Abstract
Objective: To explore the mechanism of immune regulation of Th17 in anti-NMDAR encephalitis disease. Methods: This is a retrospective study with 83 subjects included. All subjects were admitted to the Second Affiliated Hospital of Hainan Medical University from April 2018 to May 2021, including 35 patients with anti-NMDA encephalitis in an encephalitis group, and 48 patients with non-inflammatory central neuropathy in a control group. The cerebrospinal fluid (CSF) and blood samples were collected from two groups of patients, and the changes of Th17 cell, immunophenotypic characteristics, differentiation pathways and the effect were analyzed accordingly. Peripheral blood mononuclear cells (PBMCs) from patients with anti-NMDAR encephalitis were isolated and treated with different cytokines, namely IL-1β+IL-6 group, IL-1β+IL-23 group, IL-6+IL-23 group, IL-1β+IL-23+IL-6 group and TGF-β group. After co-culture, the proportion of Th17 cells and the expression level of Th17 cell-specific transcription factor RORγt mRNA were examined. Results: Th17 cells in CSF were dramatically uplifted in the encephalitis group. In terms of cell phenotype, the percentages of CD62L and CCR7 expressions in Th17 cell phenotype were significantly increased in the encephalitis group. IL-1β, IL-6, IL-7 and IL-23 mRNA in PBMCs and IL-1β, IL-6, IL-7 and IL-23 in serum were remarkably uplifted in the encephalitis group. The mRNA levels of Th17 and Th17 cell-specific transcription factor RORγ T were the highest in the IL-1β+IL-6+IL-23 group but the lowest in the TGF-β group. Th17 in CSF of patients with poor prognosis was notably higher than that of those with good prognosis. Conclusion: The proportion of Th17 cells in patients with NMDAR encephalitis and the expressions of corresponding differentiation promoting cytokines were increased, and Th17 is closely associated with patients’ treatment and prognosis. Th17 cells play crucial roles in tumorigenesis and progression of anti-NMDAR encephalitis.
Keywords: Th17 cell, NMDAR, autoimmune encephalitis, immune regulation, IL-17
Introduction
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a new type of anti-NMDAR-related autoimmune encephalitis [1]. Among the most prevalent encephalitis diseases, anti-NMDAR encephalitis has become the second most immune-mediated disease following the acute disseminated encephalomyelitis [2]. Clinical studies have shown that [3] patients received early immunosuppressive therapy and tumor resection have a good prognosis. However, it is not easy to clinically recognize encephalitis in an early stage, and there is still no “golden standard” for the diagnosis. On the contrary, it is quite difficult to make clinical diagnosis, and patients usually have long treatment cycle and high incidence of complications, which increases the possibility of long-term recurrence and a potential risk of death [4]. Therefore, early recognition and effective treatment are still important research topics in this filed.
Research has suggested that abundant immune cells are present in the cerebrospinal fluid (CSF) of patients with anti-NMDAR encephalitis, including tumor cells, lymphocytes, neutrophils and macrophages, etc. [5]. It is believed that the immune pathogenesis is due to increase of lymphocytes in CSF in the early stage, followed by the activation of autoimmune responses caused by inflammatory response of central nervous system, thereby activating B cells to produce antibodies. However, the data relating to T cells that are involved in the disease are limited. Recently, it was proposed that [6] T cells also participated in immune regulation mechanism of anti-NMDAR encephalitis. Th17 is a special type of CD4+ T cells which secrete IL-17. Th17 may be the primary cells involved in tissue inflammation and immune response, and they influence human immune diseases [7]. We previously detected Th17 cells in the celiac lymph and peripheral blood of patients with anti-NMDAR encephalitis through flow cytometry [8], and we speculated that Th17 might have a certain immunomodulatory effect on the occurrence and progression of NMDAR encephalitis. While the specific mechanism of Th17 antagonizing the immune regulation of NMDAR encephalitis has yet been revealed. To understand the disease progression and to provide corresponding basis for the early diagnosis and treatment, this study analyzed the immune regulatory mechanism of Th17 in anti-NMDAR encephalitis.
Materials and methods
Clinical data
This retrospective study included 83 subjects, and all of them were treated in the Second Affiliated Hospital of Hainan Medical University from April 2018 to May 2021, including 35 patients with anti-NMDA encephalitis in an encephalitis group and 48 patients with non-inflammatory neuropathy (25 patients with cerebrovascular disease and 23 patients with peripheral nerve disease) in a control group.
Inclusion criteria of the encephalitis group: (1) Patients met the diagnostic criteria for anti-NMDAR encephalitis (2016 edition) [9], with clinical manifestations and detection of anti-GluN1 antibodies in CSF. (2) Patients were 18 years old or above. (3) Patients signed the informed consent form of their own accord.
Inclusion criteria of control group: (1) Patients were detected negative for CSF specific antibodies. (2) Patients were 18 years old or above. (3) Patients signed the informed consent form of their own accord.
Exclusion criteria of the groups: (1) Patients underwent lumbar puncture or related treatment within 3 months prior to admission. (2) Patients had a drug history of glucocorticoids, non-steroidal anti-inflammatory drugs, or immunosuppressants. (3) Patients had other immunological disease(s).
All patients underwent lumbar puncture for CSF and blood samples, and those with anti-NMDAR encephalitis were screened for tumor at least once during hospitalization. Neurological function of patients was assessed by applying Modified Rankin Scale (mRS) at their initial admission and during follow-up at 3-6 months.
This research was carried out under the permit of Ethics Committee of The Second Affiliated Hospital of Hainan Medical University.
Differentiation by flow cytometry
Mononuclear cells were isolated from the CSF specimens of the two groups by Ficoll gradient centrifugation (Sigma Aldrich, USA, F5415). CD4+ T cells were obtained by metian Biotec magnetic bead sorting kit (Miltenyi Biotec, Germany), and the purity of the enriched CD4+ T cells was confirmed to be at least 95% by Flow cytometry (Figure 1). CD4+ T cells were inoculated with RPMI-1640 (Gbico Corporation, 12633012) medium containing 10% calf serum (Gbico Corporation, A3520502) (at a concentration of 1 × 106/mL) and cultured at 37°C and in 5% CO2. The coated anti-human CD3 antibody (2 μg/ml, eBioscience, USA), soluble anti-human CD28 mAb (1 μg/ml, eBioscience, USA), IL-6 (10 ng/ml, BD Company, USA), anti-IFNγ (1 μg/ml, American BD company), anti-IL-4 (1 μg/ml, American BD company), IL-17 (10 ng/ml, American eBioscience company) were added to the cells to stimulate CD4+ T cells for 5 d. Then, CD4+ T cells were stimulated with PMA (50 ng/ml, Sigma Company, USA), ionomycin (750 ng/ml, Sigma Company, USA) and Brefeldin A (10 mg/ml, BD Biosciences Company, USA) for 6 h. IL-17-secreting cells were separated from the above differentiated CD4+ T cells with the use of IL-17 enrichment assay kit (Miltenyi Biotec, Germany), and CD45RO (Abcam, Inc, ab210385), CD62L (Abcam, Inc, ab95140) and CCR7 (Abcam, Inc, ab155382) expressions in Th17 cells were detected by Flow cytometry.
Figure 1.
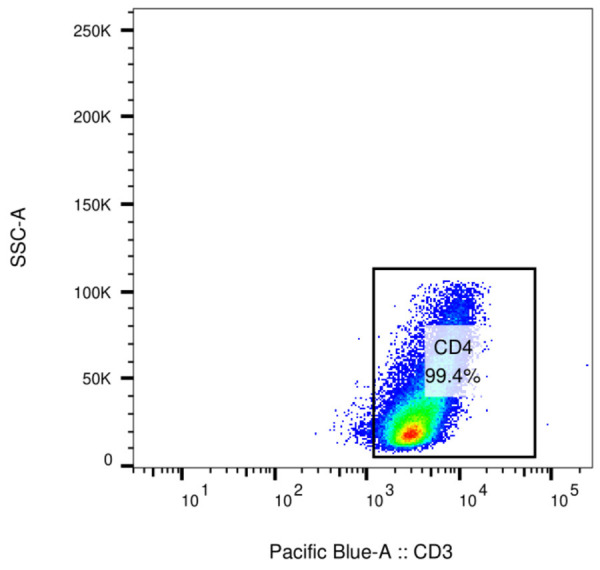
Detection of CD4+ cell purity by Flow cytometry.
Detection of cytokine expressions by RT-PCR
The patients’ peripheral blood mononuclear cells (PBMCs) were detached by Ficoll gradient centrifugation. The total RNA in PBMCs was extracted by Trizol RNA Extraction Reagent (Invitrogen company of the United States), and the purity was checked by ultraviolet spectrophotometer. TIANScript M-MLV Reverse Transcription Kit (Takara, Japan) was applied to synthesize the first strand of cDNA. Primers of IL-1β, IL-6, IL-7, IL-23, TGF-β and β-actin were designed and synthesized. SYBR Green kit (Takara Company, Japan) and Rotor-gene 3000 real-time PCR were used for detection. Rotor-gene real-time Analysis 6.0 and dual-standard curve based method were used for data analysis. The primer sequences are shown in Table 1. Amplification conditions were as follows, 94°C 60 s, 55°C 60 s, 72°C 60 s, with 35 cycles. The optical density is analyzed by an image analysis software of quantity one (bio rad company of the United States), and the mRNA expression level of the target gene was expressed by the optical density value of the target gene/optical density value of β-Actin.
Table 1.
Primer sequence
| Primer | sequence |
|---|---|
| IL-1β | forward primer 5’-ATGGCAGaAGTACCTAAGct-3’ |
| reverse primer 5’-TTAGGaAGacacaaATTGcatgg tGAactCAGT-3’ | |
| IL-6 | forward primer 5’-ATGAACTTCCTTCTCCACAAGC-3’ |
| reverse primer 5’-CTACATTTGCCGAAGAGCCCTCAGGCTGGACTG-3’ | |
| IL-7 | forward primer 5’-ATGCTTCCATGTTTCTTTTAGG-3’ |
| reverse primer 5’-AGCTTTTCTTTAGTGCCCATCAAAA TTTTATTCCAACA-3’ | |
| Il-23 | forward primer 5’-AGGGCACCCAGTCTGAGaACA-3’ |
| reverse primer 5’-CGGCCTTGCTCTTGTTTCAC-3’ | |
| TGF-β | forward primer 5’-ATGACACCACCTGAACGTCT CTTC-3’ |
| reverse primer 5’-CTACAGAGCGAAGGCTCCAAAGAAGACAGTACT-3’ | |
| β-actin | forward primer 5’-GTGGGGCGCCCCAGGCACA-3’ |
| reverse primer 5’-CTCCTTAATGTCACGcacga TTTC-3’ |
Cytokine expressions measured by ELISA
The peripheral blood was collected in both groups. The serum was separated, and the levels of IL-1β, IL-6, IL-17, IL-23 and TGF-β were measured by ELISA. All kits were purchased from R&D Company in the United States, and the operations were strictly in accordance with the instructions.
Cytokine and PBMC co-culture experiment
The patients’ PBMCs were isolated and counted. Then, 2 × 105 cells were added with IL-4 (10 μg/ml), IFN-γ (10 μG/ml) neutralizing antibodies and the following cytokine combinations, 1) IL-1β (10 ng/ml) + IL-6 (50 ng/ml), 2) IL-1β (10 ng/ml) + IL-23 (20 ng/ml), 3) IL-6 (50 ng/ml) + IL-23 (20 ng/ml), 4) IL-1β (10 ng/ml) + IL-23 (20 ng/ml) + IL-6 (50 ng/ml), 5) TGF-β (5 ng/ml), for 5 days of co-culture. The proportion of Th17 was detected by Flow cytometry, and the expression of Th17 cell-specific transcription factor RORγt mRNA was determined by real-time quantitative RT-PCR.
Statistical analysis
SPSS 26.0 was applied for data processing and analysis. The measurement data were represented by (x̅±s), and the enumeration data were expressed as percentages. For data analysis among multiple groups or multiple time points, the pairwise comparisons adopted SNK post hoc test after ANOVA. The measurement data between the two groups were compared by independent sample t-test, and the counting data were compared by chi-square test or Fisher’s precision probability test. P<0.05 indicated statistically significant.
Results
Clinical data
No statistically difference was found in sex, age, CSF-Glu and CSF chloride between the two groups (P>0.05), while CSF white blood cell count (WBC) and CSF protein in the encephalitis group exceeded those of the control group (P<0.05) (Table 2).
Table 2.
Comparison of clinical data between the two groups
| Clinical data | Encephalitis group (n=35) | Control group (n=48) | t/χ2 | P |
|---|---|---|---|---|
| Sex (n) | ||||
| Male | 19 | 27 | 0.032 | 0.859 |
| Female | 16 | 21 | ||
| Age (years old, x̅±s) | 31.2±7.1 | 32.5±9.2 | 0.636 | 0.527 |
| CSF-Glu (mmol/L, x̅±s) | 3.15±1.02 | 3.27±1.09 | 0.510 | 0.612 |
| CSF chloride (mmol/L, x̅±s) | 125.64±15.42 | 123.84±9.77 | 0.650 | 0.518 |
| CSF WBC count (/mm2) | 3.64±1.02 | 1.98±0.84 | 8.097 | 0.000 |
| CSF protein (mg/L) | 398.48±102.38 | 312.31±78.59 | 4.339 | 0.000 |
CSF: cerebrospinal fluid, WBC: white blood cell.
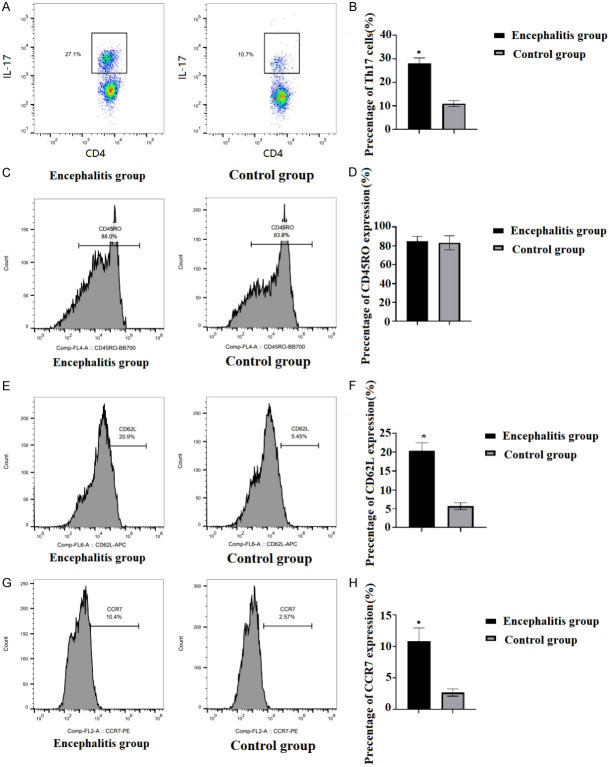
Th17 enrichment and phenotypic characteristics in CSF of the two groups
CD4+ T cells were isolated from the CSF samples of each patient, and Th17 in CSF was detected by Flow cytometry. The results revealed that Th17 cell count in the CSF of encephalitis group was obviously higher than that in the control group (P<0.05, Figure 2A and 2B). In addition, Flow cytometry was used to detect the phenotypic characteristics of Th17 cells in the CSF (Figure 2C), and the results showed that most Th17 cells in CSF of the two groups expressed CD45RO molecule, and there was no difference in CD45RO molecule expression between the two groups (P>0.05, Figure 2D). CD62L and CCR7 expressions in the encephalitis group exceeded those in the control group (P<0.05, Figure 2E-H).
Figure 2.
Enrichment and phenotypic characteristics of Th17 in the CSF of the two groups. A, B: The percentage of Th17 cells in CSF was detected by Flow cytometry. C-H: The phenotypic characteristics of Th17 cells in CSF of the two groups were detected by Flow cytometry. Compared with the control group, *P<0.05. CSF: cerebrospinal fluid.
Different cytokines inhibit Th17 cell differentiation in patients with NMDAR encephalitis
The level of IL-1β, IL-6, IL-7 and IL-23 in the PBMCs of the encephalitis group were apparently higher than those in control group (P<0.05, Figure 3A). The serum IL-1β, IL-6, IL-7, and IL-23 levels in encephalitis group were also notably higher than those in control group (P<0.05, Figure 3B). The effect of different cytokines on Th17 cells in NMADR encephalitis was analyzed. The results referred that had the Th17 cell percentage and RORγ T mRNA expression level were the highest in the IL-1β+IL-6+IL-23 group and the lowest in the TGF-β group (Figure 3C and 3D).
Figure 3.
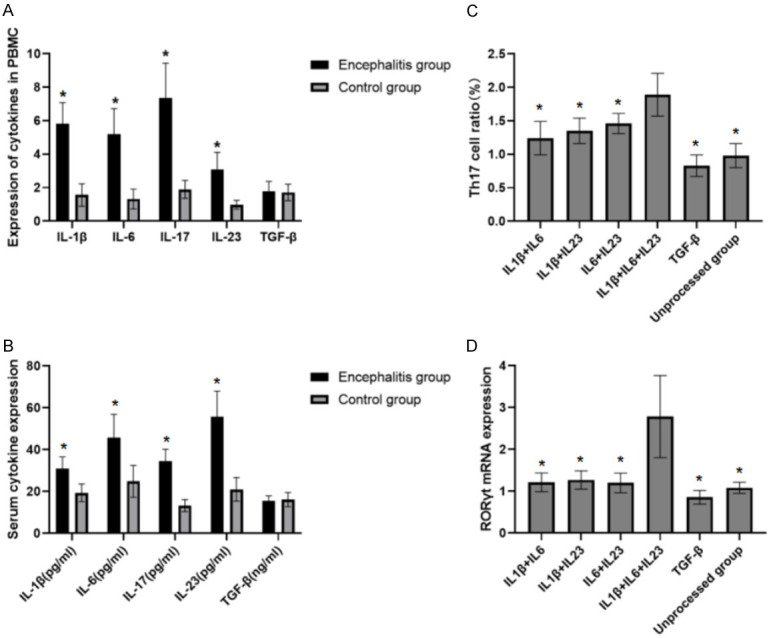
Different cytokines against Th17 cell differentiation in NMDAR encephalitis. A. The mRNA levels of IL-1β, IL-6, IL-7, IL-23 and TGF-β in the PBMCs of the two groups. Encephalitis group compared with the control group, *P<0.05. B. The serum levels of IL-1β, IL-6, IL-7, IL-23 and TGF-β. Compared with the control group, *P<0.05. C. The effect of different cytokines on Th17 cells in anti-NMADR encephalitis. Compared with IL-1β+IL6+IL23, *P<0.05. D. The effect of different cytokines on RORγt mRNA expression in anti-NMADR encephalitis. Compared with IL-1β+IL6+IL23, *P<0.05. PBMCs: peripheral blood mononuclear cells.
Prognosis prediction of Th17 against NMDAR encephalitis
The 35 patients with anti-NMDAR encephalitis were divided into good prognosis subgroup (mRS<2, n=21) and poor prognosis subgroup (mRS≥2, n=14) according to the mRS score from follow-ups. The percentage of Th17 cells in CSF in the poor prognosis subgroup was significantly higher than that in the other group [(26.18±3.17)% vs. (29.86±3.08)%] (P<0.05, Figure 4A). The predictive value of Th17 was analyzed by ROC curve, and the results showed that the area under ROC curve was 0.873 (Figure 4B).
Figure 4.
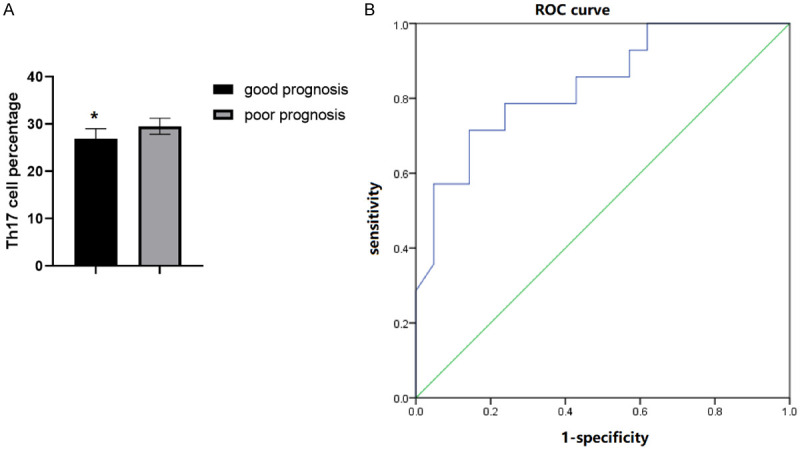
Th17 predicts the prognosis of patients with anti-NMDAR encephalitis. A. Comparison of the percentage of Th17 cells between the good prognosis group and the poor prognosis group. B. ROC curve analysis of the predictive value of Th17 cells for the poor prognosis of patients after treatment. Compared with the poor prognosis group, *P<0.05.
Discussion
It was found that patients with anti-NMDAR encephalitis carry a large number of immune cells in their CSF, including lymphocytes, macrophages, neutrophils and tumor cells, etc. [10]. Lymphocytes make up most of the immune cells in the CSF [11]. Current studies suggest that the immune pathogenesis of anti-NMDAR encephalitis is the increase of lymphocytes in CSF in the early stage, followed by the inflammatory response of central nervous system, which leads to the activation of autoimmune response, and subsequently the activation of B cells and the generation of antibodies [12]. At present, the research data on the involvement of T cells in this disease are limited. It has recently been shown that T cells are involved in the mechanism of immune regulation against NMDAR encephalitis [13]. Studies have indicated that the expression of T cell-related cytokine IL-17 in CSF increases during the progression of anti-NMDAR encephalitis [14]. Th17 is a special type of CD4+ T cells that secrete IL-17, which is considered to be the primary cell type involving tissue inflammation and immune response, and imposes a crucial effect in human autoimmune diseases [15,16].
The results of this study illustrated that the percentage of Th17 in the CSF of the encephalitis group was notably increased compared with that of the control group. This suggests that Th17 cells and the related cytokines produce a marked effect on anti-NMDAR encephalitis. Hence, the results of this as well as previous studies corroborate the hypothesis that anti-NMDAR encephalitis is an autoimmune disease associated with T cells.
The development, differentiation and biological effects of Th17 rely on a series of cytokines. Among them, IL-23 is a pro-inflammatory cytokine discovered in recent years. It accelerates the proliferation and differentiation of Th17 by binding to its receptors, and meanwhile promotes Th17 cells to secrete the characteristic inflammatory factor IL-17 in the occurrence and development of autoimmune diseases [17,18]. In this study, IL-23 mRNA expression in the PBMCs and the content in serum of patients with anti-NMDAR encephalitis were greatly increased, demonstrating that IL-23, as a differentiation promoting factor of Th17, plays a significant role in anti-NMDAR encephalitis. In addition to the direct connection between IL-17 and IL-23 and the functional status of Th17 cells, cytokines IL-1β, IL-6 and TGF-β also play an important function in regulating the differentiation of Th17 cells [19-21]. Previous studies have found that the combined action of IL-1β and IL-6 can effectively initiate the differentiation and proliferation of human Thl7 cells, but unlike rodents, TGF-β inhibits the differentiation of human Thl7 cells [22-24]. In this study, IL-1β and IL-6 mRNA in PBMCs and serum were critically increased. Therefore, together with previous results, it suggests that high expression of IL-1β, IL-6, IL-23 and TGF-β in the peripheral blood of patients with anti-NMDAR encephalitis provides an important cytokine environment for the differentiation of Th17.
RORγt as a retinoic acid-related orphan nuclear hormone receptor, plays a key role in the differentiation of Th17 and is a specific transcription factor that controls the differentiation of Th17 [25,26]. We noticed the effect of different cytokine microenvironment on the differentiation of Th17 and the difference in RORγt mRNA expression. The percentage of Th17 and the expression of RORγ T mRNA in IL-1β+IL-6+IL-23 group were the highest, which is consistent with the proportion of Th17 in peripheral blood and the increase of IL-17, IL-1β, IL-6 and IL-23.
Besides, Th17 in subjects with different outcomes was analyzed, and it showed that the percentage of Th17 in the CSF of patients with poor prognosis was higher than that in those with good prognosis. ROC curve analysis showed that Th17 cells could predict the poor prognosis of patients after treatment, and the area under ROC curve was 0.873. This suggests that the increase of IL-17 in CSF is tightly related to the progress in anti-NMDAR encephalitis. Moreover, it has been reported that there may be some dynamic changes in IL-17 levels during the occurrence and development of the disease. Th17 levels were markedly elevated in the early stages of disease, and reduced after immunosuppressive therapy. Therefore, further investigation is needed to analyze the relationship between the dynamic changes of IL-17 levels and the progression and recurrence of this disease.
The limitation of this study is the limited sample size, and the dynamic changes in Th17 cells percentage during disease progression and recurrence in patients with NMDAR encephalitis were not studied. Therefore, it is necessary to enlarge sample size and analyze the influence of Th17 in the dynamic process of disease.
In conclusion, Th17 proportion in peripheral blood and the expression of corresponding pro-differentiation cytokines increased in NMDAR encephalitis, and was closely related to the prognosis of patients. Th17 is important in the immune regulation of the occurrence and progression of NMDAR encephalitis. Th17 cell-associated cytokines might be the latent biomarker for accessing clinical outcomes of anti-NMDAR encephalitis.
Acknowledgements
This work was supported by Hainan Natural Science Foundation Project (818MS149).
Disclosure of conflict of interest
None.
References
- 1.Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, Geis C, Lancaster E, Titulaer MJ, Rosenfeld MR, Graus F. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–1057. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 2.Shi YC, Chen XJ, Zhang HM, Wang Z, Du DY. Anti-N-Methyl-d-Aspartate receptor (NMDAR) encephalitis during pregnancy: clinical analysis of reported cases. Taiwan J Obstet Gynecol. 2017;56:315–319. doi: 10.1016/j.tjog.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Shen CH, Fang GL, Yang F, Cai MT, Zheng Y, Fang W, Guo Y, Zhang YX, Ding MP. Seizures and risk of epilepsy in anti-NMDAR, anti-LGI1, and anti-GABA(B) R encephalitis. Ann Clin Transl Neurol. 2020;7:1392–1399. doi: 10.1002/acn3.51137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Wan J, Wei Z, Song C, Kang X, Du F, Jiang W, Yang F. Status epilepticus in patients with anti-NMDAR encephalitis requiring intensive care: a follow-up study. Neurocrit Care. 2022;36:192–201. doi: 10.1007/s12028-021-01283-4. [DOI] [PubMed] [Google Scholar]
- 5.Shu Y, Guo J, Ma X, Yan Y, Wang Y, Chen C, Sun X, Wang H, Yin J, Long Y, Yan X, Lu Z, Petersen F, Yu X, Qiu W. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is associated with IRF7, BANK1 and TBX21 polymorphisms in two populations. Eur J Neurol. 2021;28:595–601. doi: 10.1111/ene.14596. [DOI] [PubMed] [Google Scholar]
- 6.de Bruijn MAAM, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, van Donselaar CA, Majoie MHJM, Neuteboom RF, Sillevis Smitt PAE, Thijs RD, Titulaer MJ. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABA(B)R encephalitis. Neurology. 2019;92:e2185–e2196. doi: 10.1212/WNL.0000000000007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu HC, Su YC, Huang SC, Chiang HL, Huang PS. Anti-NMDAR encephalitis with ovarian teratomas: review of the literature and two case reports. Taiwan J Obstet Gynecol. 2019;58:313–317. doi: 10.1016/j.tjog.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazzarin SM, Vabanesi M, Cecchetti G, Fazio R, Fanelli GF, Volonté MA, Genchi A, Giordano A, Martinelli V, Colombo S, Beccaria P, Mucci M, Peccatori J, Filippi M, Minicucci F. Refractory anti-NMDAR encephalitis successfully treated with bortezomib and associated movements disorders controlled with tramadol: a case report with literature review. J Neurol. 2020;267:2462–2468. doi: 10.1007/s00415-020-09988-w. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto S, Kawai H, Okahisa Y, Tsutsui K, Kanbayashi T, Tanaka K, Mizuki Y, Takaki M, Yamada N. Anti-N-Methyl-D-aspartate receptor encephalitis in psychiatry. Acta Med Okayama. 2019;73:189–195. doi: 10.18926/AMO/56860. [DOI] [PubMed] [Google Scholar]
- 11.Forrester A, Latorre S, O’Dea PK, Robinson C, Goldwaser EL, Trenton A, Tobia A, Aziz R, Dhawan S, Brennan A, Kurukumbi M, Dong Y, Benavides DR, Offurum AI. Anti-NMDAR encephalitis: a multidisciplinary approach to identification of the disorder and management of psychiatric symptoms. Psychosomatics. 2020;61:456–466. doi: 10.1016/j.psym.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guasp M, Dalmau J. Encephalitis associated with antibodies against the NMDA receptor. Med Clin (Barc) 2018;151:71–79. doi: 10.1016/j.medcli.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Wei YC, Tseng JR, Wu CL, Su FC, Weng WC, Hsu CC, Chang KH, Wu CF, Hsiao IT, Lin CP. Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: case report and literature review. Brain Behav. 2020;10:e01540. doi: 10.1002/brb3.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai P, Zhang X, Xie Z, Liu G, Liu X, Pan S, Wang H. The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurol Scand. 2018;137:277–282. doi: 10.1111/ane.12850. [DOI] [PubMed] [Google Scholar]
- 15.Warren N, O’Gorman C, McKeon G, Swayne A, Blum S, Siskind D. Psychiatric management of anti-NMDAR encephalitis: a cohort analysis. Psychol Med. 2021;51:435–440. doi: 10.1017/S0033291719003283. [DOI] [PubMed] [Google Scholar]
- 16.Lwin S, San Yi M, Mardiana K, Woon SY, Nwe TM. Ovarian teratoma-associated anti-NMDAR encephalitis in a 12-year-old girl. Med J Malaysia. 2020;75:731–733. [PubMed] [Google Scholar]
- 17.Marques Macedo I, Gama Marques J. Catatonia secondary to anti-N-methyl-D-aspartate receptor (NMDAr) encephalitis: a review. CNS Spectr. 2020;25:475–492. doi: 10.1017/S1092852919001573. [DOI] [PubMed] [Google Scholar]
- 18.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92:e244–e252. doi: 10.1212/WNL.0000000000006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu S, Lan T, Bai R, Jiang S, Cai J, Ren L. HSV encephalitis triggered anti-NMDAR encephalitis: a case report. Neurol Sci. 2021;42:857–861. doi: 10.1007/s10072-020-04785-9. [DOI] [PubMed] [Google Scholar]
- 20.Tao S, Zhang Y, Ye H, Guo D. AQP4-IgG-seropositive neuromyelitis optica spectrum disorder (NMOSD) coexisting with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis: a case report and literature review. Mult Scler Relat Disord. 2019;35:185–192. doi: 10.1016/j.msard.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Koparal B, Çiçek S, Taner ME, Kuruoğlu A. A rapidly progressive case of anti-NMDAR encephalitis with primary psychiatric symptoms. Psychiatr Danub. 2021;33:177–179. doi: 10.24869/psyd.2021.177. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y, Zhou Z, Chen J, Peng Y, Wang H, Qiu W, Xie W, Zhang J, Wang H. Anti-NMDAR encephalitis induced in mice by active immunization with a peptide from the amino-terminal domain of the GluN1 subunit. J Neuroinflammation. 2021;18:53. doi: 10.1186/s12974-021-02107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raja P, Shamick B, Nitish LK, Holla VV, Pal PK, Mahadevan A, Thomas PT, Maya B, Saini J, Shantala H, Netravathi M. Clinical characteristics, treatment and long-term prognosis in patients with anti-NMDAR encephalitis. Neurol Sci. 2021;42:4683–4696. doi: 10.1007/s10072-021-05174-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Chen X. Recurrent anti-NMDAR encephalitis during pregnancy combined with two antibodies positive. Arch Womens Ment Health. 2021;24:1045–1050. doi: 10.1007/s00737-021-01124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Restrepo-Martinez M, Ramirez-Bermudez J, Bayliss L, Espinola-Nadurille M. Characterisation and outcome of neuropsychiatric symptoms in patients with anti-NMDAR encephalitis. Acta Neuropsychiatr. 2020;32:92–98. doi: 10.1017/neu.2019.46. [DOI] [PubMed] [Google Scholar]
- 26.Ursitti F, Roberto D, Papetti L, Moavero R, Ferilli MAN, Fusco L, Vigevano F, Curatolo P, Valeriani M. Diagnosis of pediatric anti-NMDAR encephalitis at the onset: a clinical challenge. Eur J Paediatr Neurol. 2021;30:9–16. doi: 10.1016/j.ejpn.2020.12.004. [DOI] [PubMed] [Google Scholar]