symbols: ●, PA14; ▪, PA14 rpoN::Genr.
We also tested the ability of PA14 rpoN::Genr to infect Ll-0 leaves and elicit disease symptoms without being forced into the leaf by a syringe or vacuum infiltration. Lawns of PA14 or PA14 rpoN::Genr were grown on LB-rifampin agar. Agar cylinders 3 mm in diameter were cut and placed bacterial side down on detached Arabidopsis Ll-0 leaves as described in Materials and Methods. Both PA14 and PA14 rpoN::Genr formed lesions under these assay conditions, but at relatively early stages of infection (4 days postinfection [dpi]) the wild-type strain produced significantly larger lesions than PA14 rpoN::Genr. However, by 7 dpi, we did not find any significant differences in the symptoms produced by PA14 and PA14 rpoN::Genr. At 4 dpi, the PA14 lesions were, on average, 6.3 ± 1.45 mm in diameter, whereas the diameter of the PA14 rpoN::Genr-elicited lesions were 4.4 ± 1 mm. At 7 dpi, the mean diameters of the PA14 and PA14 rpoN::Genr lesions were 7.3 ± 0.85 and 6.91 ± 1.14 mm, respectively. Interestingly, chlorotic rings around the lesions formed by the less-virulent PA14 rpoN::Genr mutant were significantly larger than around PA14 lesions (data not shown).
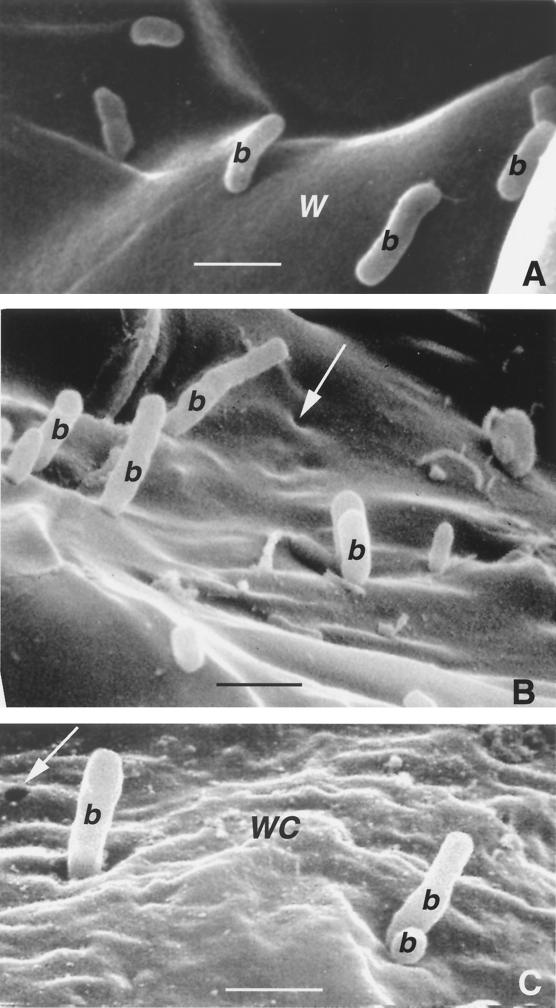
Finally, we showed in a recent publication that one of the hallmarks of PA14 pathogenesis of Arabidopsis is perpendicular attachment of PA14 cells to the leaf epidermis and to mesophyll cell walls (49). Moreover, as shown in Fig. 3B and C, PA14 causes the convolution of and the formation of small holes in mesophyll cell walls. The diameter of these holes is approximately the same as the diameter of the bacteria. In contrast to the wild type, PA14 rpoN::Genr cells did not attach perpendicularly under the conditions examined to the leaf epidermal surface 24 h postinfection (data not shown) and, as shown in Fig. 3A, rpoN::Genr cells oriented themselves parallel to mesophyll cell walls and did not cause the formation of the small holes. Thus, although we found that PA14 rpoN::Genr has diminished ability to attach to plant cell surfaces, it still has the ability to cause soft-rot symptoms. In contrast, we have shown that the P. syringae rpoN gene is absolutely required for full pathogenicity in Arabidopsis, most likely because it is required for hrp gene expression (23, 24). The hrp genes encode the components of a type III secretory system related to the Yersinia species Yop secretion system (37). P. aeruginosa has recently been shown to contain a type III secretion system as well, which is required for the export of exotoxin S (62). Our data indicate that the P. aeruginosa type III system is either rpoN independent or that a type III secretion system is not absolutely required for P. aeruginosa pathogenicity in plants.
FIG. 3.
Scanning electron micrographic visualization of freeze-fractured infected Arabidopsis Ll-0 leaves depicting the failure of P. aeruginosa PA14 rpoN::Genr cells to attach perpendicularly to and penetrate the cell walls of vessel parenchyma cells. The leaves of intact Ll-0 plants were infiltrated under vacuum, the infected plants were incubated for 72 h, and then examined by scanning electron microscopy as described in Materials and Methods. b, bacterium, W, plant wall; WC, wall convolution. (A) PA14 rpoN::Genr cells parallel to the surface of a smooth cell wall of a parenchyma vessel cell. Bar, 1 μm. (B and C) PA14 cells attached perpendicularly to the surface of a highly convoluted cell wall of a parenchyma vessel cell. Holes (arrows) in the plant cell walls with diameters similar to that of the bacteria are readily visible. Bars, 1 μm.
PA14 rpoN::Genr exhibits reduced C. elegans killing.
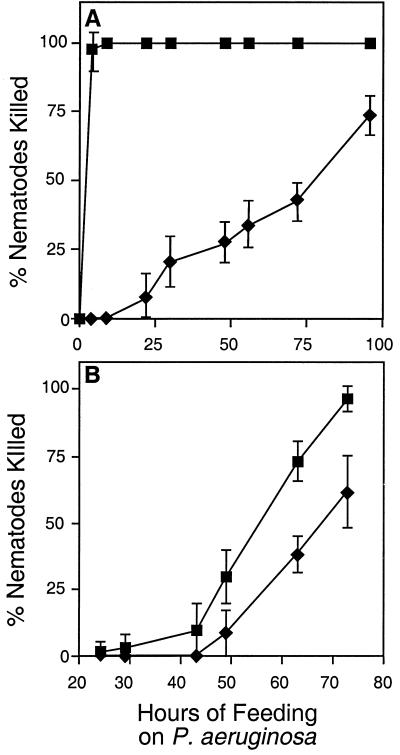
When the nematode C. elegans is fed a lawn of PA14 grown on solid medium, the nematodes die in two characteristic ways depending on the medium on which PA14 is grown (56). On a high-osmolarity but low-phosphate medium (e.g., PGS), the nematodes die rapidly (fast killing) over the course of 24 h. In contrast, nematodes that are fed PA14 grown on a low-osmolarity but high-phosphate medium (NGM) are killed at a slower rate (slow killing), dying after 2 to 3 days. Previous studies have shown that the killing of C. elegans by PA14 under these two different conditions is mediated by distinct molecular mechanisms (40, 56, 57). As shown in Fig. 4, there was significantly less killing of C. elegans by PA14 rpoN::Genr compared to killing by wild-type PA14 under both the fast- and slow-killing conditions. The killing of PA14 rpoN::Genr in the fast-killing assay was restored to wild-type levels by pPAR4SR, which carries wild-type rpoN, indicating that the loss of pathogenicity phenotype of PA14 rpoN::Genr was due to the disruption of the rpoN gene (data not shown). The decrease in fast killing by PA14 rpoN::Genr is consistent with the results described above showing that PA14 rpoN::Genr synthesizes reduced levels of pyocyanin. Previous results from our laboratory showed that pyocyanin is an important toxin mediating the fast-killing process (40).
FIG. 4.
PA14 rpoN::Genr killing of C. elegans under fast- and slow-killing conditions. (A) Fast killing. L4 stage worms were seeded onto bacterial lawns on PGS medium, and mortality was recorded as described earlier (40). Each value is the mean for three replicates. Symbols: ■, PA14; ⧫, PA14 rpoN::Genr. (B) Slow killing. L4 stage worms were seeded onto bacterial lawns on NGM media, and mortality was recorded as described previously (56). Each value is the mean for six replicates. Symbols: ●, PA14; ⧫, rpoN::Genr.
PA14 rpoN::Genr does not exhibit reduced pathogenicity in G. mellonella
A single PA14 cell is sufficient to kill a greater wax moth caterpillar when PA14 is injected into the hemolymph (30). We found that PA14 and PA14 rpoN::Genr were indistinguishable in their ability to kill fifth-instar wax moth larvae (data not shown). The LD50 in both cases was approximately one bacterial cell. This is interesting in light of the decreased virulence of PA14 rpoN::Genr in the mouse burn model. Microbial defense in insects shows interesting parallels to innate immunity in mammals and plants (8, 25, 27). Members of the cecropin class of antibacterial peptides have been found in both mammals and insects, and cecropin A in Drosophila melanogaster is regulated by a cascade similar to the one involving NF-κB activation in the mammalian inflammatory response (38). The reduction in virulence of PA14 rpoN::Genr in mice but not in wax moths suggests that rpoN does not play a significant role in the regulation of virulence factors that affect components of the host innate immune system conserved between insects and mammals.
Conclusions.
Previous work with PA14 in our laboratory has shown that PA14 utilizes a common set of virulence factors in evolutionarily disparate hosts (30, 40, 53, 57). Given this and the highly pleiotropic nature of rpoN mutants, we expected that disruption of rpoN would result in a significant impairment in plant, nematode, insect, and mouse pathogenicity. Surprisingly, a major reduction in pathogenicity of the P. aeruginosa PA14 rpoN mutant was observed only in C. elegans killing and in the elicitation of sepsis in the mouse burn model. Although decreased lesion size and a 10-fold reduction in in planta growth was observed in Arabidopsis at early stages of an infection (3 to 4 dpi), at later stages (7 dpi) the P. aeruginosa rpoN mutant elicited disease symptoms that were indistinguishable from those caused by the wild type. This contrasts with the absolute requirement for RpoN function in P. syringae for pathogenicity (23, 24). No effect of the rpoN mutation was observed in G. mellonella killing. Thus, although it has been previously reported that P. aeruginosa rpoN mutants exhibit impaired virulence in mice, the results reported here provide a more complete perspective concerning the role of RpoN in the diverse pathogenic interactions that P. aeruginosa has with several evolutionarily disparate hosts. The major conclusion is that, in contrast to our expectations, rpoN does not appear to regulate any genes that are likely to encode virulence factors universally required for pathogenicity irrespective of the host.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM48707 to F.M.A. and by grants from Hoechst AG and Aventis SA to Massachusetts General Hospital.
REFERENCES
- 1.Albright L M, Huala E, Ausubel F M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- 2.Alfano J R, Collmer A. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 2001. [Google Scholar]
- 5.Barton H A, Johnson Z, Cox C D, Vasil A I, Vasil M L. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;21:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 6.Black L K, Maier R J. IHF- and RpoN-dependent regulation of hydrogenase expression in Bradyrhizobium japonicum. Mol Microbiol. 1995;16:405–413. doi: 10.1111/j.1365-2958.1995.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 7.Bloemberg G V, O'Toole G A, Lugtenberg B J, Kolter R. Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol. 1997;63:4543–4551. doi: 10.1128/aem.63.11.4543-4551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 9.Britigan B E, Rasmussen G T, Cox C D. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect Immun. 1997;65:1071–1076. doi: 10.1128/iai.65.3.1071-1076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byng G S, Eustice D C, Jensen R A. Biosynthesis of phenazine pigments in mutant and wild-type cultures of Pseudomonas aeruginosa. J Bacteriol. 1979;138:846–852. doi: 10.1128/jb.138.3.846-852.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn L A, Weber A, Phillips T, Lory S, Kaplan M, Smith A. Pseudomonas aeruginosa infection of respiratory epithelium in a cystic fibrosis xenograft model. J Infect Dis. 2001;183:919–927. doi: 10.1086/319245. [DOI] [PubMed] [Google Scholar]
- 12.Comolli J C, Hauser A R, Waite L, Whitchurch C B, Mattick J S, Engel J N. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa N E, Lauriano C M, McGee R, Klose K E. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol Microbiol. 2000;35:743–755. doi: 10.1046/j.1365-2958.2000.01745.x. [DOI] [PubMed] [Google Scholar]
- 14.Ditta G, Stanfield S, Corbin D, Helinski D R. Broad host-range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium melliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Mindrinos M, Davis K R, Ausubel F M. Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell. 1991;3:61–72. doi: 10.1105/tpc.3.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake D, Montie T C. Flagella motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol. 1988;124:43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- 17.Essar D W, Eberly L, Hadero A, Crawford I P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galan J, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg J B, Dahnke T. Pseudomonas aeruginosa AlgB, which modulates the expression of alginate, is a member of the NtrC subclass of prokaryotic regulators. Mol Microbiol. 1992;6:59–66. doi: 10.1111/j.1365-2958.1992.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- 21.Hartig E, Zumft W G. The requirement of RpoN (sigma factor ς54) in denitrification by Pseudomonas stutzeri is indirect and restricted to the reduction of nitrite and nitric oxide. Appl Environ Microbiol. 1998;64:3092–3095. doi: 10.1128/aem.64.8.3092-3095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan H M, Fridovich I. Mechanism of the antibiotic action of pyocyanine. J Bacteriol. 1980;141:1556–1563. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrickson E L, Guevera P, Ausubel F M. The alternative sigma factor RpoN is required for hrp activity in Pseudomonas syringae pv. maculicola and acts at the level of hrpL transcription. J Bacteriol. 2000;182:3508–3516. doi: 10.1128/jb.182.12.3508-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrickson E L, Guevera P, Penaloza-Vazquez A, Shao J, Bender C, Ausubel F M. Virulence of the phytopathogen Pseudomonas syringae pv. maculicola is rpoN dependent. J Bacteriol. 2000;182:3498–3507. doi: 10.1128/jb.182.12.3498-3507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann J A. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang H-C, Lin R-H, Chang C-J, Collmer A, Deng W-L. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for hairpin Pss secretion that are arranged colinearly with Yersinia ysc homologs. Mol Plant-Microbe Interact. 1995;8:733–746. doi: 10.1094/mpmi-8-0733. [DOI] [PubMed] [Google Scholar]
- 27.Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993;9:178–183. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto K S, Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci USA. 1989;86:1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janakiraman R S, Brun Y V. Transcriptional and mutational analyses of the rpoN operon in Caulobacter crescentus. J Bacteriol. 1997;179:5138–5147. doi: 10.1128/jb.179.16.5138-5147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jander G, Rahme L G, Ausubel F M. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol. 2000;182:3843–3845. doi: 10.1128/jb.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin S, Ishimoto K, Lory S. Nucleotide sequence of the rpoN gene and characterization of two downstream open reading frames in Pseudomonas aeruginosa. J Bacteriol. 1994;176:1316–1322. doi: 10.1128/jb.176.5.1316-1322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler B, Marqués S, Köhler T, Ramos J L, Timmis K N, de Lorenzo V. Cross talk between catabolic pathways in Pseudomonas putida: XylS-dependent and-independent activation of the TOL meta operon requires the same cis-acting sequences within the Pm promoter. J Bacteriol. 1994;176:5578–5582. doi: 10.1128/jb.176.17.5578-5582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King E O, Ward M K, Raney D E. Two simple media for the demonstration of phycocyanin and fluorescin. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 34.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 35.Kohler T, Harayama S, Ramos J L, Timmis K N. Involvement of Pseudomonas putida RpoN ς factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 38.Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann J A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 39.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahajan-Miklos S, Tan M W, Rahme L G, Ausubel F M. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell. 1999;96:47–56. doi: 10.1016/s0092-8674(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 41.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 42.Merrick M J, Coppard J R. Mutations in genes downstream of the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect repression from ς54-dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 43.Meyer J, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaels J, Van Soom T, D'hooghe I, Dombrecht B, Benhassine T, de Wilde P, Vanderleyden J. The Rhizobium etli tpoN locus: DNA sequence analysis and phenotypical characerization of rpoN, ptsN, and ptsA mutants. J Bacteriol. 1998;180:1729–1740. doi: 10.1128/jb.180.7.1729-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mudgett M, Staskawicz B. Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol Microbiol. 1999;32:927–941. doi: 10.1046/j.1365-2958.1999.01403.x. [DOI] [PubMed] [Google Scholar]
- 46.Mudgett M, Staskawicz B. Protein signaling via type III secretion pathways in phytopathogenic bacteria. Curr Opin Microbiol. 1998;1:109–114. doi: 10.1016/s1369-5274(98)80150-1. [DOI] [PubMed] [Google Scholar]
- 47.O'Toole R, Milton D L, Horstedt P, Wolf-Watz H. RpoN of the fish pathogen Vibrio (Listonella) anguillarum is essential for flagellum production and virulence by the water-borne but not intraperitoneal route of inoculation. Microbiology. 1997;143:3849–3859. doi: 10.1099/00221287-143-12-3849. [DOI] [PubMed] [Google Scholar]
- 48.Pier G B, Meluleni G, Neuger E. A murine model of chronic mucosal colonization by Pseudomonas aeruginosa. Infect Immun. 1992;60:4768–4776. doi: 10.1128/iai.60.11.4768-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotnikova J M, Rahme L G, Ausubel F M. Pathogenesis of the human opportunistic pathogen Pseudomonas aeruginosa PA14 in Arabidopsis. Plant Physiol. 2000;124:1766–1774. doi: 10.1104/pp.124.4.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell B S, Court D L, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier M H, Reizer J. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem. 1995;270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- 51.Preston M J, Fleiszig S M, Zaidi T S, Goldberg J B, Shortridge V D, Vasil M L, Pier G B. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahme L G, Stevens E J, Wolfort S F, Shao J, Tompkins R G, Ausubel F M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 53.Rahme L G, Tan M W, Le L, Wong S M, Tompkins R G, Calderwood S B, Ausubel F M. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci USA. 1997;94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens E J, Ryan C M, Friedberg J S, Barnhill R L, Yarmush M L, Tompkins R G. A quantitative model of Pseudomonas aeruginosa infection in injury. J Burn Care Rehabil. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G K, Wu Z, Paulsen I T. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 56.Tan M W, Mahajan-Miklos S, Ausubel F M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan M W, Rahme L G, Sternberg J A, Tompkins R G, Ausubel F M. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA. 1999;96:2408–2413. doi: 10.1073/pnas.96.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Totten P A, Lara J C, Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Dijk K, Fouts D E, Rehm A H, Hill A R, Collmer A, Alfano J R. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol. 1999;181:4790–4797. doi: 10.1128/jb.181.16.4790-4797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods D E, Straus D C, Johanson W G, Jr, Berry V K, Bass J A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980;29:1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Z-L, Charles T C, Wang H, Nester E W. The ntrA gene of Agrobacterium tumefaciens: identification, cloning, and phenotype of a site-directed mutant. J Bacteriol. 1992;174:2720–2723. doi: 10.1128/jb.174.8.2720-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]