Abstract
Objective: To investigate the expression of single-stranded microRNAs (miRNAs) in serum and gingival crevicular fluid of patients with type 2 diabetes mellitus (T2DM) complicated by periodontal disease and its correlation with inflammatory factors. Methods: Twenty-six periodontitis patients without diabetes mellitus (periodontal group), 24 patients with T2DM (T2DM group), 22 patients with both T2DM and periodontal disease (comorbid group), and 25 healthy individuals without a history of periodontal disease (healthy group) were recruited respectively. Serum and gingival crevicular fluid specimens were collected to detect the expression levels of miRNAs and inflammatory factors including serum tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), and transforming growth factor-β (TGF-β), and their correlations were also investigated. Results: Eleven miRNAs were detected in the gingival crevicular tissue of all subjects. The expression of miR-223 and miR-200b in serum and gingival crevicular fluid was elevated higher in the comorbid group than in the other three groups (P<0.05), and their expressions in gingival crevicular fluid contributed to the differential diagnosis of periodontal disease from diabetes mellitus comorbid with periodontal disease (P<0.05). Gingival crevicular fluid miR-223 expression was positively associated with TNF-α, clinical attachment loss (CAL), and probing pocket depth (PPD) in the periodontal group, while negatively associated with IL-10 (P<0.05), and so was gingival crevicular fluid miR-200b expression with TNF-α, CAL, and PPD (P<0.05). In the comorbid group, gingival crevicular fluid miR-223 expression showed a positive correlation with TNF-α, CAL, and PPD (P<0.05), and so did gingival crevicular fluid miR-200b expression with TNF-α, CAL, and PPD (P<0.05). Conclusions: Our findings indicate a close link between the levels of miR-223 and miR-200b in serum and gingival crevicular fluid and susceptibility to T2DM as well as the pathogenesis of periodontal disease.
Keywords: T2DM, periodontal disease, gingival crevicular fluid, single-stranded microRNA
Introduction
Periodontal disease shows an annually increasing prevalence, and its risk is 2.8-3.4 times higher in diabetic patients than those without diabetes mellitus [1]. The mutual promotion of type 2 diabetes mellitus (T2DM) and periodontal disease is attributed to the connection between the capability for glycemic control and the severity of periodontal disease, since the dysregulation of periodontal microorganisms and inflammatory factors in susceptible hosts will disrupt blood glucose control [2]. Currently, despite the fact that the mechanism of interaction between T2DM and periodontal disease is of concern, there are few reliable biomarkers to identify the two diseases, so disease management strategies for periodontal disease and diabetes mellitus remain one of the most pressing clinical issues.
Excessive inflammatory immune response in patients with periodontal disease aggravates the systemic inflammatory burden, and tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) are highly sensitive in reflecting the inflammation degree [3]. In patients with only T2DM, abnormal changes in serum cytokines such as TNF-α and IL-10 can also be detected [4]. Thus, the relationship between chronic periodontal disease and systemic manifestations needs to be studied in depth from the aspects of transmitters and biomarkers. In recent years, scholars emphasized that the molecular components of gingival crevicular fluid present a clear relationship with the diagnostic or prognostic biomarkers of periodontal environmental changes. Moreover, the gingival crevicular fluid specimens have been gradually considered as clinical research objects with the characteristics of convenient collection and non-invasiveness.
Highly conserved miRNAs are non-coding members of ribonucleic acid and mediate a variety of biologic behaviors as major protein synthesis regulators. It has been found that abnormal miRNA expression specifically exists in the serum of patients with both diabetes mellitus and periodontal disease [5]. However, few reports on the mechanism of miRNAs in periodontal disease were found. Studies have shown that in gingival tissue biopsy or cell cultures of patients with chronic periodontal disease, the abnormal expression of miR-223, miR-203, and miR-200b exerts regulatory effects on gene expression [6], while the deregulation of specific miRNA is affected by a variety of factors and cytokines. We assumed that deregulated miRNAs in serum and gingival crevicular fluid are involved in the occurrence and development of periodontal disease in patients with T2DM. Thus, this study was designed to evaluate the difference of miRNA expression and its correlation with the disease and provide a new therapeutic target for the prevention and treatment of periodontal disease comorbid with T2DM.
Materials and methods
General information
A total of 26 periodontitis patients without diabetes mellitus, 24 patients with T2DM, and 22 patients with both T2DM and periodontal disease diagnosed and treated in our hospital from February 2019 to February 2021, and 25 healthy individuals without a history of periodontal disease were selected and assigned to the periodontal group, T2DM group, comorbid group, and healthy group, respectively. The research was approved by the Medical Ethics Committee (approval No. AF-SQ012), and the subjects and (or) clients signed a consent form after being fully informed of the research.
Inclusion criteria: (1) 25-65 years old; (2) Patients were diagnosed with periodontal disease of stage II based on consensus and clinical characteristics in the groups other than the T2DM group and healthy group, and healthy individuals with periodontal pockets ≤3 mm and no decrease in bleeding during exploration in the healthy group; (3) Individuals with 20 teeth or more; (4) Individuals without a history of periodontal treatment within 1 year; (5) Individuals without a history of antibiotics or hormone medications half a month before admission.
Exclusion criteria: (1) Patients complicated by systemic inflammation, immune disease, heart disease, or heart failure; (2) Patients with a history of orthodontic treatment; (3) Women during pregnancy or breastfeeding; (4) Individuals with a long-term history of smoking and alcoholism; (5) Individuals with habits that overburden the teeth, such as bruxism.
The socio-demographic characteristics, including gender, age, body mass index (BMI), family history, smoking history, drinking history, employment, education level, fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), clinical attachment level (CAL), bleeding on probing (BoP) and probing pocket depth (PPD) of subjects were collected.
Methods
Specimen collection
After the epigingival plaque was cleared, the gingival crevicular fluid sample was collected from the mesial part of the cheek side in each subject from 9:00 to 11:00 on the next morning of admission, with a depth of periodontal pocket (PD) >4 mm, a PPD ≤2 mm and no recession in the two selected sites. A Perio-paper™ patch (Oraflow Inc., USA) was inserted into the mesial periodontal pocket on the cheek side of the tooth until it encountered a slight resistance. After standing for 1 minute, three strips from the selected position (the strips contaminated with blood and saliva were discarded) were collected, transferred to Eppendorf test tubes (containing 200 µl Qiazol®) for elution, and stored at -40°C for later use.
The elbow venous blood of each subject was collected in the morning, placed in a vacuum blood collection tube containing coagulant for 30 minutes, and then centrifuged at 4000 rpm for 10 minutes to separate the serum. The separated serum was transferred into an Eppendorf test tube and stored in a refrigerator at -40°C.
miRNA analysis
The total miRNAs contained in the sample were extracted by an RNApure ultrapure total RNA rapid extraction kit (akara Bio, USA), and a TaKaRa reverse transcription kit was used to reversely transcribe the total RNA into cDNA that would then be used as the template reference primer and probe sequence for reverse transcription. The product was incubated at 37°C for 60 minutes, and at 95°C for 5 minutes to inactivate easyscript RT, and then stored at -80°C. A miScript SYBR® Green PCR Kit (Qiagen, Germany) for real-time quantitative PCR (RT-PCR) was adopted to quantify the expression levels of miR-223, miR-203, and miR-200b (The inner primers were purchased from Hepeng Biotechnology Co., Ltd., catalog number: HPBIO-Elisa2846). The expression level of target miRNAs was normalized as steady-state miRNAs expression, and the cycle number (Ct) was recorded according to the amplification curve. Finally, the relative change in miRNA expression under steady-state miRNA expression was calculated.
Detection of inflammatory factors
Instructions of the reference kit: Before the experiment, all reagents should be equilibrated to room temperature. TNF-α, IL-10, and TGF-β reagent kits were purchased from Quanzhou Ruixin Biotechnology Co., Ltd., batch No.: 20190305, 20191102, 20190305. In preparation, reagents or samples should be mixed thoroughly to avoid foaming. The specific steps were as follows: (1) Sample addition: Blank wells, standard wells, and sample wells to be tested were set, and 100 μL standard and sample diluent were added to the blank wells, and 100 μL standard or sample to be tested were added to the rest wells and shaken well. The samples were added to the bottom of the ELISA plate, without staining the wall of the well, to avoid bubbles and ensure a successful and precise result. The ELISA plate was then sealed and incubated at 37°C for 90 minutes; (2) The liquid was removed by flicking the plate over a sink. Biotinylated antibody working solution (100 μL) was added into each well (prepared within 15 minutes before use), and the ELISA plate was sealed with a sealing film, and incubated at 37°C for 1 hour; (3) The liquid in the well was removed. The plate was then washed 3 times, soaked for 1 to 2 minutes each time, about 350 μL/well, and then patted with an absorbent paper to keep the wells dry; (4) Enzyme conjugate working solution (100 μL) was added into each well, and the wells were sealed with sealing film and incubated at 37°C for 30 minutes; (5) The liquid in the well was then removed, and the plate was washed 5 times, and treated as the same as the operation in step 3; (6) Substrate solution (90 μL) was added into each well, and the ELISA plate was sealed with sealing film and incubated at 37°C for about 15 minutes in the dark; (7) Stop solution (50 μL) was added into each well to stop the reaction, and the blue color in the wells turned to yellow instantly; (8) The microplate reader was employed to measure the optical density (OD value) of each well at a wavelength of 450 nm to obtain the concentration of TNF-α, IL-10, and TGF-β.
Statistical methods
The SPSS 23.0 software was used to input and analyze the data. Measured data were expressed as “x±s”, and independent sample t-test was used for comparison between the two groups, while ANOVA was used for measured data comparison among multiple groups followed by snk-q test for pairwise comparison, and paired sample t-test was used for intragroup comparison before and after treatment. The counted data were expressed by frequency or constituent ratio and compared by chi-square exact probability method. P<0.05 was considered a significant difference.
Results
Socio-demographic characteristics of each group
There was no statistically significant difference among the four groups in terms of gender, age, body mass index (BMI), family medical history, smoking history, drinking history, employment, or education level (all P>0.05). Both the periodontal group and the comorbid group were confirmed BoP positive (+). Compared with the healthy group, the values of FBG and HbA1c in the other groups increased sharply (both P<0.05), and CAL and PPD also increased in the periodontal group and the comorbid group (both P<0.05). By contrast to the periodontal group, an elevation was observed in the values of FBG, HbA1c, CAL, and PPD in the comorbid group and in the values of FBG and HbA1c in the T2DM group (all P<0.05) but no significant changes in the values of CAL and PPD (both P>0.05). As compared to the T2DM group, the values of FBG, HbA1c, CAL, and PPD in the comorbid group showed a rise (all P<0.05). See Table 1.
Table 1.
Socio-demographic characteristics of the four groups [x̅±s/n (%)]
| Item | Periodontal group (n=26) | T2DM group (n=24) | Comorbid group (n=22) | Healthy group (n=25) | F | P |
|---|---|---|---|---|---|---|
| Gender (male/female) | 12/14 | 13/11 | 10/12 | 12/13 | 0.450 | 0.930 |
| Family medical history (yes/no) | 13/13 | 12/12 | 10/12 | 14/11 | 0.532 | 0.912 |
| History of smoking (yes/no) | 24/2 | 20/4 | 18/4 | 25/0 | 5.590 | 0.133 |
| History of drinking (yes/no) | 18/8 | 16/8 | 15/7 | 18/7 | 0.174 | 0.982 |
| Employment (yes/no) | 20/6 | 19/5 | 14/8 | 19/6 | 1.736 | 0.629 |
| Education level (high school and below/high school and above) | 19/7 | 15/9 | 14/8 | 16/9 | 0.810 | 0.847 |
| BoP (+/-) | 26/0 | 22/2 | 22/0 | 0/25 | ||
| Age (year) | 37.74±6.25 | 40.15±6.33 | 38.89±6.35 | 39.66±6.28 | 0.700 | 0.554 |
| BMI (kg/m2) | 20.01±3.11 | 19.68±3.02 | 20.44±3.14 | 20.18±3.20 | 0.242 | 0.867 |
| FBG (mmol/L) | 9.58±2.17a | 9.56±2.20a | 10.12±2.13a,b | 6.32±1.38 | 18.553 | <0.001 |
| HbA1c (%) | 8.71±1.97a | 8.22±1.96a | 9.01±2.04a,b | 5.25±1.47 | 21.048 | <0.001 |
| CAL (mm) | 6.35±1.32a | 6.33±1.30a | 8.87±1.20a,b | 1.22±0.24 | 201.747 | <0.001 |
| PPD (mm) | 4.80±0.98a | 4.86±1.01a | 5.88±1.14a,b | 1.19±0.20 | 126.065 | <0.001 |
indicates that compared with the healthy group, P<0.05.
indicates that compared with another group of patients, P<0.05.
Level of inflammatory factors in serum and gingival crevicular fluid in each group
No significant difference was found in serum TNF-α, IL-10, or TGF-β among the periodontal group, T2DM group, and healthy group (all P>0.05), and their levels in the comorbid group were higher than those in the healthy group (all P<0.05). Compared with the healthy group, the other three groups showed elevated levels of TNF-α and TGF-β, but a decreased level of IL-10 in the gingival crevicular fluid (all P<0.05). Compared with the periodontal group, the levels of the three in the gingival crevicular fluid in the T2DM group presented no changes, while those in the comorbid group increased significantly (all P<0.05). In comparison with the T2DM group, the levels of TNF-α and TGF-β in gingival crevicular fluid of the comorbid group rose greatly, but IL-10 declined significantly (all P<0.05). See Table 2.
Table 2.
Inflammatory factor levels in serum and gingival crevicular fluid in the four groups (x̅±s, pg/ml)
| Item | Periodontal group (n=26) | T2DM group (n=24) | Comorbid group (n=22) | Healthy group (n=25) | F | P | |
|---|---|---|---|---|---|---|---|
| Serum | TNF-α | 4.38±0.54 | 4.29±0.50 | 5.17±0.61 | 4.13±0.42 | 18.109 | <0.001 |
| IL-10 | 4.36±0.36 | 4.40±0.35 | 4.22±0.31 | 5.02±0.47 | 20.560 | <0.001 | |
| TGF-β | 3.66±0.47 | 3.68±0.52 | 4.44±0.62 | 3.62±0.51 | 12.586 | <0.001 | |
| gingival crevicular fluid | TNF-α | 4.77±0.54a | 4.81±0.53a | 6.03±0.66a,b | 4.26±0.44 | 43.723 | <0.001 |
| IL-10 | 4.90±0.60a | 4.96±0.62a | 4.34±0.45 | 6.22±0.71a,b | 40.476 | <0.001 | |
| TGF-β | 4.40±0.41a | 4.48±0.52a | 5.69±0.58a,b | 3.77±0.36 | 66.973 | <0.001 | |
indicates that compared with the healthy group, P<0.05.
indicates that compared with another group of patients, P<0.05.
miRNA analysis
Total RNA integrity identification
The concentration of extracted total RNA was about 2 μg/ul, and the ratio of A260/A280 was greater than 1.8, which verified that its concentration and purity met the requirements of the experiment. In addition, the total RNA electrophoresis picture showed that there was no obvious decomposition of RNA, as shown in Figure 1.
Figure 1.
Electrophoresis of total RNA extracted from periodontal tissue.
Expression of miRNA in gingival crevicular tissue
RT-PCR detected 11 miRNAs in the gingival crevicular tissues of patients with periodontal disease. Mann-Whitney U test revealed that 4 of them (miR-223, miR-200b, miR-106, miR-103) had significant differential expression in the gingival crevicular tissues between the periodontal group and the healthy group (all P<0.05). The expression of miR-223 and miR-200b increased more than 1.5 times, while the increase of miR-106 and miR-103 was lower. By contrast to the periodontal group, the expression levels of miR-223 and miR-200b in the T2DM group plummeted (all P<0.05), while those in the comorbid group surged (all P< 0.05). Compared with the T2DM group, the expression levels of miR-223 and miR-200b in the comorbid group showed a significant rise (both P<0.05). See Table 3.
Table 3.
Differential expression of miRNA in the gingival crevicular tissue of the four groups (x̅±s)
| Item | Periodontal group (n=26) | T2DM group (n=24) | Comorbid group (n=22) | Healthy group (n=25) | F | P |
|---|---|---|---|---|---|---|
| Hsa-miR-141 | 1.36±0.22 | 1.40±0.26 | 1.44±0.27 | 1.32±0.30 | 0.906 | 0.441 |
| Hsa-miR-201a | 1.17±0.18 | 1.20±0.19 | 1.19±0.20 | 1.21±0.19 | 0.206 | 0.892 |
| Hsa-miR-21c | 1.85±0.20 | 1.89±0.22 | 1.85±0.24 | 1.80±0.23 | 0.674 | 0.569 |
| Hsa-miR-22b | 1.78±0.32 | 1.79±0.33 | 1.80±0.34 | 1.82±0.35 | 0.066 | 0.978 |
| Hsa-miR-223 | 3.25±0.45a | 3.01±0.48a,b | 3.88±0.51a,b | 1.58±0.37 | 110.564 | <0.001 |
| Hsa-miR-200b | 3.06±0.21a | 2.74±0.22a,b | 3.51±0.30a,b | 2.47±0.16 | 91.841 | <0.001 |
| Hsa-miR-23a | 2.10±0.26 | 2.14±0.28 | 2.16±0.32 | 2.15±0.33 | 0.194 | 0.900 |
| Hsa-miR-106 | 2.13±0.33a | 2.20±0.28a | 2.40±0.30a,b | 1.92±0.26 | 10.671 | <0.001 |
| Hsa-miR-103 | 2.24±0.35a | 2.19±0.32a | 2.60±0.28a,b | 2.01±0.32 | 13,750 | <0.001 |
| Hsa-miR-158 | 2.30±0.33 | 2.36±0.34 | 2.40±0.32 | 2.29±0.33 | 0.582 | 0.628 |
| Hsa-miR-203 | 1.31±0.12 | 1.32±0.14 | 1.29±0.15 | 1.26±0.12 | 0.989 | 0.401 |
indicates that compared with the healthy group, P<0.05.
indicates that compared with another group of patients, P<0.05.
Analysis of serum miRNA levels in each group
Compared with the healthy group, the expression levels of miR-223, miR-200b, miR-106 and miR-103 in the other three groups presented a rise (both P<0.05). In contrast to the periodontal group, the expression levels of miR-223 and miR-200b in the T2DM group dropped (both P<0.05), while those four in the comorbid group elevated (both P<0.05). Compared with the T2DM group, the expression levels of the four in the comorbid group also increased (both P<0.05). See Table 4.
Table 4.
Differential expression of miRNA in serum of the four groups (x̅±s)
| Item | Periodontal group (n=26) | T2DM group (n=24) | Comorbid group (n=22) | Healthy group (n=25) | F | P |
|---|---|---|---|---|---|---|
| Hsa-miR-141 | 1.44±0.24 | 1.42±0.26 | 1.39±0.27 | 1.33±0.26 | 0.876 | 0.456 |
| Hsa-miR-201a | 1.22±0.20 | 1.20±0.21 | 1.25±0.20 | 1.20±0.18 | 0.327 | 0.806 |
| Hsa-miR-21c | 1.89±0.23 | 1.85±0.21 | 1.84±0.22 | 1.83±0.20 | 0.379 | 0.768 |
| Hsa-miR-22b | 1.75±0.26 | 1.80±0.30 | 1.77±0.28 | 1.74±0.30 | 0.210 | 0.889 |
| Hsa-miR-223 | 3.20±0.36a | 3.05±0.33a,b | 3.90±0.48a,b | 2.06±0.38 | 90.304 | <0.001 |
| Hsa-miR-200b | 3.11±0.17a | 2.78±0.11a,b | 3.40±0.29a,b | 2.50±0.14 | 104.023 | <0.001 |
| Hsa-miR-23a | 2.20±0.19 | 2.18±0.22 | 2.21±0.23 | 2.23±0.30 | 0.187 | 0.905 |
| Hsa-miR-106 | 2.18±0.21a | 2.19±0.24a | 2.48±0.22a,b | 1.98±0.20 | 20.728 | <0.001 |
| Hsa-miR-103 | 2.22±0.27a | 2.20±0.25a | 2.48±0.26a,b | 2.13±0.20 | 8.840 | <0.001 |
| Hsa-miR-158 | 2.23±0.21 | 2.26±0.22 | 2.30±0.23 | 2.29±0.20 | 0.534 | 0.661 |
| Hsa-miR-203 | 1.36±0.11 | 1.38±0.12 | 1.39±0.13 | 1.33±0.14 | 1.070 | 0.366 |
indicates that compared with the healthy group, P<0.05.
indicates that compared with another group of patients, P<0.05.
The predictive value of miRNA levels in serum and gingival crevicular fluid for disease
Predictive value of miRNA levels in serum and gingival crevicular fluid for periodontal disease
Table 5 demonstrates that the levels of miR-223 and miR-200b in the gingival crevicular fluid can be employed for the diagnosis of periodontal disease (AUC>0.8, P<0.05), as shown in Figure 2.
Table 5.
Efficacy indexes of miRNA levels in serum and gingival crevicular fluid in predicting periodontal disease
| Item | Sensitivity (%) | Specificity (%) | AUC (95% CI) | P |
|---|---|---|---|---|
| Serum miR-223 | 60.6 | 46.7 | 0.643 (0.529~1.758) | 0.056 |
| Serum miR-200b | 51.5 | 92.0 | 0.744 (0.637~6.851) | 0.060 |
| Gingival crevicular fluid miR-223 | 72.7 | 89.3 | 0.862 (0.780~0.945) | <0.001 |
| Gingival crevicular fluid miR-200b | 75.58 | 88.87 | 0.880 (0.625~0.996) | 0.005 |
Figure 2.
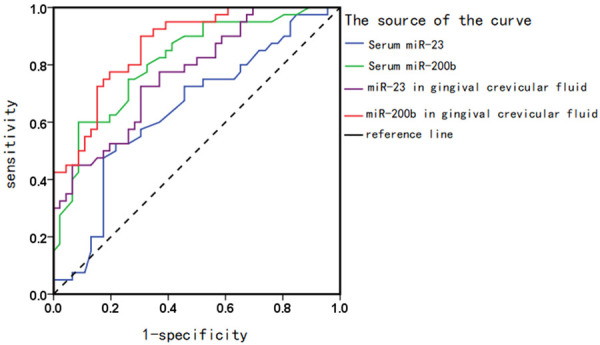
ROC curve of miRNA levels in serum and gingival crevicular fluid in predicting periodontal disease.
Predictive value of miRNA levels in serum and gingival crevicular fluid for T2DM comorbid with periodontal disease
The levels of miR-223 and miR-200b in the gingival crevicular fluid can help identify the comorbidity with diabetes mellitus and periodontal disease (AUC>0.8, P<0.05), as shown in Table 6 and Figure 3.
Table 6.
Efficacy indicators of miRNA levels in serum and gingival crevicular fluid in predicting the complication of T2DM with periodontal disease
| Item | Sensitivity (%) | Specificity (%) | AUC (95% CI) | P |
|---|---|---|---|---|
| Serum miR-223 | 47.52 | 63.02 | 0.448 (0.128~1.758) | 0.115 |
| Serum miR-200b | 58.69 | 62.35 | 0.654 (0.337~6.851) | 0.068 |
| Gingival crevicular fluid miR-223 | 74.02 | 88.02 | 0.929 (0.893~0.965) | 0.008 |
| Gingival crevicular fluid miR-200b | 77.08 | 86.03 | 0.805 (0.574~0.842) | 0.012 |
Figure 3.
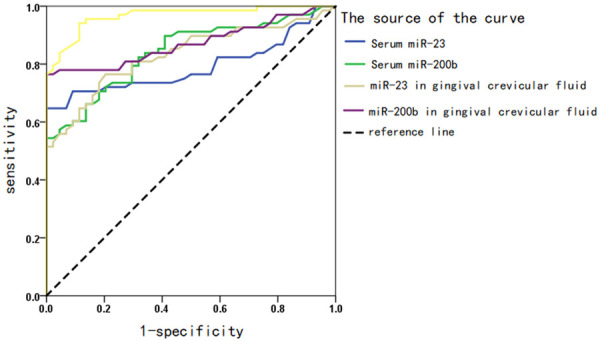
ROC curve of miRNA levels in serum and gingival crevicular fluid in predicting the complication of T2DM by periodontal disease.
Multiple linear regression analysis of miRNA levels in serum and gingival crevicular fluid
According to the absolute value of the standardized regression coefficient, the factors with an effect on the level of miR-223 in the gingival crevicular fluid were CAL, BoP, PPD in descending order (all P<0.05), and those affecting the level of miR-200b were CAL, PPD, and BoP in descending order (all P<0.05). See Table 7. Other factors were not listed in the table as they were not included in the regression equation.
Table 7.
Multiple linear regression analysis parameters of miRNA levels in serum and gingival crevicular fluid
| Independent variable | β | t | P |
|---|---|---|---|
| Constant (miR-223) | 22.360 | 7.889 | 0.001 |
| CAL | 19.968 | 6.668 | 0.015 |
| BoP | 18.553 | 0.365 | 0.019 |
| PPD | 17.625 | 0.474 | 0.024 |
| Constant (miR-200b) | 20.362 | 3.025 | 0.001 |
| CAL | 20.401 | 3.117 | 0.001 |
| BoP | 16.302 | 2.032 | 0.026 |
| PPD | 18.879 | 3.056 | 0.013 |
Correlation analysis of miRNA levels in serum and gingival crevicular fluid with inflammatory factors and periodontal related factors
With the level of miRNA in the periodontal group as a whole, the expression of miR-223 in gingival crevicular fluid was positively correlated with TNF-α, CAL, and PPD, while negatively correlated with IL-10 (P<0.05). There were positive correlations of gingival crevicular fluid miR-200b with TNF-α, CAL, and PPD (all P<0.05). See Table 8. With the level of miRNA in the comorbid group as a whole, gingival crevicular fluid miR-223 expression was positively correlated with TNF-α, CAL, and PPD (all P<0.05), and gingival crevicular fluid miR-200b expression was also positively correlated with TNF-α, CAL and PPD, while negatively correlated with IL-10 (all P<0.05). See Tables 8 and 9.
Table 8.
Correlation of miRNA levels in serum and gingival crevicular fluid with inflammatory factors and periodontal-related factors
| Relevant factors | Serum miR-223 | Serum miR-200b | Gingival crevicular fluid miR-223 | Gingival crevicular fluid miR-200b | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| r | P | r | P | r | P | r | P | |
| TNF-α | 0.117 | 0.052 | 0.132 | 0.055 | 0.229 | 0.023 | 0.247 | 0.002 |
| IL-10 | -0.040 | 0.121 | 0.135 | 0.056 | 0.102 | 0.056 | 0.060 | 0.065 |
| TGF-β | 0.005 | 0.530 | 0.101 | 0.066 | 0.808 | 0.069 | 0.041 | 0.130 |
| CAL | 0.108 | 0.112 | 0.097 | 0.154 | 0.322 | 0.014 | 0.233 | 0.012 |
| PPD | 0.097 | 0.132 | 0.060 | 0.200 | 0.203 | 0.002 | 0.220 | 0.032 |
Table 9.
Correlation of miRNA levels in serum and gingival crevicular fluid with inflammatory factors and periodontal-related factors
| Relevant factor | Serum miR-223 | Serum miR-200b | Gingival crevicular fluid miR-223 | Gingival crevicular fluid miR-200b | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| r | P | r | P | r | P | r | P | |
| TNF-α | 0.056 | 0.128 | 0.007 | 0.424 | 0.216 | 0.006 | 0.256 | 0.028 |
| IL-10 | -0.108 | 0.133 | 0.021 | 0.132 | 0.133 | 0.064 | 0.178 | 0.033 |
| TGF-β | 0.129 | 0.071 | 0.143 | 0.051 | 0.039 | 0.136 | 0.029 | 0.078 |
| CAL | 0.005 | 0.811 | 0.054 | 0.155 | 0.308 | 0.001 | 0.305 | 0.011 |
| PPD | 0.044 | 0.101 | 0.009 | 0.107 | 0.260 | 0.001 | 0.244 | 0.001 |
Discussion
Periodontal disease is characterized by inflammation and the host’s continuous immune response to non-biologic periodontal pathogens. Strong local and systemic stress responses will result in excessive destruction to periodontal tissues and increased inflammatory mediators. In the past, serum TNF-α, IL-10, and TGF-β levels have been used to determine the level of inflammation in patients with periodontal disease [7,8]. As common diagnostic techniques require the existence of active diseases and substantial damage, it is of great significance to find markers reflecting disease status for promoting the establishment of chairside diagnostic methods and helping identify risk factors. Moreover, in the immune system, the occurrence of periodontal disease is mostly accompanied by long-term persistent high blood sugar levels. Hence, the changed pattern of biomarkers related to chronic periodontal disease and T2DM has also captured extensive clinical attention. Studies have shown that there are factors that aggravate the mutual promotion between chronic periodontal disease and T2DM [9]. In addition, scholars have found that the strong expression of inflammatory factors in patients with both T2DM and periodontal disease may provide an insight into the wider use of dysregulated miRNA in new unconventional treatment strategies [10]. The effects of diabetes mellitus on periodontal disease mainly include an abnormal host response to the pathogenic microorganisms of periodontal inflammation, microvascular disease, leukocyte dysfunction, bone tissue repair disorders. Inflammatory factors are involved in the mechanism of the inflammatory stress response, which is mediated by the dysregulated miRNA profile [11].
Based on the report The dysfunctional miRNA profile was studied in the serum and gingival crevicular fluid of rat models [12], this study analyzed the correlation of the relative expression levels of miRNA in serum and gingival crevicular fluid with clinical parameters and inflammatory factors such as TNF-α, IL-10, TGF-β for the first time. After the onset of periodontal disease, the related inflammatory factors TNF-α and TGF-β increased significantly, while IL-10 decreased, and the change of the indicators was positively correlated with the intensity of the inflammatory response and the malignant progression of the disease. In this study, the periodontal group obtained higher levels of TNF-α and TGF-β, and a lower level of IL-10 when compared with the healthy group, and periodontal pateints comorbidity with T2DM had higher levels of TNF-α and TGF-β, and a lower level of IL-10 as compared to the periodontal group, indicating a trend of increase of TNF-α and TGF-β and a decline of IL-10. These results of this study were in line with previous reports [11,12] which stated that there is a high systemic inflammatory burden in the periodontal disease patient population, while the burden under influence of hyperglycemia is even intensified in patients with T2DM, leading to a further upregulated expression in inflammatory factors. The study found that abnormal miRNA profiles were detected in patients with periodontal disease, and the levels of miR-223 and miR-200b in those patients were abnormally up-regulated but showed no great disparity. Thus, it can be concluded that the independent existence of certain factors that reside in the periodontal disease and diabetic patients and promote miRNA abnormalities may not trigger the onset of the disease. Raj et al. [13] confirmed miR-223 as one of the miRNAs overexpressed in the serum of periodontal disease model rats. In patients with both diabetes mellitus and periodontal disease, higher levels of miR-223 and miR-200b were observed. With coordinated immune function, miRNA may play complex roles in different pathologies. This study found through correlation analysis that miR-223 and miR-200b were correlated with inflammatory factors TNF-α, IL-10, and TGF-β and periodontal disease-related factors CAL and PPD to varying degrees, which implied a co-regulatory effect between miRNA and inflammatory factors. In patients with both periodontal disease and diabetes mellitus, inflammatory factors and miRNA levels in the gingival crevicular fluid were significantly higher than those in corresponding serum, which indicated that gingival crevicular fluid is more sensitive to specific body inflammation response. MiR-223, as the main regulator of innate immunity, participates in tissue homeostasis and affects many diseases related to inflammation. MiR-200b participates in the differentiation of a variety of immune cells, including macrophages, neutrophils, and other cells, and plays an important role in the early stages of infection and inflammation [14]. Chronic periodontal disease is characterized by an excessive immune response to periodontal pathogens, among which neutrophils are closely related to the occurrence and development of periodontal disease. Based on the results of this paper and the report of Wei et al. [15], it was assumed that the hyper-activation of neutrophils is induced by miR-223. In addition, a large number of studies have confirmed the potential function of miR-223 expression in controlling the differentiation of osteoblasts, which can be verified by the elevated level of miR-223 in the gingival tissue of periodontal-diseased patients with the loss of alveolar tissue. The results suggest that miR-223 is highly possible to be a marker for the diagnosis of periodontal disease. On the contrary, the lower expression level of miR-203 in the T2DM group than that in the healthy group indicated a negative correlation of miR-203 with TNF-α, which was suggestive of its impact on the irreversible damage caused by the disease. The reverse effect of miR-203 and TNF-α may be attributed to the role of miR-203 in reducing inflammation by inhibiting TNF-α. MiR-200b was overexpressed in the serum and gingival crevicular fluid of the two groups of patients, which was consistent with previous reports that confirmed the increase of miR-200b in inflamed gingival tissue [16-18]. Furthermore, the significant differences in the level of miR-200b between the T2DM group and the periodontal disease group can be explained by the fact that miR-200b may participate in the inflammatory process as it is induced by inflammatory cytokines. Due to diabetes mellitus or complications, the serum miR-200b level in patients with T2DM is released from control. Studies have shown that increased miR-200b level is associated with increased pancreatic β-cell apoptosis, indicating that miR-200b plays a major role in the pathogenesis of diabetes mellitus [19,20]. This study further analyzed the ability of miR-223 and miR-200b to provide diagnostic markers, and found that the two can help the differential diagnosis of healthy individuals from patients with T2DM, and miR-223 can help the differential diagnosis of healthy individuals/periodontal disease from T2DM/T2DM comorbid with periodontal disease. It is assumed that miRNA may regulate the occurrence of periodontal disease in healthy people or people with T2DM by mediating inflammatory factors. However, due to the short follow-up time and few samples, we did not exclude other factors including but not limited to the influence of obesity on inflammatory markers. In the future, it is suggested to increase relevant dependent variables to explore the possible mechanism of miRNAs in periodontal disease, so as to fully clarify the exact molecular mechanism network of periodontal disease in systemic inflammatory burden.
In conclusion, miR-223 and miR-200b in serum and gingival crevicular fluid are expressed differentially in periodontal-diseased patients with T2DM and those without it, and miR-223 and miR-200b in the gingival crevicular fluid are highly correlated with the pathogenesis of the periodontal disease, the susceptibility to T2DM, and its serum level. Therefore, the two may be serum biomarkers for studying the molecular mechanism and pathophysiology of chronic periodontal disease.
Disclosure of conflict of interest
None.
References
- 1.Wang F, Wang XZ, Xie XL. The effect of type 2 diabetes on the content of IL-1β, IFN-γ, IL-10, PGE2 in the gingival crevicular fluid of patients with periodontitis. J Dental PulpPeriodontol. 2017;27:271–275. [Google Scholar]
- 2.Baltacioglu E, Sukuroglu E. Protein carbonyl levels in serum, saliva and gingival crevicular fluid in patients with chronic and aggressive periodontitis. Saudi Dental J. 2019;31:23–30. doi: 10.1016/j.sdentj.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng XB, Long SH, Li FJ, Hu SH. The expression and clinical significance of IL-1β, TNF-α, IL-18 and IFN-γ in gingival crevicular fluid and serum of elderly patients with chronic periodontitis and coronary heart disease. J Gerontol. 2020;40:126–129. [Google Scholar]
- 4.Huang YX, Fang L, Gao RH, Li J. Analysis of periodontitis in patients with type 2 diabetes with different levels of HbAlc. J Periodontol. 2017;5:401–403. [Google Scholar]
- 5.Abdelgawad FE. Serum vitamin B12 and homocysteine levels in type 2 diabetic patients with and without metformin therapy. J Bio Sci Engineering. 2019;12:557–570. [Google Scholar]
- 6.Guo LT, Gao ZH, Ge HQ. The expression of microRNA-155, nuclear factor-κB and soluble intercellular adhesion molecule-1 in peripheral blood of patients with type 2 diabetes and their relationship with vascular complication. J Diabetes. 2017;48:1052–1056. [Google Scholar]
- 7.Deng QY, Che YL. Changes in Th cell and cytokine levels in patients with type 2 diabetes and chronic periodontitis. Guangdong Med. 2018;39:95–98. [Google Scholar]
- 8.Zhang L, Li WM. The effect of different subgingival plaque removal methods on the balance of apoptosis factors, inflammatory factors and MMPs/TIMPs in gingival crevicular fluid. J Prat Stomatol. 2019;7:569–572. [Google Scholar]
- 9.Elazazy O, Amr K, Fattah A, Abouzaid M. Evaluation of serum and gingival crevicular fluid microRNA-223, microRNA-203 and microRNA-200b expression in chronic periodontitis patients with and without diabetes type 2. Arch Oral Bio. 2021;121:104949. doi: 10.1016/j.archoralbio.2020.104949. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Gan SL, Guo ZL. Research progress of basic periodontal therapy in regulating metabolism in patients with type 2 diabetes. J Stomatol. 2019;39:93–97. [Google Scholar]
- 11.García-Hernández AL, Muñoz-Saavedra ÁE, González-Alva P, Moreno-Fierros L, Llamosas-Hernández FE, Cifuentes-Mendiola SE, Rubio-Infante N. Upregulation of proteins of the NLRP3 inflammasome in patients with periodontitis and uncontrolled type 2 diabetes. Oral Dis. 2019;25:596–608. doi: 10.1111/odi.13003. [DOI] [PubMed] [Google Scholar]
- 12.Gomaa MA, Guindy H, El-Zamrany EA. Adjunctive subantimicrobial dose doxycycline in the treatment of chronic periodontitis in type 2 diabetic patients: a unique combination therapy. Balkan J Dent Med. 2018;22:32–37. [Google Scholar]
- 13.Raj SC, Panda SM, Dash M, Patnaik K, Praharaj K. Association of human interleukin-35 level in gingival crevicular fluid and serum in periodontal health, disease, and after nonsurgical therapy: a comparative study. Contemp Clin Dent. 2018;9:293–297. doi: 10.4103/ccd.ccd_51_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao R, He FF, Liang Y. Correlation between OPG/RANKL expression in gingival crevicular fluid and inflammatory factors, free radical production and bone metabolism in patients with diabetes and periodontitis. J Hainan Med University. 2018;24:14–17. [Google Scholar]
- 15.Huang W, Deng F, Li QL. The effect of basic periodontal treatment on the level of inflammatory factors in the gingival crevicular fluid of patients with chronic periodontitis. Chin Emerg Med. 2018;38:132–135. [Google Scholar]
- 16.Ma F, Tang CJ, Fang MF, Chen Y. Study on the effect of retinoid D1 on inflammatory factors in gingival crevicular fluid of rats with periodontitis. J Pract Stomatol. 2018;34:610–613. [Google Scholar]
- 17.Pei J, Li F, Xie Y, Liu J, Feng X. Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: lessons for a predictive, preventive, and personalized medical approach. EPMA J. 2020;11:197–215. doi: 10.1007/s13167-020-00202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo LT, Gao ZH, Ge HQ. The expression of microRNA-155, nuclear factor-κB and soluble intercellular adhesion molecule-1 in peripheral blood of patients with type 2 diabetes and its relationship with vascular complications. Chin Diabetes Mag. 2017;25:213–217. [Google Scholar]
- 19.Bagwe S, Gopalakrishnan D, Mehta V, Mathur A, Kapare K, Deshpande A. GCF and serum levels of omentin in periodontal health and disease of diabetic and nondiabetic individuals: a comparative study. Indian J Dent Res. 2020;31:520–525. doi: 10.4103/ijdr.IJDR_796_18. [DOI] [PubMed] [Google Scholar]
- 20.Yucel Z, Afacan B, Lhan HA, T Kose T, Emingil G. The trefoil factor family 1 (TFF and 3 (TFF are upregulated in the saliva, GCF and serum of periodontitis patients. Oral Dis. 2021 doi: 10.1111/odi.13820. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


