Abstract
The blood-testis barrier transfers nutrients to spermatogenic tubules to ensure the normal physiological function of the testes. It also restricts the “entry and exit” of biological macromolecules in the testicular lumen and provides a unique microenvironment for spermatogenesis. This makes the testes a safe place for some viruses and tumors, as immune factors cannot function and drugs fail to reach therapeutic concentrations in the testes. This review aimed to describe the factors regulating the structure and physiological function of the blood-testis barrier. By understanding therapeutic mechanisms of action, drugs can be developed to function in the testicles.
Keywords: Blood-testis barrier, testis, microenvironment, spermatogenesis, drugs
Introduction
The blood-testis barrier (BTB), produced by Sertoli cells (SCs) near the base of the epithelium of the seminiferous tubules, is one of the strongest blood-tissue barriers in mammals. Limiting the paracellular and transcellular diffusion of water, electrolytes, ions, hormones, paracrine factors, and other exogenous biomolecules provides a unique microenvironment for spermatogenesis and ensures the normal physiological function of the testicles [1]. The germ cells of the BTB and testicles also express many drug transporters [2], such as P-glycoprotein (P-gp), also known as multidrug-resistant protein 1 (MDR1) [3-6] and multidrug resistance-related protein 1 (MRP1) [4], which actively pump drugs out of the testicles. Overall, the barrier function of the BTB combined with the drug transport network in the testicles prevents the delivery of drugs to the testicles.
Previous studies have shown that the testicles serve as a host for several viruses, mainly human immunodeficiency virus (HIV) and, more recently, Zika virus in mice [7]. Surprisingly, HIV-infected patients who receive antiretroviral therapy have nearly undetectable concentrations of the virus in their serum, but the levels of the virus in their testicles remain very high [8]. The effectiveness of chemotherapeutics for testicular tumors, antivirals, and male contraceptives depends on their ability to bypass the BTB, whether via diffusion or passive or active transport. Therefore, understanding the complete composition of drug transporters and the mechanisms regulating the function of the BTB is important for drug development, which will ultimately help the design of chemotherapeutics, antiviral drugs, fertility drugs, and male contraceptives to reduce testicular tumor recurrence, reduce viral transmission in semen, improve male fertility, and prevent pregnancy, respectively, thereby improving patient outcomes and quality of life.
Overview of the BTB
Barrier mechanism of the BTB
The BTB is located near the basement membrane of the germinal epithelium of the seminiferous tubules and consists of tight junctions (TJs), ectoplasmic specializations (ESs), desmosomes, and gap junctions (GJs). TJs in the testes coexist and function together with ESs (Figure 1). Desmosomes and GJs exist between the regions of the plasma membrane containing TJs and basal ESs. The BTB is also composed of SCs, which are thought to act as scaffolds for germ cells. TJs are the most important component of the BTB. They prevent water, solutes, and other large molecules from passing between the paracellular spaces, thus forming the barrier. They also restrict the movement of proteins and lipids between the apical and basolateral domains, resulting in cell polarity. Desmosomes are cell-cell junctions that mediate robust adhesion, whereas GJs are cell-cell channels that enable the diffusion of metabolites, second messengers, ions, and molecules smaller than 1 kDa [9]. The BTB is also formed by ESs, testicular-specific adhesions junctions composed of hexagonally arranged filamentous actin (F-actin) microfilaments positioned between the plasma membrane and endoplasmic reticulum. ESs between SCs are defined as basal ESs, whereas those between SCs and elongated/elongated sperm cells are defined as apical ESs [10]. ES-mediated adhesion mainly consists of cadherin-catenin complexes, which regulate the actin cytoskeleton and promote cell polarity [11].
Figure 1.
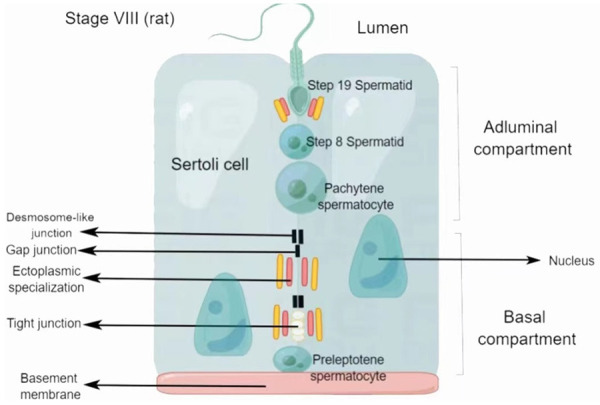
Schematic diagram of the blood-testis barrier. The blood-testis barrier is composed of tight junctions, gap junctions, ectoplasmic specializations, and desmosome-like junctions. The junction complex divides Sertoli cells into two parts: the basal compartment and the adluminal compartment. Preleptotene spermatocytes move away from the basement membrane and become pachytene spermatocytes during spermatogenesis. Then, pachytene spermatocytes rapidly complete meiosis and become sperm cells, which further differentiate into sperm by spermatogenesis.
The BTB can regulate the transport of various biological macromolecules, in which the nutrients necessary for spermatogenesis are transported into the glandular lumen. Moreover, extensive drug transport networks exist in the BTB, and efflux drug transporters are used to actively remove drugs, limiting their bioavailability in the testicles. For example, P-gp is an active drug transporter that pumps antiviral drugs, e.g., for Atazanavir, or male contraceptives, such as adjudin, out of the testicles [6,12-14]. In addition to drug transporters, SCs impart immune properties to the BTB by secreting immunosuppressive biomolecules, thus isolating the germ cells that reside in the glandular lumen from the circulatory and lymphatic systems [15]. This makes the testicles an immune-privileged site [16-21], especially in the adluminal chamber behind the BTB to isolate many spermatogenic specific antigens. These immunosuppressive biomolecules, combined with extensive networks of antiviral drug transporters and drug-metabolizing enzymes in rodent and human testicles [13,22,23], block the entry of antiviral drugs, making the testicles a safe place for certain viruses to reside [24,25].
Because of the physical and immune barrier functions of the BTB, it is difficult to introduce biological macromolecules into the seminiferous tubules for clinical treatment, contraception, and scientific research.
BTB and spermatogenesis
The TJs in the BTB divide the seminiferous epithelium into the basal and adluminal compartments. The basal compartment is adjacent to the basement membrane and contains type A spermatogonial stem cells (including A single, A paired, and A aligned spermatogonia), type B spermatogonia, and primary spermatocytes (i.e., preleptotene spermatocytes).
Nutrition
SCs are the only somatic cells in the seminiferous tubules in contact with spermatogenic cells. They participate in the BTB and are called the “nanny cells” of spermatogenic cells, as they secrete regulatory proteins, transporters, and growth factors for the differentiation and maturation of spermatogenic cells and ensure the smooth progress of spermatogenesis. When germ cells are cultured alone, they can survive for up to 24 h in vitro. When co-cultured with SCs, germ cells can survive for approximately 8 d in vitro. This phenomenon suggests that SCs provide nutrients to spermatogenic cells [26].
Spermiation
Spermiation involves a series of cellular biological events, including the cytoplasmic enveloping of spermatocytes in SCs and the expulsion of sperm cells from SCs near the luminal end.
Phagocytosis
SCs are responsible for phagocytosis and cleaning the residual components released during the metamorphosis of sperm cells into spermatozoa and degenerated germ cells during spermatogenesis [27].
Germ cell transfer
Three conserved protein modules can be detected in testicular tissue: (i) the apical Crumbs (CRB)-protein associated with Lin Seven (PALS)-Pals-associated tight junction protein (PATJ), (ii) the apicolateral partitioning-defective (PAR) 3-PAR6-aPKC complex, and (iii) the basolateral Scribble-Discs Large (DLG)-Lethal giant larvae (LGL) complex. These modules polarize the germinal epithelium of the seminiferous tubules, take part in spermatogenic cell migration, and play a guiding role in the migratory process of spermatogenic cells, thus ensuring the correct migration direction of spermatogenic cells in the seminiferous tubules [28].
Spermatogonia translocation across the BTB: Spermatocyte translocation occurs in late stage VIII/early stage IX spermatogenesis. Preleptotene spermatocytes move away from the basement membrane and become pachytene spermatocytes [29,30]. This process occurs rather rapidly, indicating that the BTB is a highly dynamic structure, possibly due to the coordinated participation of multiple signaling pathways that promote the breakdown of the old BTB and remodeling of a new BTB. The BTB is not damaged during the transport of preleptotene spermatocytes. The purpose of the BTB is to prevent harmful substances from entering the glandular cavity from the systemic circulation and disrupting the development of sperm cells after meiosis. Early studies have shown that the BTB has some unique morphological characteristics and can be dynamically reshaped to accommodate the trafficking of preleptotene spermatocytes in the seminiferous epithelium cycle, which is a cytoskeleton-dependent cellular event. The cytoskeleton in animal cells consists of actin filaments, intermediate filaments and microtubules. All three elements of the cytoskeleton are related, at least in part, to sites of intercellular attachment and may play significant roles in translocating, positioning, and anchoring spermatogenic cells in the seminiferous epithelium and in establishing and maintaining epithelial organization. In future studies, it will be necessary to understand the regulation of the transport of spermatocytes across the BTB during spermatogenesis, which will provide useful information for the design of therapeutic drugs that can reach the testicles.
Drug delivery of the BTB
Transport across the BTB
Endogenously produced bioactive peptides: Several bioactive peptides generated from structural proteins in the apical ES and basement membrane via proteolytic cleavage by matrix metalloproteinases (MMPs) can effectively modulate the function of the BTB through downstream signaling proteins [31]. Therefore, modulating BTB function by knocking out genes or interfering with protein expression is a promising approach.
Studies in the past decade have identified several bioactive polypeptides, including F5-peptide [32,33], which is derived from the laminin γ3 chain [34]. The laminin-γ3 chain, an adhesion protein specifically expressed in apical ESs [10,35], is hydrolyzed by MMP2 to generate F5-peptide, which downregulates different constitutively active phosphomimetic mutants of focal adhesion kinase (FAK), such as p-FAK-Y407E. Altered expression of p-FAK-Y407 in the testicular seminiferous epithelium activates neuronal Wiskott-Aldrich syndrome protein (N-WASP). The synergistic effects of N-WASP and actin-related protein 3 (Arp3), coupled with the epidermal growth factor receptor pathway substrate 8 (Eps8) protein, provide F-actin with the plasticity necessary to effectively change the organization of actin filaments across the SCs of the testis (Figure 2). Such a change in the filaments renders them no longer densely localized in the basal ES/BTB, leading to an increase in BTB permeability and spermatid exfoliation [32,33].
Figure 2.
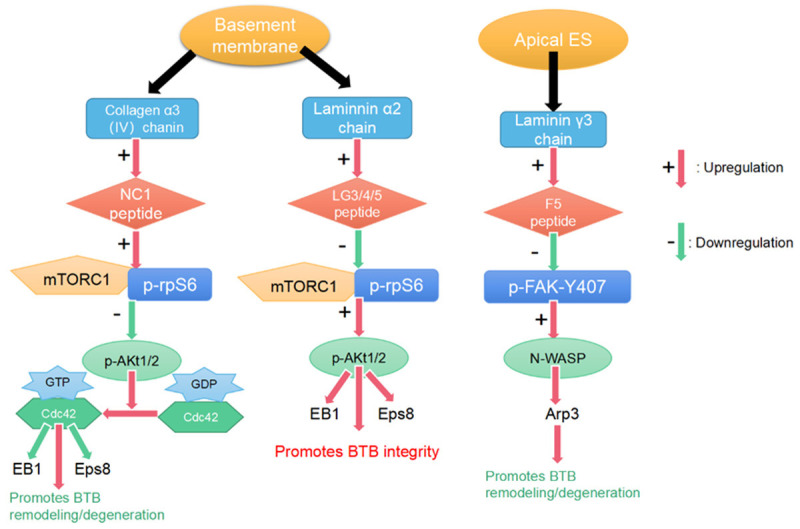
Three bioactive peptides in the testes and their downstream signaling cascades. NC1-peptide promotes blood-testis barrier remodeling/degeneration through the mTORC1/p-rpS6/p-Akt1/2/Cdc42 signaling pathway. LG3/4/5-peptide promotes BTB integrity via the mTORC1/p-rpS6/p-Akt1/2 signaling pathway. F5-peptide promotes BTB remodeling/degeneration through the p-FAK-Y407/N-WASP/Arp3 signaling pathway.
Another bioactive peptide is NC1-peptide, which consists of a non-collagenous 1 (NC1) domain [36,37] derived by the enzymatic cleavage of the collagen α3 (IV) chain (the main constituent protein [38] of the seminiferous tubule basement membrane) by MMP9 (Figure 2) [39]. NC1-peptide activates mammalian target of rapamycin complex 1 (mTORC1) [40,41] and downstream signaling protein ribosomal protein S6 (rpS6) [42] and inactivates Akt1/2 [43], also known as protein kinase B. Further activation of the cell division control protein 42 homolog (Cdc42) disrupts the organization of F-actin and MTs [43] and accelerates the degeneration and remodeling of apical/basal ESs and the BTB (Figure 2).
Additionally, bioactive laminin globular domain 3/4/5 (LG3/4/5)-peptide, derived from the C-terminus of the laminin α2 chain, is a major basement membrane protein [38]. LG3/4/5-peptide is similar to NC1-peptide and acts via the mTORC1/p-rpS6/p-Akt1/2 signaling pathway. Unlike F5- and NC1-peptides, LG3/4/5-peptide was shown to promote BTB integrity in an in vitro RNAi study (Figure 2) in which the BTB was destroyed after knocking out laminin α2 chains in the spermatogenic epithelium [44].
Overexpression of F5-peptide and rpS6 effectively enhances the transport function of the BTB. Related experiments show that the permeability of the BTB was shown to be significantly enhanced in mice in an experimental group injected with p-rpS6-MT and F5-peptide compared with control mice injected with empty pCI-neo [45]. Microscopic analysis revealed that biotin (red fluorescence) negligibly diffused into the spermatogenic tubules in control mice, but biotin diffused into the lumens of experimental mice. Actin regulatory proteins, such as Eps8 (an actin barbed end capping and bundling protein that confers actin filaments the bundled configuration at the ES) [46] and Arp3 (a branched actin polymerization protein that induces barbed end nucleation of an existing linear actin microfilament, thereby causing actin filament branching, which in turn destabilizes the ES function) [47], appeared to be abnormally positioned in the experimental group, as shown by immunofluorescence, and apical/basal ESs and TJ-related proteins were no longer specifically localized but diffusely expressed. Thus, the overexpression of p-rpS6 or F5-peptide disrupts the cytoskeleton and effectively makes the BTB leaky [45].
The overexpression of p-FAK-Y397F (a constitutively inactive p-FAK-Tyr397Phe mutant) or p-FAK-Y407E (a constitutively active p-FAK-Tyr407Glu mutant) in the SC epithelium promotes the integrity of the SC-TJ barrier, making it tighter [48]. However, the overexpression of p-FAK-Y407F (a constitutively inactive p-FAK-Tyr407Phe mutant) or p-FAK-Y307E (a constitutively active p-FAK-Tyr397Glu mutant) promotes SC-TJ barrier remodeling, making it leaky [48]. Hence, by regulating the spatiotemporal expression of these two p-FAK proteins, the immune barrier of the BTB can be made either tighter or leakier. Increasing p-FAK-Tyr407 or decreasing p-FAK-Tyr397 levels promotes the integrity of the BTB. Conversely, increasing p-FAK-Tyr397 or decreasing p-FAK-Tyr407 levels can cause the BTB to undergo remodeling in stage VIII spermatogenesis [49]. These findings suggest that these antagonistic effects maintain the dynamic balance of the BTB.
In summary, the results of animal model-based studies have shown that modulating endogenous biomolecules can alter the permeability of the BTB, further demonstrating that it is possible to deliver drugs to any tissue beyond the BTB.
Drug transporters: Drugs often need to cross biological membranes using transporters to reach their targets. There are two types of membrane transporters: (i) solute carrier transporters (SLCs), which include both facilitative transporters and secondary active transporters that do not require adenosine triphosphate (ATP) to function, and (ii) ATP-binding cassette transporters (ABCs), which are the primary active transporters. These transporters move molecules using an inverse electrochemical gradient while consuming ATP. Some transporters have been shown to play a role in transporting chemotherapeutics and antiretrovirals [50].
Even in the presence of a physiological barrier, the nutrients necessary to maintain spermatogenesis as well as toxins that target developing germ cells (e.g., cisplatin, lead, cadmium) must bypass or destroy the BTB to reach the inside of the seminiferous tubule and exert their effects. The mechanism by which such transport occurs may include a transepithelial transport pathway created by basal membrane uptake transporters and apicolateral membrane efflux transporters [51]. One mechanism by which SCs protect developing germ cells is through the expression of efflux transporters on the basal membrane [52], which also prevents therapeutic drugs from passing through the BTB. Studying transporters in the BTB is becoming increasingly important, especially for the expression and localization of SLCs and ABCs and their significance in disease research.
Several SLCs are expressed in the testes. One example is organic anion transport polypeptides (OATPs), which are bidirectional transporters of a variety of substances, including endogenous amphiphilic compounds, exogenous compounds, and pharmacological drugs [53,54]. OATP1B1 and OATP2B1 are localized in the spermatogenic epithelium and the entire testicular interstitium [55]. OATP6A1 is mainly expressed in the testes, specifically in the SCs, interstitial cells, and spermatogonia [54,56,57]. OATPs are responsible for the transport of steroid hormones, biological amines, and many drugs, including nucleoside reverse transcriptase inhibitors (e.g., tenofovir) [54]. The uptake transporters organic cation transporters 1 and 3 (OCT1 and OCT3) are present in SCs. OCT1 is located in the basement membrane, and OCT3 is located in the apical membrane [52]. Carnitine transporters are also expressed in the testes. Carnitine is essential for fatty acid oxidation and energy production for spermatogenesis. Novel OCT2 (OCTN2) is expressed in the basement membranes of SCs, and novel OCT3 (OCTN3) is expressed in the apical membranes of SCs [52]. In summary, there are various transporters in the testes, and the substances moved by these transporters have the potential to bypass/destroy the BTB for therapeutic/toxic purposes. Many drugs are substrates for these transporters; therefore, studying the expression, activity, and localization of transporters in the testes will be helpful for drug development.
The ABC family of transporters includes various specific efflux transporters that contribute to the protection of the BTB, such as P-gp, breast cancer resistance protein (BCRP), and MRPs. P-gp and MRP1 are present on the plasma membranes of Sertoli cells, germ cells, peritubular myoid cells, and endothelial cells constituting the interstitial microvasculature (Figure 3) [22,31,57]. The BCRP is also strongly expressed in interstitial microvascular endothelial cells and the lamina propria of peritubular myoid cells [55,57]. These transporters work together to actively pump drugs out of the testicles.
Figure 3.
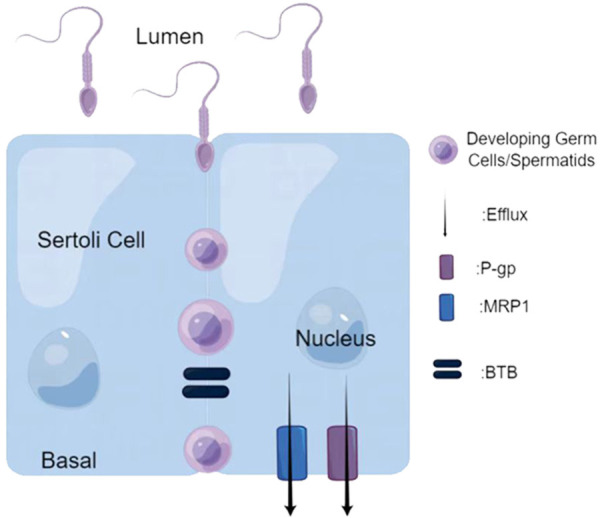
The working mechanism of active efflux transporters. P-glycoprotein (P-gp) and multidrug resistance-related protein 1 (MRP1) are present on the plasma membranes of Setroli cells. They can actively pump out biological macromolecules to ensure normal spermatogenesis.
Research has shown that the expression of drug transporters is regulated by environmental toxins (e.g., cadmium), steroids (e.g., estradiol-17 β, testosterone), and cytokines (e.g., tumor necrosis factor-α, transforming growth factor-β3) [58]. Therefore, drugs targeting these transporters can be developed to reduce the negative effects of drugs crossing the BTB, thereby improving their bioavailability.
Paracellular pathways
Paracellular barriers are also an important component of the tissue/cellular barrier in vivo to limit drug transport. Drug-modified paracellular barrier permeability has been widely studied. Reagents such as small interfering RNA (siRNA), medium-chain fatty acids, antibodies, and polypeptides have shown good regulatory effects by specifically targeting epithelial junctions [32,59]. Many peptides have been reported to interfere with the permeability of TJs by targeting the extracellular domain of TJ integral membrane proteins. For example, a 22-amino acid peptide corresponding to a stretch of the sequence of the second extracellular loop of rat occludin was shown to induce the reversible disruption of the SC-TJ barrier in vitro and in vivo [60]. Additionally, a synthetic polypeptide called C1C2 modeled after rat claudin-1 was shown to regulate the TJ function of the outer membrane of nerves in rats, thereby promoting the entry of therapeutics into the peripheral nervous system [61]. On the other hand, using a specific siRNA or short hairpin RNA to improve the bioavailability of the drugs behind the BTB may also be a viable approach. Within 48 h after the administration of claudin-5 siRNA, enhanced thyrotropin-releasing hormone uptake by neuropeptides was observed behind the blood-brain barrier in mice [62].
Transcellular approaches
Transcellular approaches increase the uptake of drug molecules by cells to improve their bioavailability. Micronization, the most widely used transcellular method, greatly reduces the size of drug particles and allows a drug to easily penetrate the cell membrane of the target organ. One technology that has been widely used in the last decade to drug micronization is supercritical fluid (SCF) technology [63]. By controlling temperature and pressure, it is now possible to produce pharmaceutical particles with small diameters (Figure 4). Furthermore, by adding an appropriate solvent, the entire process can be performed at room temperature, which is ideal for the production of heat-sensitive drugs [64]. For example, the SCF-CO2 method can produce more stable and homogeneous liposomes as drug carriers [65]. In the future, drugs can be embedded in liposomes, or SCF technology can be used to prepare small, uniform drug particles to improve their bioavailability and effectiveness.
Figure 4.
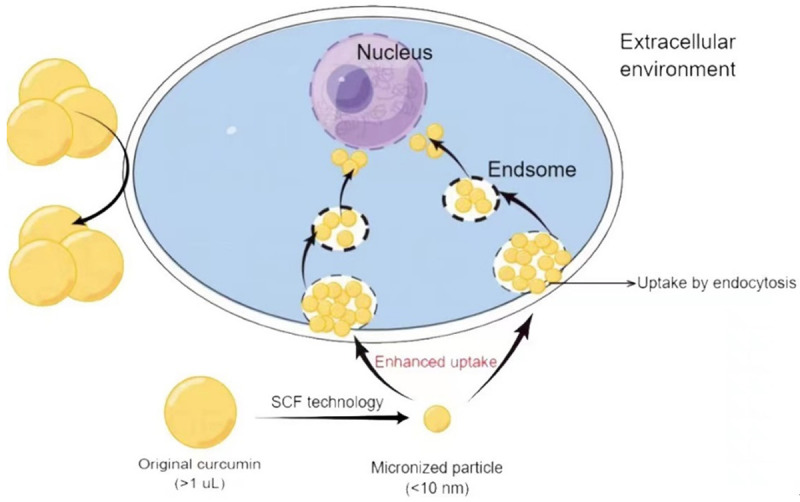
Supercritical fluid (SCF) technology facilitates drug entry into cells. Original curcumin (> 1 µL) cannot enter these cells. However, micronized particles can be taken up by endocytosis.
Nanotechnology research has also enabled the transport of drugs across the BTB. Nanoparticles (NPs), which have a particle size of 1-1,000 nm, have been widely used to transport a variety of therapeutic drugs, mainly anticancer drugs. Size control is the key to delivering drugs via NPs. NPs with a mean diameter of 60-70 nm will be excreted faster, whereas larger NPs with a mean diameter of 200 nm or greater may be sequestered by the liver or spleen. Therefore, NPs with a diameter of 70-200 nm are the most suitable for in vivo applications [66]. Traditional drug molecules are either attached to the surface of the NPs or wrapped in the core and delivered to the target organs to enhance efficacy. NPs typically consist of lipids, metals, polymers (e.g., chitosan), dendrimers, nanocrystals (e.g., semiconductor quantum dots), carbon nanotubes, mesoporous materials, or iron oxide-based magnetic nanomaterials. Of these substances, mesoporous materials are becoming the main carriers of drugs, including male contraceptives [67]. For example, mesoporous silica NPs (MSNs) are NPs 40-70 nm in diameter with ordered pores (called mesopores) of 3.8-6.1 nm, high specific surface area (700-1,100 m2/g), and large pore volumes (0.44-1.54 cm3/g) [68,69]. Amorphous nanosilica particles can be injected intravenously into mouse testes and have been demonstrated to accumulate in SCs and germ cells (e.g., spermatocytes) without causing testicular damage [70]. Additionally, mesopores can protect bioactive drugs against degradation by enzymes before reaching target cells and tissues because the inner surface of the mesopore cannot be accessed by enzymes in the systemic circulation or tissues (e.g., gut) [71]. Thus, drugs can be loaded into hexagonally arranged mesopores of magnetic MSNs for oral administration (Figure 5). They can also be specifically transported to the testicles using a magnetic field, such as by placing permanent neodymium magnets in men’s shorts (Figure 5) [72].
Figure 5.
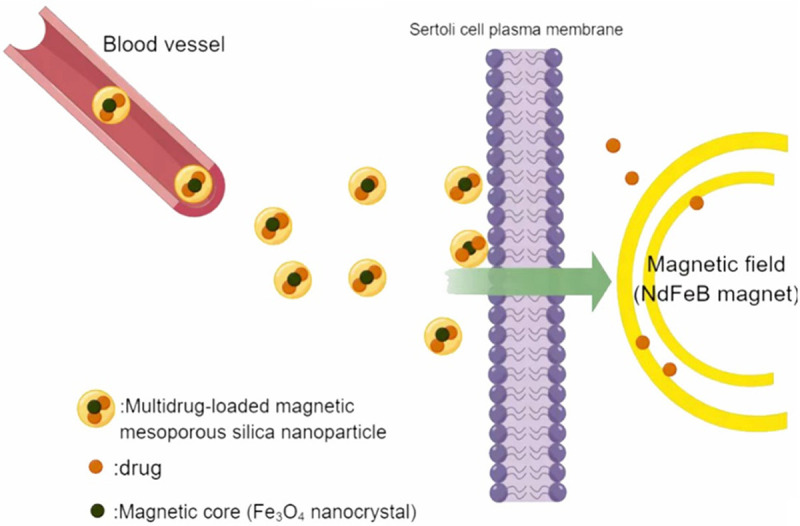
Magnetic mesoporous silica nanoparticles (MSNs) promote the entry of drug molecules into Sertoli cells. The targeted delivery of multidrug-loaded MSNs can be further improved by placing a permanent neodymium (NdFeB) magnet near the testes.
Physical delivery to the seminiferous epithelium
According to recent studies, scrotal heat stress (SHS) and pulsed unfocused ultrasound (PuFUS) can adjust the permeability of the BTB in adult mice. Mice were randomly divided into control, SHS, and PuFUS groups. Mice in the SHS group were subjected to SHS at different temperature levels (39°C, 41°C, and 43°C), and PuFUS was performed using a medical digital ultrasonic therapy instrument (ultrasonic operating frequency of 1 MHz, maximum effective sound intensity of 2.5 W/cm2, and effective area of 2 cm2) applied to the scrotal surface at different intensities (1.25 W/cm2, 1.75 W/cm2, and 2.5 W/cm2) and durations (2 min, 5 min, and 10 min). In mice in the SHS group, the BTB was effectively opened under SHS induction at 43°C for 30 min, whereas SHS induction for 30 min at 39°C or 41°C had little effect on the integrity of the BTB (Table 1). In the PuFUS group, BTB leakage levels at an intensity of 2.5 W/cm2 for 10 min were significantly higher than those in other groups (Table 2) [73]. Additionally, SHS treatment at 43°C for 120 h resulted in some biological macromolecules entering the seminiferous tubules, whereas PuFUS had no effect. Further observations of testicular tissue revealed that after SHS induction at 43°C, germ cells were shed, epithelium disappeared, and the testicular interstitium gradually thickened within 5-20 d. However, at 1.5 h, 2 d, and 7 d after the strongest PuFUS treatment (2.5 W/cm2 for 10 min), no obvious pathological changes were found in the testicular tissue [73].
Table 1.
The level of disruption of the blood-testis barrier after scrotal heat stress
| 6 h | 18 h | 48 h | 120 h | |
|---|---|---|---|---|
| 39°C | / | / | / | ++ |
| 41°C | / | / | / | ++ |
| 43°C | + | + | +++ | ++++ |
Negative: /; level of positive disruption: + < ++ < ++ < +++ < ++++.
Table 2.
The level of disruption of the blood-testis barrier after ultrasound treatment
| 2 min | 5 min | 10 min | |
|---|---|---|---|
| 1.25 W/cm2 | / | + | ++ |
| 2.5 W/cm2 | / | + | +++ |
Negative: /; level of positive disruption: + < ++ < ++ < +++ < ++++.
Normal spermatogenesis requires the testicular temperature to be lower than that of the body, which limits the application of SHS because SHS can damage testicular tissue; hence, PuFUS may be a novel approach to increase the permeability of the BTB to drugs. However, further research is needed to test these techniques and identify their potential applications in drug delivery.
Summary and prospects
Here, we summarized the physiological functions and regulation of the BTB and discussed the latest research on certain drugs crossing the BTB using specific approaches. Each method has unique mechanics and advantages.
However, there are still many challenges for drugs that must cross the BTB to elicit therapeutic function in the tissues. For example, when making the BTB leakier for drug delivery and improving drug utilization, we must also protect the integrity of the BTB. If there is irreversible damage, it can seriously affect male reproductive function. Future studies should compare and study biomolecules that can bypass the BTB and further determine whether there are specific chemical groups and characteristics that are essential to bypass the BTB. This will provide a foundation for the development of chemotherapeutics, antivirals, and male fertility drugs to reduce testicular tumor recurrence and viral infections in semen and improve male fertility. Moreover, we must consider whether men of different races and ages respond identically to the same therapeutic drug as well as control the cost of drugs, which are essential elements of the widespread use and promotion of therapeutics.
Additionally, the BTB shares features, such as structural proteins, with other blood-tissue barriers. Therefore, the drug-crossing barrier mechanisms discussed here may also play a role in the blood-brain barrier and other blood-tissue barriers.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Number: 81901532), the Natural Science Foundation of Jiangsu Province (Grant Number: BK20190188), the Gusu Health Talent Program of Suzhou (Grant Number: GSWS2020068), the Suzhou Science and Technology Development Plan (Grant Numbers: SS202060 and SZM2021010), and the Introduce Project of Clinical Medicine Experts of Suzhou Industrial Park (Grant Number: SZYQTD202104).
Disclosure of conflict of interest
None.
References
- 1.Mao B, Bu T, Mruk D, Li C, Sun F, Cheng CY. Modulating the blood-testis barrier towards increasing drug delivery. Trends Pharmacol Sci. 2020;41:690–700. doi: 10.1016/j.tips.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B. Multidrug resistance genes and P-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- 4.Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci U S A. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenabian MA, Costiniuk CT, Mehraj V, Ghazawi FM, Fromentin R, Brousseau J, Brassard P, Belanger M, Ancuta P, Bendayan R, Chomont N, Routy JP Orchid study group. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS. 2016;30:2777–2786. doi: 10.1097/QAD.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 9.Lie PP, Cheng CY, Mruk DD. The biology of the desmosome-like junction a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 11.Collins C, Nelson WJ. Running with neighbors: coordinating cell migration and cell-cell adhesion. Curr Opin Cell Biol. 2015;36:62–70. doi: 10.1016/j.ceb.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Robillard KR, Hoque MT, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2012;340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 14.Robillard KR, Chan GN, Zhang G, la Porte C, Cameron W, Bendayan R. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother. 2014;58:1713–1722. doi: 10.1128/AAC.02031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur G, Thompson LA, Dufour JM. Sertoli cells--immunological sentinels of spermatogenesis. Semin Cell Dev Biol. 2014;30:36–44. doi: 10.1016/j.semcdb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur G, Long CR, Dufour JM. Genetically engineered immune privileged Sertoli cells: a new road to cell based gene therapy. Spermatogenesis. 2012;2:23–31. doi: 10.4161/spmg.19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franca LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology. 2016;4:189–212. doi: 10.1111/andr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright K, Dziuk R, Mital P, Kaur G, Dufour JM. Xenotransplanted pig sertoli cells inhibit both the alternative and classical pathways of complement-mediated cell lysis while pig islets are killed. Cell Transplant. 2016;25:2027–2040. doi: 10.3727/096368916X692032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Deng T, Han D. Testicular immunoregulation and spermatogenesis. Semin Cell Dev Biol. 2016;59:157–165. doi: 10.1016/j.semcdb.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Gong D, Zhang C, Li T, Zhang J, Zhang N, Tao Z, Zhu W, Sun X. Are Sertoli cells a kind of mesenchymal stem cells? Am J Transl Res. 2017;9:1067–1074. [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Hoque MT, Jenabian MA, Vyboh K, Whyte SK, Sheehan NL, Brassard P, Bélanger M, Chomont N, Fletcher CV, Routy JP, Bendayan R. Antiretroviral drug transporters and metabolic enzymes in human testicular tissue: potential contribution to HIV-1 sanctuary site. J Antimicrob Chemother. 2016;71:1954–1965. doi: 10.1093/jac/dkw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian X, Cheng YH, Mruk DD, Cheng CY. Breast cancer resistance protein (Bcrp) and the testis-an unexpected turn of events. Asian J Androl. 2013;15:455–460. doi: 10.1038/aja.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darcis G, Coombs RW, Van Lint C. Exploring the anatomical HIV reservoirs: role of the testicular tissue. AIDS. 2016;30:2891–2893. doi: 10.1097/QAD.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombs RW, Lockhart D, Ross SO, Deutsch L, Dragavon J, Diem K, Hooton TM, Collier AC, Corey L, Krieger JN. Lower genitourinary tract sources of seminal HIV. J Acquir Immune Defic Syndr. 2006;41:430–438. doi: 10.1097/01.qai.0000209895.82255.08. [DOI] [PubMed] [Google Scholar]
- 26.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–263. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. doi: 10.4161/21565562.2014.979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieczynski J, Margolis B. Protein complexes that control renal epithelial polarity. Am J Physiol Renal Physiol. 2011;300:F589–601. doi: 10.1152/ajprenal.00615.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenbaum MP, Iwamori T, Buchold GM, Matzuk MM. Germ cell intercellular bridges. Cold Spring Harb Perspect Biol. 2011;3:a005850. doi: 10.1101/cshperspect.a005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 4: intercellular bridges, mitochondria, nuclear envelope, apoptosis, ubiquitination, membrane/voltage-gated channels, methylation/acetylation, and transcription factors. Microsc Res Tech. 2010;73:364–408. doi: 10.1002/jemt.20785. [DOI] [PubMed] [Google Scholar]
- 31.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su L, Mruk DD, Lie PP, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun. 2012;3:1185. doi: 10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y, Mruk DD, Lui WY, Lee WM, Cheng CY. F5-peptide induces aspermatogenesis by disrupting organization of actin- and microtubule-based cytoskeletons in the testis. Oncotarget. 2016;7:64203–64220. doi: 10.18632/oncotarget.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong EW, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis. 2013;3:e25465. doi: 10.4161/spmg.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Mruk DD, Lee WM, Cheng CY. Regulation of spermatogenesis by a local functional axis in the testis: role of the basement membrane-derived noncollagenous 1 domain peptide. FASEB J. 2017;31:3587–3607. doi: 10.1096/fj.201700052R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 39.Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 40.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Condon KJ, Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci. 2019;132:jcs222570. doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyuhas O. Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol. 2015;320:41–73. doi: 10.1016/bs.ircmb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Su W, Cheng CY. Cdc42 is involved in NC1 peptide-regulated BTB dynamics through actin and microtubule cytoskeletal reorganization. FASEB J. 2019;33:14461–14478. doi: 10.1096/fj.201900991R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, Mruk D, Chen H, Lui WY, Lee WM, Cheng CY. Regulation of the blood-testis barrier by a local axis in the testis: role of laminin α2 in the basement membrane. FASEB J. 2017;31:584–597. doi: 10.1096/fj.201600870R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao B, Li L, Yan M, Wong CKC, Silvestrini B, Li C, Ge R, Lian Q, Cheng CY. F5-peptide and mTORC1/rpS6 effectively enhance BTB transport function in the testis-lesson from the adjudin model. Endocrinology. 2019;160:1832–1853. doi: 10.1210/en.2019-00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lie PP, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci U S A. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen Q, Tang EI, Gao Y, Jesus TT, Chu DS, Lee WM, Wong CKC, Liu YX, Xiao X, Silvestrini B, Cheng CY. Signaling pathways regulating blood-tissue barriers - Lesson from the testis. Biochim Biophys Acta Biomembr. 2018;1860:141–153. doi: 10.1016/j.bbamem.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier, and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein DM, Evans KK, Hardwick RN, Dantzler WH, Wright SH, Cherrington NJ. Basolateral uptake of nucleosides by Sertoli cells is mediated primarily by equilibrative nucleoside transporter 1. J Pharmacol Exp Ther. 2013;346:121–129. doi: 10.1124/jpet.113.203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein DM, Wright SH, Cherrington NJ. Localization of multidrug resistance-associated proteins along the blood-testis barrier in rat, macaque, and human testis. Drug Metab Dispos. 2014;42:89–93. doi: 10.1124/dmd.113.054577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su L, Mruk DD, Lee WM, Cheng CY. Drug transporters and blood--testis barrier function. J Endocrinol. 2011;209:337–351. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, Hoque MT, Jenabian MA, Vyboh K, Whyte SK, Sheehan NL, Brassard P, Belanger M, Chomont N, Fletcher CV, Routy JP, Bendayan R. Antiretroviral drug transporters and metabolic enzymes in human testicular tissue: potential contribution to HIV-1 sanctuary site. J Antimicrob Chemother. 2016;71:1954–1965. doi: 10.1093/jac/dkw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fietz D, Bakhaus K, Wapelhorst B, Grosser G, Günther S, Alber J, Döring B, Kliesch S, Weidner W, Galuska CE, Hartmann MF, Wudy SA, Bergmann M, Geyer J. Membrane transporters for sulfated steroids in the human testis--cellular localization, expression pattern and functional analysis. PLoS One. 2013;8:e62638. doi: 10.1371/journal.pone.0062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein DM, Cherrington NJ. Organic and inorganic transporters of the testis: a review. Spermatogenesis. 2014;4:e979653. doi: 10.4161/21565562.2014.979653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su L, Mruk DD, Cheng CY. Regulation of drug transporters in the testis by environmental toxicant cadmium, steroids and cytokines. Spermatogenesis. 2012;2:285–293. doi: 10.4161/spmg.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM, Nguyen AT, Ozaki E, Keaney J, Blau CW, Kerskens CM, Cahalan SD, Callanan JJ, Wallace E, Grant GA, Doherty CP, Humphries P. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun. 2012;3:849. doi: 10.1038/ncomms1852. [DOI] [PubMed] [Google Scholar]
- 60.Chung NP, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 61.Zwanziger D, Hackel D, Staat C, Böcker A, Brack A, Beyermann M, Rittner H, Blasig IE. A peptidomimetic tight junction modulator to improve regional analgesia. Mol Pharm. 2012;9:1785–1794. doi: 10.1021/mp3000937. [DOI] [PubMed] [Google Scholar]
- 62.Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O’Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, Humphries P. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- 63.Deshpande PB, Kumar GA, Kumar AR, Shavi GV, Karthik A, Reddy MS, Udupa N. Supercritical fluid technology: concepts and pharmaceutical applications. PDA J Pharm Sci Technol. 2011;65:333–344. doi: 10.5731/pdajpst.2011.00717. [DOI] [PubMed] [Google Scholar]
- 64.Martín A, Cocero MJ. Micronization processes with supercritical fluids: fundamentals and mechanisms. Adv Drug Deliv Rev. 2008;60:339–350. doi: 10.1016/j.addr.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 65.Karn PR, Cho W, Hwang SJ. Liposomal drug products and recent advances in the synthesis of supercritical fluid-mediated liposomes. Nanomedicine (Lond) 2013;8:1529–1548. doi: 10.2217/nnm.13.131. [DOI] [PubMed] [Google Scholar]
- 66.Cai Q, Wang L, Deng G, Liu J, Chen Q, Chen Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am J Transl Res. 2016;8:749–764. [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang W, Chen L. In light-sensitive drug delivery system nanoparticles mediate oxidative stress. Am J Transl Res. 2020;12:1469–1480. [PMC free article] [PubMed] [Google Scholar]
- 68.Roggers R, Kanvinde S, Boonsith S, Oupický D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS PharmSciTech. 2014;15:1163–1171. doi: 10.1208/s12249-014-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Chen H, Shi J. Drug delivery/imaging multifunctionality of mesoporous silica-based composite nanostructures. Expert Opin Drug Deliv. 2014;11:917–930. doi: 10.1517/17425247.2014.908181. [DOI] [PubMed] [Google Scholar]
- 70.Morishita Y, Yoshioka Y, Satoh H, Nojiri N, Nagano K, Abe Y, Kamada H, Tsunoda S, Nabeshi H, Yoshikawa T, Tsutsumi Y. Distribution and histologic effects of intravenously administered amorphous nanosilica particles in the testes of mice. Biochem Biophys Res Commun. 2012;420:297–301. doi: 10.1016/j.bbrc.2012.02.153. [DOI] [PubMed] [Google Scholar]
- 71.Gao F, Botella P, Corma A, Blesa J, Dong L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J Phys Chem B. 2009;113:1796–1804. doi: 10.1021/jp807956r. [DOI] [PubMed] [Google Scholar]
- 72.Chen H, Mruk DD, Xia W, Bonanomi M, Silvestrini B, Cheng CY. Effective delivery of male contraceptives behind the blood-testis barrier (BTB) - lesson from adjudin. Curr Med Chem. 2016;23:701–713. doi: 10.2174/0929867323666160112122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Zafar MI, Wang X, Ding X, Li H. Heat stress and pulsed unfocused ultrasound: the viability of these physical approaches for drug delivery into testicular seminiferous tubules. Curr Drug Deliv. 2020;17:438–446. doi: 10.2174/1567201817666200514080811. [DOI] [PubMed] [Google Scholar]


