Abstract
Pancreatic cancer is characterized by inter-tumoral and intra-tumoral heterogeneity, especially in genetic alteration and microenvironment. Conventional therapeutic strategies for pancreatic cancer usually suffer resistance, highlighting the necessity for personalized precise treatment. Cancer vaccines have become promising alternatives for pancreatic cancer treatment because of their multifaceted advantages including multiple targeting, minimal nonspecific effects, broad therapeutic window, low toxicity, and induction of persistent immunological memory. Multiple conventional vaccines based on the cells, microorganisms, exosomes, proteins, peptides, or DNA against pancreatic cancer have been developed; however, their overall efficacy remains unsatisfactory. Compared with these vaccine modalities, messager RNA (mRNA)-based vaccines offer technical and conceptional advances in personalized precise treatment, and thus represent a potentially cutting-edge option in novel therapeutic approaches for pancreatic cancer. This review summarizes the current progress on pancreatic cancer vaccines, highlights the superiority of mRNA vaccines over other conventional vaccines, and proposes the viable tactic for designing and applying personalized mRNA vaccines for the precise treatment of pancreatic cancer.
Keywords: Pancreatic cancer, Precise therapy, Cancer vaccine, mRNA vaccine, Tumor antigen, Immune subtype
Background
The annual pancreatic cancer cases have doubled over the past two decades, increasing from 196,000 patients worldwide in 1990 to 441,000 in 2017 [1]. According to the 2020 global cancer statistics, there were 495,773 new cases of pancreatic cancer [2]. Given the increase in life expectancy of the global population, the incidence of pancreatic cancer is expected to continue rising over the coming decades. Surgical intervention is currently the only curative option for pancreatic cancer management in the clinic. However, only 15–20% of patients qualify for the corresponding surgery, attributed to the limited routine screening methods for detecting pancreatic cancer at an early stage [3]. Moreover, despite complete resection, local or distant recurrence of pancreatic cancer is often observed within two years after surgery [4]. Systematic chemotherapy has been the standard treatment for more than 80% of patients with locally advanced diseases or distant metastases for several decades. Even though gemcitabine plus nab-paclitaxel and FOLFIRINOX are the most recommended chemotherapeutic regimens for metastatic pancreatic ductal adenocarcinoma (PDAC) treatment, acquired resistance against these drugs is common [5–7]. Immunotherapy, targeted therapy, and other promising treatments have also been tested in preclinical studies and clinical trials; however, almost all strategies show little significant advantage over conventional chemotherapy against pancreatic cancer, together with the prevalent therapeutic resistance [8, 9]. Accordingly, the overall 5-year survival of pancreatic cancer patients is only about 10%, making the tumor is one of the leading causes of cancer-related mortality [10]. Obviously, there is an urgent need for highly effective alternatives for pancreatic cancer treatment.
Accumulating evidence indicates that the therapeutic resistance in pancreatic cancer is associated with its inter-tumoral and intra-tumoral heterogeneity, particularly as regards the genetic alteration and immune microenvironment [11–13]. For instance, SMAD4 mutation occurs in about 50% of PDAC patients [14], and this mutation promotes radiotherapeutic resistance by increasing the production of reactive oxygen species and inducing autophagy [15]. Appropriately 6% of pancreatic cancers display BRCA1/2 or PALB2 mutations [16, 17], and the lack of mutations in these genes is associated with resistance to platinum-based chemotherapy [17, 18]. Tumors without BRCA1/2 mutations are also susceptible to generating PARP inhibitor resistance [19–21]. In addition, the inter-tumoral heterogeneity in the immune microenvironment promotes resistance to immunotherapy [11]. Taking the programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) blockade as an example, its therapeutic efficacy is associated with the pre-infiltration of T cells [22]. Only < 1% of PDAC patients with high microsatellite instability that was detected the presence of neoantigen-specific T cell immunity in tumor respond to PD-1 inhibition [23–26]. In contrast, most tumors are characterized by low immunogenicity and lack of T cell infiltration and are thus resistant to immunotherapy targeting PD-1/PD-L1. Notably, pancreatic cancer is classified into distinct subtypes based on gene expression or immune characteristics [27–29]. For instance, Moffitt et al. [28] identified two stromal subtypes (normal and activated) and two tumor subtypes (basal-like and classical) based on gene expression profiles. Compared with classical subtype tumors, basal-like subtype tumors exhibit a superior response to adjuvant chemotherapy. Apart from inter-tumoral heterogeneity, increasing evidence has uncovered the intra-tumoral heterogeneity in pancreatic cancer [30]. At least three types of intra-tumoral genetic heterogeneity have been proposed [13]. Type-1 includes mutations distinguishing tumor cells within the same primary lesions, type-2 includes mutations distinguishing tumor cells within the same metastatic lesions, while type-3 includes mutations distinguishing tumor cells among different metastatic lesions. The intra-tumoral heterogeneity largely promotes adaptive resistance to cancer therapy. Single-cell sequencing for pancreatic cancer has revealed the existence of both basal-like and classical subtypes in the same tumor, partially explaining the adaptive resistance to chemotherapy [31]. These reports highlight the heterogeneity-induced therapeutic resistance, underlining the significance of developing personalized precise treatment against pancreatic cancer. Compared with the traditional monoclonal antibodies and small molecule inhibitors, cancer vaccines offer several advantages, including minimal nonspecific effects, broad therapeutic window, low toxicity, and induction of persistent immunological memory [32, 33]. Moreover, cancer vaccines can achieve precise targeting based on the characteristics in individual tumors. Therefore, vaccination is a potential approach for personalized pancreatic cancer treatment, overcoming the challenges posed by tumor heterogeneity.
Messager RNA (mRNA) vaccine has recently become one of the most potent vaccine types in prevention and treatment of multiple diseases. The successful development of mRNA vaccine is attributed to decades of relentless and intensive research. mRNA was discovered in 1961 and isolated for in vitro protein expression in 1969 [34, 35]. Until 1990, in vitro transcribed mRNA was validated able to be template to produce proteins in mouse skeletal muscle cells in vivo [36]. This was the first successful attempt for in vivo mRNA expression, setting the stage for mRNA vaccine development. Later in 1992, mRNA for vasopressin was injected and expressed in the hypothalamus, inducing physiological responses [37]. Thereafter in 1993 and 1995, mRNA was reported to induce both cellular and humoral immunity [38–40]. However, these promising findings did not attract substantial investment in the development of mRNA vaccines largely due to the perceived mRNA instability, inefficient in vivo delivery, and potential innate immunogenicity. Given the safety, simple design, and ease of manufacturing, research on mRNA continued. The technological advances in the modification and delivery of mRNA largely addressed these concerns. For instance, the application of modified nucleosides prevents mRNA recognition by pattern recognition receptors (PRRs), enhancing the translational efficacy [41]. Application of vehicles (e.g., lipid nanoparticle, polyplexes, and polymeric nanoparticles) promotes the in vivo delivery of mRNA [42]. The improvement of in vivo translational efficiency and delivery enhances the chance of clinical application of mRNA vaccines. The first application of personalized mRNA vaccine in humans was reported in 2017 against melanoma, and the vaccination induced specific immune activation, decreased the metastatic rate and prolonged the progression-free survival of patients [43]. In addition, multiple clinical trials on the efficacy of mRNA-based vaccination against human immunodeficiency virus have been completed [44]. Since its outbreak in 2019, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of the coronavirus disease 2019 (COVID-19), has infected and caused millions of deaths globally [45, 46]. Due to the threat posed by SARS-CoV-2, various treatments were rapidly developed to contain the spread of the virus. Owing to the convenience in mass production and advances in modification techniques of mRNA, two mRNA vaccines, BNT162b2 and mRNA-1273, obtained the authorization of emergency use for preventing COVID-19 [47–50]. Both achieved a protective efficacy of over 90%, and were officially approved for mass vaccination by the Food and Drug Administration against SARS-CoV-2 [49–53]. Of note, SARS-CoV-2 underwent multiple mutations, compromising the protective efficacy of BNT12b2 and mRNA-1273 [54–56]. However, this concern has currently been solved to a large extent by updating vaccine-encoded antigens accordingly, together with optimizing the administration of vaccination and boosting. The strategies used for overcoming SARS-CoV-2 variations provide valuable experience for developing and applying the personalized anti-pancreatic cancer mRNA vaccine. Together, the application of mRNA vaccine in other diseases lays a foundation for the development of personalized anti-pancreatic cancer mRNA vaccine.
This review summarizes the current advances and status of pancreatic cancer vaccines, emphasizes the superiority of mRNA-based vaccines in cancer precision treatment, and highlights the strategy for developing personalized mRNA vaccines against pancreatic cancer.
Conventional pancreatic cancer vaccines
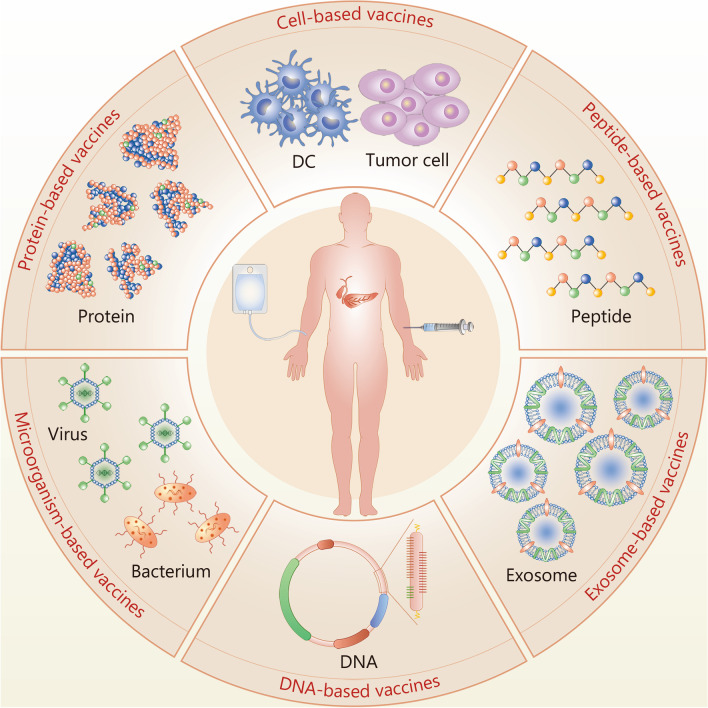
To date, multiple conventional vaccines against pancreatic cancer, including cell-based, microorganism-based, exosome-based, protein-based, peptide-based, and DNA-based forms (Fig. 1), are under development (completed clinical trials are summarized in Table 1, and ongoing clinical trials in Table 2).
Fig. 1.
Classification of existing pancreatic cancer vaccines. Multiple pancreatic cancer vaccines have been developed to date, including cell-based vaccines, microorganism-based vaccines, exosome-based vaccines, protein-based vaccines, peptide-based vaccines, and DNA-based vaccines. DC dendritic cell
Table 1.
Completed clinical trials of pancreatic cancer vaccines
| Vaccine type | NCT number | Immunogen | Additional treatment | Phase | Enrollment | Endpoint |
|---|---|---|---|---|---|---|
| Cell-based vaccine | NCT00004604 | CEA RNA-pulsed autologous DC | No | Phase I | Not provided | 2002 |
| NCT00002773 | Allogeneic pancreatic cancer cell | Cyclophosphamide, GM-CSF | Phase II | Not provided | 2004 | |
| NCT00084383 | GVAX | 5-fluorouracil, radiotherapy | Phase II | 60 | 2006 | |
| NCT00255827 | Allogeneic tumor cell expressing α-1,3 galactosyltransferase | No | Phase I/II | 7 | 2007 | |
| NCT00128622 | Autologous DC-infected with fowlpox-CEA-6D-TRICOM | Denileukin diftitox | Phase I | Not provided | 2007 | |
| NCT00027534 | Autologous DC-infected with fowlpox-CEA-6D-TRICOM | Autologous DC mixed with CMV pp65 and tetanus toxoid | Phase I | Not provided | 2007 | |
| NCT00547144 | Autologous DC | Gemcitabine, stereotactic radiosurgery | Phase I | 2 | 2008 | |
| NCT00002475 | Allogeneic or autologous tumor cell | Cyclophosphamide, GM-CSF | Phase II | Not provided | 2009 | |
| NCT00305760 | GVAX | Cetuximab, cyclophosphamide | Phase II | 60 | 2009 | |
| NCT00161187 | Allogeneic lymphocyte | No | Phase I | Not provided | 2011 | |
| NCT01410968 | Peptide-pulsed DC | Poly-ICLC | Phase I | 12 | 2016 | |
| NCT02151448 | Autologous αDC1-loaded with autologous tumor material | Celecoxib, IFN-α, rintatolimod | Phase I/II | 64 | 2019 | |
| NCT00727441 | GVAX | Surgery, cyclophosphamide | Phase II | 87 | 2019 | |
| NCT01896869 | GVAX | FOLFIRINOX, ipilimumab | Phase II | 83 | 2019 | |
| Peptide-based vaccine | NCT00006387 | RAS | Immunological adjuvant QS21 | Phase I | Not provided | 2002 |
| NCT00008099 | MUC1 | SB AS-2 | Phase I | 25 | 2004 | |
| NCT00019006 | RAS | Detox-B adjuvant | Phase I | Not provided | Not provided | |
| NCT00019331 | RAS | IL-2, GM-CSF | Phase II | Not provided | 2007 | |
| NCT00648102 | HCG-β | No | Phase I | Not provided | 2009 | |
| NCT00622622 | VEGFR2 | Gemcitabine | Phase I | 21 | 2009 | |
| NCT00709462 | HCG-β | No | Phase I | Not provided | 2010 | |
| NCT00529984 | CEA | No | Phase I/II | Not provided | 2010 | |
| NCT00425360 | Telomerase | Gemcitabine, capecitabine, GM-CSF | Phase III | Estimated 1110 | 2013 | |
| NCT00655785 | VEGFR1, VEGFR2 | Gemcitabine | Phase I/II | 17 | 2013 | |
| NCT01342224 | Telomerase | GM-CSF, gemcitabine | Phase I | 11 | 2018 | |
| Microorganism-based vaccine | NCT00003125 | ALVAC-CEA, vaccinia-CEA | IL-2, GM-CSF | Phase II | Not provided | 2004 |
| NCT00028496 | Fowlpox-CEA(6D)-TRICOM | GM-CSF | Phase I | Not provided | 2005 | |
| NCT01191684 | MVAp53 | No | Phase I | Not provided | 2013 | |
| NCT00569387 | Algenpantucel-L | Surgery, gemcitabine and 5-fluorouracil | Phase II | 73 | 2014 | |
| NCT00300950 | Yeast expressing four different mutated RAS protein | Gemcitabine | Phase II | 176 | 2015 | |
| NCT02338752 | DPT, typhoid, staphylococcus aureus, paratyphoid A and B | Surgery, chemotherapy | Phase I/II | 20 | 2015 | |
| NCT03127098 | Adenovirus [E1-, E2b-]-CEA(6D) | IL-15 | Phase I/II | Not provided | 2017 | |
| Protein-based vaccine | NCT00003025 | HSPPC-96 | No | Phase I | 16 | 2002 |
| DNA-based vaccine | NCT01486329 | VEGFR-2 DNA | No | Phase I | 72 | 2014 |
All clinical trial data were collected from ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home). CEA carcinoembryonic antigen, DC dendritic cell, GM-CSF granulocyte–macrophage colony-stimulating factor, GVAX GM-CSF gene-transfected allogeneic pancreatic cancer cell, CMV pp65 cytomegalovirus pp65, αDC1 α-type-1 polarized dendritic cell, RAS Ras GTPase-activating protein, MUC1 mucin 1, VGEFR vascular endothelial growth factor receptor, HCG-β human chorionic gonadotropin beta, SB AS-2 an immunologic adjuvant system consisting of an oil-in-water emulsion containing two immunostimulants: monophosphoryl Lipid A and a saponin derivative QS-21, MVAp53 modified vaccinia virus ankara vaccine expressing p53, DPT diphtheria, pertussis, tetanus, HSPPC-96 heat shock protein-peptide complex-96
Table 2.
Ongoing clinical trials of pancreatic cancer vaccines
| Vaccine type | NCT number | Immunogen | Additional treatment | Phase | Estimated enrollment | Status | Start point |
|---|---|---|---|---|---|---|---|
| Cell-based vaccines | NCT00389610 | GVAX | No | Phase II | 56 | Active, not recruiting | 2006 |
| NCT01088789 | GVAX | Cyclophosphamide | Phase II | 72 | Recruiting | 2010 | |
| NCT01595321 | GVAX | SBRT, FOLFIRINOX, cyclophosphamide | Not applicable | 19 | Active, not recruiting | 2012 | |
| NCT02451982 | GVAX | Cyclophosphamide | Phase II | 76 | Recruiting | 2016 | |
| NCT02648282 | GVAX | Cyclophosphamide, pembrolizumab, SBRT | Phase II | 58 | Active, not recruiting | 2016 | |
| NCT03190265 | GVAX | Cyclophosphamide, nivolumab, CRS-207, ipilimumab | Phase II | 63 | Active, not recruiting | 2017 | |
| NCT03161379 | GVAX | SBRT, nivolumab, cyclophosphamide | Phase II | 30 | Active, not recruiting | 2018 | |
| NCT03592888 | Autologous DC pulsed with mutant KRAS peptides | No | Phase I | 12 | Recruiting | 2018 | |
| NCT03006302 | GVAX | Epacadostat, pembrolizumab, CRS-207, cyclophosphamide | Phase II | 40 | Active, not recruiting | 2018 | |
| NCT03153410 | GVAX | Cyclophosphamide, pembrolizuma, IMC-CS4 | Phase I | 12 | Active, not recruiting | 2018 | |
| NCT03767582 | GVAX | SBRT, nivolumab, CCR2/CCR5 dual antagonist | Phase I/II | 30 | Recruiting | 2019 | |
| NCT04157127 | Autologous DC loaded with tumor lysate plus mRNA | No | Phase I | 43 | Recruiting | 2020 | |
| NCT04627246 | Autologous DC loaded with personalized peptides | Nivolumab, chemotherapy | Phase I | 12 | Recruiting | 2020 | |
| Peptide-based vaccines | NCT03558945 | Personalized neoantigen | Poly-ICLC | Phase I | 60 | Recruiting | 2018 |
| NCT04161755 | Personalized neoantigen | Atezolizumab, surgery, FOLFIRINOX | Phase I | 29 | Active, not recruiting | 2019 | |
| NCT04117087 | KRAS | Nivolumab, ipilimumab | Phase I | 30 | Recruiting | 2020 | |
| NCT03956056 | Personalized neoantigen and mesothelin | Poly-ICLC | Phase I | 12 | Active, not recruiting | 2020 | |
| NCT04810910 | Personalized neoantigen | Surgery, chemotherapy | Phase I | 20 | Recruiting | 2021 | |
| NCT05111353 | Neoantigen synthetic long peptide | Poly-ICLC | Phase I | 30 | Not yet recruiting | 2022 | |
| NCT05013216 | KRAS | Poly-ICLC | Phase I | 25 | Recruiting | 2022 | |
| Microorganism-based vaccines | NCT00669734 | Vaccinia, fowlpox | GM-CSF | Phase I | 18 | Active, not recruiting | 2010 |
| NCT03136406 | Recombinant saccharomyces cerevisiae yeast expressing mutant Ras | Cyclophosphamide, oxaliplatin, GI-4000, capecitabine, 5-fluorouracil, leucovorin, nab-paclitaxel, aNK, bevacizumab, avelumab, ALT-803, ETBX-011 | Phase I/II | 3 | Active, not recruiting | 2017 | |
| NCT05116917 | Influenza virus | Nivolumab, ipilimumab, SBRT | Phase II | 30 | Recruiting | 2021 | |
| DNA-based vaccines | NCT03122106 | Personalized neoantigens and mesothelin DNA | No | Phase I | 15 | Active, not recruiting | 2018 |
All clinical trial data were collected from ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home). GM-CSF granulocyte–macrophage colony-stimulating factor, GVAX GM-CSF gene-transfected allogeneic pancreatic cancer cell, SBRT stereotactic body radiation therapy, CRS-207 listeria monocytogenes-expressing mesothelin, DC dendritic cell, KRAS GTPase KRas, CCR C–C chemokine receptor, aNK NK-92 cells
Cell-based pancreatic cancer vaccines
The currently available cell-based pancreatic cancer vaccines include dendritic cell (DC)-based and tumor cell-based forms. As the most potent antigen-presenting cell (APC), DCs are usually loaded with an antigen and re-infused into patients [57]. In a phase I/II clinical trial, 12 patients with resected pancreatic and biliary cancer received mucin 1 (MUC1) peptide-loaded DC vaccine [57, 58]. Four of them survived more than 4 years after the vaccination and showed no signs of recurrence. In a related study, Wilms tumor (WT) 1-specific cytotoxic T cells were observed in seven out of eight cancer patients who received a combination of WT1-peptide-pulsed DC-based vaccine and S-1 or S-1 plus gemcitabine after surgery [59]. Of note, the procedure for developing DC vaccines is highly laborious and time-consuming and requires autologous cell preparations that do not meet economic requirement of precision therapy. Cell-based vaccines also include autologous and allogeneic tumor cell-derived vaccines [57, 59]. Even though autologous tumor cell-based vaccines are particularly suitable for personalized therapy, autologous tumor cells may be insufficient, as only 15–20% of pancreatic cancer patients are eligible for surgery [3, 57]. Therefore, allogeneic tumor cell-based vaccines, including GVAX [allogeneic granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting pancreatic cancer vaccine] and Algenpantucel-L (hyperacute-pancreatic cancer vaccine), are alternatives for pancreatic cancer treatment [57, 60, 61]. However, this approach does not consider the extensive heterogeneity of pancreatic cancer and thus is not suitable for personalized therapy. Compared with cyclophosphamide, a phase II trial revealed that GVAX did not improve the survival of patients with metastatic PDAC [60]. In one multi-institutional phase II clinical trial, the 12-month overall survival and disease-free survival rate of pancreatic cancer patients after treatment with Algenpantucel-L combined with standard adjuvant chemoradiotherapy reached 86% and 62%, respectively [60, 62].
Microorganism-based pancreatic cancer vaccines
Microorganism-based pancreatic cancer vaccines are classified into bacteria, viruses, and recombinant yeast-based forms [63, 64]. These vaccines represent a co-expressing strategy of tumor antigens and costimulatory molecules. The human adenovirus 40-based mesothelin vaccine inhibited the growth and metastasis of pancreatic cancer in mice [65]. An open-label phase I study of advanced pancreatic cancer showed that recombinant prime-boost poxviruses (targeting MUC1 and carcinoembryonic antigen prolonged the overall survival of patients with anti-MUC1 and/or carcinoembryonic antigen-specific immune responses [66]. In contrast, a phase II clinical trial revealed that compared with chemotherapy, vaccination with live-attenuated Listeria monocytogenes expressing mesothelin had no significant overall survival benefits for metastatic PDAC patients [67]. Notably, microorganism-based vaccines require complicated engineering system and elaborate fabrication, undermining their inconvenient application for personalized treatment.
Exosome-based pancreatic cancer vaccines
Tumor-derived exosomes (TEXs) are nanosized lipid bilayer encapsulating vesicles that shuttle bioactive information to the tumor microenvironment, promoting tumor progression [68, 69]. TEXs contain various tumor antigens and feature discrete sets of specific proteins that promote DC-binding and uptake of exosomes. In pancreatic cancer mouse models, DCs loaded with TEXs vaccine activated CD4+ T cells and significantly prolonged the survival of mice compared to cytotoxic drugs [70]. Notably, TEXs also include proteins and nucleic acids which have strong capability to boost the body’s immunity, and thus may cause auto-immune diseases by disrupting the immune homeostasis after vaccination, posing a challenge to safety of precision therapy [71–74].
Protein-based pancreatic cancer vaccines
Proteins for cancer vaccination are not only immunogenic but also can carry additional antigenic peptide. Proteins vaccines based on heat shock proteins, especially heat shock protein-peptide complex-96 (HSPPC-96), are currently under several clinical trials to investigate their therapeutic potential against different cancers [75–80]. A phase I pilot study revealed that 30% (3/10) of patients with resected pancreatic cancer survived for more than 5 years after vaccination with the HSPPC-96 vaccine [75]. Notably, given that HSPPC-96 must be extracted from tumor tissues of each patient, its use largely depends on the resectability of the tumors [80].
Peptide-based pancreatic cancer vaccines
Peptide-based vaccines are developed based on antigenic epitopes, the minimal immunogenic regions of antigens [81]. KRAS-targeting peptide was the first peptide-based vaccine to undergo clinical trials [82]. In a phase I/II study, GM-CSF combined with KRAS-targeting peptide vaccine-induced specific immune response in 25 of 43 (58%) patients, and the survival period was also significantly longer for responders than non-responders [83]. Another commonly tested peptide-based vaccine, the telomerase-targeting vaccine (GV1001), was well tolerated and improved patient survival in a phase I/II clinical trial [84]. However, two phase III clinical trials revealed that compared with mono-gemcitabine, a combination of GV1001 with gemcitabine did not significantly improve the overall survival of patients with advanced pancreatic cancer [85]. Aside from KRAS and telomerase-targeted peptides, clinical trials have revealed that the efficacy of survivin, gastrin, vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, WT1, and kinesin family member 20A-targeted vaccines is unsatisfactory[86–90]. Notably, the tumor peptide vaccine is major histocompatibility complex (MHC)-restricted and only activates monoclonal T cells, which may reduce the strength of anti-tumor immune response and thus do not satisfy the need of efficiency underlying precision therapy [81].
DNA-based pancreatic cancer vaccines
DNA-based vaccines serve as templates encoding antigens in transfected cells. Enolase 1 (ENO1), MUC1, survivin, and VEGFR-2-targeting DNA vaccines are examples of the DNA-based pancreatic cancer vaccines explored so far [91–95]. Preclinically, the ENO1 DNA vaccine efficiently induced the infiltration of effector T cells, antibody formation, and tumor cytotoxicity in genetically engineered mice with pancreatic cancer [92]. Moreover, combined with chemotherapy, the ENO1 DNA vaccine induced CD4+ T cell-meditated antitumor activity and strongly impaired cancer progression in mice [92]. MUC1-targeted DNA vaccine induced strong and specific cytotoxic T lymphocyte response and showed both therapeutic and prophylactic effects in mice [91]. The survivin DNA vaccine induced specific antitumor immunity and prolonged the survival period of mice [94]. Also, VXM01, an oral DNA vaccine targeting VEGFR-2, is under phase I trial for stage IV pancreatic cancer treatment [95]. Notably, DNA vaccines increase the risk of host genomic alteration, the coded antigens are expressed over a long-time, and the production of anti-DNA autoantibodies may limit their application [96, 97]. Obviously, the safety is a major concern for application of DNA vaccine in personalized pancreatic cancer treatment.
In summary, these conventional vaccines show a measure of progress in pancreatic cancer therapy. However, given the major concerns, including the safety and complexity in preparation, they are not the best options for vaccines-based personalized precise treatment of pancreatic cancer. Therefore, it is essential to select a novel kind of vaccines that meet the needs of individual pancreatic cancer patients.
Superiority of mRNA vaccine for cancer precision treatment
mRNA vaccines are emerging as potent candidates for cancer precision treatment because of their unique advantages over the above-mentioned vaccine formats. In addition to overcoming tumor heterogeneity by encoding personalized protein according to the genetic expression profile of tumor, mRNA vaccine meets the requirements of precision therapy highlighting precise targeting, high efficiency, safety, and economic cost.
Generation of natural protein products
As fore-mentioned, precise targeting is key for personalized therapy. Consistent with this point, mRNA functions as a template for protein translation, and utilizes the machinery in host cells for vaccine production. This characteristic allows for post-translational modification of the protein products, including proper folding for effective functioning [44, 98]. Also, this approach allows for the production of correctly folded and assembled multimeric proteins that cannot be generated in bioreactors; this method allows for the produced transmembrane and intracellular proteins to be translocated to the appropriate specific cellular sites. Therefore, mRNA vaccine generates protein products with endogenous characteristics, ensuring the precision of targeting.
Induction of both innate and adaptive immunities
Efficiency is another keypoint in precision therapy. Meeting the requirement, mRNA vaccine can induce both innate and adaptive immunities to exert efficient anti-tumor effects. Innate immunity forms the first line of defense against non-self antigens [97]. APCs, especially DCs, engulf foreign mRNA via pattern recognition receptors (PRRs), activating a series of proinflammation-related signaling pathways that promotes the function of innate immunity [97, 99]. For example, PRR toll-like receptor (TLR)-3 recognizes and binds double-strand RNA, regulating the secretion of cytokines and chemokines as well as the activation of the type I interferon (IFN) pathway [100]. In addition, PRR TLR-7 and TLR-8 bind single-strand RNA, activating nitric oxide synthase and the production of type I IFN [101–103]. The secretion of type I IFN is essential for the formation of an immune-stimulatory environment, wherein T cells differentiate into cytotoxic types that can eliminate tumors. Apart from innate immunity, mRNA vaccines further stimulate adaptive immunity. The protein encoded by non-self mRNA can be degraded into peptides, which are routed into the endoplasmic reticulum, loaded onto MHC-I, shuttled to the cell surface, and ultimately presented to and activate CD8+ T cells [98, 104, 105]. Meanwhile, the antigens can be transported from Golgi to endosomes and enter the MHC-II presentation pathway, where they activate CD4+ T cells [106]. Actually, the antigens can also be secreted and reinternalized and presented via MHC-II to activate CD4+ T cells or cross-presented via MHC-I to activate CD8+ T cells [104, 106]. Moreover, mRNA vaccine can upregulate the expression of costimulatory molecules (e.g., CD40 and CD86) on APCs (e.g., DCs), enhancing the antigen presentation and T cell activation [107]. Furthermore, activated APCs (e.g., macrophage and DC) present antigens to activate B cells, triggering an antibody response [108, 109]. Multiple preclinical and clinical trials have shown that mRNA vaccines induce antitumor immune responses and tumor rejection. Melanoma mouse models have revealed that mRNA-lipoplexes encoding mutant or viral neo-antigens or endogenous self-antigens trigger IFN-α release by macrophages and plasmacytoid DCs, induce strong effector and memory T-cell responses, and mediate the rejection of progressive tumors [107]. A personalized mRNA vaccine induces T cell infiltration and specific killing of melanoma [43]. Additionally, the intravenously administered liposomal RNA vaccine BNT111 mediates a durable objective response and induces strong anti-melanoma CD4+ and CD8+ T cell immunity after pretreatment with an immune checkpoint inhibitor [110]. In summary, mRNA vaccines accord with the concerns about efficiency in precision therapy, inducing both innate immunity and adaptive immunity to exert potent anti-tumor effects.
High safety in practice
Safety is also important for precision therapy. In line with this, mRNA production does not involve toxic chemicals and the risk of contamination with the adventitious virus packaged in cell cultures. Therefore, an mRNA approach averts common threats associated with other vaccine platforms (e.g., viral vectors, inactivated viruses, live viruses, and subunit protein vaccines). In addition, the rapid manufacturability of mRNA decreases opportunities for the introduction of contaminating microorganisms. In this context, it is also important to note that mRNA cannot integrate into the host genome, ruling out oncogenic potentials. Finally, mRNA can be rapidly degraded by RNA enzymes and is characterized by its adjustable half-life, which defines the controllable expression of mRNA-encoded proteins [111–113]. The first clinical study of mRNA vaccine was conducted in 2008 in melanoma patients [114]. Vaccination with naked mRNA is safe and well tolerated and does not induce World Health Organization grade III or IV adverse events. Numerous clinical trials have supported the high safety of mRNA vaccines [115–117]. For example, direct injection of protamine-protected mRNA into patients with metastatic melanoma predominantly caused local inflammatory skin reactions or fatigue, which could be easily lessened by symptomatic therapy. Therefore, mRNA vaccines are relatively safe, which is consistent with the principle of safety underlying precision therapy.
Convenience and low cost of preparation
Economic principle is last, but perhaps most important, in precision therapy. Exactly, preparation of mRNA vaccine is convenient and low cost. mRNA can be produced in vitro using a DNA template, ribonucleotide triphosphates, and recombinant enzymes [118, 119]. In this process, a plasmid DNA containing a DNA-dependent RNA polymerase promoter (e.g., T3, T7, or SP6) is first generated. It is then linearized to provide a template for mRNA synthesis using DNA-dependent RNA polymerase before degradation by DNase. A 5′cap and a 3′poly-A tail are added during the transcription step to facilitate efficient translation in vivo. Finally, free nucleotides, enzymes, truncated RNA fragments, and residual DNA are removed to obtain pure mRNA. This simple process ensures rapid mRNA production in a relatively less complex system and thus can be standardized to produce almost any encoded protein immunogen, rendering it highly suitable for constructing personalized vaccines for cancer treatment. Moreover, all reaction components and enzymes required for mRNA production are commercially available. The entire process of mRNA vaccine production takes about ten days, significantly shorter than other formats [53]. The rapid production of mRNA vaccine is a tremendous advantage for personalized therapy, meaning that treatment can be available within a short time after diagnosis. From an industrial perspective, the large-scale production of mRNA vaccines is low-cost. DNA templates are used during the transcription cycle and by scaling the in vitro transcription reaction. A very small (about 1 µg) DNA template can produce very large amounts (hundreds µg) of capped mRNA, and the product is more dependent on the transcription volume and time than on the amount of DNA [120]. In addition, the required mRNA vaccine dose is generally lower than DNA vaccines (50–100 µg for an mRNA vaccine and 1–5 mg for a DNA vaccine). Actually, an only 10 g of mRNA can generate about 100,000 vaccine doses. Together, the production of mRNA vaccines is convenient and low-cost, which is in agreement with the economic principle of precision therapy.
Development strategy of personalized mRNA vaccines for pancreatic cancer
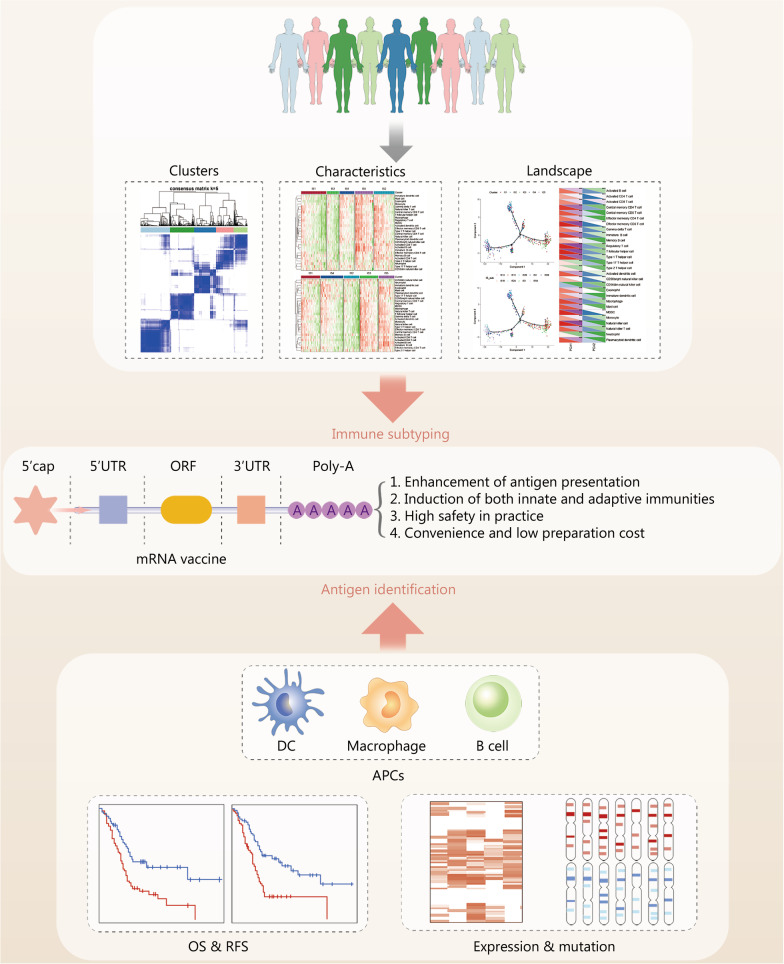
With precise targeting, efficiency, safety, and economic cost, mRNA vaccines offer promise for providing personalized pancreatic cancer treatment. Accumulating evidence suggests that the pipeline for developing personalized pancreatic cancer mRNA vaccines should be divided into three critical modules, including identifying tumor antigens, constructing mRNA vaccines, and distinguishing immune subtypes (Fig. 2).
Fig. 2.
Streamlined development of personalized mRNA vaccines for pancreatic cancer. Novel tumor antigens are identified as potent targets for the preparation of promising pancreatic cancer mRNA vaccines. Immune subtypes are identified as vital criteria for selecting applicable pancreatic cancer patients for mRNA vaccine treatment. Partial elements of this figure are adopted from Huang et al. [29] with appropriate modification. ORF open reading frame, DC dendritic cell, APCs antigen-presenting cells, OS overall survival, RFS relapse-free survival
Identification of pancreatic cancer antigens
Antigen selection is the first step in developing a vaccine. An ideal vaccine candidate should possess the following characteristics: unique to tumor cells, involved in tumorigenesis and progression, non-tolerated by the immune system, and stimulatory to the antitumor immunity [121–123]. Current immunogenic targets for cancer treatment include tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) [124]. TAAs are the most commonly targeted antigens typically expressed on normal cells but aberrantly on tumor cells. This underscores their potential as universal therapeutic targets, although they are self-antigens and thus may be immunologically tolerated and weakened vaccine potency. Unlike TAAs, TSAs are exclusively expressed in tumor cells with strong immunogenicity and a high degree of individuality and epitope diversity and thus are ideal targets for personalized vaccines. Engineering a personalized anti-pancreatic cancer mRNA vaccine begins with identifying tumor-specific non-synonymous mutations by comparing next-generation sequencing data of tumors and paired normal tissues [125]. Computational neoantigen prediction pipelines are then applied to verify the expression and predict the binding affinity of peptides generated from mutated genes onto MHC alleles. High transcript expression is related to enhanced T cell response and can compensate for the low MHC-binding affinity of mutations [126]. Furthermore, a single MHC-I-bound TSA is not sufficient, and additional MHC-II-bound TSAs are needed for effective antitumor immunity [127]. NetMHCpan and MHCflurry are tools trained for predicting the binding affinity between ligands and MHC [128–130]. Notably, for predicting immunogenicity, the stability of the neoepitope-MHC complex is more important than the binding affinity [131]. NetMHCstabpan, a tool for stability prediction, performs well in identifying immunogenic mutations [132]. In addition to the surface presentation, the interaction between peptide-MHC complex and T-cell receptor is necessary to induce an immune response, and predicting this interaction is based on amino acid side chains of the T-cell receptor facing the MHC-bound peptide [133]. Recently, a perspective pipeline for identifying tumor antigens by screening for the overexpressed and mutated genes and prognosis and APC-associated candidates has been established [29]. Notably, although the above-mentioned characteristics are based upon a sound rationale, a specific approach to weigh each of them has not been set up, and therefore, optimal candidates for mRNA vaccine development cannot be selected. Nevertheless, tumor antigen prediction is rapidly evolving thanks to the recent progress in computational biology. Accordingly, an accurate and sensitive approach for identifying potent candidates in individual pancreatic cancer will eventually be established for developing its personalized mRNA vaccines.
Construction of mRNA vaccines against pancreatic cancer
Several critical issues, including delivery, stability, translation, and immunogenicity, must be addressed before the practical application of the mRNA-based cancer vaccine [97, 98, 134, 135]. Because of its size, degradability, and charge, naked mRNA cannot efficiently pass through the cell membrane and enter the cytoplasm, except for immature DCs that can efficiently uptake mRNA via the macro-pinocytosis pathway [134, 136]. For more effective delivery of mRNA into APCs, mRNA formulations (e.g., liposomes, polyplexes, polysomes, and lipoplexes) and administration routes must be appropriately selected and optimized. After successful mRNA delivery, the half-life of mRNA transcribed in vivo must be appropriately regulated, given that several factors influence the pharmacodynamic and pharmacokinetic properties of mRNA-based therapeutics. There is a need to improve mRNA structures, including optimization of poly (A), 5′cap, poly-A tail, untranslated regions, and protein-encoding open reading frames, to enhance the stability of mRNAs [115, 137, 138]. In addition to delivery and stability, immunogenicity must also be considered. Accumulating evidence suggests that there is a negative feedback loop between mRNA and it-induced immune response. For instance, exogenous RNA stimulates the production of type I IFN by stimulating innate immunity [98], while excessive production of type I IFN inhibits translation and promotes the degradation of both ribosomal RNA and cellular mRNA [98, 134, 139, 140]. The addition of poly-A tails, optimization of sequences, and posttranscriptional purification can decrease the level of innate immunity without altering the translation of mRNA [112, 141–145]. Furthermore, increasing the immunostimulatory properties of mRNA using adjuvants promotes the potency of cancer mRNA vaccines. TriMix (mRNA encoding CD70, CD40L, and TLR4) enhances the immunogenicity of unmodified naked mRNA and improves the cytotoxicity of T lymphocyte and DC maturation [98]. Together, advances in optimization strategies for mRNA vaccine construction largely improve the efficacy of these vaccines for pancreatic cancer treatment.
Distinction of immune subtypes in pancreatic cancer
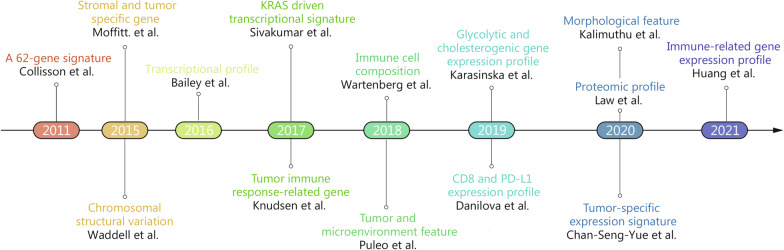
Pancreatic cancer is usually characterized by a complex immunosuppressive microenvironment, low mutational burden, and poor T cell infiltration [11, 146, 147]. Although an mRNA vaccine can activate and promote infiltration of T cells into the tumor, the entry of these cells could still be largely interfered with by the desmoplastic stroma of pancreatic cancer cells [4, 148]. Moreover, numerous immunosuppressive cells (e.g., myeloid cells, regulatory T cells, and M2 macrophages), signaling pathways (e.g., transforming growth factor beta signaling pathway, IL-10 signaling pathway, and VEGF signaling pathway), and molecules (e.g., PD-L1, T cell immunoglobulin mucin 3, T cell immunoreceptor with Ig and ITIM domains, lymphocyte activating 3, V-type immunoglobulin domain-containing suppressor of T-cell activation, and CD73) lead to multiple immunosuppression on anti-tumor immune response in the pancreatic cancer microenvironment [11, 146]. Hence, mRNA vaccines in combination with other therapies (rather than a single vaccine) and biomarkers for predicting therapeutic response of combination strategies are strongly needed for pancreatic cancer treatment. To date, diverse pancreatic cancer subtypes, defined based on different parameters, approaches, and perspectives, have been identified (Fig. 3). Multiple immunological factors, including immune-related gene expression profile and immune cell composition, are used for grouping immune subtypes of pancreatic cancer. Immune subtypes indicate the immunological status in pancreatic tumors and their microenvironment and thus are accurate biomarkers for selecting a suitable combined therapy [29, 149, 150]. For instance, immunologically "cold" pancreatic tumors generally show low immunogenicity and/or high reactive stroma, whereas immunogenic chemotherapy and stromal modulation may promote the effectiveness of an mRNA vaccine by improving tumor immunogenicity and T cell infiltration. Additionally, an mRNA vaccine combined with immune checkpoint blockade may improve T cell infiltration and function in immunologically "hot" tumors. Overall, combination therapy may enhance the efficacy of an mRNA vaccine for pancreatic cancer treatment under the guidance of immune subtypes, biomarkers for matching patients and therapeutics.
Fig. 3.
Timeline of pancreatic cancer subtyping. The timeline of pancreatic cancer subtyping, together with the distinct classification approaches and the corresponding authors, including Collisson et al. [151], Moffitt et al. [28], Waddell et al. [152], Bailey et al. [153], Sivakumar et al. [154], Knudsen et al. [155], Wartenberg et al. [150], Puleo et al. [156], Karasinska et al. [157], Danilova et al. [149], Kalimuthu et al. [158], Law et al. [159], Chan-Seng-Yue et al. [31], and Huang et al. [29], are shown as indicated. KRAS GTPase KRas, PD-L1 programmed cell death ligand 1
Concluding remarks and outlook
The mRNA vaccine is a novel and promising vehicle for developing personalized vaccines against pancreatic cancer. Identification of potent tumor antigens, optimization of immunostimulatory vaccine construction, and distinction of immune subtypes are prerequisites for the personalization of potentially effective pancreatic cancer mRNA vaccines.
At present, a first-in-human phase I study on the tolerability and safety of the mRNA-based personalized neoantigen vaccine (autogene cevumeran, also known as BNT122, RO7198457) in combination with chemotherapy and PD-L1 blockade for resected PDAC is currently underway. The preliminary findings in this trial were released for the first time on June 5, 2022 by BioNTech Company (https://investors.biontech.de/). Sixteen patients who underwent surgery and received PD-L1 inhibition were vaccinated with autogene cevumeran, and all well tolerated the treatment. Only one developed a vaccine-related grade three fever and hypertension, and no other grade three or higher adverse events were observed. In addition, half were detected de-novo neoantigen-specific T cell responses, and had a significantly longer recurrence-free survival (median not determined, but is more than 18 months) compared with those without vaccine-induced immune responses (13.4 months). Therefore, personalized mRNA vaccine is a promising strategy for pancreatic cancer treatment. Notably, only about 15–20% of pancreatic cancer patients present with localized disease that can be resected through the standard procedure [3]. In contrast, the majority of pancreatic cancers are diagnosed at the locally advanced or metastatic level and/or are poorly differentiated and ineligible for surgery, rendering the acquirement of these tumors largely dependent on biopsy. However, the inter- and intra-tumoral heterogeneity of pancreatic cancer limits the actual value of biopsy-derived samples, at least not sufficient for the personalized design and construction of mRNA vaccines as well as the distinction of patients for suitable combination therapy. Neoadjuvant therapies can be used to reduce tumor staging and eliminate micro-metastases, increasing the chance of successful surgery [160–162]. For instance, after treatment with the neoadjuvant FOLFIRINOX, 76 of 125 (60.8%) patients with unresectable pancreatic cancer qualified for tumor resection [160]. In a separate study, 141 patients with unresectable (51.1%) or borderline-resectable (48.9%) non-metastatic cancers were recruited; of these, 78% qualified and underwent surgery after FOLFIRINOX therapy [161]. Therefore, neoadjuvant therapies may facilitate the acquisition of relatively sufficient tumor samples for identifying individualized tumor antigens and immune subtypes for developing personalized mRNA vaccines.
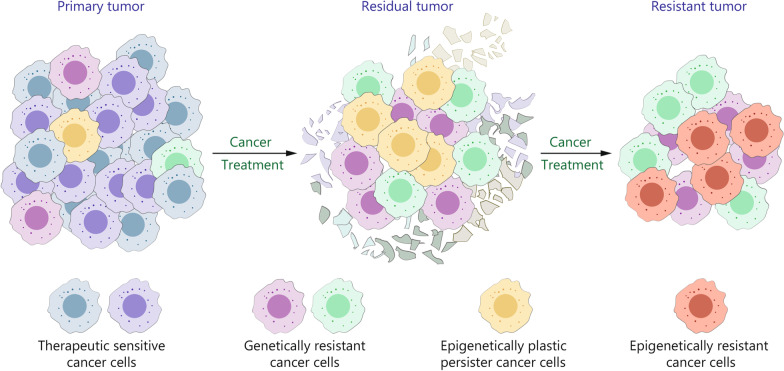
Nevertheless, treating pancreatic cancer with mRNA vaccines remains challenging. As mentioned above, pancreatic cancer is highly heterogeneous. Due to the complexity of the pancreatic tumor components, including both therapeutically sensitive cancer cells and genetically resistant cancer cells or epigenetically plastic persister cancer cells, the therapeutic stress may cause tumor evolution and consequent treatment failure (Fig. 4). Alternatively, the development of prophylactic mRNA vaccines may be another considerable strategy against this intractable disease. To date, prophylactic mRNA vaccination is mainly applied for preventing infection of viruses, since they are ectogenic and possess simple construction and antigens for vaccine development can easily be identified. In contrast, the development of prophylactic vaccines against pancreatic cancer is still in infancy, partially but not totally, due to the complexity of pancreatic cancer onset and the difficulty of assessing the effectiveness of vaccination. Research shows that PDAC arises from non-invasive precancerous lesions, microscopic pancreatic intraepithelial neoplasia (PanIN), and macroscopic intraductal papillary mucinous neoplasms (IPMNs) [163]. In processing PanIN-PDAC transformation, oncogenic mutation in KRAS gene has been detected in > 90% of the low-grade-PanINs [163]. In IPMN-PDAC progression, oncogenic mutations in KRAS and GNAS genes have been observed in 50–80% and 40–70% of IPMN, respectively [164]. KRAS and GNAS are therefore considered potential targets for preventing PDAC development. A KRAS-targeting peptide vaccine for preventing pancreatic cancer in high-risk individuals is currently under clinical test (NCT05013216), but its prophylactic efficiency is still unknown. Notably, apart from KRAS mutation, multiple genetic and epigenomic mechanisms jointly contribute to pancreatic cancer initiation. For instance, IL-33 is identified as a key factor that induces epigenetic reprogramming-mediated pancreatic oncogenesis [165, 166]. The question that whether co-targeting KRAS and IL-33 increases the probability of pancreatic cancer prevention arises and remains to be validated.
Fig. 4.
Tumor evolution in pancreatic cancer therapy. Pancreatic cancer is characterized by prevalent intra-tumoral heterogeneity and composed of therapeutic sensitive cells, genetically resistant cells, and epigenetically persister cells. Therapeutic stress in pancreatic cancer causes the transformation of tumor characteristics, leading to acquired resistance
Acknowledgements
The author Xing Huang would like to convey his sincerest and deepest gratitude to Prof. Guido Kroemer (Centre de Recherche des Cordeliers) for the technological training and ideological inspiration in his laboratory.
Abbreviations
- APC
Antigen-presenting cell
- COVID-19
Coronavirus disease 2019
- DC
Dendritic cell
- ENO1
Enolase 1
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- HSPPC-96
Heat shock protein-peptide complex-96
- IFN
Type I interferon
- IPMNs
Intraductal papillary mucinous neoplasms
- MHC
Major histocompatibility complex
- mRNA
Messager RNA
- MUC1
Mucin 1
- PanIN
Pancreatic intraepithelial neoplasia
- PDAC
Pancreatic ductal adenocarcinoma
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- PPRs
Pattern recognition receptors
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- TAAs
Tumor-associated antigens
- TEXs
Tumor-derived exosomes
- TLR
Toll like receptor
- TSAs
Tumor-specific antigens
- VEGFR
Vascular endothelial growth factor receptor
- WT1
Wilms tumor 1
Author contributions
XH, GZ, and TBL conceived the review. XH and GZ drafted and revised the manuscript. TYT and XG helped for proof-reading. All authors discussed and approved the final paper.
Funding
This work was supported by the National Natural Science Foundation of China (31970696 and 81502975, 82188102, and 81830089), Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholar (LR22H160010), National Key Research and Development Program of China (2019YFC1316000), Zhejiang Provincial Key Research and Development Program (2019C03019), and Zhejiang Provincial College Student Science and Technology Innovation Activity Plan-College Student Innovation and Entrepreneurship Incubation Program (Young Talent Program) (2022R40122).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Xing Huang and Gang Zhang contributed equally to this work.
Contributor Information
Xing Huang, Email: huangxing66@zju.edu.cn.
Gang Zhang, Email: Gang_Zhang@zju.edu.cn.
Tian-Yu Tang, Email: tangtianyu0701@zju.edu.cn.
Xiang Gao, Email: GXiangyoung@163.com.
Ting-Bo Liang, Email: liangtingbo@zju.edu.cn.
References
- 1.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493–502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 4.Hessmann E, Buchholz SM, Demir IE, Singh SK, Gress TM, Ellenrieder V, et al. Microenvironmental determinants of pancreatic cancer. Physiol Rev. 2020;100(4):1707–1751. doi: 10.1152/physrev.00042.2019. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 8.Li E, Huang X, Zhang G, Liang T. Combinational blockade of MET and PD-L1 improves pancreatic cancer immunotherapeutic efficacy. J Exp Clin Cancer Res. 2021;40(1):279. doi: 10.1186/s13046-021-02055-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Huang X, Xu J, Li E, Lao M, Tang T, et al. NEK2 inhibition triggers anti-pancreatic cancer immunity by targeting PD-L1. Nat Commun. 2021;12(1):4536. doi: 10.1038/s41467-021-24769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 11.Bear AS, Vonderheide RH, O'Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38(6):788–802. doi: 10.1016/j.ccell.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9(2):77–87. doi: 10.1038/nrgastro.2011.215. [DOI] [PubMed] [Google Scholar]
- 13.Makohon-Moore AP, Zhang M, Reiter JG, Bozic I, Allen B, Kundu D, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49(3):358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yachida S, White CM, Naito Y, Zhong Y, Brosnan JA, Macgregor-Das AM, et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin Cancer Res. 2012;18(22):6339–6347. doi: 10.1158/1078-0432.CCR-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Xia X, Yang C, Shen J, Mai J, Kim HC, et al. SMAD4 gene mutation renders pancreatic cancer resistance to radiotherapy through promotion of autophagy. Clin Cancer Res. 2018;24(13):3176–3185. doi: 10.1158/1078-0432.CCR-17-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devico Marciano N, Kroening G, Dayyani F, Zell JA, Lee FC, Cho M, et al. BRCA-mutated pancreatic cancer: from discovery to novel treatment paradigms. Cancers. 2022;14(10):2453. doi: 10.3390/cancers14102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emelyanova M, Pudova E, Khomich D, Krasnov G, Popova A, Abramov I, et al. Platinum-based chemotherapy for pancreatic cancer: impact of mutations in the homologous recombination repair and Fanconi anemia genes. Ther Adv Med Oncol. 2022;14:17588359221083050. doi: 10.1177/17588359221083050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 19.Chen A. PARP inhibitors: its role in treatment of cancer. Chin J Cancer. 2011;30(7):463–471. doi: 10.5732/cjc.011.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javle M, Shacham-Shmueli E, Xiao L, Varadhachary G, Halpern N, Fogelman D, et al. Olaparib monotherapy for previously treated pancreatic cancer with DNA damage repair genetic alterations other than germline BRCA variants: findings from 2 phase 2 nonrandomized clinical trials. JAMA Oncol. 2021;7(5):693–699. doi: 10.1001/jamaoncol.2021.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, Hingorani SR. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2017;5(11):978–991. doi: 10.1158/2326-6066.CIR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Zhang G, Liang T. Subtyping for pancreatic cancer precision therapy. Trends Pharmacol Sci. 2022;43(6):482–494. doi: 10.1016/j.tips.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development. Mol Cancer. 2021;20(1):44. doi: 10.1186/s12943-021-01310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29(9):725–738. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O'Kane GM, et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet. 2020;52(2):231–240. doi: 10.1038/s41588-019-0566-9. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoa for mRNA vaccine development. Mol Cancer. 2021;20(1):50. doi: 10.1186/s12943-021-01342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negahdaripour M, Golkar N, Hajighahramani N, Kianpour S, Nezafat N, Ghasemi Y. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol Adv. 2017;35(5):575–596. doi: 10.1016/j.biotechadv.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner S, Jacob F, Meselson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190:576–581. doi: 10.1038/190576a0. [DOI] [PubMed] [Google Scholar]
- 35.Lockard RE, Lingrel JB. The synthesis of mouse hemoglobin chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969;37(2):204–212. doi: 10.1016/0006-291X(69)90720-7. [DOI] [PubMed] [Google Scholar]
- 36.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 37.Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255(5047):996–998. doi: 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Krishnan S, Lenzen G, Magné R, Gomard E, Guillet JG, et al. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23(7):1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 39.Conry RM, LoBuglio AF, Loechel F, Moore SE, Sumerel LA, Barlow DL, et al. A carcinoembryonic antigen polynucleotide vaccine has in vivo antitumor activity. Gene Ther. 1995;2(1):59–65. [PubMed] [Google Scholar]
- 40.Conry RM, LoBuglio AF, Loechel F, Moore SE, Sumerel LA, Barlow DL, et al. A carcinoembryonic antigen polynucleotide vaccine for human clinical use. Cancer Gene Ther. 1995;2(1):33–38. [PubMed] [Google Scholar]
- 41.Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2:17056. doi: 10.1038/natrevmats.2017.56. [DOI] [Google Scholar]
- 42.Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 44.Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 45.Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 46.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: t he status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascolo S. Vaccines against COVID-19: priority to mRNA-based formulations. Cells. 2021;10(10):2716. doi: 10.3390/cells10102716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas SJ, Moreira ED, Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther. 2022;7(1):94. doi: 10.1038/s41392-022-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 55.Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 57.Salman B, Zhou D, Jaffee EM, Edil BH, Zheng L. Vaccine therapy for pancreatic cancer. Oncoimmunology. 2013;2(12):e26662. doi: 10.4161/onci.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- 59.Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, Nagai K, et al. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018;38(4):2217–2225. doi: 10.21873/anticanres.12464. [DOI] [PubMed] [Google Scholar]
- 60.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 62.Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2012;17(1):94–101. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 63.Romero P, Banchereau J, Bhardwaj N, Cockett M, Disis ML, Dranoff G, et al. The human vaccines project: a roadmap for cancer vaccine development. Sci Transl Med. 2016 doi: 10.1126/scitranslmed.aaf0685. [DOI] [PubMed] [Google Scholar]
- 64.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146(7):1784–94.e6. doi: 10.1053/j.gastro.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamasaki S, Miura Y, Davydova J, Vickers SM, Yamamoto M. Intravenous genetic mesothelin vaccine based on human adenovirus 40 inhibits growth and metastasis of pancreatic cancer. Int J Cancer. 2013;133(1):88–97. doi: 10.1002/ijc.27983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin immunotherapy for cancer: ready for prime time? J Clin Oncol. 2016;34(34):4171–4179. doi: 10.1200/JCO.2016.68.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naseri M, Bozorgmehr M, Zöller M, Ranaei Pirmardan E, Madjd Z. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology. 2020;9(1):1779991. doi: 10.1080/2162402X.2020.1779991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017;13(28):2583–2592. doi: 10.2217/fon-2017-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao L, Erb U, Zhao K, Hackert T, Zoller M. Efficacy of vaccination with tumor-exosome loaded dendritic cells combined with cytotoxic drug treatment in pancreatic cancer. Oncoimmunology. 2017;6(6):e1319044. doi: 10.1080/2162402X.2017.1319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun W, Ren Y, Lu Z, Zhao X. The potential roles of exosomes in pancreatic cancer initiation and metastasis. Mol Cancer. 2020;19(1):135. doi: 10.1186/s12943-020-01255-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A, et al. Lymphocyte-derived exosomal microRNAs promote pancreatic β cell death and may contribute to type 1 diabetes development. Cell Metab. 2019;29(2):348–61.e6. doi: 10.1016/j.cmet.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Fathollahi A, Hashemi SM, Haji Molla Hoseini M, Yeganeh F. In vitro analysis of immunomodulatory effects of mesenchymal stem cell- and tumor cell -derived exosomes on recall antigen-specific responses. Int Immunopharmacol. 2019;67:302–10. doi: 10.1016/j.intimp.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Lee ES, Sul JH, Shin JM, Shin S, Lee JA, Kim HK, et al. Reactive oxygen species-responsive dendritic cell-derived exosomes for rheumatoid arthritis. Acta Biomater. 2021;128:462–473. doi: 10.1016/j.actbio.2021.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52(8):1964–1972. doi: 10.1007/s10620-006-9205-2. [DOI] [PubMed] [Google Scholar]
- 76.Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji N, Zhang Y, Liu Y, Xie J, Wang Y, Hao S, et al. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI Insight. 2018;3(10):e99145. doi: 10.1172/jci.insight.99145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Mudgal P, Wang L, Wu H, Huang N, Alexander PB, et al. T cell receptor repertoire as a prognosis marker for heat shock protein peptide complex-96 vaccine trial against newly diagnosed glioblastoma. Oncoimmunology. 2020;9(1):1749476. doi: 10.1080/2162402X.2020.1749476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372(9633):145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 80.Tosti G, Di Pietro A, Ferrucci PF, Testori A. HSPPC-96 vaccine in metastatic melanoma patients: from the state of the art to a possible future. Expert Rev Vaccines. 2009;8(11):1513–1526. doi: 10.1586/erv.09.108. [DOI] [PubMed] [Google Scholar]
- 81.Matsui H, Hazama S, Shindo Y, Nagano H. Combination treatment of advanced pancreatic cancer using novel vaccine and traditional therapies. Expert Rev Anticancer Ther. 2018;18(12):1205–1217. doi: 10.1080/14737140.2018.1531707. [DOI] [PubMed] [Google Scholar]
- 82.Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Gedde-Dahl T, Stokke KT, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer. 1996;65(4):450–453. doi: 10.1002/(SICI)1097-0215(19960208)65:4<450::AID-IJC10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 83.Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92(3):441–450. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 84.Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Ther Adv Med Oncol. 2013;5(1):81–89. doi: 10.1177/1758834012462463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, et al. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101(2):433–439. doi: 10.1111/j.1349-7006.2009.01416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki N, Hazama S, Ueno T, Matsui H, Shindo Y, Iida M, et al. A phase I clinical trial of vaccination with KIF20A-derived peptide in combination with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37(1):36–42. doi: 10.1097/CJI.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki N, Hazama S, Iguchi H, Uesugi K, Tanaka H, Hirakawa K, et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2017;108(1):73–80. doi: 10.1111/cas.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishida S, Ishikawa T, Egawa S, Koido S, Yanagimoto H, Ishii J, et al. Combination gemcitabine and WT1 peptide vaccination improves progression-free survival in advanced pancreatic ductal adenocarcinoma: a phase II randomized study. Cancer Immunol Res. 2018;6(3):320–331. doi: 10.1158/2326-6066.CIR-17-0386. [DOI] [PubMed] [Google Scholar]
- 90.Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, et al. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012;41(3):374–379. doi: 10.1097/MPA.0b013e31822ade7e. [DOI] [PubMed] [Google Scholar]
- 91.Rong Y, Jin D, Wu W, Lou W, Wang D, Kuang T, et al. Induction of protective and therapeutic anti-pancreatic cancer immunity using a reconstructed MUC1 DNA vaccine. BMC Cancer. 2009;9:191. doi: 10.1186/1471-2407-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cappello P, Rolla S, Chiarle R, Principe M, Cavallo F, Perconti G, et al. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144(5):1098–1106. doi: 10.1053/j.gastro.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 93.Cappello P, Curcio C, Mandili G, Roux C, Bulfamante S, Novelli F. Next generation immunotherapy for pancreatic cancer: DNA vaccination is seeking new combo partners. Cancers. 2018;10(2):51. doi: 10.3390/cancers10020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu K, Qin H, Cha SC, Neelapu SS, Overwijk W, Lizee GA, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25(46):7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 95.Niethammer AG, Lubenau H, Mikus G, Knebel P, Hohmann N, Leowardi C, et al. Double-blind, placebo-controlled first in human study to investigate an oral vaccine aimed to elicit an immune reaction against the VEGF-Receptor 2 in patients with stage IV and locally advanced pancreatic cancer. BMC Cancer. 2012;12:361. doi: 10.1186/1471-2407-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fiedler K, Lazzaro S, Lutz J, Rauch S, Heidenreich R. mRNA cancer vaccines. Recent Results Cancer Res. 2016;209:61–85. doi: 10.1007/978-3-319-42934-2_5. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20(1):33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grunwitz C, Kranz LM. mRNA cancer vaccines-messages that prevail. Curr Top Microbiol Immunol. 2017;405:145–164. doi: 10.1007/82_2017_509. [DOI] [PubMed] [Google Scholar]
- 100.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 101.Veetil AT, Zou J, Henderson KW, Jani MS, Shaik SM, Sisodia SS, et al. DNA-based fluorescent probes of NOS2 activity in live brains. Proc Natl Acad Sci U S A. 2020;117(26):14694–14702. doi: 10.1073/pnas.2003034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 103.Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat Struct Mol Biol. 2015;22(2):109–115. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 104.Neefjes J, Jongsma MLM, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 105.Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022 doi: 10.1038/s41577-022-00707-2. [DOI] [PubMed] [Google Scholar]
- 106.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4):203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 108.Hasgur S, Yamamoto Y, Fan R, Nicosia M, Gorbacheva V, Zwick D, et al. Macrophage-inducible C-type lectin activates B cells to promote T cell reconstitution in heart allograft recipients. Am J Transplant. 2022;22(7):1779–1790. doi: 10.1111/ajt.17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161(3):1313–1319. [PubMed] [Google Scholar]
- 110.Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 111.Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227–234. doi: 10.1016/j.jconrel.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 112.Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsui NBY, Ng EKO, Lo YMD. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48(10):1647–1653. doi: 10.1093/clinchem/48.10.1647. [DOI] [PubMed] [Google Scholar]
- 114.Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31(2):180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 115.Beck JD, Reidenbach D, Salomon N, Sahin U, Tureci O, Vormehr M, et al. mRNA therapeutics in cancer immunotherapy. Mol Cancer. 2021;20(1):69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heine A, Juranek S, Brossart P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer. 2021;20(1):52. doi: 10.1186/s12943-021-01339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 118.Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pardi N, Muramatsu H, Weissman D, Kariko K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 120.Naik R, Peden K. Regulatory considerations on the development of mRNA vaccines. Curr Top Microbiol Immunol. 2020 doi: 10.1007/82_2020_220. [DOI] [PubMed] [Google Scholar]
- 121.Wang RF, Rosenberg SA. Human tumor antigens for cancer vaccine development. Immunol Rev. 1999;170(1):85–100. doi: 10.1111/j.1600-065X.1999.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 122.Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 2020;38(4):454–472. doi: 10.1016/j.ccell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nejo T, Yamamichi A, Almeida ND, Goretsky YE, Okada H. Tumor antigens in glioma. Semin Immunol. 2020;47:101385. doi: 10.1016/j.smim.2020.101385. [DOI] [PubMed] [Google Scholar]
- 124.Li WH, Li YM. Chemical strategies to boost cancer vaccines. Chem Rev. 2020;120(20):11420–11478. doi: 10.1021/acs.chemrev.9b00833. [DOI] [PubMed] [Google Scholar]
- 125.Lang F, Schrörs B, Löwer M, Tureci O, Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat Rev Drug Discov. 2022;21(4):261–282. doi: 10.1038/s41573-021-00387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abelin JG, Keskin DB, Sarkizova S, Hartigan CR, Zhang W, Sidney J, et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity. 2017;46(2):315–326. doi: 10.1016/j.immuni.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574(7780):696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jurtz V, Paul S, Andreatta M, Marcatili P, Peters B, Nielsen M. NetMHCpan-4.0: improved peptide-MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol. 2017;199(9):3360–8. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O'Donnell TJ, Rubinsteyn A, Bonsack M, Riemer AB, Laserson U, Hammerbacher J. MHCflurry: open-source class I MHC binding affinity prediction. Cell Syst. 2018;7(1):129–32 e4. doi: 10.1016/j.cels.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 130.Zhao W, Sher X. Systematically benchmarking peptide-MHC binding predictors: from synthetic to naturally processed epitopes. PLoS Comput Biol. 2018;14(11):e1006457. doi: 10.1371/journal.pcbi.1006457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blaha DT, Anderson SD, Yoakum DM, Hager MV, Zha Y, Gajewski TF, et al. High-throughput stability screening of neoantigen/HLA complexes improves immunogenicity predictions. Cancer Immunol Res. 2019;7(1):50–61. doi: 10.1158/2326-6066.CIR-18-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rasmussen M, Fenoy E, Harndahl M, Kristensen AB, Nielsen IK, Nielsen M, et al. Pan-specific prediction of peptide-MHC class I complex stability, a correlate of T cell immunogenicity. J Immunol. 2016;197(4):1517–1524. doi: 10.4049/jimmunol.1600582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Trolle T, Nielsen M. NetTepi: an integrated method for the prediction of T cell epitopes. Immunogenetics. 2014;66(7–8):449–456. doi: 10.1007/s00251-014-0779-0. [DOI] [PubMed] [Google Scholar]
- 134.Weng Y, Li C, Yang T, Hu B, Zhang M, Guo S, et al. The challenge and prospect of mRNA therapeutics landscape. Biotechnol Adv. 2020;40:107534. doi: 10.1016/j.biotechadv.2020.107534. [DOI] [PubMed] [Google Scholar]
- 135.Linares- Fernández S, Lacroix C, Exposito JY, Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26(3):311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 136.Diken M, Kreiter S, Selmi A, Britten CM, Huber C, Türeci Ö, et al. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011;18(7):702–708. doi: 10.1038/gt.2011.17. [DOI] [PubMed] [Google Scholar]
- 137.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Victor MP, Acharya D, Begum T, Ghosh TC. The optimization of mRNA expression level by its intrinsic properties-Insights from codon usage pattern and structural stability of mRNA. Genomics. 2019;111(6):1292–1297. doi: 10.1016/j.ygeno.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 139.De Haro C, Méndez R, Santoyo J. The eIF-2α kinases and the control of protein synthesis 1. FASEB J. 1996;10(12):1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 140.Liang SL, Quirk D, Zhou A. RNase L: Its biological roles and regulation. IUBMB Life. 2006;58(9):508–514. doi: 10.1080/15216540600838232. [DOI] [PubMed] [Google Scholar]
- 141.Batey RT, Kieft JS. Improved native affinity purification of RNA. RNA. 2007;13(8):1384–1389. doi: 10.1261/rna.528007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Karikó K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39(21):e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thess A, Grund S, Mui BL, Hope MJ, Baumhof P, Fotin-Mleczek M, et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23(9):1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weissman D, Karikó K. mRNA: fulfilling the promise of gene therapy. Mol Ther. 2015;23(9):1416–1417. doi: 10.1038/mt.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mauro VP. Codon optimization in the production of recombinant biotherapeutics: potential risks and considerations. BioDrugs. 2018;32(1):69–81. doi: 10.1007/s40259-018-0261-x. [DOI] [PubMed] [Google Scholar]
- 146.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4(6):418–428. doi: 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Danilova L, Ho WJ, Zhu Q, Vithayathil T, De Jesus-Acosta A, Azad NS, et al. Programmed cell death ligand-1 (PD-L1) and CD8 expression profiling identify an immunologic subtype of pancreatic ductal adenocarcinomas with favorable survival. Cancer Immunol Res. 2019;7(6):886–895. doi: 10.1158/2326-6066.CIR-18-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L, et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res. 2018;24(18):4444–4454. doi: 10.1158/1078-0432.CCR-17-3401. [DOI] [PubMed] [Google Scholar]
- 151.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 154.Sivakumar S, de Santiago I, Chlon L, Markowetz F. Master regulators of oncogenic KRAS response in pancreatic cancer: an integrative network biology analysis. PLoS Med. 2017;14(1):e1002223. doi: 10.1371/journal.pmed.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clin Cancer Res. 2017;23(15):4429–4440. doi: 10.1158/1078-0432.CCR-17-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155(6):1999–2013 e3. doi: 10.1053/j.gastro.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 157.Karasinska JM, Topham JT, Kalloger SE, Jang GH, Denroche RE, Culibrk L, et al. Altered gene expression along the glycolysis-cholesterol synthesis axis is associated with outcome in pancreatic cancer. Clin Cancer Res. 2020;26(1):135–146. doi: 10.1158/1078-0432.CCR-19-1543. [DOI] [PubMed] [Google Scholar]
- 158.Kalimuthu SN, Wilson GW, Grant RC, Seto M, O'Kane G, Vajpeyi R, et al. Morphological classification of pancreatic ductal adenocarcinoma that predicts molecular subtypes and correlates with clinical outcome. Gut. 2020;69(2):317–328. doi: 10.1136/gutjnl-2019-318217. [DOI] [PubMed] [Google Scholar]
- 159.Law HCH, Lagundžin D, Clement EJ, Qiao F, Wagner ZS, Krieger KL, et al. The proteomic landscape of pancreatic ductal adenocarcinoma liver metastases identifies molecular subtypes and associations with clinical response. Clin Cancer Res. 2020;26(5):1065–1076. doi: 10.1158/1078-0432.CCR-19-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(7):1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457–463. doi: 10.1097/SLA.0000000000001850. [DOI] [PubMed] [Google Scholar]
- 162.Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269(4):733–740. doi: 10.1097/SLA.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 163.Singhi AD, Wood LD. Early detection of pancreatic cancer using DNA-based molecular approaches. Nat Rev Gastroenterol Hepatol. 2021;18(7):457–468. doi: 10.1038/s41575-021-00470-0. [DOI] [PubMed] [Google Scholar]
- 164.Fischer CG, Wood LD. From somatic mutation to early detection: insights from molecular characterization of pancreatic cancer precursor lesions. J Pathol. 2018;246(4):395–404. doi: 10.1002/path.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huang X, Zhang G, Liang T. Pancreatic tumor initiation: the potential role of IL-33. Signal Transduct Target Ther. 2021;6(1):204. doi: 10.1038/s41392-021-00636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alonso-Curbelo D, Ho YJ, Burdziak C, Maag JLV, Morris JP, Chandwani R, et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature. 2021;590(7847):642–648. doi: 10.1038/s41586-020-03147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.