Abstract
Objective: To explore the mechanism of Thunberg Fritillaria in treating endometriosis (EMs) based on network pharmacology and the effect of Peiminine on the MEK/ERK pathway. Methods: We applied Chinese medicine system pharmacology analysis platform (TCMSP) database and literature search to screen the main chemical components of Fritillaria thunbergii Miq and created a Vanny map from the databases of TCMSP, GENECARDS, Online Mendelian Inheritance in Man (OMIM), and some others. The STRING database was used to construct the protein interaction network of Fritillaria thunbergii Miq and EMs. The overlapping targets and enriched pathways were discovered using the cells of the innate immune annotation database (DAVID) and the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses. To test the mechanism of Peiminine, the active ingredients of Fritillaria thunbergii, in the therapy of EMs, we designed cell assays and animal research. EMs mouse models were treated with several therapies, including fibrosis inhibitor in Peiminine by utilizing Hematoxylin-eosin staining (HE staining), MASSON staining, Immunohistochemistry, Immunofluorescence, quantitative real-time PCR (qRT-PCR) experiment, and Western blotting test. We evaluated the anti-endometriotic effects of Peiminine using 12Z human endometriotic cells. Cell Counting Kit 8 was used to assess the vitality of 12z cells (CCK8). We evaluated the migration ability of 12z cells by cell scratch test. Results: The effective active ingredients of Fritillaria thunbergii Miq in the treatment of EMs are Pelargonidin, Beta-sitosterol syringaresinol, Peimisine Pelargonidin-3, 5-diglucoside Ziebeimine Zhebeiresinol Verticine Solatubin OSI-2040 Chaksine Peiminine Peiminoside Peiminoside_qt, and 6-Methoxyl-2-acetyl-3-methyl-1, 4-naphthoquinone-8-O-beta-D-glucopyranoside. The critical targets for Fritillaria thunbergii Miq treating EMs are NOS2/PTGS1/AR/PPARG/PTGS2/NCOA2/RXRA/PGR/NR3C1/NCOA1/SLC6A4/OPRM1/BCL2 and ESR1. The results of GO function and KEGG enrichment analysis showed that the role pathway was estrogen-related signaling and thyroid hormone-related signaling. The expression of E-cadherin was decreased in EMs while MEK1/2, P-ERK, N-cadherin and vimentin were all increased in MASSON, immunofluorescence, Real-time PCR and Western blotting. In epithelial 12Z cells, high concentrations of Peiminine can block cell activity and migration, which is directly related to blocking cell fibrosis. Conclusion: Overall, this study partially verified the network pharmacological prediction that Peiminine regulates the MAPK pathway in inhibiting 12Z cell proliferation and migration, and finally protects against EMs.
Keywords: Fritillaria thunbergii Miq, endometriosis, network pharmacology, MAPK signaling pathway
Introduction
Endometriosis (EMs), affecting 6-10% of reproductive-age women [1], is defined as aberrant endometrial tissue development at ectopic places, most commonly the pelvis, and causes discomfort, dyspareunia, and infertility [2]. According to traditional Chinese medicine (TCM), the cause of Ems is Phlegm-blood Stasis Syndrome, and the ectopic endometrial tissue is regarded as the blood that has left the channels [3]. The blood leaving the meridians gathers into blood stasis, making Thoroughfare Vessel and Conception Vessel not work. Long-term blood stagnation can lead to disease. Doctors of TCM consider that the body fluid and blood are of the exact origin, so blood stasis can cause body fluid to condense into phlegm [4]. Over time, phlegm and blood stasis will mix, and finally, Phlegm-blood Stasis Syndrome is formed. Phlegm and blood stasis syndrome is a common syndrome type of EMs. The treatment of TCM is resolving phlegm and dispersing nodules. Doctors often use Fritillaria thunbergii Miq to treat EMs with phlegm and blood stasis [5]. Fritillaria thunbergii Miq, according to TCM theory, has the action of clearing away heat, resolving phlegm, alleviating cough, detoxifying, dispersing knots, and eradicating carbuncle [6].
Material and methods
Identification of candidate components and screening of active components in Fritillaria thunbergii Miq
The chemical components of Fritillaria thunbergii Miq were collected from the TCM systems pharmacology (TCMSP) database, and the practical active components were screened with drug-like (DL) as the limiting conditions [7]. Then, we obtained the targets of active components with DL greater than 0.18 from TCMSP database.
In the long-term clinical practice, TCM is usually made into decoction, powder, pill, and so on in an oral dosage form. Drug likeness (DL) is one of the essential parameters in pharmacokinetics. It is helpful for rapid screening of active ingredients in TCMSP database [8]. We screened for active ingredients of Fritillaria thunbergii Miq by conducting DL>0.18.
Prediction of drug targets for Fritillaria thunbergii Miq
The targets of Fritillaria thunbergii Miq’s active component were transformed into the gene names by the database of UniProt (https://www.uniprot.org). The Swiss Target Prediction database (http://www.swisstargetprediction.ch) was utilized to provide target information.
Screening targets of EMs
The EMs therapeutic targets were collected from the Gene Cards database (https://www.GeneCards.org/), which many researchers have used in the study of network pharmacology [9]. From the EMs-related genes and Fritillaria thunbergii Miq target genes, we screened out the overlapping genes of Fritillaria thunbergii Miq active ingredients interacting with EMs (Figure 1).
Figure 1.
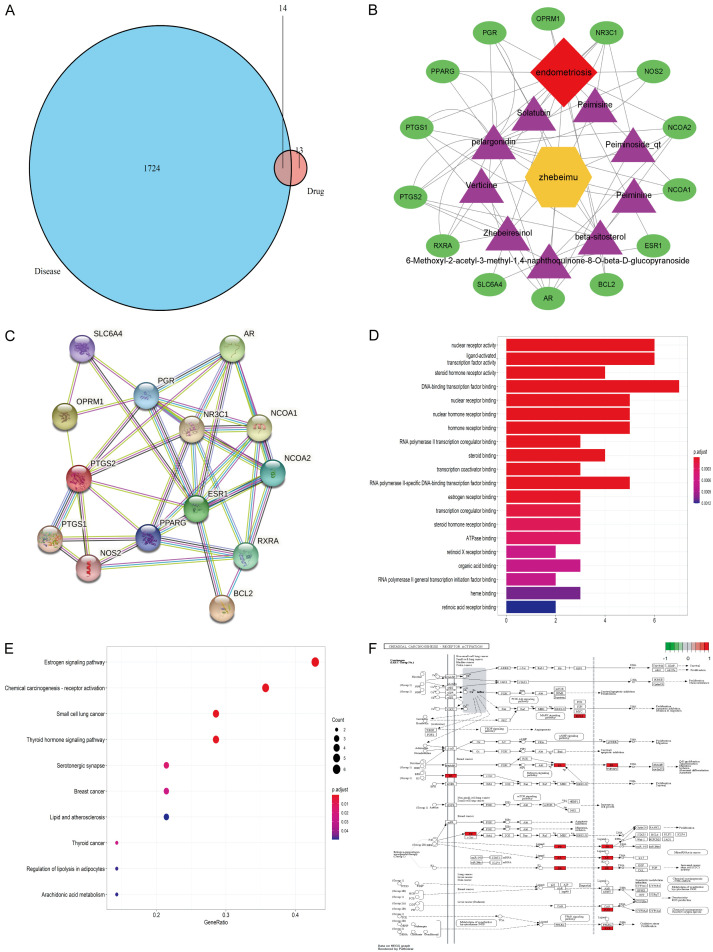
Pharmacologic analysis of Thunberg Fritillary bulb by TCM network. A: Venn diagrams: Targets matching each other of Fritillaria thunbergii Miq and Endometriosis; B: Bioactive compounds and corresponding and corresponding targets network of Fritillaria thunbergii Miq in treatment of endometriosis; C: The STRING-generated PPI network of targets. Proteins are represented by nodes. The edges indicate the PPIs; D: Analysis of the enrichment histogram of GO in the treatment of Endometriosis by Fritillaria thunbergii Miq; E: Analysis of the enrichment bubble diagram of the KEGG pathway in the treatment of Endometriosis by Fritillaria thunbergii Miq; F: KEGG pathway enrichment analysis showed that the significantly enriched pathways of Fritillaria thunbergii in the treatment of Endometriosis were estrogen-related pathway.
Construction of networks and analysis
The compound-target, target-pathway, and target-disease networks were built using Cytoscape 3.7.1 software to better investigate the molecular mechanism of Fritillaria thunbergii Miq in the treatment of EMs [10]. We were able to thoroughly comprehend the link between latent targets and effective compounds by analyzing the key effective components of Fritillaria thunbergii Miq. The nodes in the network represent genes, molecules, or proteins. Edge connects nodes representing the interaction between these active ingredients and molecules, genes and diseases.
Protein-protein interaction (PPI) networks construction
The intersection gene of the active ingredient and illness was incorporated into the string database (STRING, Version 11.0, https://string-db.org/) to develop the protein interaction network of Fritillaria thunbergii Miq in treating EMs. We input the 14 overlapping targets into the STRING database for retrieval with a minimum needed interaction score of 0.4 [11]. The PPI network was then obtained and fed into Cytoscape software to be viewed.
GO and pathway enrichment analysis for EMs-related targets of Fritillaria thunbergii Miq
The GO can describe the function of genes, and it is widely used in the field of DAVID online tool (http://david.abcc.ncifcrf.gov/). The GO function enrichment study was performed, and the results were evaluated and visualized using the R programming language (Version 3.6.0). The “cluster profiler” package was then installed, and the ID was entered into it. It then did the functional enrichment analysis of GO and KEGG using P<0.05 and Q<0.05, respectively, and outputted the findings through barplot and dotplot.
Establishment of the mouse EMs model and treatments
Sixty female 8-week-old C57/6 mice from Shanghai Laboratory Animal Center, Co., Ltd. were used to construct mouse models of EMs. The mice were housed in a temperature-controlled environment of 25±2°C and alternate darkness for 12 hours and light for 12 hours at a humidity of 55±15%.
To construct a mouse model of EMs: each mouse was intramuscularly injected with estradiol benzoate (0.1 mg/kg/d) for 7 days before surgery. Firstly, mice were injected intraperitoneally with 30 mg/mL sodium pentobarbital, and skin preparation was conducted. Secondly, we made a vertical incision of about 2 cm in the lower abdomen at a distance of 1 cm from the urethra and cut the muscle and peritoneum to expose the uterus. Thirdly, we dissociated the right uterus, ligated the proximal end 1 cm away from the right uterine horn, ligated the distal end 2 cm away from the ovary, ligated the mesenteric vessels at both ends, cut off the uterus segment about 1 cm long and placed it in saline solution. Fourthly, in the petri dish, the excess adipose tissue was removed, and the uterine segment was dissected along the longitudinal axis with ophthalmic scissors. The endometrial surface of the endometrial tissue was close to the abdominal wall peritoneum, and the serosa surface faced the abdominal cavity. The No. 7 thread was used for fixing the rich blood vessels in the inner wall of the abdominal cavity, the No. 1 thread for suturing and closing the abdominal cavity, and the skin was sutured. Postoperatively, penicillin sodium 80,000 U/d was given for three consecutive days for preventive anti-infection. After 21 days of modeling, the mice were randomly separated into six groups of 10 mice each, including Blank group (for normal saline), Model group, Peiminine low dose group (Pei-LD for Peiminine 2 mg/kg/d), Peiminine medium dose group (Pei-MD for Peiminine 4 mg/kg/d), Peiminine high dose group (Pei-HD for Peiminine 8 mg/kg/d) and Dienogest group (2 mg/day, gavage, positive control). Mice were given intragastric administration for 28 days after modeling.
Histology staining
After the sacrifice of the mice, the eutopic and ectopic endometrium tissue was fixed with 4% paraformaldehyde. Then the endometrium tissue was embedded in paraffin and cut into 4 µm-thick slices before being stained with hematoxylin and eosin (HE) and Masson staining kits (Sigma, USA).
Immunohistochemistry
The endometriotic tissue was removed and soaked in 10% formalin, embedded in paraffin, cut into an appropriate size and analyzed by Immunohistochemistry. The paraffin tissue slices were first dewaxed, and then dehydrated using a graded ethanol series. To inhibit endogenous peroxidase activity, slides were treated in 3 percent H2O2 for 10 min at room temperature. Antigen repair was accomplished by boiling for 10 min in a 0.01 M citrate buffer solution in a pressure cooker set to 120°C. The samples were treated with primary antibodies at 4°C overnight. After washing with PBS, the slides were treated with horseradish peroxidase (HRP) - coupled secondary antibodies (1:1000, Invitrogen, Carlsbad, CA, USA) for 30 minutes at room temperature. The sections were then stained with diaminobenzidine (DAB) according to the manufacturer’s technique, photographed under a microscope, and the expression levels were determined.
Immunofluorescence staining
The ectopic endometrium of mice was sliced to a thickness of about 8-10 μm with a frozen microtome. The expression of vimentin protein was detected by immunofluorescence after 30 min at room temperature. After 1 h of blocking with 2% BSA at room temperature, the primary antibody was incubated overnight at 4°C. Then, the sections were washed three times with a 0.1 mol/L phosphate buffer salt solution for 3-5 min each time. The second antibody was incubated for 1 h at room temperature in dark. After washing with 0.1 mol/L phosphate buffer salt solution for 3 times, 3-5 min each time, the sections were subjected to DAPI staining, then washed again with 0.1 mmol/L phosphate buffer solution for 3 times, each time for 3-5 min. Thereafter, anti-quenching agent was added dropwise, and the film was sealed. Finally, the samples were examined and assessed using a confocal microscope (TCS SP2; Leica Microsystems, Germany).
RNA extraction and RT-qPCR
Trizol extraction kit was used to extract RNA from eutopic and ectopic endometrium. The isolated RNA is retrotranscribed to cDNA using the GoScriptTM Reverse Transcription System kit (Promega Corporation). The SYBR-Green PCR master mix platform provided by Thermo Fisher Scientific, Inc. was used for amplification. The following thermal cycler settings were used: activation at 50°C, denaturation at 95°C (30 s), 40 amplification cycles (95°C, 5 s), and termination at 60°C (30 s). Table 1 contains the primer sequences employed in the reaction mixture.
Table 1.
List of primers used in this study
| Forward primer 5’→3’ | Reverse primer 5’→3’ | |
|---|---|---|
| MEK1/2 | CCTCTTGGTGGTCACGTCC | CCAGGTCCACTCCGAACACT |
| ERK | TCCAACCTCCTGCTGAACAC | CCAACGTGTGGCTACGTACT |
| E-cadherin | GAAGGCTTGAGCACAACAGC | CCCTGATACGTGCTTGGGTT |
| N-cadherin | AGCCAACCTAACTGTCACGG | TCCTGTAGGGTCTCCACCAC |
| Vimentin | GCAGTATGAAAGCGTGGCTG | ACCTGTCTCCGGTACTCGTT |
| ACTIN | ACCCTAAGGCCAACCGTGAAA | ATGGCGTGAGGGAGAGCATA |
Western blot test
Proteins were extracted from the samples using RIPA reagent prior to the Western blot assay (cat. no. ab7937; Abcam). Centrifuged protein-containing lysates were submitted to BCA (Bicinchoninic Acid Assay) reagent quantification (cat. no. 23227; Thermo Fisher Scientific, Inc.). Next, 50 µg of protein was electrophoretically separated on 12% SDS-PAGE (polyacrylamide gel electrophoresis), followed by loading onto Polyvinylidene fluoride membranes and 120 min of incubation in 25% skim milk. The membranes were placed in contact with primary antibody solutions directed against the identified proteins. MEK1/2 monoclonal antibody (1:1000, cat no: ab178876). Proteintech E-cadherin antibody (1:1000, cat no. 20874-1-AP), Proteintech N-cadherin monoclonal antibody (1:1000, cat no. 66219-1-Ig), Proteintech’s vimentin antibody (1:2000, cat no: 10366-1-AP), Cell Signaling Technology’s ERK Monoclonal Antibody (1E5; 1:2000; 4695), and Cell Signaling Technology’s Phosphop44/42 MAPK (Erk1/2; Thr202/Tyr204) antibody #9101 (1:1000) from ABCAM were utilized. Following incubation, the membranes were washed three times with Tris-Buffered Saline and Tween (TBST) and incubated for 60 min at 25°C with HRP linked secondary antibody solutions (1:5000; cat. no. ab7097; Abcam). The electrochemiluminescence (ECL) reagent was used to reveal the signals corresponding to the immunological complexes (cat. no. WBKLS0050; EMD Millipore). Finally, densitometric analysis of the bands was performed using Image J software (National Institutes of Health, Bethesda, MD). The Image J results were then used to calculate the relative expression of the proteins with reference to the Actin protein.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) test was used to examine cell proliferation (Vazyme, China). The 12Z cells (12Z cells derived from epithelial cells of peritoneal EMs) were seeded in three 96-well plates with 8×103 cells per well. Each plate was used to analyze the cell proliferation by adding 10 μL of CCK-8 solution and 90 μL of DMEM per well at an interval of 12 h, up to 48 h. A microplate reader was used to measure the optical density (OD) at 450 nm in each well.
Wound healing assay
A wound healing assay was used to test the capacity of cell migration. Cells were seeded on six-well plates and incubated to almost full confluence following treatment, as previously stated. With a 200-L plastic pipette tip, scratching was conducted. The 12z cells was cultured with serum-free DMEM andtreated in fresh medium with Peiminine for 6 and 24 hours. The wound closure was photographed and analyzed at 0, 6 and 24 hours. The gap area of the wound was measured by Image J software.
Statistical analysis
Data were shown as mean ± standard deviation (x̅ ± sd). Statistical comparisons between the two groups were processed by student’ t-test. The one-way ANOVA test was used for data involving more than two groups. GraphPad Prism version 9 statistical software was used to create analyses and graphics (GraphPad Software, Inc.). A value of P<0.05 was considered significant.
Results
Network pharmacology-based analysis
Components and targets of herbs in fritillaria thunbergii Miq
In the TCMSP database, we obtain fifteen active components of Fritillaria thunbergii Miq (Table 2) and 110 targets of Fritillaria thunbergii Miq. Active components include pelargonidin, beta-sitosterol, syringaresinol, Peimisine, Pelargonidin-3, 5-diglucoside, Zhebeiresinol, Ziebeimine, Verticine, 6-Methoxyl-2-acetyl-3-methyl-1, 4-naphthoquinone-8-O-beta-D-glucopyranoside, Solatubin, OSI-2040, Chaksine, Peiminine, Peiminoside and Peiminoside_qt.
Table 2.
Active Fritillaria thunbergii components
| Mol ID | Molecule Name | DL |
|---|---|---|
| MOL001004 | pelargonidin | 0.21 |
| MOL000358 | beta-sitosterol | 0.75 |
| MOL000365 | syringaresinol | 0.72 |
| MOL004440 | Peimisine | 0.81 |
| MOL004442 | Pelargonidin-3, 5-diglucoside | 0.76 |
| MOL004443 | Zhebeiresinol | 0.19 |
| MOL004444 | Ziebeimine | 0.70 |
| MOL004445 | Verticine | 0.67 |
| MOL004446 | 6-Methoxyl-2-acetyl-3-methyl-1, 4-naphthoquinone-8-O-beta-D-glucopyranoside | 0.57 |
| MOL004448 | Solatubin | 0.76 |
| MOL004449 | OSI-2040 | 0.61 |
| MOL004450 | Chaksine | 0.66 |
| MOL004451 | Peiminine | 0.67 |
| MOL004452 | Peiminoside | 0.21 |
| MOL004453 | Peiminoside_qt | 0.67 |
Prediction of drug targets for Fritillaria thunbergii Miq
From the GeneCards database, 1724 therapeutic targets of EMs were acquired. Through the Venny analysis of Fritillaria thunbergii Miq targets and EMs targets, we obtained 14 overlapping targets (Figure 1A).
Screening targets of EMs
Through the online Venn diagram tool, the target genes of the active ingredients and the genes related to EMs were intersected, and 14 related targets were obtained for the nine active ingredients of Fritillaria thunbergii in the treatment of EMs (Figure 1B). To clarify the potential mechanism of Fritillaria thunbergii Miq on EMs, Cytoscape software created a Drug-Compounds-Genes-Disease network (Figure 1B).
Construction of networks and analysis
The target genes were input into the STRING database for PPI network analysis, and the network diagram of PPI was obtained (Figure 1C). There are 14 nodes as target genes, which play an essential role in treating EMs. There are 38 edges as the connecting lines of each protein node, representing the interaction mode between each node protein.
GO and pathway enrichment analysis for EMs-related targets of Fritillaria thunbergii Miq
To investigate the biological properties of Fritillaria thunbergii Miq targets on EMs, DAVID Bioinformatics Resources 6.7’s functional annotation tool was used to undertake GO function and KEGG pathway enrichment investigations on the identified targets. The GO analysis diagram revealed that Fritillaria thunbergii Miq therapy of EMs mostly involved DNA-binding transcription factor binding, nuclear receptor activity, ligand-activated transcription factor activity, steroid hormone receptor activity, nuclear receptor binding, nuclear hormone receptor binding and other biological processes (Figure 1D). According to these findings, Fritillaria thunbergii Miq may play a part in the treatment of EMs by altering the activity of transcription factors, the binding of nuclear receptors, and the activity of steroid hormone receptors. The highly enriched pathways of Fritillaria thunbergii in the treatment of EMs, according to KEGG pathway enrichment study, were estrogen-related pathways in the Estrogen signaling pathway, Thyroid-related pathway and small cell lung cancer-related pathway (Figure 1E).
Peiminine inhibits EMs by regulating fibrosis in pathological studies
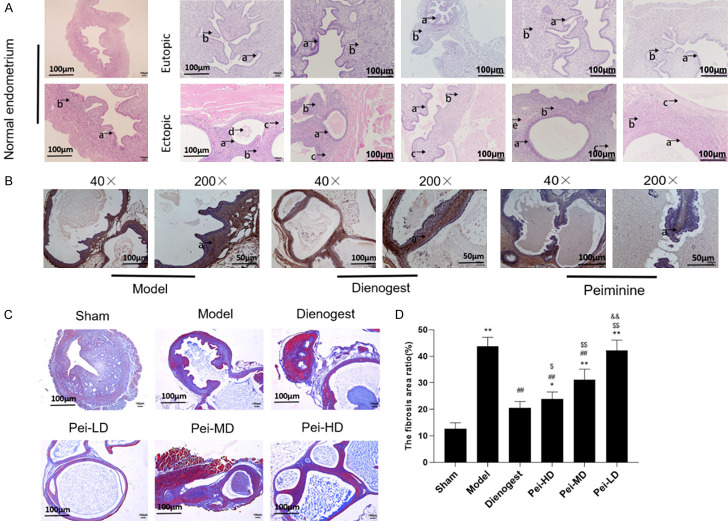
In order to further study the therapeutic mechanism of Peiminine in EMs, we carried out animal experiments in vivo. We performed HE staining on the normal endometrium in the sham group, the eutopic endometrium and ectopic endometrium in the model group and treatment groups. The treatment groups included Peiminine (low dose group, medium dose and high dose) groups and dinogestrin group. The experimental results showed that functional ectopic endometrial glands and stroma (Figure 2A) were found in the sham group, functional ectopic endometrial glands grew in the ectopic endometrium in the model group (Figure 2A). In Peiminine groups showed atrophied endometrial glands, increased cysts (Figure 2A), and there was also fibrosis tissue in the EMs mice. Expression of α-Smooth muscle actin (α-SMA), a marker protein of fibrosis, is a hallmark of mature myofibroblasts. The results of immunohistochemical detection showed that α-SMA expression was found in both eutopic endometrium and ectopic endometrium (Figure 2B) in mice, and the expression of α-SMA was higher in endometrial glands and stroma. The expression of α-SMA was weakly positive in the epithelial layer, stromal layer and glandular epithelial layer, mainly in myometrial cells. We tested the degree of fibrosis by Masson staining (Figure 2C, 2D), and found that the fiber staining area ratio of ectopic lesions at high and medium dosages of Peiminine was dramatically decreased when compared to the model control group.
Figure 2.
Peiminine inhibits endometriosis by regulating fibrosis in pathological studies. A: Representative images of hematoxylin and eosin (HE) staining for detection of normal, eutopic and ectopic endometrium. a: the glandular epithelium of the endometrium, b: the stroma of the endometrium, c: the area of fibrosis; B: Immunohistochemistry (IHC) detect α-SMA protein of ectopic endometrium from different groups; C: Representative pictures of Masson staining of normal, eutopic and ectopic endometrium from different groups. (40×, Scale bar =100 um; 200×, Scale bar =50 um). D: The fibrosis area ratio in different treatment groups. *P<0.05, compared to Sham, **P<0.01, compared to Sham; #P<0.05 compared to Model, ##P<0.01 compared to Model; $P<0.05 compared to Dienogest, $$P<0.01 compared to Dienogest; &P<0.05 compared to Pei-HD, &&P<0.01 compared to Pei-HD.
Peiminine inhibits fibrosis in EMs
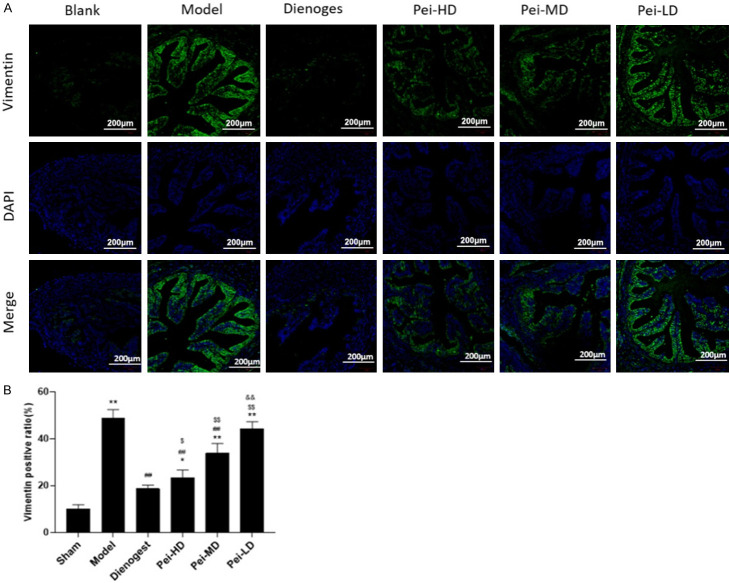
EMs is thought to be caused by epithelial-mesenchymal transition (EMT). When the menstrual blood flows counter to the outside of the uterine cavity, the microenvironment of the pelvic and abdominal cavity is unbalanced, which stimulates the secretion of cytokines and activates the molecular signal pathway to cause EMT, resulting in fibrosis. We measured the expression vimentin of a mesenchymal marker. The immunofluorescence findings revealed that the expression of vimentin fluorescence was greater in the model group’s ectopic endometrium than in the sham group’s eutopic endometrium (Figure 3A, 3B). Dienogest and Peiminine could inhibit vimentin fluorescence expression, and Peiminine inhibited vimentin fluorescence expression in a dose-dependent manner (Figure 3A, 3B).
Figure 3.
Peiminine inhibits fibrosis in endometriosis. A: Representative fluorescent photomicrographs for uterus staining with Vimentin after different treatment. Picture shows Vimentin and merge images; B: Numerical data of the positive expression of Vimentin. *P<0.05, compared to Sham, **P<0.01, compared to Sham; #P<0.05 compared to Model, ##P<0.01 compared to Model; $P<0.05 compared to Dienogest, $$P<0.01 compared to Dienogest; &P<0.05 compared to Pei-HD, &&P<0.01 compared to Pei-HD.
Peiminine inhibits EMT of EMs through the MAPK/ERK signaling pathway
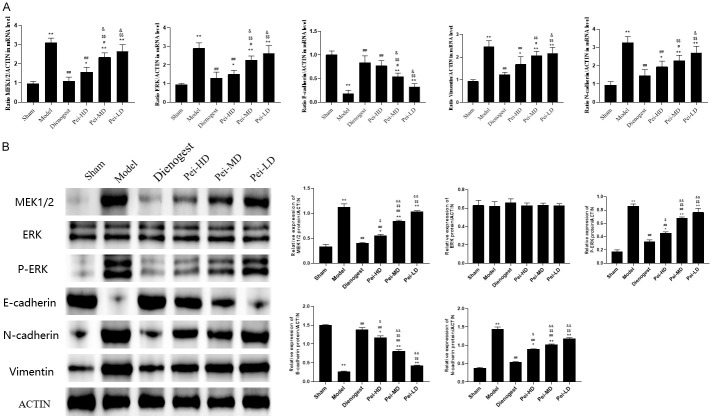
Estrogen-related MAPK signal pathway was selected from KEGG signal pathway enrichment analysis for verification. The expression levels of RNA and proteins associated to the EMT and MAPK signal pathway were investigated using Real-time PCR and Western blotting (Figure 4A, 4B). According to the results, there was no statistically significant difference between endometrial and ectopic endometrium in terms of ERK (Figure 4B). The expression of P-ERK, MEK, N-cadherin and vimentin proteins in ectopic endometrium of EMs mice were higher than that in eutopic endometrium, and Peiminine could reduce these proteins in ectopic endometrium in dose-dependent manner. The content of E-cadherin showed the opposite trend (Figure 4A, 4B).
Figure 4.
Peiminine inhibits epithelial mesenchymal transformation (EMT) of endometriosis through MAPK/ERK signaling pathway. A: mRNA expression level of MEK1/2, ERK, E-cadherin, N-cadherin, Vimentin were analyzed by RT-qPCR; B: Protein blotting images and numeric quantification of protein expression level of MEK1/2, ERK, p-ERK, E-cadherin, N-cadherin, Vimentin as detected by western blotting. *P<0.05, compared to Sham, **P<0.01, compared to Sham; #P<0.05 compared to Model, ##P<0.01 compared to Model; $P<0.05 compared to Dienogest, $$P<0.01 compared to Dienogest; &P<0.05 compared to Pei-HD, &&P<0.01 compared to Pei-HD.
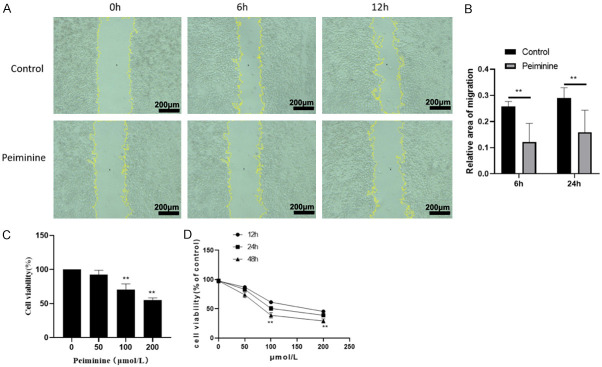
Peiminine inhibits the migration and proliferation of 12Z Cells
By using wound healing assay, Peiminine (200 μmol/mL) significantly reduced the migrations rates of 12Z Cells (Figure 5A, 5B). Peiminine inhibited the proliferation of 12Z cells. Fritillaria thunbergii Miq contains Peiminine, which is one of its active ingredients. The cell proliferation test revealed that Peiminine could suppress the proliferation of 12z cells, with the inhibitory impact proportional to the concentration (Figure 5C, 5D).
Figure 5.
Peiminine inhibits the Migration and proliferation of 12Z Cells. A: The migration image of 12Z cells were tested by wound scratch assay; B: Relative area of migration in different treatment; C: 12Z cell activity were tested by CCK8 after peiminine treatment in 48 H; D: Cell viability was determined by Cell Counting Kit-8 after treated with Peiminine on 12Z cell for 12 h, 24 h and 48 h. **P<0.01, compared to Control.
Discussion
EMs is a condition that endometrial tissue (glands and stroma) develops outside of the uterine cavity. It is more common in women in childbearing age. Pregnancy is a predominant factor. EMs has diverse etiology and symptoms, is easy to relapse and difficult to cure, and impacts on patients’ physical and mental health as well as their economic well-being [12]. At present, there is no ideal conservative treatment plan for EMs in clinical practice. The treatment is simply relying on surgery. This makes finding an effective and safe treatment plan to improve patient’s pain and infertility become the goal of reproductive endocrine disease researchers. EMs is characterized by plant growth, invasion and distant metastasis. When the ectopic endometrial “seed” encounters suitable conditions, it will stay in the pelvic cavity and pass-through “adhesion” [13]. The three steps of “invasion” and “angiogenesis” take root and sprout [14]. Once ectopic lesions are formed in the body, just like the eutopic endometrium, they will be cyclically regulated by endocrine hormones, resulting in a series of changes such as proliferation, secretion, shedding and bleeding. The difference is that the blood of ectopic lesions has no drainage channel and accumulates locally, causing surrounding tissue hyperplasia, adhesion and fibrosis. Histologically, the ectopic endometrial glands and stroma of the ovary are surrounded by dense fibrosis [15]. The pathological evolution of fibrosis is an inflammatory reaction phase, a fibrosis formation phase, a scar formation phase, and finally, the end phase. In addition, these excess fibrous tissues can cause chronic pain and affect the function of the oviduct to pick up eggs. Besides, the ovarian cortical area may also be surrounded by fibrous tissue resulting in reduced follicular reserve [16].
Fritillaria thunbergii is an herbaceous plant of Liliaceae. It grows in Xiangshan County, Zhejiang Province, China. It is a famous medicinal material in Zhejiang Province. Fritillaria thunbergii Miq is bitter in taste and cold in nature. It has the ability to remove heat, dissolve phlegm and relieve cough by softening hard, dispersing knots and eliminating carbuncle and purulent purulence. It is very useful in the treatment of EMs with phlegm and blood stasis. Experimental results showed that Fritillaria thunbergii could relieve pain and had effects in anti-inflammation, anti-oxidation and anti-tumor [17-21]. Professor Luo created Luo’s Neiyi prescription, which contains Fritillaria thunbergii. Ma Kun and others believe that the long-term blood stasis syndrome can form cysts, and Fritillaria thunbergii Miq with oyster, litchi, forsythia and orange can promote the disappearance of cysts [22].
In this work, fifteen active components of Fritillaria thunbergii Miq were obtained under screening conditions of DL>0.18, and there were 110 corresponding targets. There are 14 overlapping genes in Fritillaria thunbergii Miq and EMs. Other studies showed that pelargonidin could activate Nrf2/ARE signaling pathway, thereby reducing the inflammatory response [23,24]. Beta-Sitosterol is one of the phytosterols which widely exists in plants and drugs. It can reduce oxidative stress and cholesterol, and have effects of anti-inflammation, immune regulation and anti-tumor. It works by transforming the growth factor-β/Snail signaling pathway to inhibit EMT and to alleviate pulmonary fibrosis [25]. Peimisine has a good anti-inflammatory effect [26]. At the same time, it is one of the components of Sanjie Zhentong Capsule, which has been frequently utilized in clinical EMs therapy [27].
According to the PPI network diagram analysis, the core targets of Fritillaria thunbergii Miq for EMs treatment are as follows: NOS2, rxra, PGR, AR, PTGS2, PPARG, ncoa2, NCOA1, ESR1 and NR3C1. These targets are mostly concerned with oxidative stress, hormone dysregulation, and so on. Research indicated that nuclear steroid receptors were essential regulators of EMT and tumor growth [28,29]. EMs is an estrogen-dependent illness, according to several research [29,30].
As for the pathogenesis of EMs, steroid hormone imbalance accounts for a part of the reasons, and the pathogenesis is also closely related to thyroid hormone. In patients with EMs, almost all symptoms are usually associated with hypothyroidism [31]. Fibrosis is indicated by EMT, which has the characteristics of cell migration and invasion [32]. There is mounting evidence that EMT plays a significant role in the pathological process of EMs. EMT is often characterized by increased Vimentin and N-cadherin expression and reduced E-cadherin expression [33-35]. At the same time, EMT endows cells with the characteristics of migration and invasion [36]. This study used network pharmacology to predict that estrogen-related pathway MER/ERK signal pathway regulates the occurrence and development of EMs, and that the active ingredient of Fritillaria thunbergii has the potential to treat EMs, which was verified by experiments. First, in vitro cell experiments verified that Peiminine could inhibit the metastasis and invasion of immortalized human EMs epithelial cells and inhibit the proliferation of 12z cells. Second, Peiminine could inhibit the progression of EMs fibrosis by regulating MEK/ERK signaling pathway.
Nonetheless, the study has some limitations. First, how to develop the EMT model of 12z cells was not investigated. These cells might have several main flaws. As a result, in future research about EMs, a more convincing immortalized EMs cell line is required. Furthermore, based on the network pharmacology experimental approach, this study anticipated that the treatment of EMs with Peiminine was associated with estrogen-related pathways. Only MEK, ERK, and pERK were investigated in this experiment, but estrogen and estrogen receptor (ER) were not. More research is needed to confirm the intricate interaction between ER, MEK, MEK, and pERK and to explain the molecular mechanism.
To sum up, the study investigated the mechanism of Peiminine on EMs. Our findings suggest that blocking this mechanism, which is a regulator of the MEK/ERK pathway involves in numerous pathological processes, may mediate Peiminine’s therapeutic or protective effects. This work sheds novel insight on the pathophysiology of EMs and gives hope in the quest for medicinal agents to treat EMs. This study provides ideas and directions for further experimental research and clinical application of Fritillaria thunbergii Miq and a reference and theoretical basis for further investigation.
Acknowledgements
This project was supported by the National Youth Science and Natural Science Foundation of China (81804136), Famous TCM doctor at the hospital level (WLJH2021ZY-MZY039) and Future science and technology projects (WL-HBMS-2021003K).
Disclosure of conflict of interest
None.
References
- 1.Sarria-Santamera A, Orazumbekova B, Terzic M, Issanov A, Chaowen C, Asúnsolo-Del-Barco A. Systematic review and meta-analysis of incidence and prevalence of endometriosis. Healthcare (Basel) 2020;9:29. doi: 10.3390/healthcare9010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della Corte L, Di Filippo C, Gabrielli O, Reppuccia S, La Rosa VL, Ragusa R, Fichera M, Commodari E, Bifulco G, Giampaolino P. The burden of endometriosis on women’s lifespan: a narrative overview on quality of life and psychosocial wellbeing. Int J Environ Res Public Health. 2020;17:4683. doi: 10.3390/ijerph17134683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JX, Lin HP, Liang XF, Wang YX, Tan QQ. Effect of Yishen Zhuyu decoction combined with leuprolide acetate microspheres in the treatment of endometriosis of kidney deficiency and phlegm stasis type. China Med Her. 2021;18:93–96. 109. [Google Scholar]
- 4.Dong P, Ling L, Hu L. Systematic review and meta-analysis of traditional Chinese medicine compound in treating infertility caused by endometriosis. Ann Palliat Med. 2021;10:12631–12642. doi: 10.21037/apm-21-3425. [DOI] [PubMed] [Google Scholar]
- 5.Chen ZZ, Gong X. Effect of Hua Yu Xiao Zheng decoction on the expression levels of vascular endothelial growth factor and angiopoietin-2 in rats with endometriosis. Exp Ther Med. 2017;14:5743–5750. doi: 10.3892/etm.2017.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nile SH, Su J, Wu D, Wang L, Hu J, Sieniawska E, Kai G. Fritillaria thunbergii Miq. (Zhe Beimu): a review on its traditional uses, phytochemical profile and pharmacological properties. Food Chem Toxicol. 2021;153:112289. doi: 10.1016/j.fct.2021.112289. [DOI] [PubMed] [Google Scholar]
- 7.Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao W, Xu X, Wang X, Li B, Wang Y, Li Y, Yang L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145:1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Wang J, Shen H. Network pharmacology-based analysis of the role of traditional Chinese herbal medicines in the treatment of COVID-19. Ann Palliat Med. 2020;9:437–446. doi: 10.21037/apm.2020.03.27. [DOI] [PubMed] [Google Scholar]
- 10.Li WH, Han JR, Ren PP, Xie Y, Jiang DY. Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology. Comput Biol Chem. 2021;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. [DOI] [PubMed] [Google Scholar]
- 11.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broi MGD, Ferriani RA, Navarro PA. Ethiopathogenic mechanisms of endometriosis-related infertility. JBRA Assist Reprod. 2019;23:273–280. doi: 10.5935/1518-0557.20190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Huang D, Zhang J, Shi L, Li J, Zhang S. The effect of laparoscopic excisional and ablative surgery on ovarian reserve in patients with endometriomas: a retrospective study. Medicine (Baltimore) 2021;100:e24362. doi: 10.1097/MD.0000000000024362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farolfi A, Altavilla A, Morandi L, Capelli L, Chiadini E, Prisinzano G, Gurioli G, Molari M, Calistri D, Foschini MP, De Giorgi U. Endometrioid cancer associated with endometriosis: from the seed and soil theory to clinical practice. Front Oncol. 2022;12:859510. doi: 10.3389/fonc.2022.859510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 16.Vigano P, Candiani M, Monno A, Giacomini E, Vercellini P, Somigliana E. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod. 2018;33:347–352. doi: 10.1093/humrep/dex354. [DOI] [PubMed] [Google Scholar]
- 17.Llarena NC, Falcone T, Flyckt RL. Fertility preservation in women with endometriosis. Clin Med Insights Reprod Health. 2019;13:1179558119873386. doi: 10.1177/1179558119873386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K, Lv ZT, Zhou CH, Liang S, Huang W, Wang ZG, Zhu WT, Wang YT, Jing XZ, Lin H, Guo FJ, Cheng P, Chen AM. Peimine suppresses interleukin 1β induced inflammation via MAPK downregulation in chondrocytes. Int J Mol Med. 2019;43:2241–2251. doi: 10.3892/ijmm.2019.4141. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Kim M, Hong S, Kwon B, Song MW, Song K, Kim EY, Jung HS, Sohn Y. Anti-inflammatory effects of Fritillaria thunbergii Miquel extracts in LPS-stimulated murine macrophage RAW 264.7 cells. Exp Ther Med. 2021;21:429. doi: 10.3892/etm.2021.9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Zhong F, Yao C, Chen J, Xiang Y, Dong J, Yan Z, Ma Y. A systematic review on traditional uses, sources, phytochemistry, pharmacology, pharmacokinetics, and toxicity of fritillariae cirrhosae bulbus. Evid Based Complement Alternat Med. 2020;2020:1536534. doi: 10.1155/2020/1536534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CL, Hsia TC, Li CH, Chen KJ, Yang YH, Yang ST. Adjunctive traditional Chinese medicine improves survival in patients with advanced lung adenocarcinoma treated with first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a nationwide, population-based cohort study. Integr Cancer Ther. 2019;18:1534735419827079. doi: 10.1177/1534735419827079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Z, Zhang J, Guo Q, Chen L, Zhang W, Kang W. Pharmacological effects of verticine: current status. Evid Based Complement Alternat Med. 2019;2019:2394605. doi: 10.1155/2019/2394605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Fan YH, Zhao Y. Experience of professor Luo Yuankai in treating endometriosis with Luo’s the Neiyi prescription. Clin J Chin Med. 2016;8:105–106. [Google Scholar]
- 24.Ma K, Chen YX, Li M. Clinical experience of bushen huoxue therapy in treatment of infertility due to endometriosis. Zhongguo Zhong Yao Za Zhi. 2019;44:1094–1098. doi: 10.19540/j.cnki.cjcmm.20181225.003. [DOI] [PubMed] [Google Scholar]
- 25.Hao X, Xie J, Li Y, Chen W. Acetylated pelargonidin-3-O-glucoside exhibits promising thermostability, lipophilicity, and protectivity against oxidative damage by activating the Nrf2/ARE pathway. Food Funct. 2022;13:2618–2630. doi: 10.1039/d2fo00179a. [DOI] [PubMed] [Google Scholar]
- 26.Lee BS, Lee C, Yang S, Park EK, Ku SK, Bae JS. Suppressive effects of pelargonidin on lipopolysaccharide-induced inflammatory responses. Chem Biol Interact. 2019;302:67–73. doi: 10.1016/j.cbi.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhou BX, Li J, Liang XL, Pan XP, Hao YB, Xie PF, Jiang HM, Yang ZF, Zhong NS. β-sitosterol ameliorates influenza A virus-induced proinflammatory response and acute lung injury in mice by disrupting the cross-talk between RIG-I and IFN/STAT signaling. Acta Pharmacol Sin. 2020;41:1178–1196. doi: 10.1038/s41401-020-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ditty MJ, Ezhilarasan D. β-sitosterol induces reactive oxygen species-mediated apoptosis in human hepatocellular carcinoma cell line. Avicenna J Phytomed. 2021;11:541–550. doi: 10.22038/AJP.2021.17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Gao L, Hou W, Wu J. β-sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-κB pathways in LPS-exposed BV2 cells. Biomed Res Int. 2020;2020:7532306. doi: 10.1155/2020/7532306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan C, Zhang X, Long X, Jin J, Jin R. Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis. 2019;18:157. doi: 10.1186/s12944-019-1096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu R, Hao D, Xu W, Li J, Li X, Shen D, Sheng K, Zhao L, Xu W, Gao Z, Zhao X, Liu Q, Zhang Y. β-sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm Biol. 2019;57:161–168. doi: 10.1080/13880209.2019.1577461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajavel T, Packiyaraj P, Suryanarayanan V, Singh SK, Ruckmani K, Pandima Devi K. β-sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci Rep. 2018;8:2071. doi: 10.1038/s41598-018-20311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park YJ, Bang IJ, Jeong MH, Kim HR, Lee DE, Kwak JH, Chung KH. Effects of β-sitosterol from corn silk on TGF-β1-induced epithelial-mesenchymal transition in lung alveolar epithelial cells. J Agric Food Chem. 2019;67:9789–9795. doi: 10.1021/acs.jafc.9b02730. [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Gao X, Lan M, Li CN, Sun JM, Zhang H. Study the mechanism of peimisine derivatives on NF-κB inflammation pathway on mice with acute lung injury induced by lipopolysaccharide. Chem Biol Drug Des. 2022;99:717–726. doi: 10.1111/cbdd.14013. [DOI] [PubMed] [Google Scholar]
- 35.Du L, Du DH, Chen B, Ding Y, Zhang T, Xiao W. Anti-inflammatory activity of Sanjie Zhentong capsule assessed by network pharmacology analysis of adenomyosis treatment. Drug Des Devel Ther. 2020;14:697–713. doi: 10.2147/DDDT.S228721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu G, Tian L, Herzog SK, Rechoum Y, Gelsomino L, Gao M, Du L, Kim JA, Dustin D, Lo HC, Beyer AR, Edwards DG, Gonzalez T, Tsimelzon A, Huang HJ, Fernandez NM, Grimm SL, Hilsenbeck SG, Liu D, Xu J, Alaniz A, Li S, Mills GB, Janku F, Kittler R, Zhang XH, Coarfa C, Foulds CE, Symmans WF, Andò S, Fuqua SAW. Hormonal Modulation of ESR1 Mutant Metastasis. Oncogene. 2021;40:997–1011. doi: 10.1038/s41388-020-01563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]