Abstract
Objectives:
As the human papillomavirus (HPV) epidemic continues to grow, the number of elderly patients with oropharyngeal squamous cell carcinoma (OPSCC) is rapidly increasing. Despite this observation, this cohort remains understudied. We aimed to understand HPV status within this cohort and its impact on disease control in elderly patients.
Methods and Materials:
We identified patients aged ≥ 70 with newly diagnosed, non-metastatic, OPSCC treated with curative intent at our institution from 2007–2018. Logistic regression and survival analyses were used for outcome-specific endpoints.
Results:
In total, 88 patients were identified with a median age of 73 (interquartile range [IQR]: 71–78) and a median Charlson Comorbidity Index of 6 (IQR: 5–7). Eighty-two percent were ECOG 0 or 1 performance. Of note, 70% of the cohort had HPV+ tumors. Fifty-one percent of patients were AJCC 8th edition stage I/II and 49% were stage III/IV. Median follow-up time was 2.5 years (IQR: 0.9–4.7). Eight percent had surgery alone, 27% underwent adjuvant RT, and 64% received definitive RT. Sixty-four percent received concurrent chemotherapy. By both univariate and multivariable analyses, HPV+ status was significantly associated with improved locoregional control (LRC), overall survival (OS), and disease specific survival (DSS).
Conclusions:
In our cohort of elderly patients with OPSCC, the majority was HPV+, which was associated with improved clinical outcomes. There are many challenges when managing elderly patients with OPSCC, but as the population ages and the HPV epidemic evolves, these patients should be considered for elderly specific clinical trials to explore the role of de-intensification treatment regimens.
Keywords: Comorbidity, Elderly, Head and Neck Cancer, Human Papillomavirus (HPV), Radiotherapy, Oropharynx, Oropharyngeal squamous cell carcinoma (OPSCC)
Introduction
The clinical and epidemiological understanding of the etiology of oropharyngeal squamous cell carcinoma (OPSCC) continues to evolve as the human papillomavirus (HPV) epidemic grows and tobacco use declines [1–3]. The rise of HPV infections has caused an increase in the incidence of OPSCC in North America and Western Europe with 70% of new OPSCC being attributed to HPV [4]. Moreover, due to this increase in HPV, it is expected that OPSCC will represent at least 50% of all head and neck cancers by 2030 [5].
Between 2009–2010, the highest prevalence of HPV infection was reported in those aged 55–60 [6]. HPV+ OPSCC has generally been considered a disease primarily affecting middle-aged patients (45–64 years old) [1]. However, as the HPV+ population ages, it is leading to a new wave of older patients with HPV+ OPSCC, which will likely shift the cancer burden to a more elderly cohort within the next decade [1, 7, 8].
Due to its association with a younger cohort, and less comorbidity compared to environmentally related OPSCC, HPV+ OPSCC has classically been associated with patients with good performance status, minimal comorbidities, and longer life expectancies [9]. Aging, however, is associated with the onset of a range of illnesses, and many patients with head and neck cancer have an increased prevalence of multiple diseases associated with worse prognosis [10–12]. In addition, HPV infection itself may also be associated with certain age-related comorbidities, including cardiovascular disease [13, 14].
Despite the increased prevalence of age-related health problems, there is limited understanding of the impact such conditions have in elderly patients with HPV+ OPSCC. Similarly, medical literature and clinical trial availability are lacking for these patients and the role of treatment de-escalation remains unclear [15–17]. Hospitals and academic institutions often lack standardized protocols for these patients since existing guidelines on OPSCC treatment, based on HPV status, have generally focused on middle-aged patients without consideration of additional potential risk factors inherent to aging [18]. In one retrospective cohort study, HPV+ OPSCC patients were shown to have a superior overall survival compared to HPV− patients when accounting for comorbidities and polypharmacy [19].
Changes in the aging population may signal a paradigm shift in our understanding of HPV+ OPSCC from a disease of the middle aged to that of the elderly [1, 7, 8]. We sought to determine HPV prevalence and characteristics in an elderly cohort as well as the impact of HPV on disease and survival outcomes in this population so as to better understand how comorbidities and performance status may also influence outcomes.
Methods:
Study cohort
Study participants were identified from a retrospective database of head and neck cancer patients consecutively treated from 2007 to 2018 by the radiation oncology, ENT, and medical oncology departments at our institution. Elderly patients, aged ≥ 70 years old, with newly diagnosed non-metastatic OPSCC who underwent definitive treatment were selected. A patient must have had documentation of HPV status (criteria below) to be eligible for inclusion and a minimum follow-up of 3 months. Those with recurrent disease, prior RT to head and neck, or prior history of OPSCC were excluded from our study. Patient demographics, clinical covariates, treatment characteristics, treatment tolerability scores, and dates of follow-up and recurrence were extracted from the electronic medical record. Approval for this retrospective study was obtained from the Institutional Review Board at our institution.
Collection of clinical data
Demographic, clinicopathologic, and treatment characteristic data were collected using REDCap electronic database [20, 21]. Hematologic events during treatment were recorded and graded according to common terminology criteria for adverse events (CTCAE v5.0). Follow-up data, including adverse acute (0–6 months post treatment), subacute (6–12 months post treatment), and chronic (at least 12 months post treatment) events, were recorded and graded according to CTCAE v5.0 guidelines. Locoregional control and survival data, including cause of death and last follow-up, were obtained.
Definition of variables
Smoking status was categorized as never smokers (<100 cigarettes in lifetime), former smokers (quit smoking at least 1 years prior to diagnosis), or current smokers (smoking ≥ 1 pack a day within 1 years for ≥ 1 month). Alcohol status was categorized as never drinkers, moderate drinkers (≤1 or ≤ 2 alcoholic drinks/day for females and males respectively) or heavy drinkers (defined as ≥ 8 or ≥ 15 drinks/week for females and males respectively) [22]. Heavy drinkers were further sub-divided into former or current. All patients were staged according to AJCC 7th edition and restaged using AJCC 8th edition criteria. HPV status was defined as positive (HPV+) by PCR amplification of HPV gene loci or, in its absence, with positive p16 immunohistochemistry or in situ hybridization. HPV was defined as negative (HPV−) based on negative findings from PCR amplification of HPV gene loci or p16 immunohistochemistry.
A treatment interruption was defined as prolonged (missing at least one unexcused day of treatment) or premature discontinuation (missing at least 1 fraction or chemotherapy cycle) of prescribed treatment. A treatment interruption was then subdivided as physician initiated (e.g. hospitalization resulting in no treatment) or patient initiated (e.g. missed scheduled appointment due to weather, transport, etc.).
The Charlson comorbidity index (CCI) was calculated for each patient based on the criteria and patients’ respective comorbidities [23]. Follow-up length was calculated from end of definitive treatment to last follow-up. Data was censored at date of last follow-up or death. Follow-up survival data included: locoregional control (LRC), defined as date of treatment completion to date of last follow-up for patients with locoregional control of the primary cancer and involved lymph nodes; overall survival (OS), defined as date of treatment completion until date of last follow-up for living patients; disease specific survival (DSS), defined as date of treatment completion to date of last follow-up for living patients or until death from cause other than OPSCC. Time-to event was calculated from treatment completion instead of traditional date of diagnosis or treatment initiation due to increased treatment delays potentially affecting data in this population [19].
Statistical Analysis
Data are provided as medians with the interquartile ranges. Categorical data was tested for significance using the chi-squared or Fischer’s test and continuous and ordinal data were analyzed using the Mann-Whitney test. The association of continuous and categorical parameters with HPV status was analyzed by univariate logistic regression analysis. Multivariable regression analyses were performed to identify independent determinants. Selection of parameters entering multivariable models was derived from a stepwise selection approach. Covariates included age, race, gender, alcohol use, smoking history, CCI, and ECOG with entry and removal based on a cutoff of P<0.10.
Kaplan-Meier curves stratified by HPV status were created for the cumulative risk of locoregional failure, death, or disease-specific death. Survival curves were compared using the log-rank test for significance. All available follow-up data were used for this analysis. Cox proportional hazards models were used to generate HRs and 95% CI to estimate the association between variables of interest and LRC, OS, and DSS over the follow-up period. A stepwise selection approach was utilized to generate multivariable models based on a cutoff of P<0.25.
RESULTS:
Study cohort
Patient characteristics:
Eighty-eight patients were identified with a median age of 73 (71–78) and the majority were male (78%),white (66%), former smokers (61%), and non- or moderate drinkers (80%). Eighty-two percent of patients were ECOG 0 or 1 (42% and 40%, respectively) and had a median CCI of 6 (5–7). The majority of tumors were base of tongue (59%) or tonsillar (36%) primaries at an early stage (26% Stage I and 35% Stage II). Notably, we identified that the majority of this population was HPV+ (70%), and of these patients, 68% had PCR subtype confirmation, and 83% were serotype16. The baseline clinical and characteristics of patients are outlined in Table 1 and Supplemental Table 1.
Table 1:
Demographic and Disease Characteristics of elderly patients with HPV+ and HPV− OPSCC
| Variable | HPV+ (n=62) | HPV− (n=26) | All Patients (n =88) | p-valuea |
|---|---|---|---|---|
| Age, years | 0.56 | |||
| Median (IQR) | 74 (71–79) | 73 (71–77) | 73 (71–78) | |
| Gender, n (%) | 0.36 | |||
| Male | 47 (76) | 22 (85) | 69 (78) | |
| Female | 15 (24) | 4 (15) | 19 (22) | |
| Race/Ethnicity, n (%) | 0.007 | |||
| White | 47 (76) | 11 (42) | 58 (66) | |
| Black | 6 (10) | 7 (27) | 13 (15) | |
| Other | 4 (6) | 1 (4) | 5 (6) | |
| Hispanic | 5 (8) | 7 (27) | 12 (14) | |
| Alcohol, n (%) | 0.007 | |||
| Never/Moderate drinker | 54 (87) | 16 (62) | 70 (80) | |
| Former Heavy drinker | 3 (5) | 7 (27) | 10 (11) | |
| Current Heavy drinker | 5 (8) | 3 (12) | 8 (9) | |
| Smoking, n (%) | 0.88 | |||
| Never | 19 (31) | 7 (27) | 26 (30) | |
| Former | 38 (61) | 16 (62) | 54 (61) | |
| Current | 5 (8) | 3 (12) | 8 (9) | |
| Smoking Pack Years, n (%) | 0.99 | |||
| Never | 19 (31) | 7 (27) | 26 (30) | |
| ≤10 pack years | 11 (18) | 5 (19) | 16 (18) | |
| 10–≤20 pack years | 6 (10) | 3 (12) | 9 (10) | |
| >20 | 26 (42) | 11 (42) | 37 (42) | |
| ECOG, n (%) | 0.009 | |||
| 0 | 31 (50) | 6 (23) | 37 (42) | |
| 1 | 23 (37) | 12 (46) | 35 (40) | |
| 2 | 8 (13) | 5 (19) | 14 (16) | |
| 3 | 0 (0) | 3 (12) | 3 (3) | |
| Charleston Comorbidity Index | 0.66 | |||
| Median (IQR) | 6(5–7) | 6(5–7) | 6 (5–7) | |
| Stage (AJCC 8th edition), n (%) | <.001 | |||
| I | 23 (37) | 0 (0) | 23 (26) | |
| II | 27 (44) | 4 (15) | 31 (35) | |
| III | 12 (19) | 3 (12) | 15 (17) | |
| IVA | 0 (0) | 10 (38) | 10 (11) | |
| IVB | 0 (0) | 9 (35) | 9 (10) | |
| Primary Site, n (%) | 0.04b | |||
| Base of tongue | 35 (56) | 17 (65) | 52 (59) | |
| Palatine Tonsils | 26 (42) | 6 (23) | 32 (36) | |
| Other | 1 (2) | 3 (12) | 4 (5) | |
| HPV subtype, n (%) | n=42 | <.001 | ||
| 16 | 35 (83) | N/A | 35 | |
| 18 | 2 (5) | 2 | ||
| 33 | 1 (2) | 1 | ||
| 35 | 4 (10) | 4 |
p-values relate to HPV+ versus HPV−
p-value=0.16 when other is not included in statistical analysis
The HPV− population had a significantly greater percentage of black (27%) or Hispanic (27%) patients compared to 10% black and 8% Hispanic patients in the HPV+ cohort (P<0.05). There was a significantly greater number of former and current heavy drinkers in the HPV− cohort, as compared to the HPV+ cohort (27% and 12% respectively vs. 5% and 8% respectively; P<0.05 for both). There was a significant difference in ECOG performance between HPV+ and HPV− patients and in AJCC 8th edition staging (P<0.05 and P<0.001, respectively). There was no difference in AJCC 7th edition staging between groups (P=0.45).
Treatment characteristics:
Of the 88 patients in the cohort, 36% of patients underwent surgery and, of those patients, 77% received adjuvant RT. Of those receiving definitive RT, 56% were treated with concurrent chemotherapy typically with monotherapy (14% carboplatin, 20% cisplatin, 13% cetuximab) or a combination dual therapy (18%). Of those receiving concurrent chemotherapy, 50% underwent induction chemotherapy (n=25), most commonly with 3 cycles of docetaxel, cisplatin, and 5-fluorouracil (TPF), with no statistical difference between HPV+ and HPV− patients (P=0.87).
The median radiation dose was 70 (IQR: 64–70). Of those receiving surgery (n=31), a majority of patients had a TORS procedure (84%) and a unilateral neck dissection (58%). There was a nonsignificant trend in the number of patients who received surgical intervention based on HPV status (43% HPV+ vs. 16% HPV−; P=0.079).
Treatment characteristics of patients are outlined in Table 2 and pathological characteristics are outlined in Supplemental Table 2.
Table 2:
Treatment characteristics of elderly patients with HPV+ and HPV− OPSCC
| Variable | HPV+ (n=62) | HPV− (n=26) | All Patients (n =88) | p-valuea |
|---|---|---|---|---|
| Treatment, n (%) | 0.08 | |||
| Surgery Alone | 7 (11) | 0 (0) | 7 (8) | |
| Adjuvant RT | 15 (24) | 2 (8) | 17 (19) | |
| Adjuvant CRT | 5 (8) | 2 (8) | 7 (8) | |
| Definitive RT | 3 (5) | 4 (15) | 7 (8) | |
| Definitive CRT −Induction | 15 (24) | 10 (38) | 25 (28) | |
| Definitive CRT +Induction | 17 (27) | 8 (31) | 25 (28) | |
| Radiation Dose (Gy), n (%) | 0.06 | |||
| Median (IQR) | 66 (60–70) | 70 (68–70) | 70 (64–70) | |
| Concurrent Chemotherapy Type, n (%) | 0.10 | |||
| Cisplatin Alone | 11 (18) | 7 (27) | 18 (20) | |
| Carboplatin Alone | 11 (18) | 1 (4) | 12 (14) | |
| Cetuximab Alone | 7 (11) | 4 (15) | 11 (13) | |
| Other | 8 (13) | 8 (31) | 16 (18) | |
| TORS, n (%) | 0.40 | |||
| No | 4 (6) | 1 (4) | 5 (6) | |
| Yes | 23 (37) | 3 (12) | 26 (30) | |
| Lymph node dissection, n (%) | 0.20 | |||
| Unilateral | 17 (27) | 1 (4) | 18 (20) | |
| Bilateral | 10 (16) | 3 (12) | 13 (15) | |
| Peg tube placement, n (%) | 32 (52) | 15 (58) | 47 (53) | 0.99 |
| Prophylactic | 15 (24) | 8 (31) | 23 (26) | 0.70 |
| Reactive | 17 (27) | 7 (27) | 24 (27) | 0.70 |
| Follow-up (years) | 0.60 | |||
| Median (IQR) | 2.6 (0.9–4.7) | 1.5 (0.5–4.5) | 2.5 (0.9–4.7) |
p-values relate to HPV+ versus HPV−
Tolerance and toxicity:
Twenty-three percent of patients experienced a treatment interruption with no statistical difference between HPV+ and HPV− patients (33% HPV− vs. 19% HPV+, P=0.24). Two patients died on treatment due to an unrelated health condition (both HPV− patients). Only 5 patients prematurely discontinued RT and 12 patients were hospitalized during RT (9 HPV+ patients and 3 HPV− patients), with no direct statistical relationship to induction chemotherapy (P=0.21). Of the nonfatal treatment interruptions, 50% were patient initiated and 50% were physician initiated with no difference between HPV status (P=0.62, not shown); although HPV− patients experienced more patient-initiated interruptions and HPV+ experienced more physician-initiated. Approximately half of patients (53%) either had a prophylactic (26%) or reactive (27%) PEG tube placed for nutritional support with no significant difference between HPV+ and HPV− patients (Table 2).
Of those who completed treatment and received RT (n=79), hematologic grade 3 or 4 toxicities occurred in 10% of patients during RT (anemia, leukopenia, neutropenia, or thrombocytopenia) with no difference in HPV status (P=0.24). Acute (0–3 month) grade 3 or 4 mucositis, dysphagia, odynophagia, xerostomia, and dermatitis occurred in 34%, 48%, 47%, 14%, and 9% of patients receiving RT, respectively, with no difference between HPV+ and HPV− patients (P=0.68, 0.80, 0.91, 0.72, and 1.0, respectively). Chronic (1 year+) grade 3 or 4 toxicities occurred in 10% (n=8) of patients who received RT with no difference between HPV+ and HPV− patients (P=0.69).
Risk Factors associated with HPV
By both univariate and multivariable logistic regression analysis, race was the only clinical parameter that was found to be significantly associated with positive HPV status. Non-white patients with OPSCSS had a lower odds of being HPV+ (Univariate OR: 0.27 [95% CI: 0.10–0.71], P =0.009; Multivariable OR: 0.29 [95% CI: 0.10–0.78], P =0.008). In univariate analysis, heavy drinkers had a lower odds of having HPV+ disease, (OR: 0.24 [95% CI: 0.078–0.70], P =0.009); however, in the full model, this association approached but did not reach statistical significance despite having a similar effect size (OR: 0.32 [95% CI: 0.097–1.05], P =0.053). In univariate analysis, a higher ECOG score trended towards an association with HPV status, but failed to remain in the multivariable model based on the specified inclusion/exclusion criteria (ECOG 1 vs. ECOG 0 univariate OR: 0.37 [95% CI: 0.1–1.1], P =0.082; ECOG 2/3 vs. ECOG 1 univariate OR: 0.26 [95% CI: 0.063–1.02], P=0.053, Table 3).
Table 3.
Associations between clinical covariates and positive HPV status in univariate and multivariate logistic model
| Variable* | Univariate | Multivariateb | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
| Agea | 1.06 | 0.95–1.19 | 0.35 | |||
| Nonwhite | 0.27 | 0.10–0.71 | 0.009 | 0.29 | 0.10–0.78 | 0.008 |
| Smoking history | 0.83 | 0.29–2.26 | 0.73 | |||
| Heavy drinking | 0.24 | 0.078–0.70 | 0.009 | 0.32 | 0.097–1.05 | 0.053 |
| ECOG 1 | 0.37 | 0.11–1.10 | 0.082 | |||
| ECOG 2/3 | 0.26 | 0.063–1.02 | 0.053 | |||
| CCI ≥ 7 | 0.52 | 0.20–1.36 | 0.17 | |||
Continous variable, one interval increase
Parameters entered in multivariable model included age, race, gender, alcohol use. smoking history. CCI. and ECOG
Survival analysis
The median time to last follow-up or death was 2.6 years (0.9–4.7). Of the 86 patients who completed treatment, 68 patients (79%) had no evidence of disease at their last follow-up. Of the 21 (24%) who died post-treatment, 62% (n=13) died of disease and 38% (n=8) died of other causes. Of those still living (n=65), 6 (9%) are living with disease (3 with locoregional recurrence, 1 with distant metastasis, and 2 with both). Of those who experienced distant metastasis (n=11), the most common place of metastasis was the lung (n=7).
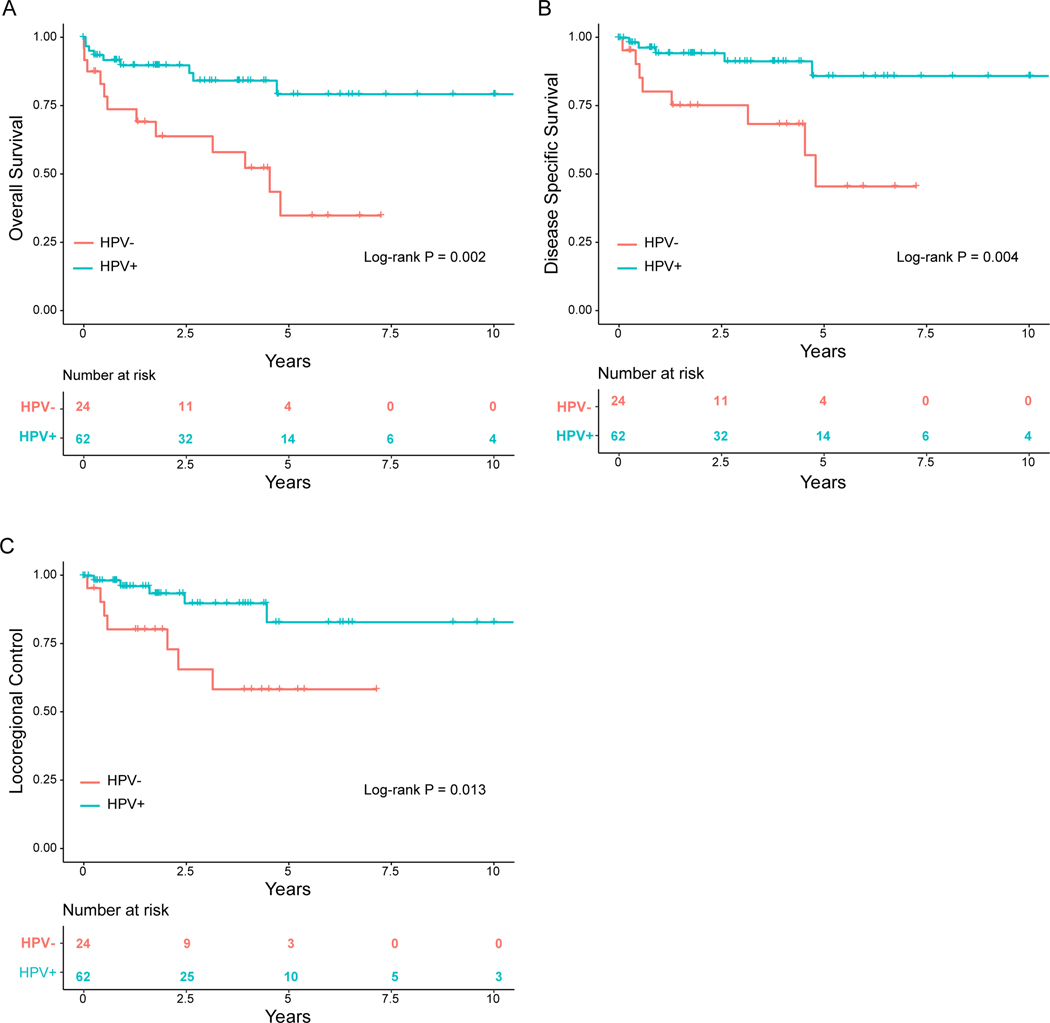
A total of 9 HPV+ patients died, with 56% (n=5) due to disease progression. A total of 12 HPV− patients died, with 75% (n=8) due to disease progression. HPV+ status was significantly associated with improved OS, DSS, and LRC (OS: HR 0.27, [95% CI: 0.11, 0.64]; P=0.003; DSS: HR 0.22, [95% CI: 0.072, 0.67], P=0.008; LRC: HR 0.26, [95% CI: 0.082, 0.82], P=0.021) when compared to HPV− patients (Table 3). At 5 years, there was a 79.2% (66.6–94.1) chance of OS for HPV+ patients compared to a 34.8% (17.2–70.5) chance for HPV−patients. Estimated LRC at 5 years was 83.0% (68.6–100.0) for HPV+ patients and 58.% (38.4–89.1) for HPV− patients. Actuarial DSS at 5 years was 86% (74.1–99.9) for HPV+ and 45.7% (23.9–87.1) for HPV− patients (Table 4).
Table 4:
Survival estimates by variables of interest
| Variable | Overall Survival (% (95% CI) | Disease Specific Survival (% (95% CI) | Locoregional Control (% (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 2.5 years | 5 years | P-valuea | 2.5 years | 5 years | P-valuea | 2.5 years | 5 years | P-valuea | |
|
| |||||||||
| HPV status | 0.002 | 0.004 | 0.013 | ||||||
| HPV− | 63.8 (46.4, 87.7) | 34.8 (17.2, 70.5) | 75.4 (58.7–96.7) | 45.7 (23.9–87.1) | 65.8 (46.3–93.5) | 58.5 (38.4–89.1) | |||
| HPV+ | 89.7 (82.2–97.9) | 79.2 (66.6–94.1) | 94.4 (88.4–100.0) | 86.0 (74.1–99.9) | 89.9 (80.6–100.0) | 83.0 (68.6–100.0) | |||
| Age | 0.16 | 0.59 | 0.72 | ||||||
| 70–80 | 84.3 (75.8–93.8) | 66.1 (52.6–83.2) | 88.7 (81.1–97.0) | 71.5 (57.6–88.7) | 84.6 (75.0–95.4) | 76.7 (63.7–92.2) | |||
| ≥80 | 73.3 (54.0–99.5) | 55.0 (28.9–100.0) | 91.7 (77.3–100.0) | 91.7 (77.3–100.0) | 73.3 (45.8–100.0) | 73.3 (45.8–100.0) | |||
| Charleston Comorbidity Index | 0.29 | 0.38 | 0.21 | ||||||
| CCI < 7 | 87.3 (78.9–96.6) | 65.2 (49.9–85.1) | 94.3 (88.3–100.0) | 72.6 (56.7–93.1) | 87.7 (77.9–98.7) | 78.0 (63.6–95.8) | |||
| CCI ≥ 7 | 70.9 (54.7–91.9) | 62.0 (42.9–89.7) | 76.9 (60.9–97.2) | 76.9 (60.9–97.2) | 72.1 (52.2–99.7) | 72.1 (52.2–99.7) | |||
| ECOG | 0.15 | 0.49 | 0.24 | ||||||
| ECOG 0 | 94.2 (86.6–100.0) | 81.5 (65.5–100.0) | 96.8 (90.8–100.0) | 83.7 (67.8–100.0) | 88.0 (73.5–100.0) | 80.0 (61.7–100.0) | |||
| ECOG 1 | 76.1 (62.9–92.1) | 55.9 (38.8–80.6) | 83.4 (71.0–97.8) | 68.8 (50.3–94.0) | 82.3 (69.1–98.0) | 82.3 (69.1–98.0) | |||
| ECOG 2 or 3 | 69.3 (48.0–99.9) | 55.4 (31.3–98.1) | 84.4 (66.6–100.0) | 67.5 (41.0–100.0) | 70.3 (45.8–100.0) | 52.8 (25.9–100.0) | |||
| Smoking | 0.71 | 0.91 | 0.41 | ||||||
| Neversmoker | 80.8 (67.0–97.4) | 56.3 (30.5–100.0) | 87.5 (75.2–100.0) | 65.6 (36.5–100.0) | 71.9 (53.2–97.0) | 71.9 (53.2–97.0) | |||
| Current or former smoker | 83.2 (73.6–94.0) | 65.5 (51.3–83.8) | 90.0 (81.9–98.8) | 74.1 (59.7–92.0) | 89.6 (81.3–98.8) | 79.6 (65.5–96.8) | |||
| Alcohol History | 1 | 0.7 | 0.64 | ||||||
| Never/Moderate alcohol use | 84.7 (76.4–94.0) | 61.3 (46.7–80.5) | 91.3 (84.3–98.9) | 71.1 (55.7–90.7) | 84.9 (74.7–96.4) | 75.1 (60.3–93.4) | |||
| Heavy alcohol use | 70.5 (49.6–100.0) | 70.5 (49.6–100.0) | 79.3 (60.9–100.0) | 79.3 (60.9–100.0) | 75.7 (54.4–100.0) | 75.7 (54.4–100.0) | |||
| Stage (8th edition) | 0.047 | 0.055 | 0.37 | ||||||
| I/II | 88.3 (79.9–97.6) | 76.5 (62.9–93.2) | 93.5 (86.7–100.0) | 84.2 (71.0–99.8) | 82.6 (70.3–97.1) | 82.6 (70.3–97.1) | |||
| III/IV | 72.7 (58.1–91.0) | 47.2 (28.7–77.9) | 81.9 (68.7–97.6) | 57.3 (36.3–90.6) | 83.3 (69.1–100.0) | 66.3 (45.2–97.3) | |||
p-value via logrank test
Positive HPV status remained significantly correlated with improved LRC, OS, and DSS when adjusted for potential confounders by multivariable modeling. Of note, HPV was the only variable that remained in regression models for LRC and DSS based on the pre-specified criteria for stepwise selection. In multivariable Cox regression for OS, HPV+ status was associated with improved survival (HR 0.23, [95% CI: 0.094, 0.56], P=0.001) while patients aged ≥ 80 had significantly worse overall survival (HR 2.88, [95% CI: 1.01, 8.18], P=0.047) as compared to those aged 70–80. All other covariates (ECOG, CCI, smoking status, alcohol history) were not significantly associated with LRC, OS, or DSS (Supplemental Table 3).
Due to collinearity, HPV status and AJCC 8th edition staging were analyzed in separate models. Kaplan-Meier analysis showed that advanced stage was associated with a worse overall survival (P=0.047). Actuarial OS at 5 years for stage III/IV was 47.2% (28.7–77.9) compared to an actuarial OS of 76.5% (62.9–93.2) for Stage I/II patients. In adjusted multivariable models, stage remained a significant predictor of OS (HR 2.55, [95% CI: 1.06, 6.13]; P=0.036). There was a trend toward an association of advanced stage with DSS in Kaplan Meier analysis (Log-rank P-value=0.055) and in adjusted model advanced stage was associated with worse DSS (DSS: HR 3.19, [95% CI: 1.02, 9.94], P=0.046). AJCC 8th edition stage was not found to be associated with LRC (Supplemental Table 4).
DISCUSSION:
Our study is one of the largest and among the first to investigate the role of HPV status in both disease and survival outcomes of elderly patients with HPV+ OPSCC while incorporating subtype information into the analysis. Prior to this report, risk factors for HPV+ OPSCC and their relationship with age-related clinical parameters had yet to be well defined. Consistent with previous studies [7, 19], the majority of elderly patients with OPSCC in our cohort were found to be HPV+, underlying the important role of HPV status in even the elderly population. Although other studies have investigated the prognostic impact of HPV in the elderly population, they employed more limited diagnostic methods for determining HPV status. Moreover, they focused on overall survival, excluding locoregional control from analysis [7, 19]. In contrast, our study— for the first time to our knowledge—incorporated DNA molecular testing to validate more accurately HPV status [7, 19]. This study supports the practice of molecular HPV testing for all patients with OPSCC, regardless of age.
In support of previous findings from epidemiological studies, race emerged as a major predictor of HPV in this population—compared to non-white patients, white patients were more likely to have HPV+ disease[1]. Although alcohol use was significant in univariate analysis, it approached but did not reach significance in the fully adjusted multivariate analysis. Nonetheless, the consistent effect sizes between the two models suggest there may be a trend between heavy alcohol use and lower odds of HPV+ OPSCC, though further studies are necessary to determine the nature of such a relationship. Previous studies have shown that HPV related OPSCC occurs in younger patients with less exposure to alcohol and smoking when compared to HPV negative OPSCC in the middle-aged population [24, 25]. In our study, both HPV+ and HPV− groups were exposed to similar amounts of smoking, however the HPV− cohort had a higher exposure to alcohol.
Although there are likely distinct risk factors for HPV−related and -unrelated OPSCC, the role of comorbidities and performance status did not influence outcome. By both univariate and multivariable analyses adjusting for age, gender, race, alcohol, tobacco use, CCI and ECOG, we show that HPV status is strongly correlated with better outcomes (survival and locoregional control). In fact, HPV status was the only clinical covariate that was shown to predict for both survival and disease outcomes in our study. Other aspects inherent to elderly patients (age, comorbidities and performance status) or common to OPSCC (smoking status and drinking history) failed to significantly predict outcomes in univariate analysis. Of note, age had no impact on disease specific outcomes. The AJCC 8th edition staging guideline incorporates HPV status into its OPSCC staging criteria [26]. In our study, higher 8th edition AJCC stage was independently associated with worse OS and DSS, providing further validation of the new staging system in an elderly cohort and the importance of HPV status in predicting outcomes.
Overall, treatment tolerability was high in this patient population with 23% of patients experiencing a treatment interruptions suggesting that age alone should not render patients ineligible for stage-appropriate standard of care. Treatment tolerability did not statistically differ between HPV+ and HPV− patients with approximately the same amount of acute and chronic toxicities as well as rate of PEG tube insertion, although HPV− patients did experience slightly more interruptions consistent with other studies looking at HPV status in all ages [27].
Our study had several limitations including those inherent to retrospective analysis and small study cohort. Additionally, a variety of treatment regimens were used in this study consistent with a lack of standardization in treating the elderly patients with OPSCC [18]. HPV testing was not routine early in the cohort and 6 patients were excluded from this study due to this issue. Other limitations include the more frequent and routine use of induction chemotherapy and concurrent carboplatin/cetuximab at our institution in locally advanced OPSCC compared to other centers, which may decrease generalizability of data.
With increasing life expectancy, and, thus, an increase in the incidence of HPV+ OPSCC in an aging population, management decisions for this elderly patient cohort should be more standardized. Elderly patients have historically been omitted from any investigational head and neck cancer trials secondary to upper age limits typically set for eligibility, excluding patients ≥ 70 years of age. An analysis of head and neck clinical trials demonstrates that elderly patients represent <5% of patients enrolled on head and neck cancer clinical trials [16, 28] despite this being a common malignancy in the elderly.
Therefore, more studies are needed to fully comprehend the best treatment for elderly patients with OPSCC. As new initiatives for de-escalation of treatment modalities emerge for HPV+ patients, the elderly are an ideal group to benefit from these efforts. This study highlights a window of opportunity to incorporate elderly patients with HPV−related cancers into these trials or design trials specifically tailored to study this cohort. It will be important to understand how to best optimize treatment for these patients as the population ages and the HPV epidemic evolves.
CONCLUSION:
The rate of HPV−positive OPSCC in the elderly is increasing and there is a gap in our understanding of outcomes in this cohort. Of note, the majority of the patients in this study were HPV−positive suggesting that it is critical to test all p16 positive elderly patients with OPSCC for HPV. As with younger patients, a positive HPV status predicted improved survival. Age alone should not render patients ineligible for standard treatment options, which are demonstrated to be well-tolerated in this modern cohort. There are many challenges when managing elderly patients with OPSCC, but efforts should be made to include elderly patients with HPV+ OPSCC in future clinical trials to explore the role of de-intensification treatment regimens in treating this growing complex, patient population.
Supplementary Material
Figure 1:
Kaplan-Meier Survival Curves. HPV positive group, n= HPV negative group, n=. (A) Overall survival for patients according to whether patients were HPV+ or HPV−. 2.5- and 5-year overall survival was 89.7% (95% CI, 82.2–97.9) and 79.2% (95% CI, 66.6–94.1) for HPV+ group respectively and 63.8% (95% CI 46.4–87.8) and 34.8% (95% CI 17.2–70.5) for HPV− group respectively (p=0.002). (B) Disease specific survival for patients according to whether patients were HPV+ or HPV−. 2.5- and 5-year disease specific survival was 94.4% (95% CI, 88.4–100.0) and 86.0% (95% CI, 74.1–99.9) for HPV+ group respectively and 75.4% (95% CI 58.7–96.7) and 45.7% (95% CI 23.9–87.1) for HPV− group respectively (p=0.004). (C) Locoregional control for patients according to whether patients were HPV+ or HPV−. 2.5- and 5-year locoregional control was 89.9% (95% CI, 80.6–100.0) and 83.0% (95% CI, 68.6–100.0) for HPV+ group respectively and 65.8% (95% CI 46.3–93.5) and 58.5% (95% CI 38.4–89.1) for HPV− group respectively (p=0.013).
The rate of HPV−positive OPSCC in the elderly is increasing
Majority of oropharynx patients aged at least 70 are HPV positive
HPV status prognosticates clinical outcomes in the elderly
Elderly patients with OPSCC tolerate treatment well
Elderly patients with HPV OPSCC necessitate clinical trial enrollment
Acknowledgements:
We acknowledge the Claude D. Older Americans Independent Pepper Center (OAIC) for providing funding [5P30AG028741-07].
Role of the funding source:
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- CCI
Charlson Comorbidity Index
- DSS
Disease Specific Survival
- ECOG
Eastern Cooperative Oncology Group
- HPV
Human Papillomavirus
- HPV−
Human Papillomavirus negative
- HPV+
Human Papillomavirus positive
- LRC
Locoregional Control
- OPSCC
Oropharyngeal squamous cell carcinoma
- OS
Overall Survival
Footnotes
Disclosures:
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Zumsteg ZS, Cook-Wiens G, Yoshida E, Shiao SL, Lee NY, Mita A, et al. Incidence of Oropharyngeal Cancer in Elderly Patients, 2000–2012. JAMA Oncology. 2016;2:1617–23. [DOI] [PubMed] [Google Scholar]
- [2].Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking Prevalence and Cigarette Consumption in 187 Countries, 1980–2012Global Smoking Prevalence and Cigarette ConsumptionGlobal Smoking Prevalence and Cigarette Consumption. JAMA. 2014;311:183–92. [DOI] [PubMed] [Google Scholar]
- [3].Louie KS, Mehanna H, Sasieni P. Trends in head and neck cancers in England from 1995 to 2011 and projections up to 2025. Oral Oncology. 2015;51:341–8. [DOI] [PubMed] [Google Scholar]
- [4].Gooi Z, Chan JYK, Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. The Laryngoscope. 2016;126:894–900. [DOI] [PubMed] [Google Scholar]
- [5].Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gillison ML, Broutian T, Pickard RKL, Tong Z-y, Xiao W, Kahle L, et al. Prevalence of Oral HPV Infection in the United States, 2009–2010. JAMA. 2012;307:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Windon MJ, D’Souza G, Rettig EM, Westra WH, van Zante A, Wang SJ, et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer. 2018;124:2993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rettig EM, Fakhry C, Khararjian A, Westra WH. Age Profile of Patients With Oropharyngeal Squamous Cell CarcinomaIncreasing Age of Patients With Oropharyngeal Squamous Cell CarcinomaLetters. JAMA Otolaryngology–Head & Neck Surgery. 2018;144:538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marur S, D’Souza G, Westra WH, Forastiere AA. HPV−associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology. 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bøje CR. Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma – A systematic review. Radiotherapy and Oncology. 2014;110:81–90. [DOI] [PubMed] [Google Scholar]
- [11].Reid BC, Alberg AJ, Klassen AC, Samet JM, Rozier RG, Garcia I, et al. Comorbidity and survival of elderly head and neck carcinoma patients. Cancer. 2001;92:2109–16. [DOI] [PubMed] [Google Scholar]
- [12].Sanabria A, Carvalho AL, Vartanian JG, Magrin J, Ikeda MK, Kowalski LP. Comorbidity Is a Prognostic Factor in Elderly Patients with Head and Neck Cancer. Annals of Surgical Oncology. 2007;14:1449–57. [DOI] [PubMed] [Google Scholar]
- [13].AddisoD, SeidelmanB, JanjuA, EmamH, StaziakV, HalletR, et al. Human Papillomavirus Status and the Risk of Cerebrovascular Events Following Radiation Therapy for Head and Neck Cancer. J Am Heart Assoc. 2017;6:e006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Joo E-J, Chang Y, Kwon M-J, Cho A, Cheong Hae S, Ryu S. High-Risk Human Papillomavirus Infection and the Risk of Cardiovascular Disease in Korean Women. Circulation Research. 2019;124:747–56. [DOI] [PubMed] [Google Scholar]
- [15].Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct Risk Factor Profiles for Human Papillomavirus Type 16–Positive and Human Papillomavirus Type 16–Negative Head and Neck Cancers. JNCI: Journal of the National Cancer Institute. 2008;100:407–20. [DOI] [PubMed] [Google Scholar]
- [16].Pignon J-P, Al Maître, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiotherapy and Oncology. 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
- [17].Bhatia A, Burtness B. Human Papillomavirus–Associated Oropharyngeal Cancer: Defining Risk Groups and Clinical Trials. Journal of Clinical Oncology. 2015;33:3243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dave E, Su W, Gupta V, Miles B, Demicco E, Soriano T, et al. Human Papilloma Virus-positive Oropharyngeal Squamous Cell Carcinoma in the Elderly. Anticancer Res. 2017;37:1847–51. [DOI] [PubMed] [Google Scholar]
- [19].Caparrotti F, O’Sullivan B, Bratman SV, Ringash J, Lu L, Bayley A, et al. Exploring the Impact of Human Papillomavirus Status, Comorbidity, Polypharmacy, and Treatment Intensity on Outcome of Elderly Oropharyngeal Cancer Patients Treated With Radiation Therapy With or Without Chemotherapy. International Journal of Radiation Oncology*Biology*Physics. 2017;98:858–67. [DOI] [PubMed] [Google Scholar]
- [20].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington, DC: December 2015. [Google Scholar]
- [23].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Clinical Epidemiology. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [24].D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case–Control Study of Human Papillomavirus and Oropharyngeal Cancer. New England Journal of Medicine. 2007;356:1944–56. [DOI] [PubMed] [Google Scholar]
- [25].Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- [26].Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. 2018;16:479–90. [DOI] [PubMed] [Google Scholar]
- [27].Hess CB, Rash DL, Daly ME, Farwell DG, Bishop J, Vaughan AT, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus-positive vs human papillomavirus-negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:4–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.