Abstract
The high efficacy mu-opioid receptor (MOR) agonist methadone is an effective opioid use disorder (OUD) medication used exclusively in opioid-dependent patients. However, methadone has undesirable effects that limit its clinical efficacy. Intermediate efficacy MOR agonists may treat OUD with fewer undesirable effects. We compared the effects of methadone with the intermediate efficacy MOR agonist TRV130 (oliceridine) on fentanyl-vs.-food choice and somatic withdrawal signs in opioid-dependent and post-opioid-dependent rats. Male rats (n = 20) were trained under a fentanyl-vs.-food choice procedure. Rats were then provided extended fentanyl (3.2 µg/kg/infusion) access (6 p.m.–6 a.m.) for 10 days to produce opioid dependence/withdrawal. Rats were treated with vehicle (n = 7), TRV130 (3.2 mg/kg; n = 8), or methadone (3.2 mg/kg; n = 5) three times per day after each extended-access session (8:30 a.m., 11 a.m., 1:30 p.m.). Withdrawal sign scoring (1:55 p.m.) and choice tests (2–4 p.m.) were conducted daily. Vehicle, TRV130, and methadone effects on fentanyl choice were redetermined in post-opioid-dependent rats. Vehicle-, TRV130-, and methadone-treated rats had similar fentanyl intakes during extended access. Vehicle-treated rats exhibited increased withdrawal signs and decreased bodyweights. Both methadone and TRV130 decreased these withdrawal signs. TRV130 was less effective than methadone to decrease fentanyl choice and increase food choice in opioid-dependent rats. Neither methadone nor TRV130 decreased fentanyl choice in post-opioid-dependent rats. Results suggest that higher MOR activation is required to reduce fentanyl choice than withdrawal signs in fentanyl-dependent rats. Additionally, given that TRV130 did not precipitate withdrawal in opioid-dependent rats, intermediate efficacy MOR agonists like TRV130 may facilitate the transition of patients with OUD from methadone to lower efficacy treatments like buprenorphine.
Subject terms: Reward, Pharmacology
Introduction
Opioid-overdose fatalities have become the most common cause of death for Americans 18–45 years of age [1]. Opioid use disorder (OUD) medications (MOUDs) are intended to promote abstinence from illicit opioids and prevent fatal overdose. Currently available MOUDs include naltrexone, buprenorphine, and methadone. Of these, methadone is the only medication that can be administered without risk of precipitating withdrawal symptoms in patients who have used other high efficacy opioids in the preceding days. Methadone is effective to decrease illicit opioid use and retain patients in treatment [2–5]. However, methadone is underutilized, with <15% of patients receiving this MOUD [5, 6]. Reasons for the limited use of methadone include stringent regulations owing to concerns of overdose, abuse liability, and other undesirable effects [2, 4, 7]. Development of MOUDs that maintain the effectiveness of methadone but possess fewer undesirable effects may increase treatment-utilization rates for opioid-dependent patients and address Helping to End Addiction Long-term initiatives.
GTPγS binding studies illustrate that methadone is a high efficacy mu-opioid receptor (MOR) agonist [8], corresponding with methadone’s ability to produce side effects that require high levels of MOR activation. For example, methadone can produce fatal respiratory depression at-or-near therapeutic doses, particularly during the induction phase of OUD treatment and/or in people with only moderate levels of opioid dependence [7, 9–11]. Additional, albeit non-MOR mediated, undesirable methadone effects include increased risk for QTc interval prolongation, which can lead to fatal cardiac arrhythmia [12]. In contrast to methadone, buprenorphine possesses lower MOR efficacy [8], and its effects on respiratory depression accordingly plateau at sub-lethal levels [13–15]. Buprenorphine also produces fewer effects on QTc prolongation than methadone [16]. However, the low MOR efficacy of buprenorphine can precipitate opioid withdrawal in highly opioid dependent people [17, 18], which may pose a significant barrier to OUD treatment. Therefore, MOR agonists with efficacy between buprenorphine and methadone may balance high enough MOR efficacy to avoid precipitated withdrawal in highly opioid-dependent patients and low enough MOR efficacy to decrease the risk of fatal respiratory depression and other undesirable effects.
TRV130 (oliceridine) is one MOR agonist with efficacy between buprenorphine and methadone [19–22]. TRV130 produces weaker respiratory-depressant effects than morphine [23–26] and does not elicit QTc prolongation or cardiac arrhythmias following supra-therapeutic dosing [27]. In addition, the MOR efficacy of TRV130 is sufficient to produce analgesia and is available in the United States for the acute management of severe pain [27]. Overall, available data suggest that TRV130 or a related orally bioavailable compound TRV734 may represent a potential alternative MOUD to methadone [28]. However, whether TRV130 produces a methadone-like decrease in opioid self-administration in opioid-dependent subjects is unknown.
Because one primary treatment goal for methadone-maintenance therapy is to decrease illicit opioid use in opioid-dependent people, preclinical evaluation of methadone-like MOUDs should determine treatment effects on opioid self-administration in opioid-dependent subjects (see [29, 30]). Furthermore, candidate medications should not decrease self-administration of non-opioid reinforcers (e.g., food) and should instead promote a reallocation of behavior away from opioid use and toward responding for non-opioid reinforcers. This focus on behavioral selectivity of medication effects helps protect against false-positive outcomes due to undesirable candidate-medication effects such as impairment of operant behavior and can be accomplished using preclinical opioid-vs.-food “choice” procedures [31, 32]. For example, research using opioid-dependent nonhuman primates has shown that MOR agonists decrease opioid choice and increase food choice in a MOR efficacy dependent manner (methadone ≥ morphine > buprenorphine) [33–36]. This literature illustrates the sensitivity of choice procedures to MOR efficacy and aligns with the clinical effectiveness of these MOUDs [2, 37–39]. Accordingly, the goal of our study was to compare the effect of TRV130 and methadone on fentanyl-vs.-food choice in opioid-dependent and post-opioid-dependent rats. Somatic withdrawal signs and bodyweights were also examined as secondary measures of opioid withdrawal.
Materials and methods
Subjects
Twenty male Sprague-Dawley rats were acquired at 10 weeks of age (Envigo Laboratories, Frederick, MD) and surgically implanted with vascular access ports (Instech, Plymouth Meeting) and custom-made jugular catheters as described previously [40]. Once catheterized, rats were assigned to one of three groups: Vehicle (n = 8), TRV130 (n = 7), or Methadone (n = 5). Rats were singly housed in a temperature and humidity-controlled vivarium that was maintained on a 12-h light/dark cycle (lights off at 6:00 p.m.). Water and food (Teklad Rat Diet, Envigo) were freely available in the home cage. Rat maintenance and research were conducted in accordance with the 2011 guidelines for the care and use of laboratory animals and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Only male rats were used for these studies because our previous research [41] showed that methadone robustly decreases fentanyl-vs.-food choice in opioid-dependent male, but not female, rats (see “Discussion”).
Drugs
Fentanyl HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). (±)-Methadone HCl was purchased from Spectrum Chemicals (Gardena, CA). (+)-TRV130 2HCl was synthesized and provided by the laboratory of Bruce Blough (RTI). Fentanyl and methadone were dissolved in sterile water and diluted with sterile saline. TRV130 was dissolved in a vehicle of 1:1:8 ethanol:cremophor:sterile water at a stock concentration of 25 mg/ml. The TRV130 stock solution was diluted to 3.2 mg/ml with sterile saline. Accordingly, rats receiving “vehicle” injections were administered an equivalently saline-diluted solution of 1:1:8 ethanol:cremophor:sterile water. Therefore, both the TRV130 and vehicle injections contained 1.3% ethanol. Solutions were passed through a 0.22-micron sterile filter (Millex GV, Millipore Sigma, Burlington, MA) before administration. Drug doses are expressed as the salt forms and delivered based on bodyweight.
Procedure
Rats were trained to respond under a fentanyl-vs.-food choice procedure as described previously [40]. Briefly, choice sessions were conducted each weekday from 2–4 p.m., and each choice session consisted of five, 20-min response components wherein the available unit fentanyl dose increased by 0.5 log unit increments between each component (0–10 µg/kg/injection), and the food reinforcer remained constant (0.1 ml of 32% Ensure®). Food availability was signaled by illuminating a red stimulus light above the left lever, and fentanyl availability was signaled by illuminating a green stimulus light above the right lever. The green light turned on and off in 3-s cycles, with longer light-on flashes signaling availability of larger unit doses of fentanyl during successive components of the session. During each component, the rats could complete up to 10 ratio requirements (fixed-ratio (FR) 5) between the food- and fentanyl-associated levers. A 20-s timeout (TO) period followed each earned reinforcer presentation, during which the levers retracted and the stimulus lights were extinguished. Choice was considered stable after at least five choice sessions and when the smallest unit dose of fentanyl that maintained at least 80% of completed ratio requirements was within a 0.5 log-unit range for three consecutive days with no upward or downward trends (i.e., stability criteria). Data collected during the final training day served as “pre-extended-access baseline” values for subsequent analyses.
Following stability, a regimen of overnight (6:00 p.m.–6:00 a.m.) extended fentanyl (3.2 μg/kg/infusion) access (FR5, TO 10-s schedule of reinforcement) began. Fentanyl availability was signaled by illuminating a green stimulus light above the right lever. After each extended access session, the rats were subcutaneously (SC) injected three times per day at 150-min intervals (8:30 a.m., 11:00 a.m., 1:30 p.m.) with either vehicle (1 ml/kg), TRV130 (3.2 mg/kg), or methadone (3.2 mg/kg). The TRV130 dose and dosing regimen was selected based on previous evidence that 3.2 mg/kg and smaller TRV130 doses produced antinociception [42, 43], fentanyl-like discriminative stimulus effects [44], suppression of food-maintained responding [44], and suppression of intracranial self-stimulation [45]. The methadone dose and dosing regimen was selected based on our published study showing that 3.2 mg/kg methadone as the minimally effective acute dose to decrease fentanyl choice in male opioid-dependent rats [41]. The functional equivalence of effects and time courses for these TRV130 and methadone doses was further evaluated in antinociception studies described below.
During the session immediately preceding the opioid-withdrawal experiments (i.e., pre-opioid-dependence baseline) the rats were observed for 30 s for the presence of nine somatic withdrawal signs (see Supplementary Materials) and weighed before beginning the daily choice test (2:00 p.m.). Withdrawal sign scoring was also conducted 8 h after each extended access self-administration session (~1:55 p.m.), and 25 min after the third daily SC vehicle/TRV130/methadone treatment. Extended access fentanyl self-administration sessions occurred Sunday–Thursday nights for two consecutive weeks (i.e., Week 1 and Week 2). A timeline of the daily procedures during this portion of the experiment is shown in Fig. 2D. The rationale for the timing of the injection intervals was to provide multiple dosing interventions with a relatively high dose of TRV130 or methadone within the daily 8 h abstinence period between the conclusion of the overnight self-administration session (6:00 a.m.) and the beginning of the choice session (2:00 p.m.). Published pK data from rats show that TRV130 and methadone each have half-lives of ~1.5 h [46, 47], suggesting that three doses spread in 2.5-h intervals might be sufficient to decrease opioid withdrawal without significant drug accumulation between injections.
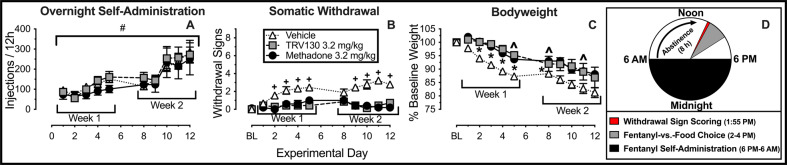
Fig. 2. TRV130 and methadone decrease somatic withdrawal sign expression and weight loss following withdrawal from extended access to fentanyl self-administration.
A Number of fentanyl injections (3.2 μg/kg/infusion unit dose) self-administered during each 12-h session (6 p.m.–6 a.m.). B Number of somatic withdrawal signs (maximum of nine) observed 8 h after the conclusion of each extended access self-administration session. C Change in bodyweight expressed as a percentage of baseline. Points represent mean ± SEM. #Denotes a significant main effect of time. D Timeline of daily procedures. *Denotes significant difference between TRV130 and vehicle treatment. ^Denotes significant difference between methadone and vehicle treatment. +Denotes significantly greater withdrawal sign expression relative to both TRV130 and methadone treatment (separate Mann–Whitney U tests corrected with Benjamini–Hochberg false discovery procedure), p < 0.05. See Table 1 in Supplementary Materials for statistics relevant to each panel. Vehicle: n = 8; TRV130: n = 7; Methadone: n = 5.
Following the 2-week regimen of extended fentanyl access, a 1-week “washout” period occurred wherein daily withdrawal sign scoring, weight collection, and fentanyl-vs.-food choice tests continued. Data collected on the Friday of this week served as “post-extended access baseline” values for subsequent analyses. Following this washout period, rats were injected SC with vehicle (1 ml/kg), TRV130 (3.2 mg/kg), or methadone (3.2 mg/kg) once per day for five consecutive days (1:30 p.m., M-F) prior to withdrawal sign scoring, weight measurement, and fentanyl-vs.-food choice tests.
To examine the functional equivalence for effects and time courses for the TRV130 and methadone doses used in our study, a time course of TRV130 and methadone antinociceptive effects was determined using a warm-water tail-withdrawal procedure as a final experiment in the rats of the Methadone group following an additional washout week. A water bath (Precision, 280 Series Water Bath, Winchester, VA) was maintained at 50 °C (±1 °C). Each session began by wrapping the rat with a towel, leaving the tail exposed. The distal 5 cm of the tail was immersed in the heated water, and the latency to fully remove the tail was recorded with a digital chronograph with 0.01 s resolution (Sports Timer, Fisher Brand, Hampton, NH). If the rat did not remove its tail by 20 s, the experimenter removed the tail, and a latency of 20 s was assigned. Following two baseline latency determinations (−10 and 0 min prior to injection), TRV130 (3.2 mg/kg) or methadone (3.2 mg/kg) was administered subcutaneously. Tail-withdrawal latencies were determined 10, 30, 100, 150, and 180 min after injection. Tail-withdrawal tests were conducted on Monday and Wednesday, with the testing order of TRV130 and methadone randomized across rats.
Data analysis
See Supplementary Table 1 for a complete listing of all statistical tests and factors. For the fentanyl-vs.-food choice studies, the primary dependent measures were percent of choices completed on the fentanyl-associated lever, the number of choices completed per component, and the number of choices completed per session (food, fentanyl, or food + fentanyl). Other dependent measures included the number of fentanyl injections earned during extended-access sessions, the number of withdrawal signs present, and changes in bodyweight relative to the pre-extended-access baseline. Effects of repeated vehicle, TRV130, and methadone administration on choice were averaged across the 5 days of each experimental week. For the tail-withdrawal tests, raw latency to remove the tail was converted to percent maximum possible effect (%MPE) using the following equation:
Here, test latency was the latency after TRV130 or methadone administration, and baseline latency was the latency recorded during the second baseline observation prior to drug administration on that particular day. Data were compared by one- or two-way ANOVA as appropriate, and a significant ANOVA was followed by appropriate post hoc tests. For all statistical tests, the criterion for significance was p < 0.05.
Results
Fentanyl vs. food choice and somatic withdrawal in dependent rats
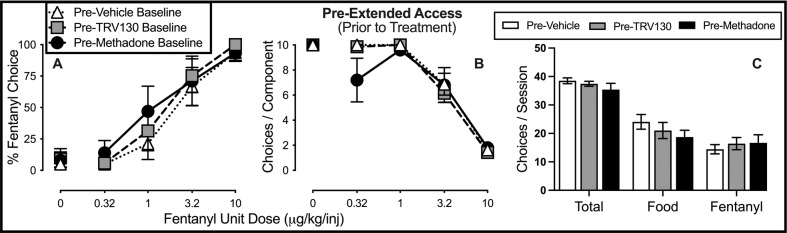
Figure 1 shows fentanyl-vs.-food choice in each group before extended fentanyl access. At baseline, liquid food was almost exclusively chosen when no fentanyl or the smallest unit dose (0.32 μg/kg/infusion) was available (Fig. 1A). As the fentanyl dose increased, lever pressing was reallocated away from food and toward fentanyl, and the largest unit fentanyl dose (10 μg/kg/injection) maintained near-exclusive choice. In addition, choices per component decreased as a function of increasing unit dose of fentanyl (Fig. 1B). No baseline group differences were detected for percent fentanyl choice (Fig. 1A), the number of choices completed per component (Fig. 1B), or the total number of choices completed per session (Fig. 1C).
Fig. 1. Baseline on fentanyl-vs.-food choice prior to extended access fentanyl self-administration.
A Percent fentanyl choice. x-axis: Intravenous unit fentanyl dose in μg/kg/infusion. B Percentage of completed ratio requirements on the fentanyl-associated lever. x-axis: Intravenous unit fentanyl dose in μg/kg/infusion. C Number of choices completed per session. Points represent mean ± SEM. See Table 1 in Supplementary Materials for statistics relevant to each panel. Vehicle: n = 8; TRV130: n = 7; Methadone: n = 5.
Figure 2 compares effects of vehicle, TRV130, and methadone treatment on fentanyl consumption, withdrawal signs, and bodyweights during extended fentanyl access (see Fig. 2D for a timeline of daily experiments). The number of fentanyl infusions earned during the 2 weeks of extended access increased across time (Fig. 2A; time: F1.8,30.6 = 26.8, p < 0.0001). In addition, somatic withdrawal signs increased (Fig. 2B; time: F5.0,89.3 = 2.5, p = 0.04) and bodyweights decreased over time (Fig. 2C; time: F1.2,20.8 = 69.4, p < 0.0001). Relative to vehicle treatment, neither TRV130 nor methadone treatments altered overnight rates of extended access fentanyl self-administration; however, both TRV130 and methadone significantly decreased the expression of withdrawal signs determined immediately before daily choice sessions and 25 min after the last daily TRV130 or methadone injection (Fig. 2B; interaction: F18,162 = 2.3, p = 0.003). TRV130 and methadone also decreased withdrawal-induced weight loss (Fig. 2C; treatment: F1,17 = 5.8, p = 0.01).
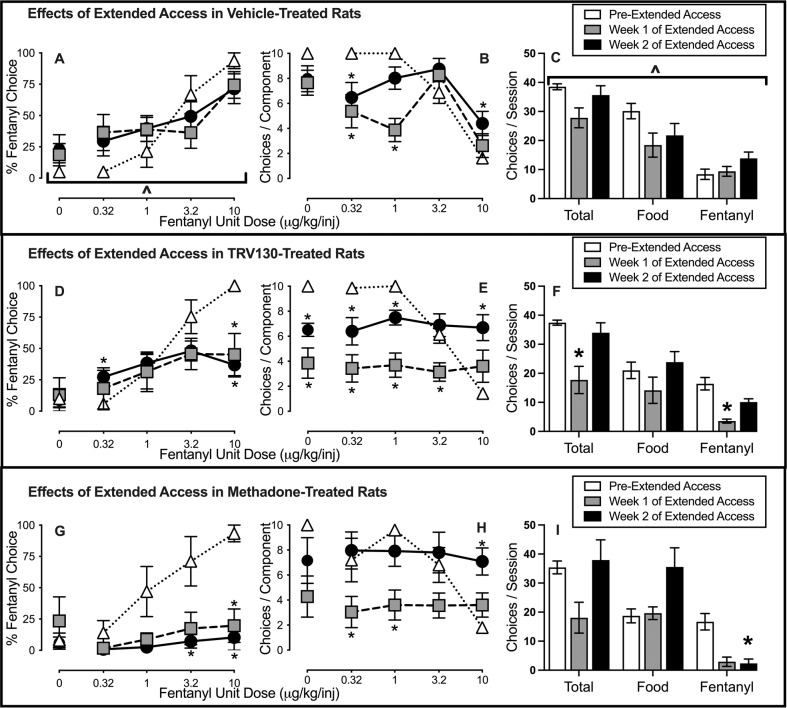
Figure 3 compares effects of treatment with vehicle, TRV130, and methadone on fentanyl-vs.-food choice during extended fentanyl access. In vehicle-treated rats, extended fentanyl access and associated fentanyl dependence tended to increase fentanyl choice and reduce food choice. A fentanyl unit dose × time interaction was detected for percent fentanyl choice across the 2 weeks of extended fentanyl access (Fig. 3A: F2.9,19.6 = 4.2, p = 0.02), although post hoc analysis did not detect significant effects following multiple-comparison corrections. Figure 3B shows that the number of choices completed per component decreased when small (0.32, 1 μg/kg/injection) unit fentanyl doses were available and increased when 10 μg/kg/injection fentanyl was available. Additionally, a time × reinforcer type interaction was detected in vehicle-treated rats (Fig. 3C: F4,42 = 3.2, p = 0.02).
Fig. 3. Effects of repeated vehicle, TRV130, and methadone treatment on fentanyl-vs.-food choice in male opioid-dependent rats assessed 8 h after overnight (6:00 p.m.–6:00 a.m.) extended access fentanyl self-administration sessions.
A, D, G Opioid-withdrawal effects on percent fentanyl choice. x-axis: Intravenous unit fentanyl dose in μg/kg/infusion. B, E, H Percentage of completed ratio requirements on the fentanyl-associated lever. x-axis: Intravenous unit fentanyl dose in μg/kg/infusion. C, F, I Number of choices completed per session. Points represent mean ± SEM. Data of week 1 and week 2 show averaged results of the choice sessions within the particular week. *Denotes significant difference relative to pre-extended access. ^Denotes either a significant time × unit dose interaction or a time × reinforcer type interaction, p < 0.05. See Table 1 in Supplementary Materials for statistics relevant to each panel. Vehicle: n = 8; TRV130: n = 7; Methadone: n = 5.
Relative to the pre-opioid-dependence baseline, both TRV130 (Fig. 3D) and methadone (Fig. 3G) decreased fentanyl choice during weeks 1 and 2 of extended fentanyl access. However, when compared between groups, methadone produced significantly greater decreases in fentanyl choice than TRV130 in week 2 (Supplementary Fig. 1D). Furthermore, Supplementary Fig. 1D shows TRV130 was not significantly different from vehicle. During week 1, both TRV130 and methadone decreased the number of choices completed per component when small unit fentanyl doses were available (Fig. 3E, H). During week 2, decreased choices per component were detected for TRV130, but not methadone, at small unit doses of fentanyl, and both TRV130 and methadone increased choices completed when 10 μg/kg/infusion fentanyl was available (Fig. 3E, H). TRV130 decreased the total number of fentanyl choices (irrespective of unit dose) only during week 1 (Fig. 3F). In contrast, methadone virtually eliminated fentanyl choices during week 2 (Fig. 3I).
In conclusion, in fentanyl-dependent rats, methadone and TRV130 had a similar inhibitory effect on somatic withdrawal signs while methadone had a stronger inhibitory effect on fentanyl vs. food choice than TRV130, particularly during the 2nd week of the experiment.
Fentanyl vs. food choice in post-dependent rats
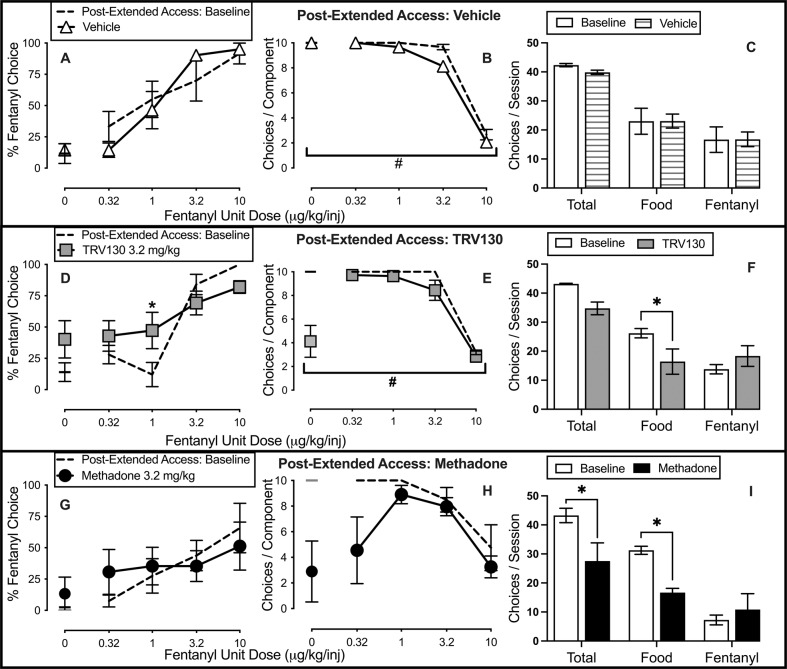
Figure 4 compares effects of repeated daily treatment with vehicle, TRV130, and methadone on fentanyl-vs.-food choice in post-dependent rats after a 1-week washout from extended fentanyl access. At this time, withdrawal signs were no longer present before the daily choice sessions. In contrast to their effects during fentanyl dependence, neither TRV130 nor methadone decreased fentanyl choice in this post-dependent stage. Dashed lines of Fig. 4 show the post-extended access (i.e., post-opioid dependence) baseline fentanyl choice dose-effect functions. No experimental group differences in baseline percent fentanyl choice were detected (Supplementary Fig. 2). During this post-dependent stage, percent fentanyl choice was unaffected by either repeated vehicle (Fig. 4A) or 3.2 mg/kg methadone (Fig. 4G). However, choice of 1 μg/kg/infusion fentanyl significantly increased during repeated 3.2 mg/kg TRV130 treatment (Fig. 4D; interaction: F2.2,8.6 = 4.7, p = 0.04). TRV130 decreased only the number of food choices completed per session (Fig. 4F; interaction: F2,12 = 6.2, p = 0.01), whereas methadone decreased both total choices and food choices completed per session (Fig. 4I; interaction: F2,18 = 4.3, p = 0.03).
Fig. 4. Effects of repeated vehicle, TRV130, and methadone treatment on fentanyl-vs.-food choice in male post-opioid-dependent rats.
A, D, G Treatment effects on percent fentanyl choice. x-axis: Intravenous unit fentanyl dose in μg/kg/infusion. B, E, H Percentage of completed ratio requirements on the fentanyl-associated lever. Abscissa: Intravenous unit fentanyl dose in μg/kg/infusion. C, F, I Number of choices completed per session. Points represent mean ± SEM. Vehicle, TRV130, and methadone data show averaged results of the choice sessions within the particular week. *Denotes significant difference relative to baseline. #Denotes a significant main effect of treatment, p < 0.05. See Table 1 in Supplementary Materials for statistics relevant to each panel. Vehicle: n = 8; TRV130: n = 7; Methadone: n = 5.
Finally, Supplementary Fig. 3 shows that SC injections of TRV130 (3.2 mg/kg) and methadone (3.2 mg/kg) produced similar magnitudes and time courses of thermal antinociception (time: F1.1,4.4 = 19.5, p = 0.009). TRV130 and methadone antinociceptive effects peaked at the 30-min timepoint and dissipated by the 100-min timepoint. No main effect of treatment or treatment × time interaction was detected. These results support the use of identical doses and inter-dose intervals for choice studies as described above.
Discussion
We compared the effects of repeated administration of TRV130 (intermediate MOR efficacy) and methadone (high MOR efficacy) on fentanyl-vs.-food choice in opioid-dependent and post-dependent male rats. We found that extended access to fentanyl self-administration established opioid dependence as evidenced by increased expression of somatic withdrawal signs and decreased body weights. These results are consistent with our previous findings and the published literature. Both TRV130 and methadone similarly decreased opioid-withdrawal effects on somatic withdrawal signs and weight loss. However, methadone was more effective than TRV130 to decrease fentanyl choice in opioid-dependent rats. In addition, neither methadone nor TRV130 decreased fentanyl choice in post-dependent rats. These results have two implications. First, opioid-dependence status is an important determinant of MOR agonist effectiveness to decrease opioid reinforcement. Second, higher MOR activation is necessary to decrease fentanyl choice in opioid-dependent rats than to decrease expression of somatic withdrawal signs and withdrawal-induced weight loss. Whereas TRV130 produced methadone-like decreases in withdrawal signs, TRV130 was less effective than methadone to decrease fentanyl choice in opioid-dependent rats. This partial dissociation suggests that the mechanisms of somatic withdrawal signs and fentanyl choice are not the same and that withdrawal-induced increases in fentanyl choice are not solely maintained by the negative reinforcing effects of somatic opioid withdrawal.
Opioid-withdrawal effects on fentanyl choice
Opioid withdrawal has been shown to increase opioid reinforcement and decrease behaviors maintained by non-opioid reinforcers (e.g., food or electrical brain stimulation), thus promoting increased opioid choice at the expense of non-drug reinforcement [30]. Consistent with previous findings, the number of choices completed per component decreased during the withdrawal regimen in vehicle-treated rats (Fig. 3B). However, despite robust evidence that fentanyl intake during the extended access sessions was sufficient to produce opioid dependence, there was only a nonsignificant trend in the present study for opioid withdrawal to increase fentanyl choices and reduce food choices in vehicle-treated rats (Fig. 3A, C). Previous studies in both male nonhuman primates and male rats have reported withdrawal-associated increases in opioid-choice behavior (see [30] for review). It is presently unknown why opioid withdrawal did not robustly enhance opioid choice in vehicle-treated rats in the present study; one possibility is that choice behavior was affected by the 1.3% ethanol concentration in the daily vehicle injections. Two additional environmental variables were different between the present study and our previous study reporting withdrawal-associated increases in fentanyl choice in male rats [41]. First, the liquid food concentration in these studies was 32% diluted Ensure whereas the food concentration was 18% in the previous study. The liquid food concentration was increased for these studies because the formulation of Ensure was changed by Abbott Laboratories, which resulted in an apparent reduction in palatability. Second, our laboratory moved to a different building between the previous and present study such that rats in the previous study were subjected to being moved on an elevator between sessions whereas rats in the present study were not. All other environmental variables such as operant box, food, housing conditions, were similar. Although the robustness of opioid withdrawal effects on opioid choice requires further examination, the absence of robust withdrawal-associated increases in fentanyl choice does not preclude the examination of candidate MOUD effects on fentanyl choice in opioid-dependent rats, as the within-subject design compared treatment effects relative to pre-opioid-dependence baselines.
One limitation of the current study was the use of only male rats. This decision was made based on our previous observation that opioid withdrawal decreased fentanyl choice in female rats [41]. Furthermore, this previous study was unable to detect decreases in fentanyl choice following methadone treatment, even when methadone virtually eliminated the remnant levels of fentanyl choice [41]. Therefore, available data suggest our procedure is insensitive to methadone as a “positive control” compound in female opioid-withdrawn rats, diminishing the ability to compare the relative effectiveness of a candidate medication (e.g., TRV130) to a current standard of care. Nevertheless, we propose the spontaneous-withdrawal-associated decreases in fentanyl choice in female rats is an intriguing topic for further research, as it mirrors the methadone-induced decreases in fentanyl choice in opioid-withdrawn male rats. These behavioral sex differences, coupled with emerging evidence of sex differences in opioid-withdrawal effects on reward-related brain structures (e.g., [41, 48]) provides opportunity for future work to study mechanisms of the interaction between opioid withdrawal and sex on the decision to self-administer fentanyl over an alternative reinforcer.
Candidate MOUD effectiveness depends on level of opioid dependence
Current MOUDs vary in MOR efficacy (naltrexone < buprenorphine < methadone). Naltrexone (MOR antagonist) is only used in patients that are post-opioid dependent or have very low levels of opioid dependence because naltrexone and other MOR antagonists readily precipitate opioid withdrawal in opioid-dependent people. Buprenorphine (low efficacy MOR agonist) and methadone (high efficacy MOR agonist) are indicated only for opioid-dependent patients, although buprenorphine can precipitate opioid withdrawal in people with higher degrees of opioid dependence. Consistent with clinical observations, the level of opioid dependence is also a determinant of MOUD effectiveness to decrease opioid self-administration in preclinical studies. Naltrexone and buprenorphine decrease opioid self-administration in both non-opioid-dependent monkeys [49, 50] and rats [51, 52], but when opioid dependence has been established, both treatments can precipitate withdrawal and increase opioid choice [53, 54]. In contrast, methadone decreases opioid self-administration in opioid-dependent nonhuman primates undergoing withdrawal [34, 35] but in non-opioid-dependent or post-dependent nonhuman primates, methadone decreases opioid self-administration only at doses that markedly suppress many other behaviors [35, 55]. The present methadone effects on fentanyl choice are consistent with the nonhuman primate literature and extend these findings to rats. Also consistent with these previous nonhuman primate studies, methadone-induced decreases in opioid choice in opioid-dependent subjects correlated with methadone-induced decreases in withdrawal signs and other measures of opioid withdrawal. Overall, the effectiveness of methadone to decrease fentanyl-vs.-food choice in male opioid-dependent rats undergoing withdrawal supports the validity of this procedure to evaluate candidate MOUDs such as TRV130.
TRV130 was similarly effective to methadone in decreasing expression of somatic withdrawal signs and withdrawal-induced weight loss. However, the effectiveness of TRV130 to decrease fentanyl choices was weaker during the second week of the opioid-withdrawal regimen. One interpretation of the fentanyl-vs.-food choice results is that the degree of opioid dependence increased across the two weeks of the opioid-withdrawal regimen and TRV130 was most effective to decrease fentanyl choice when opioid dependence was at lower levels during week 1. Because we observed no evidence that TRV130 precipitated opioid withdrawal in opioid-dependent rats, these results suggest that TRV130 may be more effective in patients with lower levels of opioid dependence.
The results of the antinociception timecourse experiment (Supplementary Fig. 3) suggest that 3.2 mg/kg TRV130 and 3.2 mg/kg methadone are similarly effective. Moreover, it should be noted that the 3.2 mg/kg TRV130 is at or above the Emax dose where TRV130 dose-effect curves plateau on other endpoints in rats (e.g., hot-plate antinociception and respiratory depression, see [42]; depression of ICSS, see [45]; opioid discriminative-stimulus and rate-decreasing effects, see [44]), suggesting that the dose of TRV130 examined in the current study was large. However, the possibility remains that larger doses of TRV130 could produce effects similar to those observed following methadone treatment in the current study. In either case, the relatively modest effectiveness of TRV130 to decrease opioid choice in opioid-dependent subjects suggests that the level of MOR activation produced by the current TRV130 dosing regimen may be too low to promote methadone-like reallocation of behavior away from opioid self-administration and toward food self-administration at the level of opioid dependence achieved in the current study. Conversely, the lack of TRV130 effects in non-opioid-dependent subjects demonstrates that TRV130 MOR efficacy is too high to function as an antagonist of fentanyl self-administration. Overall, the differential effectiveness of TRV130 on the dependent measures of somatic withdrawal sign expression and fentanyl choice has implications for our understanding of the neurobiological mechanisms mediating opioid reinforcement during withdrawal.
Implications for preclinical evaluation of MOUDs for opioid-dependent people
During the 2-week period of extended fentanyl access, daily 12-h sessions of fentanyl availability were followed by daily 8-h withdrawal periods before the subsequent fentanyl-choice session, and rats treated only with vehicle during these daily withdrawal periods showed increasing levels of withdrawal signs and weight loss prior to daily choice sessions. Methadone treatment in fentanyl-dependent rats virtually eliminated withdrawal signs and decreased weight loss while also nearly eliminating fentanyl choice. We reported similar results with methadone previously [41], and morphine also displayed similar effectiveness to reduce opioid choice and withdrawal signs in opioid-dependent monkeys undergoing withdrawal [53]. These findings might initially seem to raise the possibility that fentanyl choice in opioid-dependent rats and monkeys may be maintained partially or completely by negative reinforcing effects of somatic withdrawal (i.e., dependent rats chose fentanyl to alleviate opioid withdrawal) [51], but our findings with TRV130 suggest otherwise. Specifically, the TRV130 dosing regimen was as effective as methadone to reduce somatic withdrawal signs and alleviate weight loss, but it was less effective than methadone to reduce fentanyl choice.
The dissociation in TRV130 effects on somatic signs vs. fentanyl choice has at least two implications. First, lower levels of MOR activation may be sufficient to reduce the unconditioned opioid withdrawal signs measured here (i.e., somatic withdrawal signs, withdrawal-associated weight loss), while higher levels may be required to reduce operant-conditioned fentanyl choice in opioid-dependent subjects. This illustrates that intermediate efficacy MOR agonists may block withdrawal signs in opioid-dependent people without blocking opioid self-administration, similar to non-MOR MOUDs like clonidine [56]. Reciprocally, a second implication is that fentanyl choice could be maintained in part by relief of withdrawal signs more subtle than those detected by our observational somatic withdrawal signs. For example, previous studies have shown that opioid antagonists like naloxone are more potent in opioid-dependent subjects to elicit some “affective” signs of withdrawal (e.g., conditioned place aversion) than to elicit grossly observable withdrawal signs [57]. Like these other “affective” signs of withdrawal, fentanyl choice may be sensitive to even mild levels of withdrawal. For example, naltrexone is more potent to increase opioid choice in monkeys [54] than to elicit a range of different unconditioned withdrawal signs [58, 59]. Accordingly, reductions in opioid choice in opioid-dependent people undergoing withdrawal may require very high levels of MOR activation sufficient not only to block somatic signs of opioid withdrawal, but also to block even these mild and residual withdrawal signs.
Supplementary information
Author contributions
EAT, MLB, SSN, DHE, and YS designed and conceptualized the study. EAT carried out the experiments. EAT, MLB, and SSN performed the data analyses. BEB synthesized TRV130. DHE and SSN secured funding. All authors reviewed the content and approved the final version prior to submission.
Funding
Research was supported by the National Institute on Drug Abuse of the National Institutes of Health under R01DA026946 (PI, SSN), P30DA033934 (PI, William L. Dewey), and NIH Bench-to-Bedside Grant (PI, DHE). The research was supported [in part] by the Intramural Research Program of the NIH, NIDA. The National Institute on Drug Abuse had no role in study design, collection, analysis or interpretation of the data, in the writing or decision to submit the manuscript for publication. The manuscript content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. YS is an associate editor for Neuropsychopharmacology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01393-3.
References
- 1.Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. Center for Disease Control and Prevention: National Center for Health Statistics; 2022.
- 2.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 2010;29:200–16. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009:CD002209. [DOI] [PubMed]
- 4.Buprenorphine/Naloxone Versus Methadone for the Treatment of Opioid Dependence: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness and Guidelines [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016 Sep 2. [PubMed]
- 5.Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3:e1920622. doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105:e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modesto-Lowe V, Brooks D, Petry N. Methadone deaths: risk factors in pain and addicted populations. J Gen Intern Med. 2010;25:305–9. doi: 10.1007/s11606-009-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selley DE, Liu Q, Childers SR. Signal transduction correlates of Mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharm Exp Ther. 1998;285:496–505. [PubMed] [Google Scholar]
- 9.Caplehorn JR. Deaths in the first two weeks of maintenance treatment in NSW in 1994: identifying cases of iatrogenic methadone toxicity. Drug Alcohol Rev. 1998;17:9–17. doi: 10.1080/09595239800187551. [DOI] [PubMed] [Google Scholar]
- 10.Zador D, Sunjic S. Deaths in methadone maintenance treatment in New South Wales, Australia 1990-5. Addiction. 2000;95:77–84. doi: 10.1046/j.1360-0443.2000.951778.x. [DOI] [PubMed] [Google Scholar]
- 11.Zador DA, Sunjic SD. Methadone-related deaths and mortality rate during induction into methadone maintenance, New South Wales, 1996. Drug Alcohol Rev. 2002;21:131–6. doi: 10.1080/09595230220139028. [DOI] [PubMed] [Google Scholar]
- 12.Cruciani RA. Methadone: to ECG or not to ECG…That is still the question. J Pain Symptom Manag. 2008;36:545–52. doi: 10.1016/j.jpainsymman.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth. 2005;94:825–34. doi: 10.1093/bja/aei145. [DOI] [PubMed] [Google Scholar]
- 14.Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627–32. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 15.Moss LM, Algera MH, Dobbins R, Gray F, Strafford S, Heath A, et al. Effect of sustained high buprenorphine plasma concentrations on fentanyl-induced respiratory depression: a placebo-controlled crossover study in healthy volunteers and opioid-tolerant patients. PLoS ONE. 2022;17:e0256752. doi: 10.1371/journal.pone.0256752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sessler NE, Walker E, Chickballapur H, Kacholakalayil J, Coplan PM. Disproportionality analysis of buprenorphine transdermal system and cardiac arrhythmia using FDA and WHO postmarketing reporting system data. Postgrad Med. 2017;129:62–8. doi: 10.1080/00325481.2016.1271698. [DOI] [PubMed] [Google Scholar]
- 17.Spadaro A, Sarker A, Hogg-Bremer W, Love JS, O’Donnell N, Nelson LS, et al. Reddit discussions about buprenorphine associated precipitated withdrawal in the era of fentanyl. Clin Toxicol (Phila). 2022;60:694–701. Epub 2022 Feb 4. [DOI] [PMC free article] [PubMed]
- 18.Soyka M. Transition from full Mu opioid agonists to buprenorphine in opioid dependent patients—a critical review. Front Pharm. 2021;12:718811. doi: 10.3389/fphar.2021.718811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgueno J, Pujol M, Monroy X, Roche D, Varela MJ, Merlos M, et al. A complementary scale of biased agonism for agonists with differing maximal responses. Sci Rep. 2017;7:15389. doi: 10.1038/s41598-017-15258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yudin Y, Rohacs T. The G-protein-biased agents PZM21 and TRV130 are partial agonists of mu-opioid receptor-mediated signalling to ion channels. Br J Pharm. 2019;176:3110–25. doi: 10.1111/bph.14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal. 2020;13. [DOI] [PubMed]
- 22.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–90.. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155:1829–35.. doi: 10.1016/j.pain.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Ayad S, Demitrack MA, Burt DA, Michalsky C, Wase L, Fossler MJ, et al. Evaluating the incidence of opioid-induced respiratory depression associated with oliceridine and morphine as measured by the frequency and average cumulative Duration of dosing interruption in patients treated for acute postoperative pain. Clin Drug Investig. 2020;40:755–64. doi: 10.1007/s40261-020-00936-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahan A, van Dam CJ, Niesters M, van Velzen M, Fossler MJ, Demitrack MA, et al. Benefit and risk evaluation of biased mu-receptor agonist oliceridine versus morphine. Anesthesiology. 2020;133:559–68. doi: 10.1097/ALN.0000000000003441. [DOI] [PubMed] [Google Scholar]
- 26.Bergese S, Berkowitz R, Rider P, Ladouceur M, Griffith S, Segura Vasi A, et al. Low incidence of postoperative respiratory depression with oliceridine compared to morphine: a retrospective chart analysis. Pain Res Manag. 2020;2020:7492865. doi: 10.1155/2020/7492865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food and Drug Administration. Oliceridine briefing document: FDA advisory committee meeting. 2018.
- 28.Ramos KA, James IE, Skobieranda F, Soergel DG, Ruff D, Fossler MJ. Two-part phase 1 multiple-ascending-dose study to evaluate the safety, tolerability, pharmacodynamics, and pharmacokinetics of TRV734 in healthy adults. Clin Pharm Drug Dev. 2022;11:51–62. doi: 10.1002/cpdd.1016. [DOI] [PubMed] [Google Scholar]
- 29.Banks ML, Townsend EA, Negus SS. Testing the 10 most wanted: a preclinical algorithm to screen candidate opioid use disorder medications. Neuropsychopharmacology. 2019;44:1011–2. doi: 10.1038/s41386-019-0336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsend EA, Negus SS, Banks ML. Medications Development for Treatment of Opioid Use Disorder. Cold Spring Harb Perspect Med. 2021;11:a039263. [DOI] [PMC free article] [PubMed]
- 31.Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharm Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends Pharm Sci. 2017;38:181–94. doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spragg SDS. Morphine addiction in chimpanzees. Baltimore: Johns Hopkins Press; 1940. p. 132.
- 34.Griffiths RR, Wurster RM, Brady JV. Choice between food and heroin: effects of morphine, naloxone, and secobarbital. J Exp Anal Behav. 1981;35:335–51. doi: 10.1901/jeab.1981.35-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharm Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 36.Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014:CD002207. [DOI] [PubMed]
- 38.Klimas J, Gorfinkel L, Giacomuzzi SM, Ruckes C, Socias ME, Fairbairn N, et al. Slow release oral morphine versus methadone for the treatment of opioid use disorder. BMJ Open. 2019;9:e025799. doi: 10.1136/bmjopen-2018-025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noble F, Marie N. Management of opioid addiction with opioid substitution treatments: beyond methadone and buprenorphine. Front Psychiatry. 2018;9:742. doi: 10.3389/fpsyt.2018.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend EA, Schwienteck KL, Robinson HL, Lawson ST, Banks ML. A drug-vs-food “choice” self-administration procedure in rats to investigate pharmacological and environmental mechanisms of substance use disorders. J Neurosci Methods. 2021;354:109110. doi: 10.1016/j.jneumeth.2021.109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ. Opioid withdrawal produces sex-specific effects on fentanyl-versus-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci. 2021. 10.1016/j.bpsgos.2021.04.009. [DOI] [PMC free article] [PubMed]
- 42.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, et al. G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharm Exp Ther. 2013;344:708–17. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 43.Austin Zamarripa C, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend. 2018;192:158–62. doi: 10.1016/j.drugalcdep.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, et al. Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology. 2019;150:200–9. doi: 10.1016/j.neuropharm.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 2017;31:730–9. doi: 10.1177/0269881116689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Wang Y, Zuo A, Li C, Wang W, Jiang W, et al. Synthesis, biological, and structural explorations of a series of mu-opioid receptor (MOR) agonists with high G protein signaling bias. Eur J Med Chem. 2022;228:113986. doi: 10.1016/j.ejmech.2021.113986. [DOI] [PubMed] [Google Scholar]
- 47.Ling GS, Umans JG, Inturrisi CE. Methadone: radioimmunoassay and pharmacokinetics in the rat. J Pharm Exp Ther. 1981;217:147–51. [PubMed] [Google Scholar]
- 48.Liu SX, Gades MS, Swain Y, Ramakrishnan A, Harris AC, Tran PV, et al. Repeated morphine exposure activates synaptogenesis and other neuroplasticity-related gene networks in the dorsomedial prefrontal cortex of male and female rats. Drug Alcohol Depend. 2021;221:108598. doi: 10.1016/j.drugalcdep.2021.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Townsend EA, Bremer PT, Jacob NT, Negus SS, Janda KD, Banks ML. A synthetic opioid vaccine attenuates fentanyl-vs-food choice in male and female rhesus monkeys. Drug Alcohol Depend. 2021;218:108348. doi: 10.1016/j.drugalcdep.2020.108348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Townsend EA, Negus SS, Poklis JL, Banks ML. Lorcaserin maintenance fails to attenuate heroin vs. food choice in rhesus monkeys. Drug Alcohol Depend. 2020;208:107848. doi: 10.1016/j.drugalcdep.2020.107848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Townsend EA, Blake S, Faunce KE, Hwang CS, Natori Y, Zhou B, et al. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology. 2019;44:1681–9. [DOI] [PMC free article] [PubMed]
- 52.Hammerslag LR, Hofford RS, Kang Q, Kryscio RJ, Beckmann JS, Bardo MT. Changes in fentanyl demand following naltrexone, morphine, and buprenorphine in male rats. Drug Alcohol Depend. 2020;207:107804. doi: 10.1016/j.drugalcdep.2019.107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2008;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dean RL, Bilsky EJ, Negus SS. Opiate receptors and antagonists: from bench to clinic. New York, NY: Humana Press; 2009. xxiii, 757 p. 6.
- 55.Jones BE, Prada JA. Effects of methadone and morphine maintenance on drug-seeking behavior in the dog. Psychopharmacology. 1977;54:109–12. doi: 10.1007/BF00426764. [DOI] [PubMed] [Google Scholar]
- 56.Ling W, Amass L, Shoptaw S, Annon JJ, Hillhouse M, Babcock D, et al. Buprenorphine Study Protocol G. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharm Exp Ther. 1994;271:1391–8. [PubMed] [Google Scholar]
- 58.Valentino RJ, Katz JL, Medzihradsky F, Woods JH. Receptor binding, antagonist, and withdrawal precipitating properties of opiate antagonists. Life Sci. 1983;32:2887–96. doi: 10.1016/0024-3205(83)90325-9. [DOI] [PubMed] [Google Scholar]
- 59.Becker GL, Gerak LR, Koek W, France CP. Antagonist-precipitated and discontinuation-induced withdrawal in morphine-dependent rhesus monkeys. Psychopharmacology. 2008;201:373–82. doi: 10.1007/s00213-008-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.