Abstract
Objective: To evaluate the efficacy of rituximab combined with GDP regimen (gemcitabine + cisplatin + dexamethasone) in the treatment of non-Hodgkin lymphoma and its impact on the immune function of patients. Methods: Clinical data of 88 patients with non-Hodgkin lymphoma (NHL) treated in Affiliated Hospital of Yan’an University from February 2017 to February 2019 were analyzed retrospectively. Among them, 40 patients treated with the second-line regimen (gemcitabine + cisplatin + dexamethasone) were served as the control group, and 48 patients received additional rituximab were as the observation group. The therapeutic effect, incidence of adverse reactions, levels of complement (C3, C4) and immunoglobulin [immunoglobulin (Ig) G, IgM, IgA] before and after treatment were compared between the two groups. Cox regression analysis was used to analyze the prognostic factors of patients. Results: The total response rate of patients in observation group was higher than that in the control group (P<0.05). There was no significant difference in the incidence of adverse reactions (hair loss, nausea and vomiting, thrombocytopenia, anemia and bone marrow suppression) between the two groups (P>0.05). After treatment, the levels of C3 and C4 in both groups were lower than those before treatment, and the decrease in observation group were more evident than that in control group (P<0.05). No notable fluctuation was observed in the levels of IgG, IgM and IgA in both groups between before and after treatment (P>0.05). Cox regression analysis found that Ann Arbor stage and pretreatment disease status were the factors affecting the prognosis of patients. Conclusion: Rituximab combined with GDP regimen has a significant effect on the treatment of non-Hodgkin lymphoma, and Ann Arbor stage and pretreatment disease state are prognostic factors for patients with NHL.
Keywords: Rituximab, gemcitabine, cisplatin, dexamethasone, non-Hodgkin’s lymphoma, immune function
Introduction
Non-Hodgkin lymphoma (NHL) is a type of clinical malignant tumor originating from the malignant transformation of various cells in the human immune system [1]. Statistics showed that [2] approximately 510,000 people were diagnosed with NHL worldwide in 2018 (from NHL’s world epidemiological data), and about 250,000 people died from the disease. Although the incidence of NHL is relatively low, the poor prognosis still leads to high mortality and needs therapeutic improvement [3]. NHLs can be divided into B cell, T cell and natural killer cell lymphomas [4]. B-cell NHL (B-NHL) is clinically the most common one, accounting for 70-85% of all NHLs. It can be further divided into diffuse large B-cell lymphoma, follicular lymphoma and mantle cell lymphoma according to the pathological type [5]. The pathogenesis of NHL is currently mainly considered to be related to factors such as human immunodeficiency and exposure to radioactive substances [6]. With the advent of anti-CD20 monoclonal antibodies, the combined application of anti-CD20 monoclonal antibodies and traditional chemotherapy drugs has greatly improved the survival rate of patients with B-NHL, but the cytotoxicity caused by chemotherapy is also very common, and there are still some patients with primary resistance to immunochemotherapy or experiencing relapse after treatment [7]. Therefore, it is an urgent task to find a high-efficiency and low-toxicity treatment strategy to prolong the survival period of patients and improve the life quality of patients.
At the current stage, many clinical treatment methods are available for NHL, including first-line treatment, second-line treatment [8] and surgical treatment, among which the former two are more commonly applied [9]. Studies have shown that patients with NHL are often in poor physical status, and treatment regimens inevitably bring strong side effects that negatively affect the long-term survival rate and life quality of patients [10]. Prolonged chemotherapy leads to an accumulation of drug toxicity, resulting in unsatisfactory physical condition that makes it challenging for intensive chemotherapy and hematopoietic stem cell transplantation [11]. Rituximab is currently the most widely used anti-CD20 monoclonal antibody, and is applied in B-NHL treatment by targeting the CD20 antigen on B lymphocytes [12]. However, few reports have focused on the combined efficacy of rituximab and GDP regimen in the treatment of B-NHL.
In this study, we aimed to analyze the efficacy of rituximab combined with GDP regimen in the treatment of NHL and their impact on the immune function of patients, and to find the influencing factors that affect the prognosis of patients, so as to provide reference for clinical treatment plans.
Materials and methods
Clinical information
A retrospective analysis was conducted on 88 patients with NHL admitted to Affiliated Hospital of Yan’an University from February 2017 to February 2019. Among them, 40 patients treated with the second-line regimen (gemcitabine + cisplatin + dexamethasone) were served as the control group, and 48 patients received additional rituximab were as the observation group. This study was approved by the Medical Ethics Committee of Affiliated Hospital of Yan’an University (approval number: 2020LL0335).
Inclusion and exclusion criteria
Inclusion criteria: Patients who met the World Health Organization (WHO) criteria for pathology and genetics of hematopoietic and lymphohistiomas [13]; patients who were confirmed to have B-NHL by lymph node biopsy or surgical histopathology; patients with an expected survival time over 3 months; patients with complete clinical data; patients gave consent to the participation of present study. All patients were initially treated B-NHL patients.
Exclusion criteria: Patients with severe liver, kidney or cardiac insufficiency; patients with acute infection or other malignant tumors; patients with central nervous system involvement or immune system diseases; patients with intolerance to the experimental drugs; patients with systemic diseases; patients who were pregnant or breastfeeding.
Therapeutic plans
Patients in the control group were treated with the second-line regimen as follows: Gemcitabine (Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd., H20093404, specification: 1.0 g) 1000 mg/(m2·d), intravenous drip on the 1st and 8th days; cisplatin (Qilu Pharmaceutical, H20023461, specification: 20 mg) 25 mg/(m2·d), intravenous drip on the 1st and 8th days; Dexamethasone (Chenxin Pharmaceutical Co., Ltd., H37021969, specification: 5 mg) 20 mg~40mg/(m2·d), intravenous drip on the 1st and 8th days. On the basis of treatment in control group, patients in the observation group were offered additional rituximab (Shanghai Fuhong Henlius Biopharmaceutical, S20190021, specification: 100 mg) 375 mg/(m2·d), intravenous drip on the first day. All patients were treated consecutively for 6 weeks.
Fluid complement and immunoglobulin testing
Complement 3 (C3, National machinery injection 20162405278), complement 4 (C4, National machinery injection 20162405275), immunoglobulin G (IgG, National machinery injection 20192400291), immunoglobulin A (IgA, National machinery injection 20182402399), immunoglobulin M (IgM, National machinery injection 20182402331) were detected using Siemens automatic analyzer (Atellica® SI automatic chemiluminescence immunoassay analyzer). Detection kits were provided by Siemens Healthcare Diagnostics Inc.
Observation Indicators
Main outcome measures: The clinical efficacy of two groups after treatment was classified as follows. It was seen as complete remission (CR) if the tumor lesions disappeared, the long axis of the lymph nodes was reduced to less than 10 mm, the results of fine needle aspiration were negative or no palpable lymph nodes were found, and the results of histological and morphological examinations of the bone marrow were normal. It was seen as partial remission (PR) if the sum of the diameters of the target lesions decreased by at least 30% compared with the baseline level. It was seen as progressive disease (PD) if the sum of the diameters of the target lesions increased by over 20%, and its absolute value increased by 5 mm, or new lesions appeared. It was seen as stable disease (SD) if the extent of target lesion was intermediate between PR and PD. Objective response rate (ORR): (CR + PR) cases/total cases × 100%. Cox regression analysis was used to analyze the influencing factors of patients’ prognosis.
Secondary outcome measures: Clinical data were compared between two groups, and levels of C3, C4, IgG, IgA and IgM were compared between two groups before and after treatment. According to the acute and subacute toxicity classification standards of anticancer drugs from WHO, the disease was divided into grade 0-IV, and the adverse reactions during the treatment were recorded.
Statistical analysis
SPSS 20.00 software was used to analyze the data, and GraphPad Prism 8 software was to visualize figures. The enumeration data were expressed as percentage (%) and compared by Chi-square test. The measurement data were expressed as the mean ± standard deviation, and inter-group and intra-group comparisons were conducted with the use of independent sample t test and paired t test, respectively. Rank data were tested by value sum test, denoted by Z. Kaplan-Meier was used in survival analysis to draw the survival curve, calculate the survival rate and perform the Log-rank test. Cox regression analysis was to analyze the independent prognostic factors affecting patients. The statistical significance level was set at P<0.05.
Results
Comparison of baseline data
Subjects were comparable between two groups due to insignificant differences observed regarding age, sex, Ann Arbor stage, B symptoms, pathological type, pretreatment disease state and lactate dehydrogenase level (P>0.05, Table 1).
Table 1.
Comparison of baseline data
| Factors | Control group (n=40) | Observation group (n=48) | χ2/Z | P |
|---|---|---|---|---|
| Age | ||||
| ≥60 years old | 24 | 20 | 2.933 | 0.086 |
| <60 years old | 16 | 28 | ||
| Sex | ||||
| Male | 22 | 28 | 0.098 | 0.753 |
| Female | 18 | 20 | ||
| Ann Arbor Stage | ||||
| Stage II | 16 | 22 | -0.180 | 0.858 |
| Stage III | 12 | 10 | ||
| Stage IV | 12 | 16 | ||
| B Symptoms | ||||
| Existed | 7 | 10 | 0.155 | 0.693 |
| Non-existed | 33 | 38 | ||
| Pathological type | ||||
| Follicular Lymphoma | 28 | 33 | 0.016 | 0.899 |
| Other | 12 | 15 | ||
| Pretreatment disease state | ||||
| Initial Treatment | 20 | 20 | 0.611 | 0.434 |
| relapsed/refractory | 20 | 28 | ||
| LDH Level | ||||
| Normal | 7 | 5 | 0.929 | 0.335 |
| Above Normal | 33 | 43 |
Lactate dehydrogenase (LDH).
Comparison of clinical efficacy
Comparing the clinical efficacy of two groups, it was found that the observation group held a higher ORR (better clinical efficacy) than that of the control group (P<0.05, Table 2).
Table 2.
Comparison of clinical efficacy
| Items | CR | PR | PD | SD | ORR |
|---|---|---|---|---|---|
| Control group (n=40) | 8 (20.00) | 15 (37.50) | 8 (20.00) | 9 (22.50) | 23 (57.50) |
| Observation group (n=48) | 12 (25.00) | 26 (54.17) | 8 (16.67) | 2 (4.17) | 38 (79.17) |
| χ2/Z | -1.984 | 4.816 | |||
| P | 0.047 | 0.028 | |||
Note: complete remission (CR); partial remission (PR); progressive disease (PD); stable disease (SD); objective response rate (ORR).
Changes of C3 and C4 before and after treatment
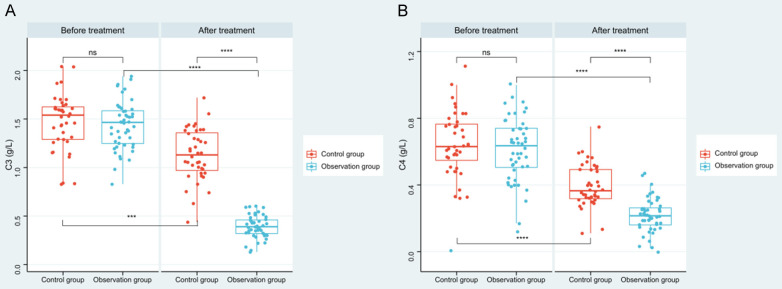
After treatment, levels of serum C3 and C4 in both groups went evidently lower than those before treatment (P<0.001), and the decrease in the observation group were more obvious when compared with that of the control group (P<0.001, Figure 1).
Figure 1.
Changes of C3 and C4 in patients before and after treatment. A. Comparison of changes in C3 levels before and after treatment between the two groups. B. Comparison of changes of C4 levels before and after treatment between the two groups. Note: ns indicates P>0.05; *** indicates P<0.001; **** indicates P<0.0001. complement 3 (C3), complement 4 (C4).
Changes in immunoglobulin before and after treatment
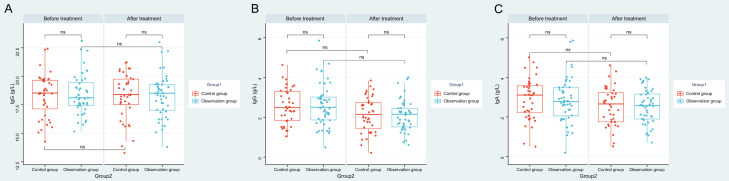
After treatment, there was no obvious fluctuation in terms of serum IgG, IgM and IgA levels in both groups (P>0.05), and levels of those three were roughly the same in observation group as those in control group (P>0.05, Figure 2).
Figure 2.
Changes of immunoglobulins in patients before and after treatment. A. Comparison of IgG levels before and after treatment between the two groups. B. Comparison of IgM levels before and after treatment between the two groups. C. Comparison of IgA levels before and after treatment between the two groups. Note: ns indicates P>0.05; *** indicates P<0.001; **** indicates P<0.0001. immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM).
Comparison of adverse reactions
There was no statistical difference in the incidence of adverse reactions between two groups (P>0.05, Tables 3, 4 and 5).
Table 3.
Adverse reactions in the control group
| Adverse reactions | Grading | ||||
|---|---|---|---|---|---|
|
| |||||
| Total Score | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Decreased White Blood Cell Count | 34 | 8 | 19 | 7 | 0 |
| Decreased Neutrophil Count | 32 | 9 | 10 | 12 | 1 |
| Decreased Platelet Count | 14 | 6 | 4 | 3 | 1 |
| Anemia | 19 | 8 | 7 | 4 | 0 |
| Increased Alt | 26 | 13 | 11 | 1 | 1 |
| Increased Ast | 19 | 8 | 9 | 1 | 1 |
| Increased Ggt | 13 | 6 | 3 | 1 | 3 |
| Increased Blood Bilirubin | 13 | 5 | 6 | 2 | 0 |
| Increased Creatinine | 5 | 2 | 2 | 1 | 0 |
| Hyperuricemia | 11 | 6 | 5 | 0 | 0 |
| Upper Respiratory Tract Infection | 8 | 4 | 0 | 3 | 1 |
| Lung Infection | 9 | 5 | 0 | 2 | 2 |
| Agranulocytosis with Fever | 4 | 2 | 0 | 1 | 1 |
| Catheter-Related Infection | 2 | 1 | 0 | 1 | 0 |
| Itching | 6 | 0 | 3 | 3 | 0 |
| Feeling Abnormal | 1 | 1 | 0 | 0 | 0 |
| Fatigue | 11 | 7 | 3 | 1 | 0 |
| Diarrhea | 1 | 0 | 1 | 0 | 0 |
| Thromboembolic Events | 1 | 0 | 1 | 0 | 0 |
Table 4.
Adverse reactions of patients in the observation group
| Adverse reactions | Grading | ||||
|---|---|---|---|---|---|
|
| |||||
| Total Score | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Decreased White Blood Cell Count | 34 | 10 | 18 | 6 | 0 |
| Decreased Neutrophil Count | 39 | 12 | 15 | 10 | 2 |
| Decreased Platelet Count | 15 | 7 | 3 | 2 | 3 |
| Anemia | 22 | 10 | 8 | 3 | 1 |
| Increased Alt | 29 | 12 | 15 | 2 | 0 |
| Increased Ast | 19 | 7 | 10 | 2 | 0 |
| Increased Ggt | 11 | 6 | 2 | 1 | 2 |
| Increased Blood Bilirubin | 14 | 5 | 7 | 1 | 1 |
| Increased Creatinine | 4 | 2 | 1 | 1 | 0 |
| Hyperuricemia | 12 | 7 | 4 | 1 | 0 |
| Upper Respiratory Tract Infection | 7 | 3 | 2 | 1 | 1 |
| Lung Infection | 8 | 5 | 0 | 2 | 1 |
| Agranulocytosis with Fever | 4 | 2 | 1 | 0 | 1 |
| Catheter-Related Infection | 2 | 0 | 1 | 1 | 0 |
| Itching | 7 | 0 | 3 | 3 | 1 |
| Feeling Abnormal | 1 | 0 | 1 | 0 | 0 |
| Fatigue | 12 | 8 | 3 | 1 | 0 |
| Diarrhea | 1 | 0 | 0 | 1 | 0 |
| Thromboembolic Events | 1 | 0 | 0 | 1 | 0 |
Table 5.
Comparison of adverse reactions between the two groups
| Adverse reactions | Control group (n=40) | Observation group (n=48) | χ2 value | P value |
|---|---|---|---|---|
| Decreased White Blood Cell Count | 34 | 34 | 1.589 | 0.207 |
| Decreased Neutrophil Count | 32 | 39 | 0.021 | 0.882 |
| Decreased Platelet Count | 14 | 15 | 0.138 | 0.709 |
| Anemia | 19 | 22 | 0.024 | 0.876 |
| Increased Alt | 26 | 29 | 0.195 | 0.658 |
| Increased Ast | 19 | 19 | 0.557 | 0.455 |
| Increased Ggt | 13 | 11 | 1.010 | 0.314 |
| Increased Blood Bilirubin | 13 | 14 | 0.114 | 0.735 |
| Increased Creatinine | 5 | 4 | 0.412 | 0.520 |
| Hyperuricemia | 11 | 12 | 0.070 | 0.790 |
| Upper Respiratory Tract Infection | 8 | 7 | 0.452 | 0.501 |
| Lung Infection | 9 | 8 | 0.476 | 0.490 |
| Agranulocytosis with Fever | 4 | 4 | 0.073 | 0.786 |
| Catheter-Related Infection | 2 | 2 | 0.034 | 0.851 |
| Itching | 6 | 7 | 0.003 | 0.956 |
| Feeling Abnormal | 1 | 1 | 0.017 | 0.896 |
| Fatigue | 11 | 12 | 0.070 | 0.790 |
| Diarrhea | 1 | 1 | 0.017 | 0.896 |
| Thromboembolic Events | 1 | 1 | 0.017 | 0.896 |
| Total incidence | 229 (30.13) | 242 (26.53) | 2.650 | 0.103 |
Effect of different treatment regimens on patient survival
We followed up patients for 3 years and compared the 3-year survival of two groups of patients, and found that there was no statistical difference in the survival rate between the two groups (P=0.353, Figure 3).
Figure 3.
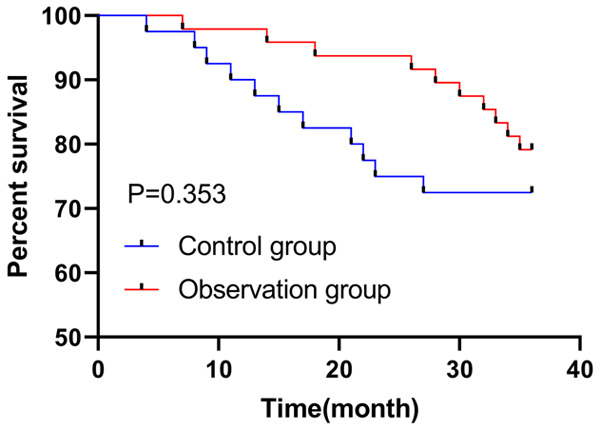
Effect of different treatment options on the 3-year survival of patients.
Prognostic analysis
Cox regression was applied to analyze the clinical factors affecting the prognosis of patients. In univariate Cox regression, it was found that Ann Arbor stage (P<0.001, HR: 2.879, 95% CI: 1.602-5.174) and pretreatment disease status (P=0.005, HR: 5.755, 95% CI: 1.694-19.556) were factors affecting the prognosis of patients. Further multivariate Cox regression analysis also indicated that Ann Arbor stage (P<0.001, HR: 1.630, 95% CI: 1.630-5.320) and pretreatment disease status (P=0.004, HR: 1.739, 95% CI: 1.739-20.134) were independent factors affecting the prognosis of patients (P<0.05, Table 6).
Table 6.
Cox regression analysis
| Factors | Univariate Cox | Multifactor Cox | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P | HR | 95% CI | P | HR | 95% CI | |
| Age | 0.774 | 1.134 | 0.481~2.670 | |||
| Sex | 0.610 | 1.258 | 0.521~3.035 | |||
| Ann Arbor stage | <0.001 | 2.879 | 1.602~5.174 | <0.001 | 1.630 | 1.630~5.320 |
| B Symptoms | 0.940 | 0.959 | 0.323~2.852 | |||
| Pathological Type | 0.776 | 0.877 | 0.354~2.173 | |||
| Pretreatment Disease State | 0.005 | 5.755 | 1.694~19.556 | 0.004 | 1.739 | 1.739~20.134 |
| Ldh Levels | 0.227 | 3.447 | 0.463~25.691 | |||
| Treatment Plan | 0.357 | 1.496 | 0.635~3.524 | |||
Discussion
B-NHL, a common clinical type of NHL, is a malignant hematological disease with high incidence, strong invasiveness and poor clinical prognosis [14]. Chemotherapy is effective and essential for the treatment of patients with NHL, but there are still some refractory patients who cannot achieve ideal therapeutic effects after several courses of first-line chemotherapy [15].
Rituximab, an artificial chimeric anti-CD20 monoclonal antibody, can bind to the CD20 antigen on B lymphocytes and directly inhibit tumor cell growth or induce tumor cell apoptosis [16]. In recent years, studies have found that rituximab could bind to complement C1q to fix the surface of tumor cells surrounded by antibodies and complement proteins, mediate complement-dependent cytotoxicity, thereby increasing the sensitivity of tumor cells to chemotherapeutic drugs [17]. In this study, we observed the efficacy of the second-line regimen combined with rituximab in patients with B-NHL, and found that the overall clinical efficacy of the observation group was improved after treatment, with a markedly higher ORR than that of the control group, indicating that the second-line treatment regimen combined with rituximab could improve the efficacy of the patients. In the study of Wang et al. [18], it was found that the clinical ORR of patients with B-NHL after second-line treatment was 49.50%, which was relatively lower than what we have observed. However, the discrepancy might be due to different sample inclusion, for most samples they collected were all relapsed or refractory patients, and our sample included treatment-naive patients. But we did improve the ORR of patients with the combination therapy, and we believe that this is because rituximab can specifically bind to the CD20 antigen, kill lymphoma cells through antibody and complement-dependent cytotoxicity, and improve the therapeutic effect.
To confirm our hypothesis, we also examined the changes of serum C3 and C4 in patients. The body’s complement system is the first-line immune defense for the host against foreign pathogens. Complements are mainly synthesized and secreted by the liver and stay relatively stable in normal conditions, but when certain pathological changes occur in the liver, its level may fluctuate to varying degrees [19]. When the body is invaded by pathogens, the complement system can form a membrane attack complex by adhering to the surface of pathogens, which can promote the lysis of pathogen-infected cells, and meanwhile release anaphylatoxins to cause inflammatory response [20].
C3 and C4 have various functions such as sterilization, opsonization, immunity and inflammatory mediators. C3 is the core of the complement system and located in the center of the system, being able to reflect the potential complement activation [21]. In our study, it was found that levels of C3 and C4 of patients after treatment were decreased evidently, and the decline in the observation group was comparatively higher than that of the control group. This is mainly because C3 and C4 are involved in the physiological progression and activation of tumors, and when patient’s immune function decreases, the humoral immunity will correspondingly increase, resulting in an increase in the level of complements. Additionally, Rituximab injection normally acts in a complement-dependent manner, thereby reducing the complement level [22]. Interestingly, no difference was found in the expressions of immunoglobulins between two groups of patients in our results, suggesting that rituximab had a lesser effect on patient immune function. Not only that, we conducted adverse reactions analysis on patients and found that there was no difference regarding adverse reactions between the two groups, which indicated that rituximab would not increase the occurrence of adverse reactions in patients and was safe to a certain extent.
Finally, we analyzed and compared the 3-year prognosis of the patients and found no notable difference in terms of survival rate between the two groups. In the study of Morschhauser et al. [23], it was also found that no significant difference existed between combination therapy group (n=513) and monotherapy group (n=517) in term of the 3-year survival rate (78% vs. 77%), which suggested that the combination therapy had no effect on patient survival, but it improved patient outcomes in our study, without increasing adverse reactions. Furthermore, we indicated in Cox regression analysis that Ann Arbor stage and pretreatment disease status were prognostic factors affecting patient survival. The higher the Ann Arbor stage, the worse the prognosis, and the prognosis of newly treated patients was higher than that of relapsed or refractory patients, which is consistent with previous findings [24].
In this study, we determined the efficacy of rituximab combined with GDP regimen in the treatment of NHL and its impact on patients’ immune function. However, this study still has certain limitations. First, due to the limitation of time and research methods, we only obtained the results of 3-year survival of patients. Whether the combination therapy has an effect on the long-term survival of patients still needs to be further verified. Second, as a retrospective study, there may be some bias in the results of statistical analysis. In the future, we hope to perform prospective studies with long-term follow-up to refine our findings.
In conclusion, rituximab combined with GDP regimen had a significant effect on the treatment of NHL. This treatment could effectively delay the progression of the disease and ensure the safety of patients’ lives.
Disclosure of conflict of interest
None.
References
- 1.Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390:298–310. doi: 10.1016/S0140-6736(16)32407-2. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.McCarten KM, Nadel HR, Shulkin BL, Cho SY. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol. 2019;49:1545–1564. doi: 10.1007/s00247-019-04529-8. [DOI] [PubMed] [Google Scholar]
- 4.Bowzyk Al-Naeeb A, Ajithkumar T, Behan S, Hodson DJ. Non-Hodgkin lymphoma. BMJ. 2018;362:k3204. doi: 10.1136/bmj.k3204. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch BJ, Chang SJ, Le A. Non-Hodgkin lymphoma metabolism. Adv Exp Med Biol. 2018;1063:95–106. doi: 10.1007/978-3-319-77736-8_7. [DOI] [PubMed] [Google Scholar]
- 6.Tun AM, Ansell SM. Immunotherapy in Hodgkin and non-Hodgkin lymphoma: innate, adaptive and targeted immunological strategies. Cancer Treat Rev. 2020;88:102042. doi: 10.1016/j.ctrv.2020.102042. [DOI] [PubMed] [Google Scholar]
- 7.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, Lewis DJ, Sureda Balari A, Cunningham D, Oliveri RS, Elliott B, DeMarco D, Azaryan A, Chiu C, Li T, Chen KM, Ahmadi T, Lugtenburg PJ. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398:1157–1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 8.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM, Munoz J, Farooq U, van Meerten T, Reagan PM, Sureda A, Flinn IW, Vandenberghe P, Song KW, Dickinson M, Minnema MC, Riedell PA, Leslie LA, Chaganti S, Yang Y, Filosto S, Shah J, Schupp M, To C, Cheng P, Gordon LI, Westin JR All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene ciloleucel as second-line therapy for large B-Cell lymphoma. N Engl J Med. 2022;386:640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 9.Batgi H, Basci S, Dal MS, Kizil Cakar M, Uncu Ulu B, Yigenoglu TN, Ozcan N, Kilinc A, Merdin A, Yildiz J, Bakirtas M, Sahin D, Darcin T, Iskender D, Baysal NA, Altuntas F. Gemcitabine, dexamethasone and cisplatin (GDP) is an effective and well-tolerated mobilization regimen for relapsed and refractory lymphoma: a single center experience. Turk J Med Sci. 2021;51:685–692. doi: 10.3906/sag-2008-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Wang B, Tao W, Si Y, Lin G, Zhang Y, Liu R, Yuan W. Comparison of the efficacy and impact of GEMOX and GDP in the treatment of patients with non-Hodgkin’s lymphoma. J BUON. 2020;25:1042–1049. [PubMed] [Google Scholar]
- 11.Batgi H, Merdin A, Dal MS, Kizil Cakar M, Yildiz J, Basci S, Uncu Ulu B, Yigenoglu TN, Darcin T, Sahin D, Bakirtas M, Tetik A, Iskender D, Altuntas F. The effect of gemcitabine, dexamethasone, and cisplatin chemotherapy in relapsed/refractory NHL and HL patients: a single center experience. J Oncol Pharm Pract. 2020;26:1857–1863. doi: 10.1177/1078155220905654. [DOI] [PubMed] [Google Scholar]
- 12.Salles G, Barrett M, Foa R, Maurer J, O’Brien S, Valente N, Wenger M, Maloney DG. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34:2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreyling M, Ghielmini M, Rule S, Salles G, Ladetto M, Tonino SH, Herfarth K, Seymour JF, Jerkeman M ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:298–308. doi: 10.1016/j.annonc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Kesavan M, Eyre TA, Collins GP. Front-line treatment of high grade b cell non-Hodgkin lymphoma. Curr Hematol Malig Rep. 2019;14:207–218. doi: 10.1007/s11899-019-00518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novo M, Castellino A, Nicolosi M, Santambrogio E, Vassallo F, Chiappella A, Vitolo U. High-grade B-cell lymphoma: how to diagnose and treat. Expert Rev Hematol. 2019;12:497–506. doi: 10.1080/17474086.2019.1624157. [DOI] [PubMed] [Google Scholar]
- 16.Ineichen BV, Moridi T, Granberg T, Piehl F. Rituximab treatment for multiple sclerosis. Mult Scler. 2020;26:137–152. doi: 10.1177/1352458519858604. [DOI] [PubMed] [Google Scholar]
- 17.Bondza S, Marosan A, Kara S, Losing J, Peipp M, Nimmerjahn F, Buijs J, Lux A. Complement-dependent activity of CD20-specific IgG correlates with bivalent antigen binding and C1q binding strength. Front Immunol. 2020;11:609941. doi: 10.3389/fimmu.2020.609941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Bai Y. Effect of second-line chemotherapy in treating relapsed or refractory diffuse large B cell lymphoma and prognosis analysis. J BUON. 2020;25:2003–2010. [PubMed] [Google Scholar]
- 19.Zinellu A, Mangoni AA. Serum complement C3 and C4 and COVID-19 severity and mortality: a systematic review and meta-analysis with meta-regression. Front Immunol. 2021;12:696085. doi: 10.3389/fimmu.2021.696085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copenhaver M, Yu CY, Hoffman RP. Complement components, C3 and C4, and the metabolic syndrome. Curr Diabetes Rev. 2019;15:44–48. doi: 10.2174/1573399814666180417122030. [DOI] [PubMed] [Google Scholar]
- 21.Ling M, Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin Lab Med. 2019;39:579–590. doi: 10.1016/j.cll.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein A, Alexander RV, Zack DJ. A review of complement activation in SLE. Curr Rheumatol Rep. 2021;23:16. doi: 10.1007/s11926-021-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, Fruchart C, Libby EN, Casasnovas RO, Flinn IW, Haioun C, Maisonneuve H, Ysebaert L, Bartlett NL, Bouabdallah K, Brice P, Ribrag V, Daguindau N, Le Gouill S, Pica GM, Martin Garcia-Sancho A, Lopez-Guillermo A, Larouche JF, Ando K, Gomes da Silva M, Andre M, Zachee P, Sehn LH, Tobinai K, Cartron G, Liu D, Wang J, Xerri L, Salles GA RELEVANCE Trial Investigators. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934–947. doi: 10.1056/NEJMoa1805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadi I, Schummer A, Dreyling M, Eze C, Bodensohn R, Roengvoraphoj O, Belka C, Li M. Effectiveness and tolerability of radiotherapy for patients with indolent non-Hodgkin’s lymphoma: a monocenter analysis. Sci Rep. 2021;11:22586. doi: 10.1038/s41598-021-01851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]