Abstract
Background
Laryngeal squamous cell carcinoma (LSCC) is a prevalent malignant tumor of the head and neck with a dismal prognosis. Keratin17 (KRT17) has been proven to serve as an oncogene in various cancers, but it has never been explored in LSCC. We proposed to assess the impact and possible mechanisms of KRT17 in the development of LSCC.
Methods
Quantitative reverse transcription-PCR (qRT-PCR) was utilized to examine the mRNA levels. The Kaplan–Meier method was used to calculate the relationship between KRT17 expression and survival curves in LSCC patients. Cell counting kit-8 (CCK-8), colony formation, and flow cytometry assays were utilized to estimate LSCC cell proliferation. The migration and invasion abilities of LSCC cells were ascertained by wound-healing and transwell assays. Immunohistochemical and western blot assays were utilized to appraise protein levels. The xenograft tumor model was used to determine the effect of KRT17 on tumor growth.
Results
In the present study, KRT17 was extremely high in LSCC tissues and cells and correlated with a poor prognosis. Inhibition of KRT17 weakens cell proliferative, migratory, and invasive abilities in LSCC and contributes to cell cycle arrest. Besides, we approved that knockdown of KRT17 extraordinarily restrained the xenograft tumor growth in vivo. We preliminarily investigated the role of KRT17 on the AKT/mTOR and Wnt/β-catenin signaling axes and found that these signaling pathways were largely blocked by KRT17 deletion.
Conclusion
Collectively, we uncovered that exhaustion of KRT17 suppresses LSCC progression through coordinating AKT/mTOR and Wnt/β-catenin signaling axes, illustrating KRT17 as a promising biomarker for making strides in LSCC treatment.
1. Introduction
Laryngeal squamous cell carcinoma (LSCC) is the second largest sort of head and neck malignancy, with extending recurrence and mortality [1–3]. In spite of the ceaseless enhancement of treatment and demonstrative strategies, the overall 5-year survival rate for LSCC patients remains unsatisfactory, especially for those with advanced or metastatic disease [4, 5]. Subsequently, there is a pressing need to address the essential molecular regulatory pipelines of LSCC pathogenesis.
Keratin, one of the intermediate filament of the protein family, has a molecular weight of about 40–70 kDa and forms the cytoskeleton [6, 7]. Keratin can be separated into sort I keratin and sort II keratin, of which KRT17 is commonly found in epithelial cells and belongs to sort I keratin [8, 9]. KRT17 is not present in the epidermis of normal skin but can be initiated under stressful conditions such as skin scratching [10]. Studies have confirmed that KRT17 is a typical marker of hyperproliferation in psoriatic skin, and extracts of Curcuma amada, Humulus lupulus, and Hypericum perforatum can inhibit the expression of KRT17 in psoriatic skin [11]. KRT17, as a multifunctional promoter and oncogene, has appeared to play an imperative role in advancing the proliferation, metastasis, and consequent deadly results of malignant tumors [12–16]. In the meantime, other confirmations have also appeared that KRT17 expression was expanded in tumor tissues but not in nontumor regions [17]. In addition, the high expression level of KRT17 has been shown to be closely relevant to the advancement of epithelial-mesenchymal transformation (EMT), proposing another crucial portion in accelerating cancer cell survival and metastasis [18]. A later study utilized RNA sequencing for gene expression profiling in human normal mucosal and LSCC tissues and recognized 50 genes with the highest upregulation in LSCC, of which KRT17 was described as one [19]. In any case, it is not explicit whether KRT17 can play a part in LSCC and its mechanism.
In this study, we uncovered that KRT17 is elevated in LSCC tissues and is possibly associated with poor outcomes. KRT17 accelerates LSCC proliferation and invasion by means of enacting AKT/mTOR and Wnt/β-catenin signaling axes. Our revelations propose that KRT17 may be an imperative marker of LSCC progression and unfavorable survival.
2. Materials and Methods
2.1. Patient Samples
A total of 42 human LSCC and correspondent nontumor normal samples (5 cm distant from the tumor boundary) were obtained from the LSCC patients who experienced surgical resection in the First Affiliated Hospital, Huzhou University, The First People's Hospital of Huzhou hospital. All participants did not undergo preoperative radiotherapy or chemotherapy and signed an informed consent form. This study was endorsed by the Institutional Ethics Committee of the First Affiliated Hospital, Huzhou University, The First People's Hospital of Huzhou Hospital, and was undertaken in compliance with the Declaration of Helsinki.
2.2. Quantitative Reverse Transcription-PCR (qRT-PCR)
The cells and tissues were lysed utilizing Trizol reagent (Sangon Biotech (Shanghai) Co., Ltd., China). The PCR examination was accomplished utilizing PrimeScript RT reagent kit (Takara, Dalian, China) and SYBR Premix ExTaq II (TaKaRa). The primer sequences are included in Table 1.
Table 1.
Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| KRT17 | 5′-GATCCGTGACTGGTACCAG-3′ | 5′-TGTGAGGATCTTGTTCTGCA -3′ |
| GAPDH | 5′-TCAAGATCATCAGCAATGCC-3′ | 5′-CGATACCAAAGTTGTCATGGA-3′ |
Protein-protein interaction (PPI) network construction.
A PPI network was constructed using the STRING (https://string-db.org) database based on key KRT17 coregulated genes in order to assess potential interactions between proteins of interest [20]. Interactions with a medium confidence score ≥0.4 were considered as significant.
2.3. Western Blot
A RIPA lysate (Beyotime, China) was utilized to extricate total proteins, which were quantified by a BCA kit (Solarbio, China), followed by subjecting them to an SDS-PAGE gel and transferring them to PVDF membranes (GE Healthcare Life, USA). Afterwards, blocking blots were performed with 5% fat-free milk, and the process was followed by incubation with the primary antibodies against KRT17 (AF5480, Affinity, USA), E-cadherin (AF0131, Affinity), N-cadherin (AF4039, Affinity), β-catenin (AF6266, Affinity), AKT (AF6261, Affinity), p-AKT (AF0016, Affinity), mTOR (AF6308, Affinity), p-mTOR (AF3308, Affinity c-Myc (AF0358, Affinity), cyclin D1 (AF0931, Affinity), cyclin E (AF0144, Affinity), CDK2 (AF6237, Affinity), CDK4 (DF6102, Affinity), Snail (AF6032, Affinity), MMP7 (AF0218, Affinity), and GAPDH (AF7021, Affinity). Then, the membranes were encoded with a secondary antibody conjugated to HRP and finally subjected to measurement with an ECL kit (Pierce, Waltham, MA, USA).
2.4. Cell Culture Conditions and Transfection Procedure
Human LSCC cells (TU686, TU177, AMC-HN-8, and TU212) and bronchial epithelioid cells (16HBE) were obtained from iCell Bioscience Inc. (Shanghai, China) and cultured under the appropriate media and conditions as required by the instructions. Following the operational requirements of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), KRT17 shRNAs, and an appropriate negative control (GenePharma, Shanghai, China) were transfected into AMC-HN-8 and TU177 cells.
2.5. Cell Counting Kit-8 (CCK-8) Assay
5000 AMC-HN-8 and TU177 cells were inoculated in each well of a 96-well plate, followed by the addition of 10 μl CCK-8 (Beyotime) at the time of 24, 48, 72, and 96 h of incubation and then incubated for 4 h and detection of absorbance values at 450 nm using an enzyme reader (Molecular Devices, USA).
2.6. Colony Formation Assay
AMC-HN-8 and TU177 cells were inoculated into a 6-well plate and cultured for 2 weeks until the cells shaped into large clones. The complete medium was supplanted every 3 days. According to the requirements of crystal violet staining, clones were dyed, counted, and photographed.
2.7. Cell Cycle Analysis
AMC-HN-8 and TU177 cells were cultivated for 48 h, harvested by centrifugation, and fixed with 70% ethanol at 4°C for 12 h. DNA staining solution (propidium iodide and RNase A, BD Biosciences, USA) and 5 μL osmotic solution were included under dark conditions, and cell cycle distribution detection was conducted using a flow cytometer (BD Biosciences).
2.8. Cellular Wound-Healing Assay
When 6-well plate cell growth reached 90% confluence, the scratches were delivered with a 200 ul pipette tip and cultured without fetal bovine serum, and the gap size was measured 24 h later.
2.9. Transwell Assay
The abilities of cells to migrate or invade were determined in transwell chambers (Corning, USA) without or pre-surfaced Matrigel. The AMC-HN-8 and TU177 cells (1 × 105 cells/well) were included in the top chambers without serum medium, whereas the complete medium was included in the bottom chambers. After 24 h of incubation, cells moving to the bottom chambers were fixated with 4% paraformaldehyde, subsequently dyed with crystal violet, counted, and photographed.
2.10. Tumor Xenograft Model
This study involved animal experiments that were endorsed by the institutional guidelines of the Committee of the First Affiliated Hospital, Huzhou University, The First People's Hospital of Huzhou Hospital, and were undertaken in compliance with the Declaration of Helsinki. 2 × 106 LSCC cells transfected with sh-KRT17 or sh-NC were separately injected into the right flank of each female nude mouse with 6 mice (4–5 weeks old) randomized in each group. Tumor volume was monitored at 3-day intervals utilizing a vernier caliper. Thirty-one days after injection, tumors were resected and weighed. Then, tumor tissues were fixed in formalin, embedded in paraffin, and cut into 4-μm-thick slices. The sections were incubated with the primary anti-Ki-67 (AF0198, Affinity) and anti-KRT17 (AF0190, Affnity) antibodies at 4°C for 12 h after dewaxing and antigen repair. In this way, HRP-conjugated secondary antibody was added, and the signals were generated with diaminobenzidine and photographed with microscopy.
2.11. Statistical Analysis
Data are stated in terms of mean ± SD from at least three individual replicate trials. Statistics were obtained with SPSS 16.0 software, and graphical plots were generated with GraphPad Prism 8.0 software. Differences between two or among more groups were performed via Student's t-test or one-way analysis of variance (ANOVA). LSCC patients were assigned to either low or high KRT17 expression groups based on the median value of KRT17 expressed in LSCC tissues, and their overall survival curves were ascertained following the Kaplan–Meier method. P < 0.05 was affirmed as representing statistical significance.
3. Results
3.1. KRT17 Expression Is Elevated in LSCC Tissues and Cells and Is Linked to Poor Prognosis
Previous studies have appeared that increased KRT17 expression in LSCC tissues [19]. Consistently, KRT17 mRNA expression in LSCC tissues was essentially enhanced by contrast to nontumor tissues (Figure 1(a)). In comparison to nontumor tissues, KRT17 mRNA expression was relatively low in 7 LSSC tissues, while KRT17 mRNA expression was relatively high in 35 LSSC tissues (Figure 1(b)). Western blot also confirmed the reliable result that KRT17 is elevated in LSCC tissues (Figure 1(c)).
Figure 1.
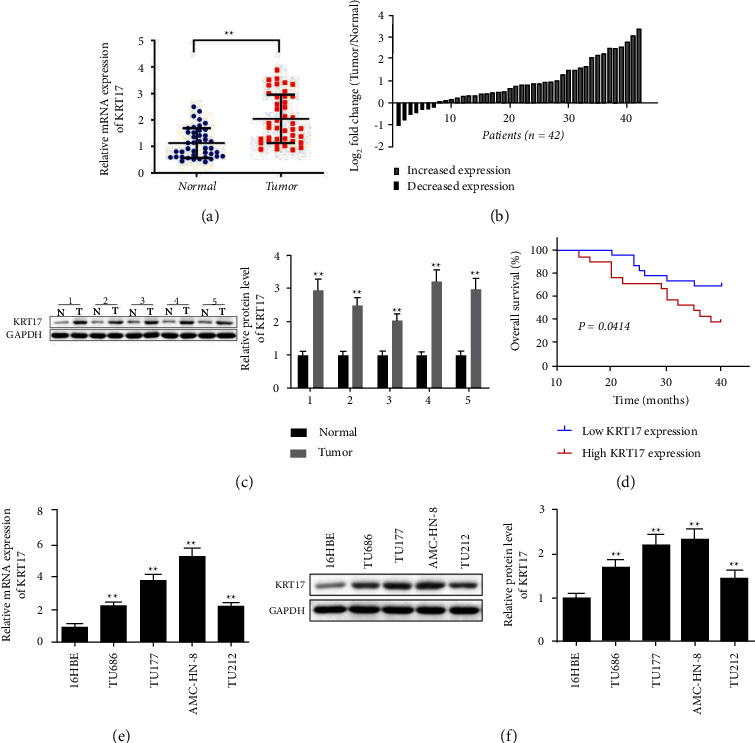
KRT17 expression is elevated in LSCC tissues and cells and is linked to poor prognosis. (a, b) QRT-PCR detected KRT17 mRNA expression in LSCC and nontumor tissues (n = 42). (c) Western blot detected KRT17 protein level in LSCC and nontumor tissues (n = 5). (d) Overall survival of LSCC patients with high or low level of KRT17. (e) qRT-PCR assessed KRT17 mRNA in AMC-HN-8, TU686, TU177, TU212, and 16HBE cells. (f) Western blot detected KRT17 protein level in AMC-HN-8, TU686, TU177, TU212, and 16HBE cells. ∗A significant difference compared with the normal group or 16HBE group. ∗∗P < 0.01. Abbreviations: N, nontumor tissues; T, LSCC tissues.
Analysis of the correlation between KRT17 and clinic-pathological features of LSCC patients (Table 2) uncovered that KRT17 expression was remarkably correlated with differentiation (P < 0.001), T classification (P < 0.01), lymph node metastasis (P < 0.05), and clinical stage (P < 0.05). In any case, gender and primary position were not associated with KRT17 expression. To assist in assessing the clinical importance of KRT17 expression in LSCC, survival curves were utilized to compare the difference in overall survival between low and high KRT17 expression groups (P=0.414). The results uncovered that LSCC patients with high KRT17 expression had significantly lower overall survival than those with low KRT17 expression (Figure 1(d)). Furthermore, the KRT17 mRNA and protein levels were significantly overexpressed in AMC-HN-8, TU686, TU177, and TU212 cells with respect to the noncancer 16HBE cells (Figures 1–(e)1(f)). Since the expression of KRT17 was higher in AMC-HN-8 and TU177 cells, they were chosen for subsequent knockdown experiments.
Table 2.
Correlation between KRT17 and clinicopathological features of LSCC patients (n = 42).
| Characteristic | No. (n = 42) | KRT17 expression level | P value | |
|---|---|---|---|---|
| Low (n = 23) | High (n = 19) | |||
| Gender | 0.845 | |||
| Female | 17 | 9 | 8 | |
| Male | 25 | 14 | 11 | |
|
| ||||
| Primary location | 0.248 | |||
| Supraglottic | 27 | 13 | 14 | |
| Glottic | 15 | 10 | 5 | |
|
| ||||
| T classification | 0.001∗∗∗ | |||
| T1 + T2 | 20 | 17 | 3 | |
| T3 + T4 | 22 | 6 | 16 | |
|
| ||||
| Differentiation | 0.002∗∗ | |||
| High | 20 | 6 | 14 | |
| Moderate + poor | 22 | 17 | 5 | |
|
| ||||
| Lymph node metastasis | 0.016∗ | |||
| Yes | 18 | 6 | 12 | |
| No | 24 | 17 | 7 | |
|
| ||||
| Clinical stage | 0.03∗ | |||
| I + II | 21 | 15 | 6 | |
| III + IV | 21 | 8 | 13 | |
∗ A significant difference compared with the KRT17 low expression group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3.2. Depletion of KRT17 Represses the Growth of LSCC Cells
The findings of qRT-PCR and western blot appeared to show that sh-KRT17#1 and sh-KRT17#2 effectively diminished the expression of KRT17 both in AMC-HN-8 and TU177 cells (Figures 2(a)-2(b)), and sh-KRT17#1 was selected for and sh-KRT17#1 was chosen for subsequent trials due to its high knockdown efficiency. The CCK-8 and colony assays uncovered that inhibition of KRT17 in AMC-HN-8 and TU177 cells exhibited decreased proliferative capacities (Figures 2(c)–2(d)). Moreover, we performed a flow cytometry assay to determine the impact of sh-KRT17 on the LSCC cell cycle. The results implied that in AMC-HN-8 and TU177 cells, the cell cycle was stalled in the G1 phase after KRT17 knockdown (Figure 2(e)).
Figure 2.
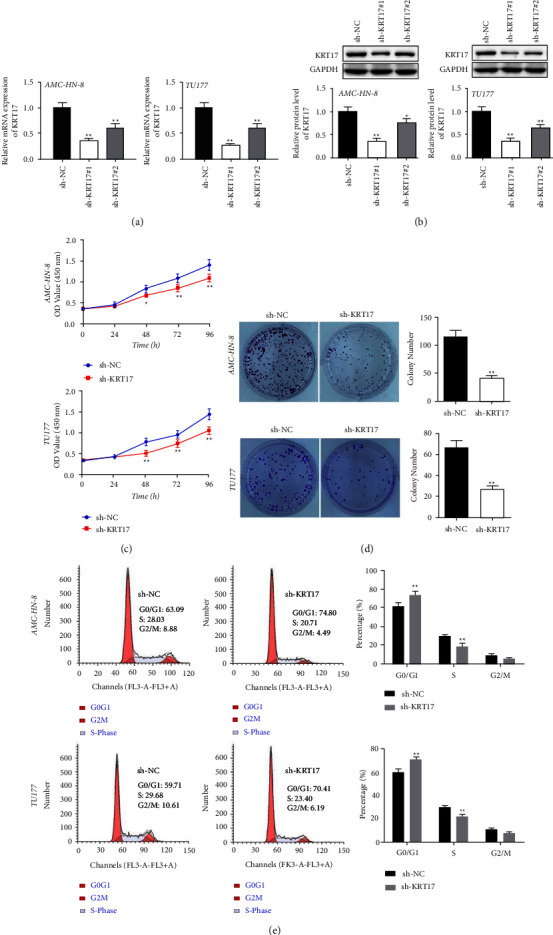
Depletion of KRT17 represses the growth of LSCC cells. (a) KRT17 was silenced by shRNAs determined by RT-qPCR. (b) The KRT17 protein level was estimated by western blot. (c) Cell viabilities were ascertained using CCK-8 assay. (d) The number of clones formed by AMC-HN-8 and TU177 cells was checked by a 2-week colony formation assay. (e) The cell cycle distribution ratios were determined analyzed using flow cytometry. ∗A significant difference compared with the sh-NC group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3.3. Depletion of KRT17 Hinders LSCC Cell Xenograft Tumor Growth In Vivo
The results of nude mouse xenograft models transfected with AMC-HN-8 and TU177 cells exhibited dramatically lower tumor volume and weight in the sh-KRT17 group than in the sh-NC group (Figures 3(a)–3(c)). At the same time, the immunohistochemical assay proposed that the levels of Ki-67 and KRT17 were markedly down-regulated in the sh-KRT17 group in contrast to the sh-NC group (Figure 3(d)). Collectively, these findings uncovered that knockdown of KRT17 hinders tumorigenesis in an in vivo LSCC cell xenograft model.
Figure 3.
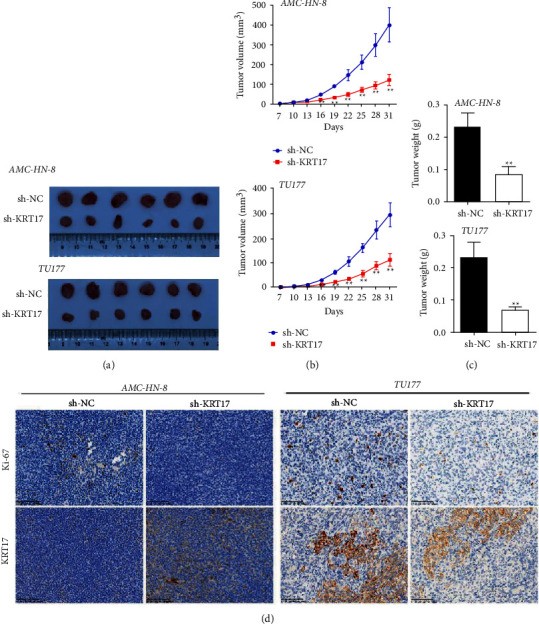
Depletion of KRT17 hinders in vivo LSCC cell xenograft tumor growth. (a) Images of tumor xenografts of mice injected with AMC-HN-8 and TU177 cells. (b) Tumor volume curves at 3-day intervals. (c) Tumor weight of mice tumor xenografts after 31 days after inoculation. (d) Representative immunohistochemical images of Ki-67 and KRT17 in tumor xenografts (scale bar = 100 μm). ∗A significant difference compared with the sh-NC group. ∗P < 0.05 and ∗∗P < 0.01.
3.4. Depletion of KRT17 Represses Migratory and Invasive Abilities of LSCC Cells
Wound healing data revealed that AMC-HN-8 and TU177 cells depleted of KRT17 had significantly reduced scratch healing abilities compared to sh-NC cells, indicating slower cell motility. The metastatic capacities of AMC-HN-8 and TU177 cells were further detected by a transwell assay, which showed a significant reduction in the number of migrating and invading cells after the knockdown of KRT17. Meanwhile, downregulation of KRT17 essentially expanded the expression of E-cadherin but hindered N-cadherin compared with the sh-NC group (Figure 4(d)).
Figure 4.
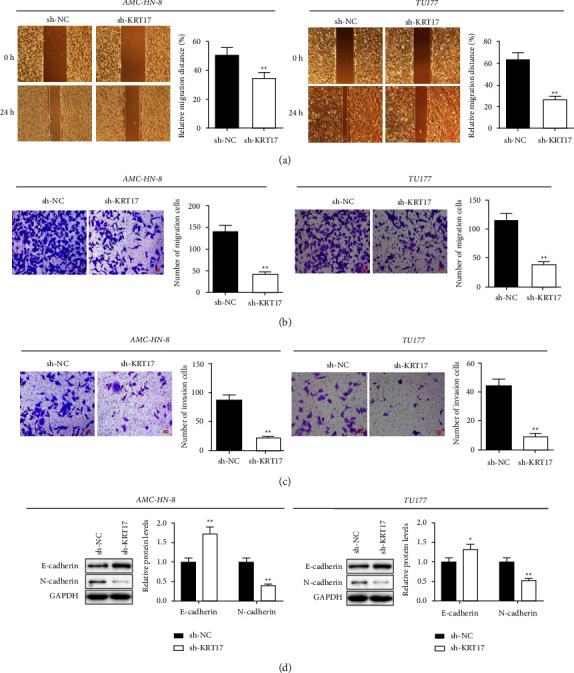
Depletion of KRT17 represses migratory and invasive abilities of LSCC cells. (a) Cell migratory abilities were assessed via the wound-healing assay (scale bar = 200 μm). (b, c) Migratory and invasive abilities was examined using Transwell assay (scale bar = 50 μm). (d) Protein levels were estimated by Western blot. ∗A significant difference compared with the sh-NC group. ∗∗P < 0.01.
3.5. KRT17 Regulates LSCC Progression by Affecting AKT/mTOR and Wnt/β-Catenin Axes
Next, we explored the mechanism by which KRT17 mediates LSCC progression. The previous pieces of evidence have unraveled the accelerating impacts of KRT17 on AKT/mTOR and Wnt/β-catenin axes [21–24]. To further explore the effects of KRT17 in the AKT/mTOR and Wnt/β-catenin signaling pathway, we used the STRING database (https://www.string-db.org/) to identify KRT17-related genes. As shown in Figure 5(a), we generated a PPI network with a total of 14 nodes containing KRT17 and AKT/mTOR and Wnt/β-catenin signaling-related proteins. Subsequently, we verified KRT17-mediated changes in the levels of proteins related to the above pathways in AMC-HN-8 and TU177 cells by western blot assay. The results recommended that exhaustion of KRT17 restrained the critical proteins of AKT/mTOR and Wnt/β-catenin pathways (Figures 5(b)-5(c)). Depletion of KRT17 also diminished the downstream proteins included within Wnt/β-catenin pathway, such as cyclin D1, c-Myc, and MMP7. Importantly, the western blot assay also found the expression of mesenchymal marker (Snail) and cell cycle-related proteins, counting CDK2, CDK4, and Cyclin E, was diminished in the sh-KRT17 group compared to the sh-NC group. Taken together, our discoveries uncovered that KRT17 actuated LSCC progression, at least in part by means of enactment of AKT/mTOR and Wnt/β-catenin axes.
Figure 5.
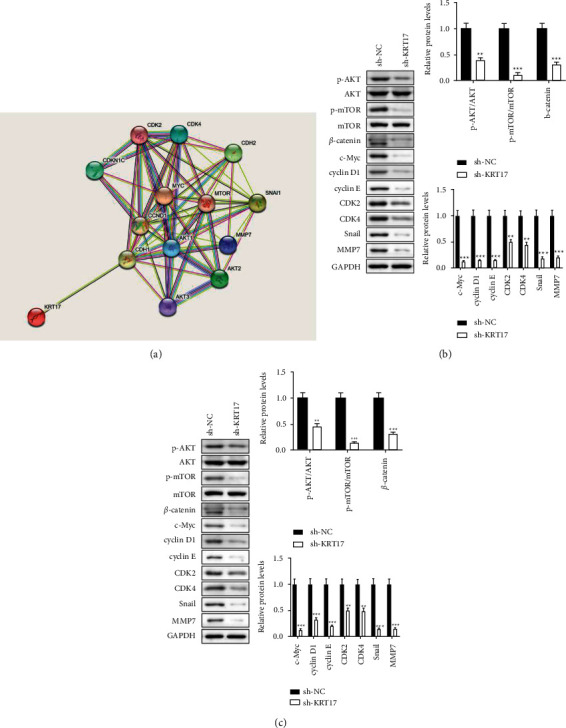
KRT17 regulates LSCC progression by affecting AKT/mTOR and Wnt/β-catenin axes (a). The protein-protein interaction (PPI) network of the KRT17 from STRING online database (https://string-db.org) was constructed (b). The protein levels in AMC-HN-8 cells were evaluated using western blot (c). The protein levels in TU177 cells were estimated using western blot. ∗A significant difference compared with the sh-NC group. ∗∗P < 0.01 and ∗∗∗P < 0.001.
4. Discussion
Patients diagnosed with LSCC can be treated by surgery, chemotherapy, or radiotherapy, but the overall therapeutic impact is still not palatable, the long-term survival rate is low, and the prognosis is destitute. The occurrence and development of LSCC is considered to be the result of the imbalance between oncogenes and tumor suppressor genes. In this manner, effectively seeking gene-level therapeutic targets and potential mechanisms has become the focus of current research. In this study, we examined the relevance of KRT17 to LSCC. Our research recommended that KRT17 regulates growth, migration, and invasion in LSCC through the AKT/mTOR and Wnt/β-catenin axes. These discoveries uncover that KRT17 plays a part in the advancement of LSCC and is probably a prospective therapy candidate.
Studies have appeared that KRT17 can directly participate in the regulation of an assortment of tumors. For example, KRT17 impacts the pathogenesis of cervical cancer by promoting the nuclear transport and degradation of protein P27 (Kip1) [25]. Mihaela et al. detailed that knockdown of KRT17 diminished cell motility and invasion capacity and retards tumor growth through p38, AKT/mTOR and ERK/JNK signaling in gastric cancer [12]. Yan et al. revealed that KRT17 regulates osteosarcoma cell proliferation, glycolysis, and tumor development through AKT/mTOR/HIF1α pathway [21]. KRT17 was found to play a key role in advancing cervical cancer development and paclitaxel-induced mediate resistance [26]. Moreover, KRT17 advances carcinogenesis of skin cancer [27], non-small-cell lung cancer [22], renal cell carcinoma [28], pancreatic cancer [29], and hepatocellular carcinoma [16]. Li et al. detailed that KRT17 was included in areca-induced oral cancer [18], and Khanom et al. showed that KRT17 promoted oral cancer tumor growth [17]. These discoveries imply an oncogenic role of KRT17, but its exact function in LSCC remains to be clarified.
We obtained high expression of KRT17 mRNA in LSCC by comparing 42 pairs of LSCC tissues with normal adjacent tissues, which is consistent with the present study [19]. KRT17 has been proven to be a prognostic marker for a multitude of cancers, including lung cancer [22], colon cancer [30], cervical cancer [31], bladder cancer [32], etc. The ability to predict outcomes and to identify key players in biological mechanisms that lead to poor outcomes are two important objectives in cancer research [15]. In LSCC, we ascertained that expression of KRT17 in a panel of LSCC samples (42 cases) was positively correlated with the differentiation, T-typing, lymph node metastasis, and clinical stage. Noticeably, overall survival analysis indicated that patients with high KRT17 expression levels exhibited a remarkable shorter survival duration than those displaying low KRT17 expression levels, recommending its potential as a prognostic marker of LSCC. We further affirmed the part of KRT17 in LSCC cells. Depletion of KRT17 hindered cell proliferative, migratory, invasive abilities and contributed to cell cycle arrest. Additionally, in vivo xenograft tumor studies inferred that knockdown of KRT17 clearly repressed tumorigenesis, i.e., lower tumor volume and weight, and Ki-67 level. Hence, KRT17 plays an imperative part in advancing LSCC proliferation and metastasis.
Activation of AKT/mTOR and Wnt/β-catenin axes in LSCC has been extensively studied [33–36]. Past studies have suggested that KRT17 mediates cancer progression through the AKT/mTOR or Wnt/β-catenin axis [21, 22]. In this manner, we focused on KRT17 in LSCC mechanisms through the above-mentioned signals. Western blot revealed that downregulation of KRT17 essentially diminished the expression of p-AKT, p-mTOR, and β-catenin, which affirmed the inactivation status of the AKT/mTOR and Wnt/β-catenin axes. Consistently, the downstream proteins of the Wnt/β-catenin axis and the cell cycle-related proteins were also diminished after the exhaustion of KRT17.
5. Conclusion
In conclusion, our study demonstrates that the knockdown of KRT17 restrains proliferation, migration, and invasion partly through AKT/mTOR and Wnt/β-catenin axes, proposing KRT17 as a novel biological target in LSCC and giving unused considerations for the alter of treatment of LSCC.
Acknowledgments
The work was supported by the Project of Zhejiang Provincial Education Department (No. Y202147030) and Project of Huzhou Science and Technology Bureau (No. 2021GY40).
Data Availability
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. AJCacjfc: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Steuer C. E., El-Deiry M., Parks J. R., Higgins K. A., Saba N. F. An update on larynx cancer. CA: A Cancer Journal for Clinicians . 2017;67(1):31–50. doi: 10.3322/caac.21386. [DOI] [PubMed] [Google Scholar]
- 3.Bradford C. R., Ferlito A., Devaney K. O., Mäkitie A. A., Rinaldo A. Prognostic factors in laryngeal squamous cell carcinoma. Laryngoscope Investigative Otolaryngology . 2020;5(1):74–81. doi: 10.1002/lio2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marur S., Forastiere A. A. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clinic Proceedings . 2016;91(3):386–396. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen N., Bellile E., Thomas D., et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head and Neck . 2016;38(7):1074–1084. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob J. T., Coulombe P. A., Kwan R., Omary M. B. Types I and II keratin intermediate filaments. Cold Spring Harbor Perspectives in Biology . 2018;10(4) doi: 10.1101/cshperspect.a018275.a018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toivola D. M., Boor P., Alam C., Strnad P. Keratins in health and disease. Current Opinion in Cell Biology . 2015;32:73–81. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa I., Takahashi K., Moll I., Moll R. Expression of keratins in cutaneous epithelial tumors and related disorders–distribution and clinical significance. Experimental Dermatology . 2011;20(3):217–228. doi: 10.1111/j.1600-0625.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Zhang S., Wang G. Keratin 17 in disease pathogenesis: from cancer to dermatoses. The Journal of Pathology . 2019;247(2):158–165. doi: 10.1002/path.5178. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Yin M., Zhang L.-J. Keratin 6, 16 and 17—critical barrier alarmin molecules in skin wounds and psoriasis. Cells . 2019;8(8):p. 807. doi: 10.3390/cells8080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendrisch F., Haarhaus B., Krieger N., Quirin K.-W., Schempp C. M., Wölfle U. The effect of herbal medicinal products on psoriasis-like keratinocytes. Biomolecules . 2021;11(3):p. 371. doi: 10.3390/biom11030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chivu-Economescu M., Dragu D. L., Necula L. G., et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer . 2017;20(6):948–959. doi: 10.1007/s10120-017-0712-y. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs R. P., Batazzi A. S., Han M. C., Coulombe P. A. Loss of keratin 17 induces tissue-specific cytokine polarization and cellular differentiation in HPV16-driven cervical tumorigenesis in vivo. Oncogene . 2016;35(43):5653–5662. doi: 10.1038/onc.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bournet B., Pointreau A., Souque A., et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology . 2012;12(1):27–34. doi: 10.1016/j.pan.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Hu H., Xu D. H., Huang X. X., et al. Keratin17 promotes tumor growth and is associated with poor prognosis in gastric cancer. Journal of Cancer . 2018;9(2):346–357. doi: 10.7150/jca.19838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Tian Z.-C., Zhou D.-H., et al. KRT17 Serves as an Oncogene and a Predictor of Poor Survival in Hepatocellular Carcinoma Patients . 2020. [Google Scholar]
- 17.Khanom R., Nguyen C. T. K., Kayamori K., et al. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS One . 2016;11(8) doi: 10.1371/journal.pone.0161163.e0161163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y.-C., Chiang C.-H., Wu C.-C., et al. Proteomics analysis reveals involvement of Krt17 in areca nut-induced oral carcinogenesis. Journal of Proteome Research . 2016;15(9):2981–2997. doi: 10.1021/acs.jproteome.6b00138. [DOI] [PubMed] [Google Scholar]
- 19.Gao W., Zhang Y., Luo H., et al. Targeting SKA3 suppresses the proliferation and chemoresistance of laryngeal squamous cell carcinoma via impairing PLK1–AKT axis-mediated glycolysis. Cell Death and Disease . 2020;11(10):p. 919. doi: 10.1038/s41419-020-03104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szklarczyk D., Gable A. L., Nastou K. C., et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Research . 2021;49(D1):D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X., Yang C., Hu W., et al. Knockdown of KRT17 decreases osteosarcoma cell proliferation and the warburg effect via the AKT/mTOR/HIF1α pathway. Oncology Reports . 2020;44(1):103–114. doi: 10.3892/or.2020.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Yang M.-Q., Lei L., et al. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Management and Research . 2019;11:7485–7497. doi: 10.2147/cmar.s218926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji R., Ji Y., Ma L., et al. Keratin 17 upregulation promotes cell metastasis and angiogenesis in colon adenocarcinoma. Bioengineered . 2021;12(2):12598–12611. doi: 10.1080/21655979.2021.2010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z., Yu S., Ye S., et al. Keratin 17 activates AKT signalling and induces epithelial-mesenchymal transition in oesophageal squamous cell carcinoma. Journal of Proteomics . 2020;211 doi: 10.1016/j.jprot.2019.103557.103557 [DOI] [PubMed] [Google Scholar]
- 25.Escobar-Hoyos L. F., Shah R., Roa-Peña L., et al. Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Research . 2015;75(17):3650–3662. doi: 10.1158/0008-5472.can-15-0293. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Chen Q., Deng Z., et al. KRT17 confers paclitaxel-induced resistance and migration to cervical cancer cells. Life Sciences . 2019;224:255–262. doi: 10.1016/j.lfs.2019.03.065. [DOI] [PubMed] [Google Scholar]
- 27.DePianto D., Kerns M. L., Dlugosz A. A., Coulombe P. A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nature Genetics . 2010;42(10):910–914. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarlos D. P., Yusenko M. V., Peterfi L., Szanto A., Kovacs G. Dual role of KRT17: development of papillary renal cell tumor and progression of conventional renal cell carcinoma. Journal of Cancer . 2019;10(21):5124–5129. doi: 10.7150/jca.32579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D., Ni X.-F., Tang H., et al. KRT17 functions as a tumor promoter and regulates proliferation, migration and invasion in pancreatic cancer via mTOR/S6k1 pathway. Cancer Management and Research . 2020;12:2087–2095. doi: 10.2147/cmar.s243129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ujiie D., Okayama H., Saito K., et al. KRT17 as a prognostic biomarker for stage II colorectal cancer. Carcinogenesis . 2020;41(5):591–599. doi: 10.1093/carcin/bgz192. [DOI] [PubMed] [Google Scholar]
- 31.Escobar-Hoyos L. F., Yang J., Zhu J., et al. Keratin 17 in premalignant and malignant squamous lesions of the cervix: proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Modern Pathology . 2014;27(4):621–630. doi: 10.1038/modpathol.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J., Xu H., Ji H., et al. Low expression of keratin17 is related to poor prognosis in bladder cancer. OncoTargets and Therapy . 2021;14:577–587. doi: 10.2147/ott.s287891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao R., Tian L., Zhao B., et al. FADS1 promotes the progression of laryngeal squamous cell carcinoma through activating AKT/mTOR signaling. Cell Death and Disease . 2020;11(4):p. 272. doi: 10.1038/s41419-020-2457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang N., Hui L., Wang Y., Yang H., Jiang X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncology Reports . 2014;31(6):2651–2659. doi: 10.3892/or.2014.3120. [DOI] [PubMed] [Google Scholar]
- 35.Sun S., Gong C., Yuan K. LncRNA UCA1 promotes cell proliferation, invasion and migration of laryngeal squamous cell carcinoma cells by activating Wnt/β-catenin signaling pathway. Experimental and Therapeutic Medicine . 2019;17(2):1182–1189. doi: 10.3892/etm.2018.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao R., Wang S., Liu J., et al. KLK11 acts as a tumor-inhibitor in laryngeal squamous cell carcinoma through the inactivation of Akt/Wnt/β-catenin signaling. Journal of Bioenergetics and Biomembranes . 2021;53(1):85–96. doi: 10.1007/s10863-020-09870-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


