Abstract
Background: Currently, there is no optimal treatment strategy for ostial left anterior descending (LAD) or ostial left circumflex artery (LCx) lesions. This study explored effectiveness and safety of drug-coated balloons (DCB) in individuals presenting with ostial LAD or LCx lesions. Methods: A total of 137 patients with de novo ostial LAD or LCx lesions scheduled for DCB treatment were prospectively recruited into the study. After mandatory lesion preparation, DCB-only or hybrid strategy [DCB + drug-eluting stent (DES)] were performed on 120 patients (87.59%). The primary endpoint was the rate of 2-year target lesion revascularization (TLR). Rates of major adverse cardiovascular events (MACE), cardiac death, target vessel myocardial infarction (TVMI), and vessel thrombosis were explored as the secondary outcomes. Quantitative coronary angiography software was used to analyze coronary angiograms. Results: Of the participants, 58 were treated with DCB-only and 62 with hybrid strategy. Relative to the DCB-only group, patients in the hybrid group had longer target lesions (15.47 ± 10.08 vs. 36.85 ± 9.46 mm, P<0.001) and higher Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) scores (23.47 ± 5.22 vs. 29.98 ± 3.18, P<0.001). During follow-up (731 ± 64 days), neither the primary endpoint (TLR) nor the secondary endpoints (including MACE, cardiac death, TVMI, and vessel thrombosis) differed statistically between the two groups (all P > 0.05). Treatment strategy (DCB-only or hybrid) was not a significant risk factor for TLR. Patients who underwent DCB-only exhibited less late lumen loss compared with the patients who underwent hybrid strategy (-0.26 ± 0.59 vs. 0.42 ± 0.47 mm, P<0.001) at 1-year angiographic follow-up. Conclusions: With regards to safety and efficacy, the strategy of DCB as a standalone therapy was similar in comparison with the hybrid strategy of DCB + DES for ostial LAD and ostial LCx lesions. This approach might be effective and technically easy in treating ostial LAD and LCx diseases.
Keywords: Drug-coated balloon, ostial LAD, ostial LCx, de novo
Introduction
Coronary stenosis is often complicated with ostium of the left anterior descending (LAD) or circumflex (LCx) artery. Nonetheless, therapy is challenging owing to the complicated effects of the distal left main (LM) coronary artery, as revealed by prior intravascular ultrasound (IVUS) studies [1]. Ostial stenting, crossover stenting, and other solutions have been studied. However, the findings have not been convincing owing to the ostium’s existing struts. Several studies have investigated the use of focal stenting; yet, the proximal stent edge may protrude to the ostium of the adjacent artery or it may not completely cover the lesion. Aside from that, the adjacent vessel may be affected by plaque shift [2,3]. Despite the fact that stenting from the main vessel (MV) to the LM (crossover method) is linked with improved outcomes [4], it invariably results in complications such as the side branch (SB) ostium being covered by metal struts, SB occlusion, or severe stenosis owing to carina shift. Therefore, the optimal approach remains disputed.
Without the requirement for permanent struts, drug-coated balloons (DCB) efficiently treat in-stent restenosis [5,6]. Several clinical studies reveal that DCB is also advantageous for coronary de novo lesions [7-10], with considerable benefits for small-vessel disease. Numerous studies [11,12] have evaluated the effectiveness and safety of DCB in the treatment of coronary bifurcation lesions.
To our knowledge, there are no reports on the application of DCB to ostial LAD or LCx lesions. Our multicenter study aims to prospectively examine the impact of treatment with DCB-only or hybrid [DCB + drug-eluting stent (DES)] therapy on the 2-year outcomes of patients with ostial LAD or LCx stenosis.
Material and methods
Study subjects
From June 2015 to May 2019, individuals were prospectively recruited in the present research. Patients with a) 2.5-4.0 mm sized coronary vessel and b) ostial LAD or LCx lesions (diameter stenosis ≥ 50%) (scilicet, Medina 0,1,0, or 0,0,1) were included in the study. Exclusion criteria included patients with a) concomitant distal LM stenosis > 30% diagnosed via angiography, b) Medina 0,1,1 LM bifurcation, c) nonadherence to optimization procedures as indicated in the reports review and angiographic films, d) acute myocardial infarction (AMI), and e) cardiogenic shock or unstable hemodynamics (Figure 1).
Figure 1.
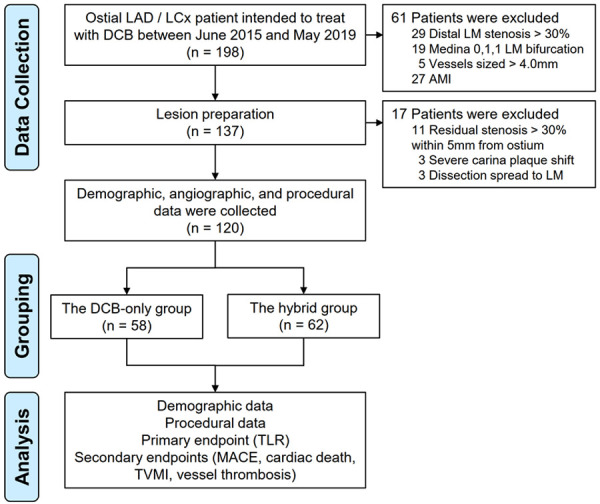
Study flowchart. LAD denotes left anterior descending, LCx left circumflex, DCB drug-coated balloon, LM left main, AMI acute myocardial infraction, TLR target lesion revascularization, MACE major adverse cardiovascular events, and TVMI target vessel myocardial infarction.
Prior to the intervention, patients received 300 mg of aspirin or underwent long-term aspirin treatment. A loading dosage of 600 mg of clopidogrel or 180 mg of ticagrelor was given. Dual antiplatelet treatment (DAPT) was administered to patients treated with DCB-only for 1 to 3 months after the procedure, while those with stent implantation were given DAPT for the duration suggested by guidelines [13,14]. Patients having a history of hypersensitivity or contraindications to DAPT, heparin, paclitaxel, or -limus were excluded, as were women of reproductive age and those with a survival expectancy of less than one year. The research was authorized by the institutional Ethics Committee of the First Affiliated Hospital of Zhengzhou University. All participants provided their written, informed permission. The information was recorded using conventional computerized case report forms.
Study procedures
Prior to DCB therapy, appropriate lesion preparation was given special attention. Mandatory pre-dilatation was performed using a plain balloon, a non-compliant balloon, and a scoring balloon [containing non-slip element (NSE) scoring balloon and cutting balloon] at a balloon-to-vessel ratio of 0.8 to 1.0. Subsequently, DCB angioplasty was performed only in the absence of a large, flow-limiting dissection [< Type C according to the National Heart, Lung, and Blood Institute (NHLBI) classification [15]] and when the residual stenosis was ≤ 30% based on at least two perpendicular angiographic views. If there remaining residual stenosis ≤ 30% just in the proximal 5 mm following lesion preparation, irrelevant of other segments, a hybrid technique was first adopted. A DCB angioplasty was performed first, followed by a stent deployment to guarantee full coverage of the dissection and severe elastic recoil segment (residual stenosis > 30%). To decrease carina plaque shifting, the distance between the proximal end of the stent and the ostium of the vessel must be more than 3 mm. After lesion preparation, individuals with residual stenosis > 30% within 5 mm of the vascular ostium or extensive carina plaque displacement resulting in SB involvement were eliminated. A paclitaxel/iopromide matrix was coated on the DCB utilized in the present investigation (SeQuent™ Please, B. Braun, Melsungen, Germany). To prevent a geographic mismatch, the length of the DCB was set to surpass the target lesion by at least 2 mm. The DCB sizes were fitted to the diameters of the reference vessels using a balloon-to-vessel ratio of 0.80-1.00. At a pressure of > 7 bars, the recommended inflation time is at least 30 seconds. New-generation DESs were implanted in situations where the outcomes of DCB treatment were not adequate owing to significant residual stenosis or dissections (Figures 2 and 3).
Figure 2.
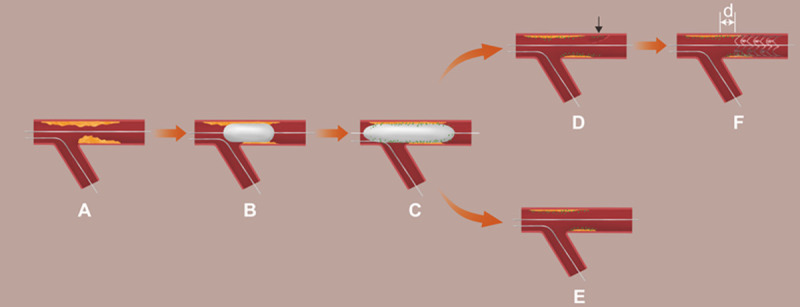
Procedural steps. A. Wiring both branches; B. Lesion preparation: cutting/scoring balloon with balloon/vessel = 0.8~1.0; C. Drug-coated balloon angioplasty; D. Results after DCB angioplasty in hybrid group (or planned DCB-only, but ≥ Type C dissection happened after DCB angioplasty); E. Final result in DCB-only group; F. Stent deployment. Black arrow: Dissection (≥ type C or flow-limited). d: the distance between the proximal end of the stent and the ostium of the vessel ≥ 3 mm.
Figure 3.
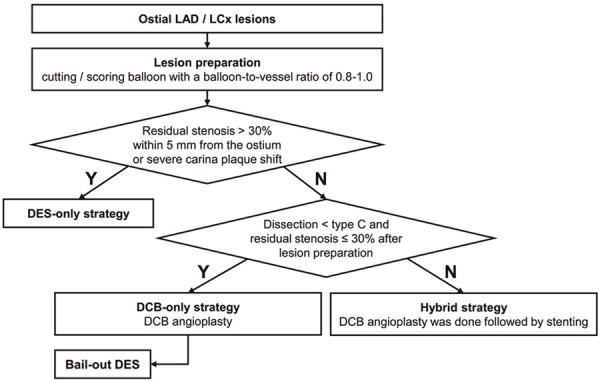
Proposed pathway for the use of DCB in ostial LAD/LCx lesions. LAD denotes left anterior decreasing, LCx left circumflex, DCB drug-coated balloon, DES drug-eluting stent.
Follow-up
Telephone contact or office visits were undertaken for clinical follow-up at 1 month and every 3 months up to 24 months. Follow-up coronary angiography was conducted 12 months after the index operation (after the determination of the main clinical outcome), unless clinical indicators suggested that it be performed prematurely.
Endpoint and definitions
The target lesion revascularization (TLR) rate at 2 years served as the primary endpoint of this study. TLR indicated as coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) because of thrombosis or target lesion restenosis, encompassing distal and proximal edge segments and the ostium of the side branches. The frequencies of major adverse cardiovascular events (MACE) [defined as the composite outcome of cardiac death, TLR, target vessel myocardial infarction (TVMI), and vessel thrombosis], cardiac death, TVMI, and vessel thrombosis were also evaluated. Periprocedural myocardial infarction (MI) (within 48 hours) was defined as cardiac troponin readings that were at least five times the assay’s 99th percentile upper reference limit (URL) plus either of the following: 1) new ischemic electrocardiogram (ECG) alterations or new pathological Q waves; 2) imaging evidence of new loss of viable myocardium or new regional wall motion abnormalities; or 3) angiographically verified graft or coronary artery obstruction or new severe stenosis with thrombosis. Spontaneous myocardial infarction (after 48 h) was defined as a clinical symptom compatible to MI with cardiac troponin levels > 1 × URL and new ST-segment elevation or depression or other abnormalities as described previously [16]. Unless there was convincing proof to the contrary, all MIs were deemed to be TVMIs [17]. The Academic Research Consortium definition of vessel thrombosis was used to identify it [17]. When the cause of death was unknown or indeterminable, cardiogenic causes of death were assumed.
Quantitative coronary angiographic evaluation
For quantitative coronary angiographic (QCA) analysis, edge detection methods and a bifurcation algorithm (QAngio XA version 7.3, Medis Medical Imaging, Leiden, Netherlands) were utilized [18]. The reference for calibration was the guiding catheter. Baseline, post-procedure, and follow-up parameters were evaluated. Using the location of the lesion, the main branch vessel was identified. (a) lesion length; (b) reference vessel diameter (RVD); (c) minimum lumen diameter (MLD); (d) percentage of diameter stenosis; (e) percentage of area stenosis; (f) acute luminal gain (MLD immediately after the procedure minus the MLD before the procedure); and (g) late lumen loss (LLL, MLD immediately after the procedure minus the MLD at follow up). The RVD was the vessel segment that came just after the main branch ostial lesion.
Sample size
TLR is the primary endpoint of the current study. The sample size was determined using the following formula:
N = ((Zα+Zβ)/δ)2 × π 0 × (1 - π 0)
where π is incident rate of TLR of DES in LADo PCI, π0 is incident rate of DCB in de novo large vessel lesions, δ = |π-π0|. Based on previous studies, the incident rate of TLR is 12.2% in DES treatment [4] and 4.3% in DCB treatment [7], respectively. Hence 25 patients were required for a power of 0.8 with α = 0.05.
Statistical analysis
All of the findings were analyzed using R version 3.6.1 (The R Foundation, Vienna, Austria; http://www.r-project.org). Continuous data were reported as means ± standard deviations or [median (interquartile range)], whilst categorical variables were provided as frequencies (or percentages). Using Fisher’s exact test for categorical variables and independent sample t test or Mann-Whitney-Wilcox nonparametric test for continuous variables, the DCB-only and hybrid groups were compared. The time-to-event data were represented by Kaplan-Meier curves and compared using log-rank tests. Multivariate regression analysis was performed to evaluate risk factors for TLR after treatment of ostial LAD or LCx lesions. The following variables were included in model: strategies (DCB-only or hybrid), diabetes, hypertension, hyperlipidemia, acute coronary syndrome (ACS), and diffuse lesion. All statistical significance was assessed using a P-value <0.05.
Results
Baseline clinical, angiographic, and procedural characteristics
A total of 137 ostial LAD/LCx stenosis patients intended for treatment with DCB were identified. After lesion preparation, 17 patients (11 with residual stenosis > 30% within 5 mm from the vessel ostium, 3 with side branch involvement due to severe carina plaque shift, and 3 with dissection spread to LM) were excluded, leaving 120 patients (87.59%) who met the inclusion criteria. Of these, 58 (48.33%) received DCB-only treatment (Figure 1). Baseline features are shown in Table 1. The participants’ mean age was 59.75 ± 11.29 years, 71.67% were male, 39 (32.50%) were diabetic, 59 (49.17%) had hypertension, and 30 (25%) had hyperlipidemia. Clinical parameters did not differ significantly between the DCB-only versus the hybrid group. The incidence of intervention in non-target vessels during the same procedure were similar between the two groups (P > 0.05). Relative to the hybrid group, the proportion of patients with PCI history and left ventricular ejection fraction (LVEF) and were significantly higher in the DCB-only group (P = 0.016 and P = 0.026, respectively).
Table 1.
Demographic characteristics*
| Variable | All patients (n =120) | DCB-only group (n = 58) | Hybrid group (n = 62) | P value |
|---|---|---|---|---|
| Age (years) | 59.75 ± 11.29 | 58.79 ± 10.51 | 60.65 ± 11.99 | 0.351 |
| Sex (Male) | 86 (71.67%) | 41 (70.69%) | 45 (72.58%) | 0.818 |
| Diabetes mellitus | 39 (32.50%) | 18 (31.03%) | 21 (33.87%) | 0.740 |
| Hypertension | 59 (49.17%) | 28 (48.28%) | 31 (50.00%) | 0.850 |
| Hyperlipidemia | 30 (25.00%) | 19 (32.76%) | 11 (17.74%) | 0.058 |
| History of smoking | 45 (37.50%) | 22 (37.93%) | 23 (37.10%) | 0.925 |
| Renal insufficiency | 6 (5.00%) | 3 (5.17%) | 3 (4.84%) | 1.000 |
| Stable angina | 45 (37.50%) | 20 (34.48%) | 25 (40.32%) | 0.509 |
| Unstable angina | 75 (62.50%) | 38 (65.52%) | 37 (59.68%) | |
| Previous MI history | 11 (9.17%) | 5 (8.62%) | 6 (9.68%) | 0.841 |
| Previous PCI history | 19 (15.83%) | 14 (24.14%) | 5 (8.06%) | 0.016 |
| Previous CABG history | 4 (3.33%) | 2 (3.45%) | 2 (3.23%) | 1.000 |
| Family history of CAD | 25 (20.83%) | 14 (24.14%) | 11 (17.74%) | 0.389 |
| LVEF | 61.50 (59.00-64.00) | 62.00 (59.00-64.00) | 59.00 (59.00-63.00) | 0.026 |
| Other vessel treated during the same procedure | 26 (21.67%) | 10 (17.24%) | 16 (25.81%) | 0.255 |
Plus-minus values are means ± SD. CABG denotes coronary artery bypass grafting, CAD coronary artery disease, DES drug-eluting stent, DM diabetes mellitus, LVEF left ventricular ejection fraction, MI myocardial infarction, and PCI percutaneous coronary intervention.
Procedural and angiographic characteristics are presented in Table 2. Over the course of the study, 89 (74.17%) of the target lesions were located in the LAD, and 31 (25.83%) in LCx. Overall, the studied lesions were relatively complex. Extremely tortuous lesions, diffuse disease, lesions with heavy calcification, multi-vessel diseases and total occlusions were included. Average Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) score was 26.83 ± 5.38. Compared to the DCB-only group, lesions in the hybrid group were more diffuse (P<0.001).
Table 2.
Procedural and angiographic characteristics*
| Variable | All patients (n = 120) | DCB-only group (n = 58) | Hybrid group (n = 62) | P value |
|---|---|---|---|---|
| Vascular access | 0.672 | |||
| Trans-radial | 115 (95.83%) | 55 (94.83%) | 60 (96.77%) | |
| Trans-femoral | 5 (4.17%) | 3 (5.17%) | 2 (3.23%) | |
| Treated vessel | 0.208 | |||
| Left anterior descending artery | 89 (74.17%) | 40 (68.97%) | 49 (79.03%) | |
| Left circumflex artery | 31 (25.83%) | 18 (31.03%) | 13 (20.97%) | |
| Feature of lesion | ||||
| Total occlusion | 9 (7.50%) | 2 (3.45%) | 7 (11.29%) | 0.165 |
| Diffuse vessel disease | 58 (48.33%) | 4 (6.90%) | 54 (87.10%) | < 0.001 |
| Calcified lesions | 9 (7.50%) | 6 (10.34%) | 3 (4.84%) | 0.312 |
| RVD | 3.43 ± 0.39 | 3.44 ± 0.38 | 3.43 ± 0.40 | 0.933 |
| Diameter stenosis (by QCA) | 0.70 ± 0.13 | 0.70 ± 0.11 | 0.70 ± 0.14 | 0.348 |
| Area stenosis (by QCA) | 0.89 ± 0.07 | 0.90 ± 0.06 | 0.89 ± 0.08 | 0.348 |
| Lesion length (mm) | 26.52 ± 14.48 | 15.47 ± 10.08 | 36.85 ± 9.46 | < 0.001 |
| SYNTAX score | 26.83 ± 5.38 | 23.47 ± 5.22 | 29.98 ± 3.18 | < 0.001 |
| Lesion preparation | 120 (100%) | 58 (100%) | 62 (100%) | - |
| POBA | 94 (78.33%) | 58 (100.00%) | 36 (58.06%) | < 0.001 |
| NSE scoring balloon | 27 (22.50%) | 11 (18.97%) | 16 (25.81%) | 0.370 |
| Cutting balloon | 81 (67.50%) | 43 (74.14%) | 38 (61.29%) | 0.133 |
| Non-compliant balloon | 22 (18.33%) | 11 (18.97%) | 11 (17.74%) | 0.863 |
| ROTA | 1 (0.83%) | 0 (0.00%) | 1 (1.61%) | 1.000 |
| Maximum pre-dilation balloon diameter (mm) | 3.10 ± 0.35 | 3.14 ± 0.36 | 3.06 ± 0.33 | 0.204 |
| Maximum pre-dilation balloon diameter/RVD ratio | 0.91 ± 0.07 | 0.92 ± 0.08 | 0.90 ± 0.07 | 0.125 |
| DCB use | ||||
| Number of DCBs used (per lesion) | 1.04 ± 0.24 | 1.09 ± 0.34 | 1.00 ± 0.00 | 0.036 |
| DCB diameter (mm) | 3.14 ± 0.40 | 3.21 ± 0.44 | 3.07 ± 0.35 | 0.120 |
| DCB diameter/RVD ratio | 0.92 ± 0.08 | 0.93 ± 0.08 | 0.90 ± 0.08 | 0.017 |
| Length of DCB (mm) | 20.00 (17.00-20.00) | 20.00 (17.00-26.00) | 17.00 (15.00-20.00) | 0.002 |
| Inflation pressure (bar) | 8.00 (8.00-8.00) | 8.00 (8.00-10.00) | 8.00 (8.00-8.00) | 0.129 |
| Coronary dissection after DCBA | < 0.001 | |||
| None | 55 (45.83%) | 35 (60.34%) | 20 (32.26%) | |
| Type A | 25 (20.83%) | 15 (25.86%) | 10 (16.13%) | |
| Type B | 16 (13.33%) | 5 (8.62%) | 11 (17.74%) | |
| Type C | 13 (10.83%) | 2 (3.45%) | 11 (17.74%) | |
| Type D | 10 (8.33%) | 1 (1.73%) | 9 (14.52%) | |
| Type E | 1 (0.83%) | 0 (0.00%) | 1 (1.61%) | |
| Type F | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Bailout stenting | 4 (3.33%) | 4 (6.90%) | 0 (0.00%) | 0.052 |
Plus-minus values are means ± SD. DCB denotes drug-coated balloon, NSE non-slip element, SYNTAX Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery, POBA plain old balloon angioplasty, ROTA rotational atherectomy, and RVD reference vessel diameter.
Lesion preparation was performed in all lesions, and nearly 90% of the lesions were pre-dilatation with scoring balloons. The ratio for maximum pre-dilation balloon diameter to RVD was 0.91 ± 0.07. Total DCB length was 20.97 ± 8.58 mm in each lesion (mean diameter: 3.14 ± 0.40 mm, DCB diameter/RVD ratio: 0.92 ± 0.08). Mean inflation pressure was 8.47 ± 1.50 bars. Coronary dissection after DCB angioplasty in the hybrid group was much more serious compared with that in DCB-only group (P<0.001). The proportion of ≥ type C dissection reached 20% in the total population. Four (6.9%) patients underwent bailout stenting in DCB-only group. Regardless of whether it is a bailout stent in the DCB-only group or a stent in the hybrid group, the proximal edge is more than 3 mm away from the ostium. Additionally, 64 (53.33%) patients underwent IVUS check during procedure.
Clinical outcomes
A total of 114 patients (95.00%) had clinical follow-up data available after 24 months, during which, 2 cardiac deaths (1.75%), 2 TVMI (1.75%), and no probable or definite vessel thrombosis occurred (Table 3). TLR was happened in 6 (5.26%) of the followed patients. In 2 of the 6 patients, restenosis occurred at the proximal margin of the MV; in 1 patient, restenosis occurred within the SB; in 2 patients, restenosis occurred in both MV and SB; the remaining patient had restenosis within the distal segment of stent in hybrid group. And MACE occurred in 9 patients (7.89%). The overall TLR-free survival rate was 95.00% at 2 years (Figure 4). Multivariate regression analysis showed that none of the factors we included was significant risk factor for 2-year TLR (all P > 0.05) (Table S1).
Table 3.
Risk of Primary and Secondary Outcomes at 2-year follow-up*
| Endpoints | All patients (n = 120) | DCB-only group (n = 58) | Hybrid group (n = 62) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Patients with clinical follow-up | 114 (95.00%) | 56 (96.55%) | 58 (93.55%) | - | - |
| TLR | 6 (5.26%) | 2 (3.57%) | 4 (6.90%) | 0.500 (0.088-2.845) | 0.679 |
| MACE† | 9 (7.89%) | 4 (7.14%) | 5 (8.62%) | 0.815 (0.207-3.207) | 1.000 |
| Cardiac death | 2 (1.75%) | 2 (3.57%) | 0 (0.00%) | - | 0.439 |
| TVMI | 2 (1.75%) | 0 (0.00%) | 2 (3.45%) | - | 0.496 |
| Periprocedural | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - | - |
| Non-periprocedural | 2 (1.75%) | 0 (0.00%) | 2 (3.45%) | - | 0.496 |
| Vessel thrombosis | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - | 1.000 |
| Definite | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - | - |
| Probable | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | - | - |
CI denotes confidence interval, DCB drug-coated balloon, DES drug-eluting stent, MACE major adverse cardiovascular events, TLR target lesion revascularization, and TVMI target vessel myocardial infarction.
MACE defined as the composite outcome of cardiac death, TLR, TVMI, and vessel thrombosis.
Figure 4.
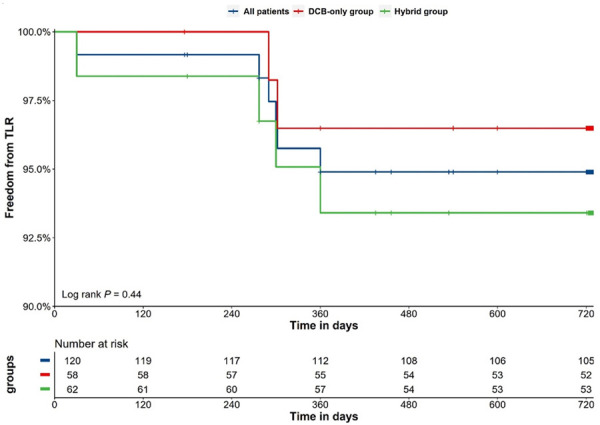
Kaplan-Meier analysis for freedom from target lesion revascularization. DCB denotes drug-coated balloon, TLR target lesion revascularization.
Quantitative coronary analysis
The mean RVD was 3.43 ± 0.39 mm, with a mean target lesion length of 26.52 ± 14.48 mm (15.47 ± 10.08 mm in DCB only group vs. 36.85 ± 9.46 mm in hybrid group, P<0.001). Target vessel intervention resulted in acute luminal gain of 1.62 ± 0.49 mm. Follow-up angiography at a mean interval of 12 months revealed late lumen loss of -0.26 ± 0.59 in DCB-only group and 0.22 ± 0.47 mm in hybrid group (P<0.001) (Table 4).
Table 4.
Quantitative coronary analysis*
| Variable | All patients (n = 120) | DCB-only group (n = 58) | Hybrid group (n = 62) | P value |
|---|---|---|---|---|
| Pre-intervention MLD (mm) | 1.05 ± 0.48 | 1.06 ± 0.54 | 1.03 ± 0.41 | 0.518 |
| Post-intervention MLD (mm) | 2.66 ± 0.39 | 2.58 ± 0.33 | 2.75 ± 0.42 | 0.025 |
| Acute lumen gain (mm) | 1.62 ± 0.49 | 1.53 ± 0.50 | 1.71 ± 0.46 | 0.024 |
| Patients with angiographic follow-up | 87 (72.50%) | 42 (72.41%) | 45 (72.58%) | |
| Follow up MLD (mm) | 2.69 ± 0.63 | 3.03 ± 0.62 | 2.37 ± 0.47 | <0.001 |
| Late lumen loss (mm) | -0.01 ± 0.58 | -0.26 ± 0.59 | 0.22 ± 0.47 | <0.001 |
DCB denotes drug-coated balloon, MLD minimal lumen diameter.
Discussion
The most important conclusion of this research is that lesions at the ostium of the LAD or LCx may be treated safely with DCB, resulting in a minimal incidence of TLR. Lesion lengths that are shorter make it simpler to achieve “stentlessness” with DCB-only therapy. The 2-year clinical follow-up revealed that the outcomes of lesions treated with DCB alone, including TLR and other secondary endpoints, were comparable to those of the hybrid strategy. Multivariate regression showed that whether the final strategy was DCB-only or hybrid did not affect the primary endpoint of the study. The QCA demonstrated that the DCB-only group had late lumen enlargement, while the hybrid group exhibited lumen decrease at one year angiographic follow-up.
Ostial LAD lesion (LM 0,1,0) is conventionally regarded as intractable to percutaneous intervention owing to the technical complexity and danger of severe consequences. Notably, 62% of the arteries had continuous plaque from the LM into both the LAD and LCx arteries, 90% of the arteries had plaque from the LM into the proximal LAD artery, and 66.4% of the arteries had plaque from the LM into the LCx artery. A total of 9.3 and 17.1% of LAD and LCx arteries presented with plaques which did not involve the distal LM, respectively [1].
Stenting the LM towards the LAD and precise stent placement at the LAD ostium level are the primary techniques commonly used to treat this subset of lesions [19]. Cases with a significant bifurcation angle and in which IVUS reveals the lack of disease in the distal LM may be efficiently treated with LAD ostium precise stenting. The precise LAD ostial stenting technique involves covering the counter-carina with a stent protrusion reaching to the circumflex ostium. This approach has limitations, including a) if positioned too proximally, the device protrudes into the LM and may damage LCx, complicating repeat intervention, and b) when the ostial LAD lesion is not entirely covered by the stent, late restenosis and acute recoil may occur [19]. Therefore, appropriate stent location is crucial. In addition, the distal LM is commonly implicated, threatening partial lesion coverage if the affected LM is not covered by the stent. Several studies suggest that LAD and LCx ostial disease should be treated percutaneously with a stent from the LM to the affected MV and provisional SB stenting. In contrast to precise ostial stenting, our technique can guarantee that plaques extending from the target vessel to LM are entirely covered by DCB without any loss of geographic coverage. Simultaneously, the precision requirements for stent positioning are drastically lowered. The essence of the technical solution we proposed is that the distance between the ostium of the vessel and the proximal end of the stent must be ≥ 3 mm (Figure 2) so that the SB ostium and angiographic carina are not covered by stent struts and carina plaque shift is avoided, thereby preserving SB patency.
In a recent study, precise stenting at the LAD ostium was compared to crossover stenting, and the results indicated that crossover stenting was superior to ostial stenting method, with less restenosis [4]. Therefore, based on current understanding, ostial stenting should be avoided unless anatomical conditions are very favorable (un-diseased LM, rectangular angle between LAD-LCx and perfect SB takeoff visualization). In all other situations, crossover stenting should be done, followed by proximal optimization technology (POT) and final kissing (provisional or “inverted” provisional [20] is preferred, if required). Notably, in clinical practice, determining whether to do SB dilation following crossover stenting in an LM intervention is problematic. Despite this, the 5-year cumulative incidence of target lesion revascularization in patients who had crossover stenting from the LM to LAD was not substantially different between the non-kissing and kissing balloon groups [21]. It should be highlighted, however, that the frequency of long-term clinical sequelae associated with accurate LAD ostial stenting, crossover stenting, or SB dilation following crossover stenting remains inadequate.
Beatriz et al. [22] performed a retrospective study to evaluate the safety and efficacy of second-generation drug-coated balloons in patients with SB ostial lesions. 49 patients with a de novo Medina 0,0,1 lesion aggravated by cardiac ischemia were treated with a balloon catheter of the second generation DCB-Dior II. The subjects with LM bifurcation lesions were excluded from the trial. Angiographic success was determined to be 86% (14% of patients presented with acute recoil [n = 5] or had suffered a coronary dissection of type B or more [n = 2]; these patients were treated by insertion of a bare metal stent). At a mean follow-up of 12.2 ± 2.2 months, the risk of MACE was 14.3% (1 myocardial infarction, 0 cardiac deaths, 7 target lesion revascularizations). The results revealed no instances of occlusion or thrombosis. At a mean angiographic follow-up of 7.2 ± 1.1 months, the rate of binary restenosis was 22.5% (n = 7) and a late loss of 0.32 ± 0.73 mm was detected. The absence of ostial LAD/LCx lesions in the research and the relatively small RVD (2.18 ± 0.34 mm) may account for the relatively high TLR rate. Obviously, the fact that the DCB used in this research is Dior II may also be a significant factor.
According to our knowledge, little study has been undertaken on the use of DCB for the treatment of ostial LAD lesions. This type of lesion can be safely and effectively treated using the method we propose (Figures 2 and 3). Only 17 (12.41%) participants were excluded from this research owing to unsatisfactory lesion preparation results. Due to proper and sufficient lesion preparation, the DCB-only method was successful in approximately half of all subjects. In addition, after DCB angioplasty, only a few participants had bailout stenting. Importantly, the 2-year clinical and 1-year angiographic follow-up demonstrated that TLR and MACE incidence rates were much lower than in prior trials using precise stenting or crossover technique. In addition, the present investigation revealed that no patient presented with thrombotic events. And it is well awareness that thrombotic events in the LAD or LCx ostium are disastrous.
DCB effectiveness is substantially impacted by drug transfer, decreased transit time, and bioavailability. This is contingent upon adequate lesion preparation and meticulous angioplasty with a balloon with a diameter between 0.8 and 1.0 × RVD for more than 30 seconds. Appropriate lesion preparation improves acute gain, remodeling, and prevents flow-limiting dissection. Tanaka et al. [23] demonstrated that insufficient pre-dilatation prior DCB predict TLR results. Ostial lesions contain a higher calcium and fibrous tissue composition, as well as a greater elasticity. Notably, over 90% of lesions in our research were prepared with scoring balloons for improved plaque modification. In order to attain the aim of 30% residual stenosis within 5 mm of the vessel ostium, the average balloon-vessel diameter ratio reached 0.91, considerably lengthening the period of balloon expansion. Consequently, after DCB angioplasty, the proportion of dissection > type B reached 20%. Obviously, this did not impair our acute procedure success rate, since we used a hybrid method in this circumstance. Whether a bailout stent or a stent was employed in the DCB-only group or the hybrid group, the proximal edge was more than 3 mm from the ostium. A gap of 3 to 5 mm assures that the MV will not abruptly occlude as a result of severe dissection or hemorrhage (Figures 2, 3). Importantly, following the treatment there are no struts at the ostium, allowing for multiple future intervention options. Therefore, the adoption of this unique approach achieves a very high success rate while avoiding the deficiencies of DES-only solutions.
We also discovered that the overall length of DCB+DES is much longer than the length of the lesion, suggesting that DCB and DES overlapped extensively during intervention. This may be one of the reasons why follow-up TLR and LLL are favorable in the hybrid group despite longer lesions [24]. And paclitaxel and -limus synergy may also play a role. Earlier studies [25,26] have established the overlap between DCB and DES is safe. We did not observe late-acquired malapposition or thrombosis of the stents.
Angiography has a number of limitations for estimating plaque load and distribution, as well as actual luminal size, and it provides little information on plaque composition. IVUS may lead to improved results and facilitate the selection of an appropriate PCI approach [27]. Here, IVUS improved the procedure’s success rate and safety. We evaluated initial plaque distribution, severity of dissection/hematoma, and side branch involvement following lesion preparation or DCB angioplasty.
Our proposed approach is equally relevant to LM 0, 1, 1 bifurcation; however, in these cases, more complex 2-stent techniques may be required when utilizing DES-only. This technique may also be used to other ostial lesions of large branches. Ostial SB lesions are especially significant because they may result in the development of a new MV lesion. Although isolated SB ostial lesions (0, 0, 1) are rare, they are challenging to treat (especially in narrow, “Y” shape angulations). Each operator should be aware of the so-called “sad story of the ostial diagonal lesion” and proceed cautiously, since too vigorous treatment of these lesions may induce trauma to the LAD, resulting in the formation of a new stenosis on the vessel. This issue will be greatly mitigated with the use of the suggested approach.
As an observational research with a limited sample size, the present study has a number of limitations. In addition, 21.67% of the participants received DES implantation in a separate coronary artery during the same procedure owing to the intricacy of the lesions (mean SYNTAX score: 26.83 ± 5.38), which may have an impact on clinical results. Lack of a control arm is the primary drawback of the research. In addition, prospective and randomized studies should be done to assess the efficacy of DES-only vs DCB.
Conclusion
In terms of TLR, MACE, cardiac death, TVMI, and vessel thrombosis, the hybrid approach of DCB + DES was comparable to the DCB-only strategy for de novo ostial LAD and ostial LCx patients. It may be an alternative to stenting or possibly the preferred therapy for individuals who qualify. Consequently, a ‘stent-less’ technique using DCB on these lesions may be beneficial even in the present PCI age.
Acknowledgements
The authors thank the physicians who helped this research (especially Guanghui Liu, Luosha Zhao, Youyou Du, Zhenwen Huang, Feifei Zhang, Xule Wang, and Xiaolin Zheng) and other colleagues who participated in data collection (including Peng Qin, Sen Guo, Yanjun Zhou, Wencai Zhang, Shuai Zhou, Ran Li and Qiangwei Shi). Supported by the Medical Science and Technique Research Plan of He’nan Province (Provincial and Ministerial Co-construction Project) (Grant No. SB201901027).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Oviedo C, Maehara A, Mintz GS, Araki H, Choi SY, Tsujita K, Kubo T, Doi H, Templin B, Lansky AJ, Dangas G, Leon MB, Mehran R, Tahk SJ, Stone GW, Ochiai M, Moses JW. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–112. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]
- 2.Park SJ, Lee CW, Hong MK, Kim JJ, Park SW. Stent placement for ostial left anterior descending coronary artery stenosis: acute and long-term (2-year) results. Catheter Cardiovasc Interv. 2000;49:267–271. doi: 10.1002/(sici)1522-726x(200003)49:3<267::aid-ccd9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Asakaura Y, Takagi S, Ishikawa S, Asakura K, Sueyoshi K, Sakamoto M, Takatsuki S, Oda T, Nakagawa M, Furukawa Y, Oyamada K, Iwanaga S, Ogawa S, Hinohara T. Favorable strategy for the ostial lesion of the left anterior descending coronary artery: influence on narrowing of circumflex coronary artery. Cathet Cardiovasc Diagn. 1998;43:95–100. doi: 10.1002/(sici)1097-0304(199801)43:1<95::aid-ccd28>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Rigatelli G, Zuin M, Baracca E, Galasso P, Carraro M, Mazza A, Lanza D, Roncon L, Daggubati R. Long-term clinical outcomes of isolated ostial left anterior descending disease treatment: ostial stenting versus left main cross-over stenting. Cardiovasc Revasc Med. 2019;20:1058–1062. doi: 10.1016/j.carrev.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Zhu Y, Han Z, Wang X, Wang X, Qiu C. Drug-coated balloon in combination with bare metal stent strategy for de novo coronary artery disease: a PRISMA-compliant meta-analysis of randomized clinical trials. Medicine (Baltimore) 2017;96:e6397. doi: 10.1097/MD.0000000000006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Lu W, Wang X, Pan L, Fu W, Liu Q, Han Z, Sun G, Qin X, Li R, Zheng X, Shan Y, Qiu C. Drug-coated balloon angioplasty: predicting outcomes based on different patterns of drug-eluting stent restenosis. Int J Cardiovasc Imaging. 2020;36:171–178. doi: 10.1007/s10554-019-01681-y. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Zhu Y, Han Z, Sun G, Qin X, Wang Z, Liu G, Xi W, Wang X, Pan L, Qiu C. Short-term outcomes from drug-coated balloon for coronary de novo lesions in large vessels. J Cardiol. 2019;73:151–155. doi: 10.1016/j.jjcc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Farah A, Ohlow MA, Mangner N, Mobius-Winkler S, Weilenmann D, Wohrle J, Stachel G, Markovic S, Leibundgut G, Rickenbacher P, Osswald S, Cattaneo M, Gilgen N, Kaiser C, Scheller B BASKET-SMALL 2 Investigators. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504–1510. doi: 10.1016/S0140-6736(20)32173-5. [DOI] [PubMed] [Google Scholar]
- 9.Ali RM, Degenhardt R, Zambahari R, Tresukosol D, Ahmad WA, Kamar H, Kui-Hian S, Ong TK, bin Ismail O, bin Elis S, Udychalerm W, Ackermann H, Boxberger M, Unverdorben M. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;7(Suppl K):K83–92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Lu W, Han Z, Pan S, Wang X, Shan Y, Wang X, Zheng X, Li R, Zhou Y, Qin P, Shi Q, Zhou S, Zhang W, Guo S, Zhang P, Qin X, Sun G, Qin Z, Huang Z, Qiu C. Clinical outcomes of drug-coated balloon in coronary lesions: a real-world, all-comers study. Clin Res Cardiol. 2022;111:732–741. doi: 10.1007/s00392-021-01895-y. [DOI] [PubMed] [Google Scholar]
- 11.Lopez Minguez JR, Nogales Asensio JM, Doncel Vecino LJ, Sandoval J, Romany S, Martinez Romero P, Fernandez Diaz JA, Fernandez Portales J, Gonzalez Fernandez R, Martinez Caceres G, Merchan Herrera A, Alfonso Manterola F BABILON Investigators. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10:50–57. doi: 10.4244/EIJV10I1A10. [DOI] [PubMed] [Google Scholar]
- 12.Worthley S, Hendriks R, Worthley M, Whelan A, Walters DL, Whitbourn R, Meredith I. Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-month results of the multicenter BIOLUX-I study. Cardiovasc Revasc Med. 2015;16:413–417. doi: 10.1016/j.carrev.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 14.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 15.Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. 1991;68:467–471. doi: 10.1016/0002-9149(91)90780-o. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 17.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 18.Collet C, Onuma Y, Cavalcante R, Grundeken M, Genereux P, Popma J, Costa R, Stankovic G, Tu S, Reiber JHC, Aben JP, Lassen JF, Louvard Y, Lansky A, Serruys PW. Quantitative angiography methods for bifurcation lesions: a consensus statement update from the European Bifurcation Club. EuroIntervention. 2017;13:115–123. doi: 10.4244/EIJ-D-16-00932. [DOI] [PubMed] [Google Scholar]
- 19.Burzotta F, Lassen JF, Lefevre T, Banning AP, Chatzizisis YS, Johnson TW, Ferenc M, Rathore S, Albiero R, Pan M, Darremont O, Hildick-Smith D, Chieffo A, Zimarino M, Louvard Y, Stankovic G. Percutaneous coronary intervention for bifurcation coronary lesions: the 15(th) consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307–1317. doi: 10.4244/EIJ-D-20-00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burzotta F, Lassen JF, Louvard Y, Lefevre T, Banning AP, Daremont O, Pan M, Hildick-Smith D, Chieffo A, Chatzizisis YS, Dzavik V, Gwon HC, Hikichi Y, Murasato Y, Koo BK, Chen SL, Serruys P, Stankovic G. European Bifurcation Club white paper on stenting techniques for patients with bifurcated coronary artery lesions. Catheter Cardiovasc Interv. 2020;96:1067–1079. doi: 10.1002/ccd.29071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida K, Toyofuku M, Morimoto T, Ohya M, Fuku Y, Higami H, Yamaji K, Muranishi H, Yamaji Y, Furukawa D, Tada T, Ko E, Kadota K, Ando K, Sakamoto H, Tamura T, Kawai K, Kimura T AOI LMCA Stenting Registry Investigators. Prognostic impact of final kissing balloon technique after crossover stenting for the left main coronary artery: from the AOI-LMCA registry. Cardiovasc Interv Ther. 2019;34:197–206. doi: 10.1007/s12928-018-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaquerizo B, Fernandez-Nofreiras E, Oategui I, Suarez de Lezo J, Rumoroso JR, Martin P, Routledge H, Tizon-Marcos H. Second-generation drug-eluting balloon for ostial side branch lesions (001-Bifurcations): mid-term clinical and angiographic results. J Interv Cardiol. 2016;29:285–292. doi: 10.1111/joic.12292. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka A, Latib A, Jabbour RJ, Kawamoto H, Giannini F, Ancona M, Regazzoli D, Mangieri A, Mattioli R, Chieffo A, Carlino M, Montorfano M, Colombo A. Impact of angiographic result after predilatation on outcome after drug-coated balloon treatment of in-stent coronary restenosis. Am J Cardiol. 2016;118:1460–1465. doi: 10.1016/j.amjcard.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Ielasi A, Buono A, Pellicano M, Tedeschi D, Loffi M, Donahue M, Regazzoli D, De Angelis G, Danzi G, Reimers B, Tespili M. A Hybrid approach evaluating a drug-coated balloon in combination with a new-generation drug-eluting stent in the treatment of De Novo diffuse coronary artery disease: the hyper pilot study. Cardiovasc Revasc Med. 2021;28:14–19. doi: 10.1016/j.carrev.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Cortese B, Silva Orrego P, Agostoni P, Buccheri D, Piraino D, Andolina G, Seregni RG. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. 2015;8:2003–2009. doi: 10.1016/j.jcin.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Mitomo S, Jabbour RJ, Mangieri A, Ancona M, Regazzoli D, Tanaka A, Giannini F, Carlino M, Montorfano M, Chieffo A, Latib A, Colombo A. Mid-term clinical outcomes after bailout drug-eluting stenting for suboptimal drug-coated balloon results: insights from a milan registry. Int J Cardiol. 2018;263:17–23. doi: 10.1016/j.ijcard.2018.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Legutko J, Yamawaki M, Costa RA, Costa MA. IVUS in bifurcation stenting: what have we learned? EuroIntervention. 2015;11(Suppl V):V55–58. doi: 10.4244/EIJV11SVA12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


