Abstract
Background
Caffeine is widely used in preterm infants for apnea control. It has no effect on sleep in the only existing polysomnographic study including ten preterm infants Behavioral and polygraphic studies have conflicting results.
Methods
We studied 21 late-preterm infants at a median gestational age of 36 weeks. Polysomnography was performed twice, at baseline on day 1 and on the day after the onset of caffeine treatment (20 mg/kg loading and 5 mg/kg morning maintenance dose).
Results
Caffeine acted short term as a breathing stimulant with reduction of apneas, improved baseline SpO2 (p < 0.001), and decreased 95 percentile of end-tidal carbon dioxide level (p < 0.01). It also increased arousal frequency to SpO2 desaturations of more than 5% (p < 0.001). Caffeine did not affect sleep stage distribution, sleep efficiency, frequency of sleep stage transitions, appearance of REM periods, or the high number of spontaneous arousals. The median spontaneous arousal count was 18 per hour at baseline, and 16 per hour during caffeine treatment (p = 0.88).
Conclusions
In late-preterm infants, caffeine has a clear short-term respiratory stimulant effect, and it increases the arousal frequency to hypoxia. However, caffeine does not appear to act as a central nervous system stimulant, and it has no acute effect on sleep quality.
Impact
Effects of caffeine on sleep in preterm infants has previously been investigated with only one full polysomnographic study including ten preterm infants. The study showed no effect.
The current study shows that caffeine acts short term as a respiratory stimulant and increases arousal frequency to hypoxia.
Although a potent central nervous system (CNS) stimulant in adults, caffeine does not seem to have similar acute CNS effect in late-preterm infants.
The onset of caffeine treatment has no short-term effect on sleep stage distribution, sleep efficiency, frequency of sleep stage transitions, appearance of REM periods, or the high number of spontaneous arousals.
Introduction
Caffeine is globally the most widely used psychoactive stimulant1. In addition to its central nervous system (CNS) stimulant effect, caffeine and other methyxanthines are respiratory stimulants. Due to its effects on breathing, caffeine is used in preterm infants for treatment and prevention of apneas2,3. In preterm infants, caffeine effectively decreases long central and mixed apneas and periodic breathing3,4. For infants born at less than 32–34 weeks of gestation, caffeine treatment is commonly started during the first day of life and discontinued at around 34–35 weeks of gestational age3,5. However, some preterm infants may continue to express apneas and periodic breathing, and could benefit from continuing treatment4. As used for prevention and treatment of apneas in preterm infants, caffeine improves long-term neurological outcome6–9. The mechanism of this positive effect is not properly confirmed, although the most readily available explanation is the reduction of apneas and repetitive hypoxia3,4,10. Another potential mechanism is direct neuroprotective action of caffeine through adenosine receptors11–13.
Sleep is important for the developing brain14. Disturbances in sleep distribution may cause neurological sequelae during this time of rapid brain maturation15,16. In animal studies, rapid eye-movement (REM) sleep deprivation has proven harmful14,17. In adults and adolescents, as a CNS stimulant, caffeine alters sleep quality by reducing total sleep time and sleep efficiency, prolonging sleep latency, and reducing subjective sleep quality1. In clinical practice, infants seem to sleep well even when on high doses of caffeine for apnea treatment. The effect of caffeine on sleep in preterm and term infants remains controversial and not widely studied18–25. The aim of this study was to investigate short-term effects of caffeine on sleep in late preterm infants with polysomnography (PSG).
Methods
Study design and patients
We performed PSG recordings in 21 infants born preterm in the neonatal units of Helsinki University Hospital, Helsinki, Finland. At the time of the study, the infants were clinically stable with no respiratory support or caffeine treatment. The studied infants were considered by the clinician in charge to need caffeine treatment for apneas with desaturations, or excessive periodic breathing. The study infants underwent full PSG studies to investigate respiratory events and sleep. On day 1, a baseline recording was performed followed by administration of a caffeine citrate loading dose of 20 mg/kg. Caffeine treatment was continued with a daily dose of 5 mg/kg. On day 2, after onset of caffeine treatment, a second recording was performed.
The Helsinki University ethics committee approved the study. Parents provided written consent forms and did not receive any monetary compensation for participation.
Polysomnography
The PSG setup followed the recommendations of the American Academy of Sleep Medicine (AASM)26. It comprised monitoring of electroencephalography (EEG) channels (C4-M1, Cz-Fz, Cz-O2, and O2-M1), left and right electro-oculography (EOG) channels, nasal airflow (pressure sensor), respiratory movements (abdominal band), chin and diaphragm electromyography (EMG), electrocardiography (ECG, lead II position), pulse oximeter oxygen saturation (SpO2) with a 4-s averaging interval, and end-tidal carbon dioxide (EtCO2). The PSG recordings were done using Siesta PSG equipment (Compumedics, Abbotsford, Australia).
The PSG recordings were converted into European Data Format (EDF) and transferred into Embla® RemLogic™ PSG software (Natus Medical Inc., Pleasanton, CA) for both visual (T.K.) and automatic scoring analysis. The completion of data analysis was done by an extensive research special purpose software.
The sleep stage analysis was performed visually, recognizing wakefulness, non-REM (NREM) sleep, and REM sleep. An arousal was defined as a period of 3 s or more with a sustained increase in chin EMG with or without changes in the EEG signals. Heart rate was not used as an indicator. Central, obstructive, and mixed apneas, and periodic breathing were recognized. We determined respiratory pauses of 4 s or more as apneas. Apnea and periodic breathing definitions, as well as arousal definitions used in this study are presented in Table 1 and have also been previously described in detail4,27. We scored breathing effort visually from the PSG analysis based on the diaphragm EMG on a scale of none (0) to maximal (2) effort in apneas with obstructive breaths. Heart rate variability (HRV) was measured from periods of deep NREM sleep.
Table 1.
Apnea and arousal definitions used in the current study.
| Apnea | Definition | Duration |
|---|---|---|
| Central apnea | Apnea with no breathing movements | Pause in breathing lasting: >2 breathing cycles and ≥4 s |
| Obstructive apnea | Apneas with breathing movements but no airflow | All obstructive pauses of breathing |
| Mixed apnea | Apneas commencing as central but showed obstructive respiratory movements | All mixed apneas |
| Apnea of prematurity |
Apnea with: Heart rate decrease to <100 beats per minute, or drop in SpO2 to <80%, or apnea length of >20 s |
|
| Arousal | Definition | Duration |
| Arousal |
A sustained increase in chin and diaphragm EMG from baseline recording excluding sucking of the dummy, or gross body movements causing artefacts in ECG, EEG, respiratory signal |
≥3 s |
| Apnea arousal | An arousal appearing during an apnea, or within 5 s after the end of an apnea | ≥3 s |
| Long arousal or awakening | Same as arousal | ≥15 s |
EMG electromyogram, ECG electrocardiogram, EEG electroencephalogram.
Statistical methods and analysis
We used the non-parametric Wilcoxon signed-rank test for pairwise comparison as the number of study infants was limited to 21 and the majority of the dependent variables were not normally distributed. Results were noted significant at p < 0.05. For the statistical analysis we used SPSS® Statistics software versions 27 (IBM, Armonk, NY).
Results
At the time of the study, the median age of the 21 infants was 4.7 (interquartile range, IQR 2.8–7.1) weeks, and gestational age 36 (IQR 35–36) weeks. They were born at a median of 31 (IQR 28 to 33) weeks of gestation with a median birth weight of 1.610 (IQR 1.140–2.190) kg. None of the infants received respiratory support or supplemental oxygen directly before the study. Sixty-seven percent of the infants had previous caffeine treatment, which was discontinued at a median of 8 days before the study onset. The demographic data are presented in more detail in Table 2.
Table 2.
Demographic data of the study infants.
| Infants (n) | 21 |
| Female | 13 (62%) |
| Gestational age at birth (weeks) | 31.1 (28.4–33.6) |
| Weight at birth (kg) | 1.610 (1.140–2.190) |
| BPD | 5 (24%) |
| GMV-IVH | 3 (14%) |
| Grade 1 to 2 | 2 (9.5%) |
| Grade 4 | 1 (4.8%) |
| Age at study (weeks) | 4.7 (2.8–7.1) |
| Gestational age at study (weeks) | 35.7 (35.0–36.3) |
| Weight at study (kg) | 2.240 (2.015–2.595) |
| Infants with caffeine previously | 14 (67%) |
| Caffeine-free period before study (days) | 8 (7–11) |
| Gestational age (weeks) at cessation of previous caffeine treatment | 34.0 (33.7–35.0) |
BPD bronchopulmonary dysplasia, GMH-IVH germinal matrix or intraventricular hemorrhage. Results presented as median (interquartile range) or number (percentage).
Effect of caffeine on breathing
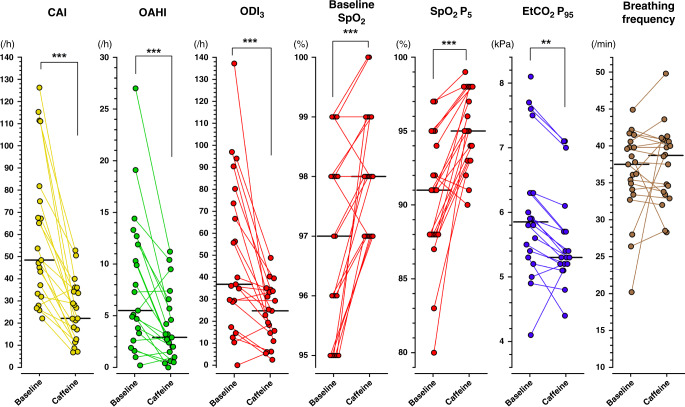
Caffeine acted as a short-term breathing stimulant (Fig. 1). Caffeine reduced the number of apneas (p < 0.001), frequency of oxygen desaturation (p < 0.001), increased median SpO2 levels (p < 0.001), and decreased the high 95th percentile EtCO2 level (p < 0.01), but caffeine did not show significant effect on breathing frequency (Supplementary Table S1).
Fig. 1. Apnea and respiratory results presented as individual changes.
Caffeine acted as a ventilatory stimulant in late-preterm infants. Caffeine treatment decreased the central apnea index (CAI), the obstructive apnea-hypopnea index (OAHI), and oxygen desaturation of over 3% from baseline (ODI3). Baseline median pulse oximeter oxygen saturation (SpO2) and the 5th percentile SpO2 level increased with caffeine treatment. Breathing frequency remained unchanged, but the end-tidal carbon dioxide (EtCO2P95) level decreased with caffeine. See also Supplementary Table S1 for more specific data on EtCO2 and breathing frequency values. /h per hour of sleep, /min per minute, kPa kilopascal, **P < 0.01, ***P < 0.001.
Sleep characteristics and spontaneous arousals
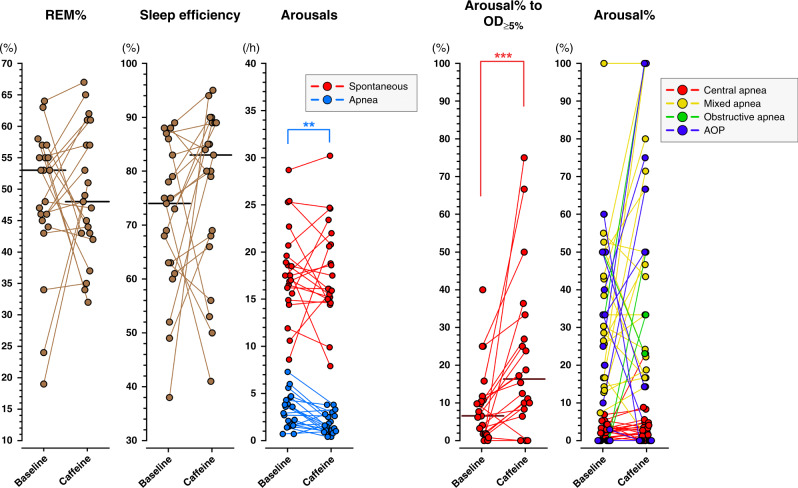
Sleep characteristics and arousal data are presented in Tables 3 and 4 and Fig. 2. Baseline PSG recordings lasted longer than recordings after the onset of caffeine treatment (p = 0.002) but there was no significant difference in sleep efficiency (p = 0.16). All the main sleep attributes remained similar in both study phases (Table 3); sleep stage distribution, frequency of sleep stage transitions, REM sleep latency, and other characteristics of REM sleep showed no significant changes during caffeine treatment. Spontaneous arousals were frequent and more common in REM than in NREM sleep both during baseline PSG recording and after the onset of caffeine treatment (Table 4). Figure 2 shows individual changes in sleep and arousal parameters.
Table 3.
Polysomnographic recording times and sleep characteristics.
| 1. Baseline | 2. Caffeine | P value | |
|---|---|---|---|
| Recording time (minutes) | 223 (188–284) | 183 (165–199) | 0.002 |
| Sleep efficiency (%) | 74.0 (61.5–84.5) | 83.0 (67.0–89.0) | 0.16 |
| TST (minutes) | 166 (135–200) | 152 (120–164) | 0.054 |
| REM of TST (%) | 53.0 (44.0–56.0) | 48.0 (42.5–59.0) | 0.63 |
| NREM of TST (%) | 47.0 (44.0–55.5) | 52.0 (41.0–57.0) | 0.64 |
| REM latency | 1.0 (0.0–20.8) | 0.0 (0.0–11.0) | 0.55 |
| Sleep stage transitions per hour | 21.9 (18.45–27.6) | 24.50 (18.9–28.7) | 0.65 |
| REM periods (minutes) | |||
| Shortest | 4.5 (4.0–6.0) | 4.5 (4.0–6.3) | 0.97 |
| Longest | 25.5. (16.0–38.8) | 23.0 (15.5–37.3) | 0.95 |
| Average time | 11.0 (8.3–15.2) | 12.5 (9.0–16.2) | 0.38 |
| Average interval | 21.4 (13.3–29.7) | 17.4 (14.1–27.1) | 0.39 |
Main sleep parameters remained unchanged during caffeine treatment. TST total sleep time, REM rapid eye-movement sleep, NREM non-rapid eye-movement sleep. Results presented as median (interquartile range).
P = significance according to Wilcoxon signed-rank test of two related samples
Table 4.
Number of arousals from sleep.
| 1. Baseline | 2. Caffeine | P value | |
|---|---|---|---|
| Total arousal count per hour | |||
| TST | 20.5 (18.6–24.5) | 19.3 (16.4–23.1) | 0.20 |
| NREM | 14.0 (9.1–17.0) | 11.6 (8.8–14.9) | 0.51 |
| REM | 29.1 (23.0–35.2) | 26.2 (22.6–29.1) | 0.048 |
| P NREM vs. REM | <0.001 | <0.001 | |
| Spontaneous arousal count per hour | |||
| TST | 17.5 (15.3–20.2) | 16.1 (14.9–21.4) | 0.88 |
| NREM | 11.8 (7.0–14.2) | 11.3 (8.5–14.5) | 0.31 |
| REM | 26.5 (19.1–29.3) | 23.2 (20.0–26.2) | 0.24 |
| P NREM vs. REM | <0.001 | <0.001 | |
| Apnea arousal count per hour | |||
| TST | 2.9 (1.6–4.5) | 1.5 (1.0–2.7) | 0.002 |
| NREM | 2.2 (1.3–4.2) | 0.9 (0.0–1.3) | <0.001 |
| REM | 4.4 (2.4–6.4) | 2.1 (1.3–4.7) | 0.012 |
| P NREM vs. REM | 0.005 | 0.001 | |
TST total sleep time, NREM non-rapid eye-movement sleep, REM rapid eye-movement sleep, AOP apnea of prematurity.
Results presented as median (interquartile range).
P = significance according to Wilcoxon signed-rank test of two related samples.
The rate of spontaneous arousals from sleep did not differ with caffeine treatment. Apnea arousals were less frequent during caffeine treatment due to the decrease of apneas. Arousals were more common in REM than in NREM sleep.
Fig. 2. Sleep and arousal results presented as individual changes.
Sleep parameters remained unchanged with caffeine treatment, and caffeine did not act as a central nervous system stimulant in late-preterm infants. The amount of rapid-eye-movement (REM) sleep, sleep efficiency, and the frequency of spontaneous arousals did not change with caffeine treatment. Apnea arousals decreased with caffeine treatment due to the reduction in apneas. Caffeine treatment increased the rate of arousal to desaturation of a minimum 5% units (OD≥5%), but it had no effect on arousal to apneas. See also Table 3 for more specific data on sleep parameters, Table 4 for arousal percentages also in varying sleep stages, Table 5 for arousal to desaturation, and supplementary Table S2 for data on arousal to varying types of apneas. /h per hour of sleep, AOP apnea of prematurity, **P < 0.01, ***P < 0.001.
Arousal to apneas and hypoxia
Periodic breathing, and central, mixed, and apnea of prematurity defined apneas decreased with caffeine (Fig. 1), as we have previously shown4. Arousal responses to apneas were low during both baseline and caffeine (Fig. 2). The median percentage of all apneas that led to arousal was 5% at baseline and 8% during caffeine treatment (p = 0.10). Caffeine treatment did not affect the frequency of arousal responses to different types on apnea (Fig. 2 and Supplementary Table S2). Similar to the frequency of spontaneous arousals, apnea arousals were more common in REM sleep than in NREM sleep (p < 0.01) (Table 4).
Desaturation was not a potent factor in inducing arousals in these preterm infants. However, caffeine treatment increased the arousal percentage to desaturations of over 5% units (Fig. 2, Arousal% to OD≥5%), especially in REM sleep (p < 0.001). Also, arousal to desaturations to SpO2 of less than 90% was increased in REM sleep (p = 0.03), and to less than 95% both in REM and NREM sleep (p < 0.001 and p = 0.01, respectively) (Table 5).
Table 5.
Percentage of desaturations leading to arousal.
| 1. Baseline | 2. Caffeine | P value | |
|---|---|---|---|
| Arousal to desaturations to <90% | |||
| TST | 3.6 (0.0–13.4) | 14.8 (0.0–32.1) | 0.011 |
| NREM | 0.6 (0.0–8.2) | 12.1 (0.0–44.6) | 0.20 |
| REM | 4.4 (0.0–16.7) | 20.0 (0.0–36.4) | 0.028 |
| P NREM vs. REM | 0.75 | 0.17 | |
| Arousal to desaturations to <95% | |||
| TST | 5.3 (1.8–8.5) | 14.9 (7.3–31.1) | <0.001 |
| NREM | 1.3 (0.0–5.4) | 12.1 (0.0–44.6) | 0.011 |
| REM | 8.0 (5.1–12.2) | 16.5 (11.1–30.4) | <0.001 |
| P NREM vs. REM | 0.007 | 1.0 | |
| Arousal to desaturations of ≥5% | |||
| TST | 6.6 (1.7–11.3) | 16.3 (8.8–31.7) | <0.001 |
| NREM | 0.9 (0.0–6.2) | 3.3 (0.0–33.3) | 0.11 |
| REM | 10.6 (4.1–16.3) | 24.0 (16.8–46.6) | <0.001 |
| P NREM vs. REM | 0.059 | 0.19 | |
TST total sleep time, NREM non-rapid eye-movement sleep, REM rapid eye-movement sleep.
Results presented as median (interquartile range).
P = significance according to Wilcoxon signed-rank test of two related samples
Caffeine increased the arousal rate to desaturations of SpO2 to less than 90% and 95%, and to desaturation of a minimum 5% units from baseline SpO2 level.
Heart rate variability
Low-frequency variability (LFV), high-frequency variability (HFV), and total power (TP) showed no differences from baseline to caffeine treatment (Supplementary Table S3).
Discussion
Our study shows that although caffeine acts as a respiratory stimulant in late-preterm infants, it does not seem to have a clear short-term CNS stimulant effect. Caffeine reduced the number of apneas, increased the frequency of arousals to hypoxia, improved SpO2 baseline, and decreased the 95th percentile EtCO2 level. The effect on hypoxia-related arousals was more pronounced in REM than in NREM sleep. Caffeine treatment did not have a clear short-term effect on sleep. After the onset of caffeine treatment, sleep stage distribution, sleep efficiency, frequency of sleep stage transitions, appearance of REM periods, and the high number of spontaneous arousals remained unaffected. We suggest that the increase in arousal tendency to hypoxia is due to an increase in hypoxic ventilatory drive rather than an increased general arousability.
The effect of caffeine on sleep
It is generally assumed that after 27–28 weeks of gestational age premature infants start to exhibit defined sleep states or stages17,28,29. REM sleep is clearly established in infants older than 30 weeks of gestation30. Sleep is an important state in the development of infants. In animal studies, especially the development of REM sleep is necessary for normal brain development. Deprivation of REM sleep in newborn rats causes, for example, anxiety and disturbed sleep, and a lack of brain plasticity in adulthood14.
Although caffeine has a clear effect on sleep quality in adults1, the impact on sleep in preterm infants is not as evident. There are some studies concentrating on this topic showing contradictory results18–25. An observational study by Thoman et al.18 implied that preterm infants previously treated with theophylline spend less time in active sleep than controls or full-term infants. Similar findings with caffeine were noted by Koch et al.23 during caffeine treatment with an observational study setup. They found caffeine to increase wakefulness and alertness by decreasing active sleep while the amount of quiet sleep remained unchanged during the first five days of caffeine treatment. In contrast, a polygraphic study by Dietrich et al.19 in nine preterm apneic infants showed just the opposite finding shortly after acute caffeine administration. Hayes et al.24 demonstrated in a video and polygraphic setting that preterm infants who had been treated with methyxanthines for more than 5 days had lower arousal rates and shorter duration of sleep-related spontaneous movements than controls.
A PSG based study of Curzi-Dascalova et al.20 performed during maintenance caffeine treatment had findings similar to our short-term results showing that caffeine treatment does not have a clear effect on sleep. A loading dose of caffeine was given a minimum 3 days in advance. The findings of Curzi-Dascalova et al. are supported by other small studies both with investigations of chronic and short-term methylxanthine treatment20–22. The CAP (caffeine for apnea of prematurity) trial showed with neurocognitive testing, PSG, actigraphy, and sleep questionnaires that caffeine treatment for apnea of prematurity (AOP) and AOP prevention has positive long-term neurocognitive action without long-term effects on sleep at ages 5–12 years7–9,31.
Most of the few studies of the effects of methylxanthines on sleep in preterm infants have been conducted during maintenance methylxanthine treatment20,21,23–25. Only a few have investigated short-term effects of caffeine as in the current study19,22,23. Koch et al.23 studied both short-term and maintenance treatment of five days. At the beginning of caffeine treatment, due to slow elimination in (preterm) infants, caffeine levels accumulate. After 5–7 days, caffeine concentration starts to slowly decrease due to an increase in caffeine clearance32.
The effect of caffeine on arousal to apneas and desaturation
We show that caffeine significantly reduces the number of apneas and hypoxic episodes. Arousal responses to apneas remain unaffected but caffeine increased the frequency of arousals to hypoxic events. Thoppil et al.25 found similar results with maintenance theophylline treatment. However, in the current study, even during caffeine treatment the rate of arousal to desaturation of a minimum 5% (units) from baseline SpO2 was only 24% in REM sleep and 3% in NREM sleep. Our results concur with previous studies showing hypoxia often to fail in causing arousals in infants, especially in NREM sleep27,33,34. As the number of spontaneous arousals remained unaffected by caffeine, we suggest the increased arousal frequency to hypoxia to be due to an increase in hypoxic ventilatory drive instead of an increase in general arousability.
Caffeine mechanism of action
Caffeine is a CNS and respiratory stimulant1. Both actions are supposedly mediated though antagonizing adenosine receptors A1 and A2A1,35. In adults and adolescents, caffeine alters sleep quality by reducing total sleep time, reducing sleep efficiency, prolonging sleep latency, and reducing subjective sleep quality1. Preterm and term infants are commonly exposed to caffeine during fetal life through the placenta, and after birth through breast milk. Caffeine treatment is currently a part of common practice for prevention and treatment of apneas in preterm infants born at less than 34 weeks of gestation5. As treatment for apneas or their prevention, caffeine doses are at least 10-folds greater than through exposure from breast milk. With these high dosages, caffeine likely affects adenosine A3 receptors and GABA receptors in addition to A1 and A2A receptors in the CNS36.
Caffeine is metabolized and excreted differently in preterm and term infants than in older children and adults. Preterm infants excrete most caffeine through the kidneys without significant liver metabolism. The hepatic metabolism takes over the direct excretion by 6–8 months of life. Because unmetabolized caffeine is excreted slowly, the half-life of caffeine in preterm and term infants ranges from 40–230 h, decreasing to adult levels of 2–5 h during the first year of life37,38. We find it unlikely that delayed excretion and a longer half-life would explain the differences in action of caffeine between preterm infants and adults. Yet, the differences in direct CNS mechanism of action seems evident.
Limitations of the study
Sleep stages in term and preterm infants may be hard to differentiate. Accurate evaluation of different sleep stages generally requires EEG, eye movement (with EOG), and chin EMG confirmation39. With preterm infants, sleep stage definition is more complex. The EEG of preterm infants is immature. However, even among preterm infants, sleep stages may be assessed by respiration, heart-beat parameters, movements, and EMG tone26,29,40.
A consensus for arousal definition in preterm infants is lacking. Therefore we have in part applied the criteria of The International Pediatric Workgroup on Arousals for infants aged 1–6 months27,41.
Long-term follow-up in preterm infants previously treated with caffeine showed no effect on sleep and assures the safety of caffeine treatment in these infants31. We studied only short-term effects of caffeine and suggest caution when extrapolating these effects for long-term use. However, it is likely that the effects remain similar.
Conclusions
Caffeine has a clear respiratory stimulant effect in late-preterm infants but it does not appear to act as a CNS stimulant. A high loading dose of caffeine 20 mg/kg does not affect sleep stage distribution, sleep efficiency, frequency of sleep stage transitions, appearance of REM sleep periods, or the high number of spontaneous arousals. Caffeine increases the arousal frequency to SpO2 desaturations. This is suggested to be caused by an increase in hypoxic ventilatory drive.
Supplementary information
Acknowledgements
We sincerely thank the study infants and their families for taking part in our study. We thank the staff of the Helsinki University Hospital neonatal wards for assistance in recruiting the study infants and providing aid during the PSG recordings. We also thank the staff at the Helsinki University Hospital Children´s neurophysiology department for aid and assistance. We thank the Finnish Foundation for Pediatric Research in financial support of this study.
Author contributions
M.S.-M., S.A., and T.K. contributed to the study plan. M.S.-M. and T.K. performed and analyzed the sleep studies and statistics. M.S.-M. and T.K. have been the main writers of the manuscript with contribution of S.A. All authors reviewed and accepted the final draft of the manuscript.
Funding information
This study was supported by the Finnish Foundation for Pediatric Research. Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Competing interests
The authors declare no competing interests.
Consent statement
Parents/guardians provided informed written consent prior to participation in the study.
Footnotes
The original online version of this article was revised: The legends of Figures 1 and 2 have been corrected.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent statement: Parents/guardians provided informed written consent prior to participation in the study.
Change history
5/16/2022
A Correction to this paper has been published: 10.1038/s41390-022-02092-x
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-021-01794-y.
References
- 1.Clark, I. & Landolt, H. P. Coffee, caffeine, and sleep: a systematic review of epidemiological studies and randomized controlled trials. Sleep Med. Rev. 31, 70–78 (2017). [DOI] [PubMed]
- 2.Schmidt B, et al. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006;354:2112–2121. doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 3.Henderson-Smart, D. J. & De Paoli, A. G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. CD000140 (2010). [DOI] [PMC free article] [PubMed]
- 4.Seppa-Moilanen M, Andersson S, Rantakari K, Mikkola K, Kirjavainen T. Caffeine and supplemental oxygen effectively suppress periodic breathing with only minor effects during long episodes of apnoea in preterm infants. Acta Paediatr. 2019;108:443–451. doi: 10.1111/apa.14541. [DOI] [PubMed] [Google Scholar]
- 5.Eichenwald EC. Apnea of prematurity. Pediatrics. 2016;137:1–7. doi: 10.1542/peds.2015-3757. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, et al. Reduction in developmental coordination disorder with neonatal caffeine therapy. J. Pediatr. 2014;165:356–359. e352. doi: 10.1016/j.jpeds.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, et al. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the cap randomized clinical trial. JAMA Pediatr. 2017;171:564–572. doi: 10.1001/jamapediatrics.2017.0238. [DOI] [PubMed] [Google Scholar]
- 10.Kumar, V. H. S. & Lipshultz, S. E. Caffeine and clinical outcomes in premature neonates. Children (Basel)6, 118 (2019). [DOI] [PMC free article] [PubMed]
- 11.Eichenwald EC. National and international guidelines for neonatal caffeine use: are they evidenced-based? Semin. Fetal Neonatal Med. 2020;25:101177. doi: 10.1016/j.siny.2020.101177. [DOI] [PubMed] [Google Scholar]
- 12.Rivkees SA, Wendler CC. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr. Res. 2011;69:271–278. doi: 10.1203/PDR.0b013e31820efbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle LW, et al. Caffeine and brain development in very preterm infants. Ann. Neurol. 2010;68:734–742. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- 14.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med. Rev. 2003;7:321–334. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 15.Huppi PS, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 16.Serag A, et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59:2255–2265. doi: 10.1016/j.neuroimage.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 17.Graven S. Sleep and brain development. Clin. Perinatol. 2006;33:693–706. doi: 10.1016/j.clp.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Thoman EB, et al. Theophylline affects sleep-wake state development in premature infants. Neuropediatrics. 1985;16:13–18. doi: 10.1055/s-2008-1052537. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich J, Krauss AN, Reidenberg M, Drayer DE, Auld PA. Alterations in state in apneic pre-term infants receiving theophylline. Clin. Pharmacol. Ther. 1978;24:474–478. doi: 10.1002/cpt1978244474. [DOI] [PubMed] [Google Scholar]
- 20.Curzi-Dascalova L, Aujard Y, Gaultier C, Rajguru M. Sleep organization is unaffected by caffeine in premature infants. J. Pediatr. 2002;140:766–771. doi: 10.1067/mpd.2002.124383. [DOI] [PubMed] [Google Scholar]
- 21.Chardon K, et al. Effect of caffeine on peripheral chemoreceptor activity in premature neonates: interaction with sleep stages. J. Appl. Physiol. 2004;96:2161–2166. doi: 10.1152/japplphysiol.01160.2003. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel M, Witolla C, Albani M. Sleep and aminophylline treatment of apnea in preterm infants. Eur. J. Pediatr. 1978;128:145–149. doi: 10.1007/BF00444299. [DOI] [PubMed] [Google Scholar]
- 23.Koch G, et al. Caffeine preserves quiet sleep in preterm neonates. Pharmacol. Res. Perspect. 2020;8:e00596. doi: 10.1002/prp2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes MJ, et al. Apneic preterms and methylxanthines: arousal deficits, sleep fragmentation and suppressed spontaneous movements. J. Perinatol. 2007;27:782–789. doi: 10.1038/sj.jp.7211820. [DOI] [PubMed] [Google Scholar]
- 25.Thoppil CK, Belan MA, Cowen CP, Mathew OP. Behavioral arousal in newborn infants and its association with termination of apnea. J. Appl. Physiol. 1991;70:2479–2484. doi: 10.1152/jappl.1991.70.6.2479. [DOI] [PubMed] [Google Scholar]
- 26.Berry, R. B., Quan, S. F., Abreu, A. R., Bibbs, M. L. & DelRosso, L. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6, Vol. 2.6 (American Academy of Sleep Medicine, 2020).
- 27.Seppä-Moilanen M, Andersson S, Kirjavainen T. Spontaneous and apnea arousals from sleep in preterm infants. Pediatr. Res. 2021;89:1261–1267. doi: 10.1038/s41390-020-1068-2. [DOI] [PubMed] [Google Scholar]
- 28.Curzi-Dascalova L, et al. Sleep state organization in premature infants of less than 35 weeks’ gestational age. Pediatr. Res. 1993;34:624–628. doi: 10.1203/00006450-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Palmu K, Kirjavainen T, Stjerna S, Salokivi T, Vanhatalo S. Sleep wake cycling in early preterm infants: comparison of polysomnographic recordings with a novel EEG-based index. Clin. Neurophysiol. 2013;124:1807–1814. doi: 10.1016/j.clinph.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Darnall RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin. Perinatol. 2006;33:883–914. doi: 10.1016/j.clp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Marcus CL, et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am. J. Respir. Crit. Care Med. 2014;190:791–799. doi: 10.1164/rccm.201406-1092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch G, et al. Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm neonates. J. Pediatr. 2017;191:50–56. doi: 10.1016/j.jpeds.2017.08.064. [DOI] [PubMed] [Google Scholar]
- 33.Darnall RA. The carotid body and arousal in the fetus and neonate. Respir. Physiol. Neurobiol. 2013;185:132–143. doi: 10.1016/j.resp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne RS, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir. Physiol. Neurobiol. 2005;149:257–271. doi: 10.1016/j.resp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-B. [DOI] [PubMed] [Google Scholar]
- 36.Adén, U. Methylxanthines during pregnancy and early postnatal life. In Methylxanthines. Handbook of Experimental Pharmacology. (ed. Fredholm, B. B.) 373–389 (Springer, Berlin, Heidelberg, 2011). [DOI] [PubMed]
- 37.Moschino, L. et al. Caffeine in preterm infants: where are we in 2020? ERJ Open Res. 6, 00330–2019 (2020). [DOI] [PMC free article] [PubMed]
- 38.Nehlig A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev. 2018;70:384–411. doi: 10.1124/pr.117.014407. [DOI] [PubMed] [Google Scholar]
- 39.de Weerd AW, van den Bossche RA. The development of sleep during the first months of life. Sleep. Med. Rev. 2003;7:179–191. doi: 10.1053/smrv.2002.0198. [DOI] [PubMed] [Google Scholar]
- 40.Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J. Clin. Sleep. Med. 2016;12:429–445. doi: 10.5664/jcsm.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Paediatric Work Group on Arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J. Sleep. Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.