Abstract
Biomphalaria snails, namely B. pfeifferi and B. sudanica, are the principal intermediate hosts for Schistosoma mansoni infection in Ethiopia. Epidemiological studies of Biomphalaria snails and their infection status with S. mansoni is vital for public health planning. This study aimed to assess the spatial and seasonal abundance of Biomphalaria snails as well as their infection status with S. mansoni around Lake Tana, northwest Ethiopia. Malacological survey was conducted from January 2021 to December 2021 in ten different collection sites in and around Lake Tana. Snail collection was performed for 20 min from each collection site seasonally (four times in a year) using a standard scoop and handpicking from aquatic vegetation. All collected snails were carefully examined based on their morphological features and all live Biomphalaria snails were subjected to cercariae shedding experiment. Descriptive statistics were used to determine the prevalence of S. mansoni infection and its relationship with snail collection sites and seasons. A total of 3886 freshwater snails were collected from ten collection sites around Lake Tana. Out of the total snails collected, 1606 (41.3%; 95% CI 39.77–42.89%) were Biomphalaria spp. The highest (374) and the lowest numbers (98) of Biomphalaria snails were collected from Shinne River and Qunzela Lakeshore, respectively. Out of the 1375 live Biomphalaria snails, 14.4% (95% CI 12.59–16.37%) snails shed cercariae, but only 4.87% (95% CI 3.79–6.15%) were cercariae of S. mansoni. The infection prevalence of S. mansoni ranged from 10.59% at the Cherechera site to 1.49% at Gumara River. Biomphalaria snail infections with S. mansoni cercariae were observed throughout the season, the highest and the lowest infection rates being in the spring and summer seasons. Significant differences in the prevalence of S. mansoni infection in Biomphalaria snails were observed across study sites and seasons (p < 0.05). Biomphalaria snails were the most abundant freshwater snails found in nearly all of snail collection sites throughout the year. It was revealed that nearly five percent of Biomphalaria snails were infected with S. mansoni cercariae. This study highlights the importance of appropriate snail control strategies to support the ongoing prevention and control of schistosomiasis around Lake Tana.
Subject terms: Zoology, Diseases
Introduction
Schistosomiasis is one of the neglected tropical diseases (NTD) that is widely distributed in Africa, South America, the Middle East and Southeast Asia1,2. The prevalence of the disease varies among regions depending on the socio-economic level, environmental conditions, human water contact behaviour of the community as well as on the level of control strategies employed in the country. The disease is severe in Africa, particularly in sub-Saharan Africa, due to the suitability of the climatic condition and socio-economic development of the region. It is estimated that 85–95% of the global schistosomiasis are in sub-Saharan Africa with the highest prevalence among school-aged childre3,4 In Ethiopia, the prevalence of schistosomiasis could reach as high as 90% in some localities, particularly for Schistosoma mansoni5. Although Ethiopia launched a school-based deworming program in 2015 to control schistosomiasis6, the prevalence of the disease is still high in several localities7–10.
Schistosoma mansoni uses freshwater snails of the genus Biomphalaria as an intermediate host to complete its life cycle11. Malacological studies have indicated the presence of several snail groups in Ethiopia. Biomphalaria species, namely B. pfeifferi and B. sudanica, serve as intermediate hosts for S. mansoni12,13 while Bulinus snails serve as intermediate hosts for S. haematobium14,15. B. pfeifferi and B. sudanica are the principal intermediate hosts for S. mansoni in Ethiopia. However, limited information is available about the distribution, abundance and diversity of these snails in several endemic foci of the country. The distribution of schistosomiasis in any endemic foci is directly correlated with the distribution of snail vectors16,17. Information regarding the distribution and abundance of Biomphalaria snails around Lake Tana is dated back to the beginning of the 1990s18. An updated and in-depth investigation of snail intermediate hosts of S. mansoni is vital to designing cost-effective snail control strategies in the area.
The infection prevalence of Biomphalaria snails with S. mansoni varied from 319 to 58%20 in Ethiopia. Our previous review showed that about 15% of Biomphalaria snails of Ethiopia were positive for S. mansoni cercariae21. However, the infection status of Biomphalaria snails around Lake Tana has not been investigated. A recent evidence showed a high prevalence (35%) of S. mansoni infection in humans7 in the study area despite the ongoing deworming program. In the present study it was hypothesized that there might be a high-level of Biomphalaria snails infected with S. mansoni around Lake Tana. Knowing the infection status of freshwater snails with S. mansoni serves as one of the important criteria to determine the transmission dynamics of S. mansoni in the study area. In addition, assessment of natural snail infection with S. mansoni is important to elucidate the level of environmental contaminations with fecal matter from humans as well as from other non-human primates.
Epidemiological studies on the abundance, distribution, and infection status of Biomphalaria snails are vital for policymakers to design appropriate schistosomiasis prevention and control strategies. However, there is no recent information on the abundance and distribution of Biomphalaria snails and their infection status around Lake Tana. . Therefore, this study aimed to investigate the spatial and seasonal abundance of Biomphalaria snails and their infection status with S. mansoni in and around Lake Tana, northwestern Ethiopia.
Material and methods
Description of the study areas
Lake Tana is located in the north-western part of Ethiopia at 12°0.00' N and 37°0.14' E. Lake Tana is the largest lake in Ethiopia and the major source of the Blue Nile River. The lake consists of more than 37 islands and peninsula and some of them serve for human habitation22. Lake Tana covers an area of 3020 km2 and a maximum depth of 15 m. Lake Tana is rich in biodiversity with several species of birds, fish, amphibians, macro-invertebrates, and micro-invertebrates. The lake and islands on the lake serve as homes for several species of birds including the endemic ones. Lake Tana consists of 28 known species of fish, of which 68% of them are endemic23. As a result of its rich biodiversity, the United Nations Educational, Scientific and Cultural Organization (UNESCO) recognized Lake Tana as a Biosphere reserve in 201524. The present study was conducted at different sites of Lake Tana shores (Dek, Cherechera, Gorgora, Zegie and Qunzela) and tributary rivers of Lake Tana (Enferanz, Gumara, Garno, Shinnie and Robit). The selection of the collection sites was based on human habitation and the frequent human-water contact behaviour of the community.
Operational definitions of words or phrases
Winter is a dry season in Ethiopia that span from December to February. Spring is span from March to May. There may be occasional rain in most parts of Ethiopia. Summer is the major rainy season in Ethiopia that span from June to August. Autumn is the major harvesting season in Ethiopia that spans from September to November.
Study design
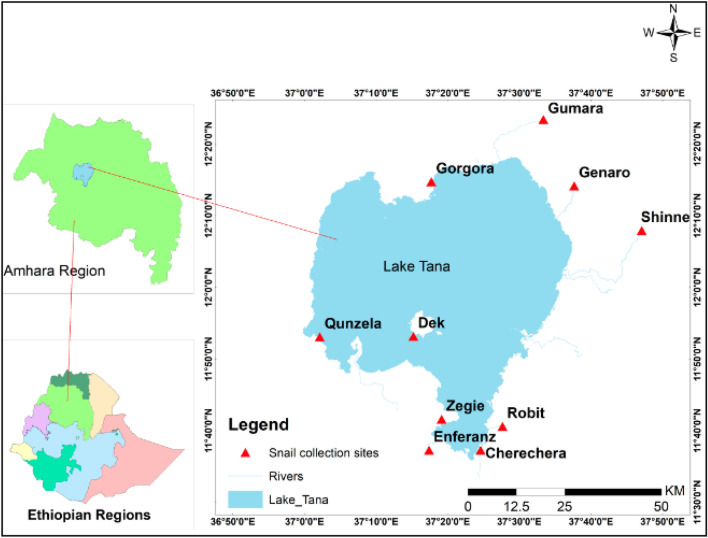
Malacological surveys were conducted from January 2021 to December 2021 to assess the distribution and seasonal abundance of Biomphalaria snails from the shorelines of Lake Tana (Dek, Cherechera, Gorgora, Zegie and Qunzela) and its tributary rivers, namely Enferanz, Gumara, Garno, Shinnie and Robit (Fig. 1). From each site, snails were sampled from two different points at least 200 m apart. The specific sample collection sites were selected based on the frequency of human-water contact during water fetching, washing clothes, bathing, swimming, fish processing and other domestic activities. The geographical coordinate of each sampling site was taken using a global positioning system (GPS) and it was properly recorded.
Figure 1.
Map of the study areas around Lake Tana. The map was prepared using ArcGIS online software.
Snail collection
Freshwater snails were collected and examined using a standard protocol as described elsewhere25. Snails were sampled using standard scoops (2 mm mesh size) and forceps from water bodies or picked with gloved hands from aquatic vegetation at the shoreline of Lake Tana as well as from the rivers that fed the lake. The snail collection sites were selected based on close proximity to human settlement and high level of open defecation. The scooping was performed for 20 min from each site between 8:00 AM and 10:00 AM on a seasonal basis (four times a year) by the same individual. Samplings were conducted in areas about 10 m along the shorelines of Lake Tana, selected rivers, and from an area of ca.5m2 from lake water at each sampling point. Each collected snail was kept separately in a wide-mouth glass bottle filled with water and aquatic vegetation from the same area. The snail samples were transported to the Biomedical Sciences Laboratory of the Department of Biology, Bahir Dar University. All collected snails were sorted, counted and identified in the laboratory.
Morphological identification of Biomphalaria snails
All the collected snails were carefully examined based on morphological features using standard identification keys to at least a genus level as described elsewhere25–27. The common criteria to distinguish snail species include shell shape, shell size, nature of aperture, color and banding pattern of the shell28. Once the morphological identification was completed, the snails were kept at dark for 48 h and then they were used for cercariae shedding experiment.
Testing of snails for S. mansoni infections and identification
Individual snails were carefully transferred into shedding vials that contained 10 ml of natural spring water29 with a neutral pH. The shedding of S. mansoni and other trematodes cercariae was induced by exposure to artificial light (60 watts) for about two hours at room temperature in the morning (10:00–12:00 AM30. Each snail was observed under a dissecting microscope to determine the presence of shedding trematodes cercariae. The water in the shedding vial was carefully examined for the presence of cercariae using a dissecting microscope.
Live cercariae shedding from each snail were transferred to a microscopic slide and covered with a coverslip. The cercariae were carefully observed using a microscope with 40 × magnification power and identified based on their morphological features using a standard identification key31–33. The types and the numbers of cercariae discharged from each snail were properly recorded.
Data analysis
The data generated during the study were analysed using SPSS version 23. Descriptive statistics was used to determine the proportion of Biomphalaria snails and their infection prevalence across study sites and study seasons. Analysis of variance (ANOVA) was used to determine the differences in the abundance of Biomphalaria snails across study sites and seasons. Chi-square test was used to asssess the relationship between Biomphalaria snail infection with S. mansoni and studies sites and seasons. The geographic coordinate of snail collection sites was taken using a global positioning system (GPS) from each sampling point. Mapping of the snail collection site was prepared using ArcGIS online free software. For all statistical analyses, a p-value below 0.05 was used to declare statistical significance..
Ethics approval and consent to participate
This study was conducted after obtaining ethical clearance from the Ethical Review Committee of College of Science, Bahir Dar University with Ref. No. PGRCSVD/155/2020. The objective of the study was explained to the local administration before snail collection.
Results
The study was conducted at a latitudinal range of 11.6181–12.3909° E and a longitudinal range of 37.0343–37.7848°N. The collection sites were classified as periphery of Lake Tana and its tributary rivers. All snails were collected from the area where there is frequent human-water contact for various activities such as washing clothes, bathing, swimming, fetching water and fish processing (Table 1). The snail collection sites had either muddy or stony substrates with clean or turbid water. Some of the images of snail collection sites are presented in Fig. 2.
Table 1.
Sampling points, GPS coordinates and other basic information of the snail collection sites.
| Study area | Sampling point | Elevation (masl) | GPS coordinate | Human activity | Nature of substrate | Nature of the water | Vegetation type | Habitat classification | |
|---|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | ||||||||
| Cherechera | a | 1793 | 11.62069 | 37.40997 | Swimming, bathing and fish processing | Muddy | Turbid water | Floating vegetation | Lake periphery |
| b | 1789 | 11.61805 | 37.41066 | ||||||
| Dek Island | a | 1787 | 11.88602 | 37.25337 | Bathing, washing cloth & fish processing | Muddy | Turbid water | Papyrus and other floating vegetation | Lake periphery |
| b | 1788 | 11.88646 | 37.25124 | ||||||
| Zegie Peninsula | a | 1787 | 11.69279 | 37.31926 | Swimming, bathing and washing cloth | Rocky | Clear | Floating vegetation | Lake periphery |
| b | 1782 | 11.69217 | 37.31730 | ||||||
| Qunzela town | a | 1806 | 11.88451 | 37.03577 | Bathing, fetching & washing cloth | Muddy | Turbid water | Floating vegetation | Lake periphery |
| b | 1790 | 11.88241 | 37.03426 | ||||||
| Gorgora Peninsula | a | 1802 | 12.24575 | 37.29543 | Bathing, washing cloth & fish processing | Rocky | Clean | Floating vegetation | Lake periphery |
| b | 1790 | 12.24256 | 37.29681 | ||||||
| Enferanz river | a | 1820 | 11.62090 | 37.28981 | Fetching and washing cloth | Muddy | Turbid water | Floating vegetation | River |
| b | 1807 | 11.62208 | 37.28934 | ||||||
| Shinie river | a | 1957 | 12.13247 | 37.78487 | Bathing & washing cloth | Rocky | Clean water | Covered with green algae | River |
| b | 1950 | 12.13083 | 37.78442 | ||||||
| Robit river | a | 1849 | 11.67600 | 37.46081 | Bathing & washing cloth | Rocky | Clean water | Algae & floating vegetation | River |
| b | 1841 | 11.67606 | 37.45956 | ||||||
| Gumara river | a | 1906 | 12.39091 | 37.55574 | Bathing, fetching & washing cloth | Rocky | Turbid water | Covered with algae | River |
| b | 1903 | 12.39083 | 37.55716 | ||||||
| Garno river | a | 1859 | 12.23636 | 37.62763 | Bathing and washing cloth | Rocky | Clean water | Covered with algae | River |
| b | 1856 | 12.23685 | 37.62878 | ||||||
Figure 2.
Image taken from some of the snail collection sites around Lake Tana; (a) Enferanz river (b) Cherechera site (c) Garno river (d) Qunzela port (e) Gorgora port (f) Shinne river (g) Dek Island (h) Robit river. All the images were taken by the corresponding author (TH).
The abundance of freshwater snails in Lake Tana and tributary rivers
A total of 3886 freshwater snails were collected from 20 sampling points at ten study sites during the study period. Five freshwater snail genera, namely Biomphalaria, Lymnaea, Bulinus, Melanoides and Bellamya snails, were recorded from the study areas (Fig. 3). The dominant snail genus observed in the study area was Biomphalaria (41.33%, 95% CI 39.77–42.89%) followed by Lymnaea (Table 2). Lymnaea snails were identified from all snail collection sites while Biomphalaria snails were observed from nine snail collection sites. Melanoides and Bellamya were observed from limited study sites, mainly from the Lake Tana periphery.
Figure 3.
Freshwater snail genera collected from Lake Tana; (a) Biomphalaria snails (right and left side view), (b) Bulinus snails (apertural and abapertural view), (c) Lymnaea snails (ventral and dorsal view), (d) Bellamya snails (ventral and dorsal view) and (e) Melanoides snails (ventral and dorsal view). All the images were taken by the corresponding author (TH).
Table 2.
Diversity and abundance of freshwater snails of Lake Tana and its tributary rivers, 2021/22.
| Collection sites | Freshwater snail Genus observed in the study area | |||||
|---|---|---|---|---|---|---|
| Biomphalaria snails No. (%) | Bulinus snails No. (%) | Lymnaea snails No. (%) | Melanoides snails No. (%) | Bellamya snails No. (%) | Total No. (%) | |
| Cherechera | 194 (12.1) | 87 (19.6) | 40 (3.0) | 139 (70.2) | 71 (23.0) | 531 (13.70) |
| Dek Island | 173 (10.8) | 119 (26.8) | 151 (11.4) | 0 | 34 (11.0) | 477 (12.3) |
| Zegie Peninsula | 99 (6.2) | 65 (14.6) | 63 (4.7) | 37 (18.7) | 76 (24.6) | 340 (8.7) |
| Qunzela town | 98 (6.1) | 68 (15.3) | 173 (13.0) | 9 (4.6) | 58 (18.8) | 406 (10.5) |
| Gorgora Peninsula | 0 | 17 (3.8) | 56 (4.2) | 0 | 38 (12.3) | 111 (2.8) |
| Enferanze river | 128 (7.9) | 45 (10.1) | 168 (12.6) | 12 (6.1) | 32 (10.4) | 385 (9.9) |
| Shinie river | 374 (23.3) | 0 | 240 (18.1) | 0 | 0 | 614 (15.8) |
| Robit river | 240 (14.9) | 42 (9.5) | 319 (24.0) | 0 | 0 | 601 (15.5) |
| Gumara river | 162 (10.1) | 1 (0.23) | 60 (4.5) | 0 | 0 | 223 (5.7) |
| Garno river | 138 (8.6) | 0 | 59 (4.4) | 1 (0.5) | 0 | 198 (5.1) |
| All sites | 1606 (41.33) | 444 (11.43) | 1329 (34.20) | 198 (5.10) | 309 (7.94) | 3886 (100) |
Spatial and seasonal abundance of Biomphalaria snails
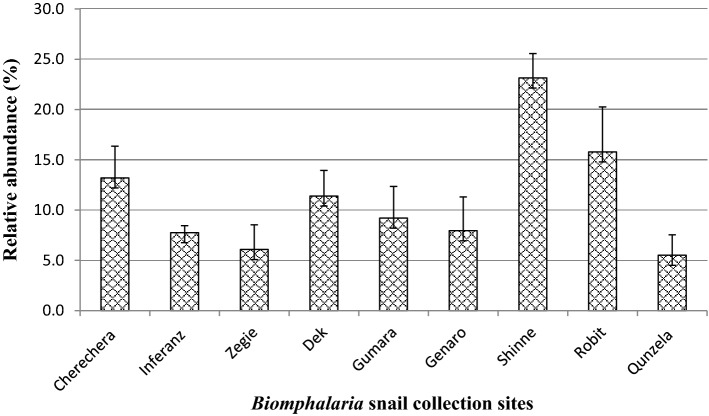
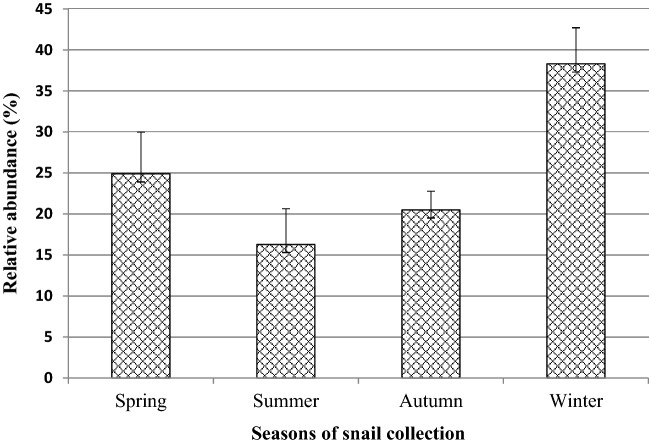
Biomphalaria snails were collected from 18 sampling points around Lake Tana. A total of 1606 Biomphalaria snails were collected from the nine study sites around Lake Tana. The highest (23.29%; 95% CI 21.2–25.4%) and the lowest (6.1%; 95% CI 4.9–7.4%) number of Biomphalaria snails were collected from Shinne River and Qunzela lakeshore, respectively (Fig. 4). Similarly, the abundance of Biomphalaria snails varied across snail collection seasons. The seasonal distribution showed that the winter season had the highest Biomphalaria snail abundance, 36.43% (95% CI 34.07–38.83%), while the summer season showed the lowest abundance of Biomphalaria snail, 18.06% (95% CI 16.21–20.03%) (Fig. 5). There was a significant difference in the abundance of Biomphalaria across study sites and seasons (p < 0.05).
Figure 4.
Relative abundance of Biomphalaria snails at different collection sites.
Figure 5.
Relative abundance of Biomphalaria snails on a seasonal basis.
Comparison of Biomphalaria snails by habitat
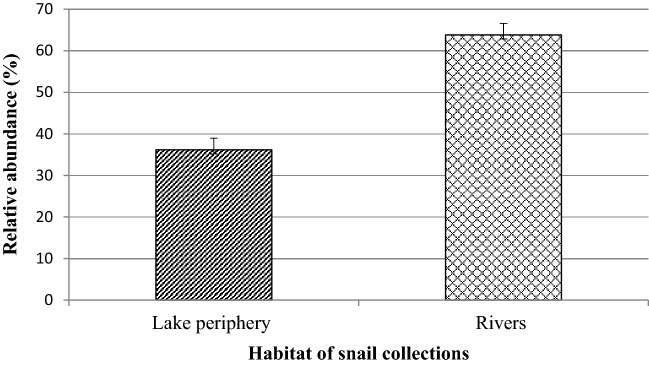
Freshwater snails were collected from two types of habitats: tributary rivers and Lake periphery. Biomphalaria snails were more common in rivers with a sandy and stony basement than in lakeshores (Fig. 6). There was a significant difference in the number of Biomphalaria snails between the lakeshore and riverine areas (p = 0.026).
Figure 6.
Relative abundance of Biomphalaria snails at study habitat.
Infection status of Biomphalaria snails of Lake Tana and its tributary rivers
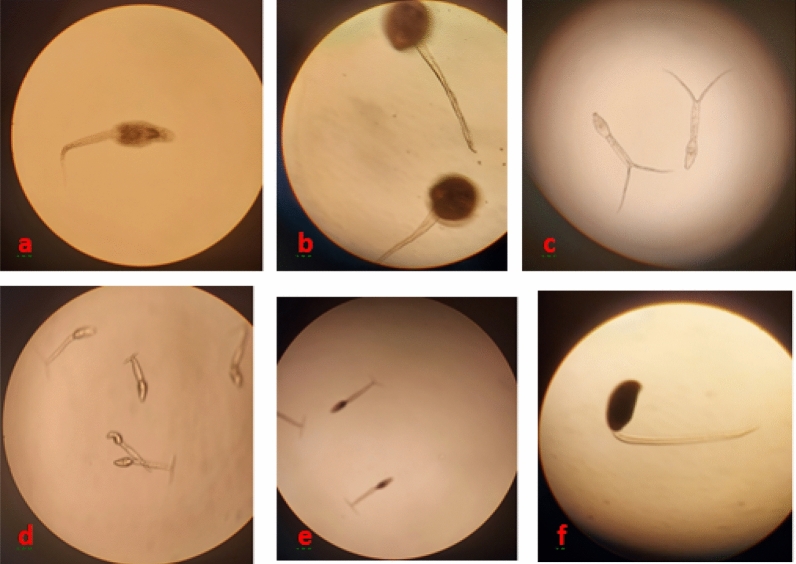
A total of 1375 live Biomphalaria snails were tested for trematode infection. Among these snails, 14.40% (95% CI 12.59–16.37%) snails shed trematode cercariae, but only 4.87% (95% CI 3.79–6.15%) were cercariae of S. mansoni (Table 3). The common trematodes observed in this study consisted of cercariae of Schistosoma mansoni, Amphistome, Echinostome, Brevifurcate apharyngeate distome and unidentified cercaria (Fig. 7).
Table 3.
Prevalence of Schistosoma mansoni and other trematodes infection among Biomphalaria snail species.
| Snail collection sites | Snail count | Snail examined for cercaria | S. mansoni cercaria | Other trematodes cercaria | Total trematodes cercaria |
|---|---|---|---|---|---|
| Number | Number | Number (%) | Number (%) | Number (%) | |
| Cherechera | 194 | 170 | 18 (10.59) | 23 (13.53) | 41 (24.12) |
| Inferanz river | 128 | 109 | 6 (5.50) | 24 (22.02) | 30 (27.52) |
| Zegie Peninsula | 99 | 84 | 2 (2.38) | 6 (7.14) | 8 (9.52) |
| Dek Island | 173 | 143 | 7 (4.90) | 19 (13.29) | 28 (19.58) |
| Gumara river | 162 | 134 | 2 (1.49) | 6 (4.48) | 8 (5.97) |
| Genaro river | 138 | 127 | 2 (1.57) | 12 (9.45) | 14 (11.02) |
| Shinne river | 374 | 313 | 18 (5.75) | 23 (7.35) | 41 (13.10) |
| Robit river | 240 | 206 | 6 (2.91) | 12 (5.83) | 18 (8.74) |
| Qunzela town | 98 | 89 | 6 (6.74) | 4 (4.49) | 10 (11.24) |
| Total | 1606 | 1375 | 67 (4.87) | 129 (9.38) | 198 (14.40) |
Figure 7.
Trematode cercaria shed by Biomphalaria snails 100 × magnification; (a) Echinostome cercaria, (b) Amphistome cercaria, (c) Brevifurcate-apharyngeate diastome cercaria, (d, e) Schistosome cercaria, (f) Unidentified cercaria. Echinostome and Schistosome cercaria observed from all study sites while Brevifurcate diastome cercariae were observed in Cherechera, Enferanze, Robit and Dek. Unidentified cercaria was observed from Dek Island.
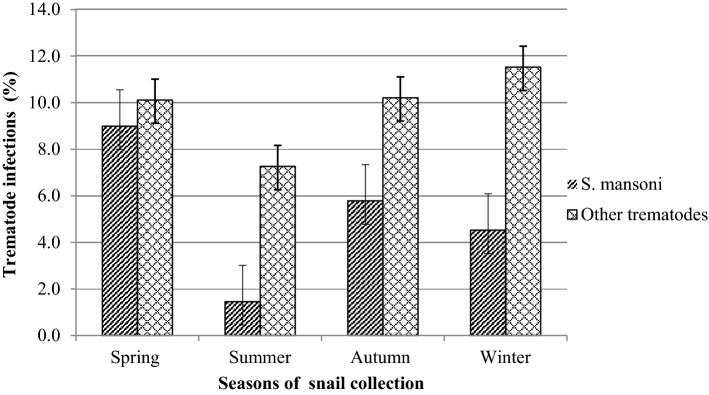
The highest S. mansoni infection was observed from the Cherechera site (10.59%) followed by Qunzela site (6.74%) while the lowest S. mansoni infection was observed from the Gumara River (1.49%). A significant difference in the infection prevalence was observed across study sites (p = 0.004). The study was conducted in all seasons and the highest and the lowest S. mansoni cercariae were observed during the spring and summer seasons, respectively (Fig. 8). There was a significant difference in the infection prevalence of Biomphalaria snails across study seasons (p < 0.001).
Figure 8.
Seasonal variations of S. mansoni and other trematodes infection.
Discussion
Epidemiological studies about the snail intermediate snail host species are vital for policymakers to design appropriate schistosomiasis control strategies. The principal intermediate host for S. mansoni in Ethiopia is Biomphalaria species12,13. Assessment of abundance, distribution and infectiction status of Biomphalaria snails contributes a lot to the prevention and control of schistosomiasis in the country. Schistosomiasis control strategies might not be effective without considering the snail intermediate hosts. In line with this, the present study aimed to determine the abundance, distribution and infection status of Biomphalaria snails with S. mansoni cercariae in and around Lake Tana.
The present study was conducted at lakeshores and tributary rivers of Lake Tana.This study revealed the presence of Biomphalaria, Bulinus, Lymnaea, Melanoides and Bellamya snails in the study sites. Among these snail genera, Biomphalaria snail was the predominant snail genus and it was recorded from nine study sites, which is in agreement with reports from studies conducted at Gibe River Basin, Ethiopia34, Kenya and Tanzania35. Biomphalaria snails were more common in rivers than in the lakeshores, which is in line with reports from studies conducted in Ethiopia36, East Africa37, South Africa38, Nigeria39 and Kenya40. This shows that Biomphalaria snails prefer rivers and streams that have clear water with sandy and gravel substrates to lakeshores that have muddy substrates.
Seasonal variation of Biomphalaria snails
The abundance and distribution of Biomphalaria snails varied significantly across study seasons. Biomphalaria snails were dominant during winter and spring as compared with other seasons. Similar observations were reported from Egypt41. Several studies have shown that the abundance of Biomphalaria snails was higher in the dry season than in wet season42–44. In contrast to our finding, Biomphalaria snails were more abundant during the wet seasons than in the dry season in South Africa29. This might be associated with the water temperature, velocity, turbidity and other environmental parameters of the study area. High rainfall, water velocity, and turbidity during the rainy season affect the natural habitats of snails in Ethiopia. As a result of these environmental conditions, the abundance of Biomphalaria snails may decline in the study area. This suggests that Biomphalaria snails may prefer stable habitat for survival.
The spatial variation of Biomphalaria snails
The abundance of Biomphalaria snails varied across study sites. Biomphalaria snails were recorded from all study sites except Gorgora. Although we attempted several times to search for Biomphalaria snails from Gorgora peninsula, we could not find Biomphalaria snails. The abundance of Biomphalaria snails varied from 6.1% to 23.3% in the different study sites. Spatial variations in the abundance of Biomphalaria snails across study sites were well documented35,40,45,46. It is known that snail abundance varies from area to area depending on different environmental and biotic factors. In the present study, the difference in abundance of snails across sites might be associated with the nature of study sites, Biomphalaria snails being more abundant in rivers than in lakeshores. A study in Senegal showed that Biomphalaria snails preferred clean rivers and streams having stony and gravel substrates47. The overall variation in the abundance of Biomphalaria snails might be associated with the nature of the water, types of aquatic vegetation, nature of water substrate, geographical locations and other environmental parameters.
Infection status of Biomphalaria snails
The current study showed that 14.4% of Biomphalaria snails shed different types of trematodes cercariae, which is in agreement with reports from studies conducted in Egypt48 and Tanzania49. In contrast to the present finding, only 4.6% of Biomphalaria snails were infected with trematodes around Omo Gibe River Basin in Ethiopia34. The present study revealed that 4.87% Biomphalaria snails were infected with S. mansoni, which is in agreement with reports from studies conducted in different parts of Ethiopia50,51 as well as with finding in systematic review and meta-analysis from African countries 52. In contrast, high prevalence of Schistosoma mansoni cercariae in Biomphalaria snails were reported from different parts of Ethiopia13,20,53, Tanzania49,54 and Nigeria55. The proportions of Biomphalaria snails infected with schistosome cercariae reported from Kenya were even lower than our findings40,45. The difference in infection status of Biomphalaria snails observed across studies is mainly linked to the types of diagnostic methods used. Superior detection of S. mansoni infection from Biomphalaria snails was obtained using PCR compared to cercarial shedding experiments. For example, 12% vs. 47% was reported from Tanzania49 and 5% vs. 27% shown in review paper in African countries52. In addition, anthropogenic activities, geographical locations, water quality, types of aquatic vegetation and other environmental factors might have contributed to the observed differences.
Significant variation of Biomphalaria infection with S. mansoni was observed across the study seasons. The highest infection rate was observed during the dry season as compared to the wet season, which is in line with reports from Tanzania56, Sudan43,57 and Nigeria58. High levels of open-field defecation, human-water contact activities, and stable water conditions are observed during the dry seasons of the year in Ethiopia. These conditions might contribute to the long-term survival of Biomphalaria snails leading to high chance of infection with S. mansoni miracidia.
The infection status of Biomphalaria snails varied across study sites. In this study, it was revealed that the proportion of infected Biomphalaria snails was higher along the Lake periphery than in rivers. In contrast to this finding, more number of infected Biomphalaria snails were reported from lakeshores as compared to rivers and streams in western Kenya40. These variations are mainly linked to the level of anthropogenic activities such as human-water contact activities and open-field defecation. High Biomphalaria snail infection was observed in the area where there is frequent human- water contact activities associated with washing clothes, swimming, bathing, fetching water and fish processing.
Schistosomiasis control strategies in sub-Saharan African countries including Ethiopia focus on mass-drug administration to school-aged children, with little or no emphasis on snail control. In this study, Biomphalaria snails were shown to be sources of S. mansoni infection and therefore it is an appropriate area for intervention to support the ongoing schistosomiasis prevention and control in the study area. Therefore, policymakers are advised to revisit the current schistosomiasis control and prevention strategies in Ethiopia.
Limitation of the study
Water quality and its association with snail abundance were not assessed in the present study. Water quality may have an impact on the abundance of Biomphalaria snails at different study sites as well as across seasons. The study was conducted at ten different sites and four seasons making it difficult to collect information for water quality analysis. In the present study, we used a cercarial shedding experiment which has lower sensitivity compared to PCR approaches49. This might have led to the underestimation of the true prevalence of S. mansoni cercariae in Biomphalaria snails.
Conclusion
Lake Tana and its tributary rivers serve as suitable habitats for freshwater snails particularly for Biomphalaria snails. Biomphalaria species were abundant freshwater snails and they were present in varied numbers in nearly all of snail collection sites throughout the year. In this study it was revealed that nearly five percent of Biomphalaria snails were infected with S. mansoni cercariae. The prevention and control of schistosomiasis in Ethiopia totally rely on mass-drug administration without giving due consideration to snail control. This study showed that Biomphalaria snails were important sources of S. mansoni infection to humans living in the nearby snail habitats. Therefore, policymakers, regional administrators, and other stakeholders working on schistosomiasis need to incorporate appropriate snail control strategies to support the ongoing schistosomiasis prevention and control strategies.
Acknowledgements
We would like to thank the International Foundation for Science (IFS) and Institute of Biotechnology (IoB), Bahir Dar University, for financial support for this study.
Abbreviations
- IoB
Institute of biotechnology
- GPS
Global positioning system
- SPSS
Statistical package for social science
- MASL
Meter above sea level
- NTD
Neglected tropical diseases
- UNESCO
United Nations Educational, Scientific and Cultural Organization
- PCR
Polymerase chain reaction
- CI
Confidence interval
- MDA
Mass drug administration
Author contributions
T.H. was involved in the design, data collection, processing, interpretation of the findings, and drafting of the manuscript. All the images were taken by T.H. E.N. and A.M. were involved in the conception of the idea, drafting the manuscript, reviewing and editing the manuscript.
Funding
The study was funded by the International Foundation for Science (IFS grant number: I2-A-6545-1) and Institute of Biotechnology (IoB), Bahir Dar University. The funders have no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou X. Schistosomiasis. Nat. Rev. Dis. Primers. 2018;4(1):13. doi: 10.1038/s41572-018-0013-8(2018). [DOI] [PubMed] [Google Scholar]
- 2.WHO. Schistosomiasis. 2022 [cited 2022 19 of March ]; Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
- 3.Mazigo, H. D. Participatory integrated control strategies and elimination of schistosomiasis in sub-Saharan Africa, Lancet Global Health7, E999 10.1016/S2214-109X(19)30271-2 (2019). [DOI] [PubMed]
- 4.Onasanya A, Bengtson M, Oladepo O, Van Engelen J, Diehl JC. Rethinking the top-down approach to schistosomiasis control and elimination in sub-saharan Africa. Front Public Health. 2021 doi: 10.3389/fpubh.2021.622809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worku L, Damte D, Endris M, Tesfa H, Aemero M. Schistosoma mansoni Infection and associated determinant factors among school children in Sanja town Northwest Ethiopia. J. Parasito. Res. 2014 doi: 10.1155/2014/792536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Ethiopian School-Based Deworming Campaign Targets 17 Million Children. 2015. (2015).
- 7.Hailegebriel T, Nibret E, Munshea E, Ameha Z. Prevalence, intensity and associated risk factors of Schistosoma mansoni infections among schoolchildren around Lake Tana, northwestern Ethiopia. PLoS NTDs. 2021;15(10):e0009861. doi: 10.1371/journal.pntd.0009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayalew J, Addisu A, Tegegne Y. Prevalence, intensity, and associated factors of Schistosoma mansoni among school children in northwest Ethiopia. J. Parasitol. Res. 2020 doi: 10.1155/2020/8820222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bereket A, Zewdneh T, Fiseha W, Dawit L, Liang S, Berhanu E. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone Southern Ethiopia. BMC Public Health. 2017;17(1):587. doi: 10.1186/s12889-017-4499-x(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teshome B, Hu W, Liang S, Berhanu E. Transmission of Schistosoma mansoni in Yachi areas, southwestern Ethiopia: new foci. Infect. Dis. Poverty. 2019;8(1):1. doi: 10.1186/s40249-018-0513-5(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X-T, Gu Q-Y, Limpanont Y, Song L-G, Wu Z-D, Okanurak K, et al. Snail-borne parasitic diseases: An update on global epidemiological distribution, transmission interruption and control methods. Infect Dis. Poverty. 2018;7(1):28. doi: 10.1186/s40249-018-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erko B, Balcha F, Kifle D. The ecology of Biomphalaria sudanica in Lake Ziway. Ethiopia Afr. J. Ecol. 2006;44(3):347–352. doi: 10.1111/j.1365-2028.2006.00615.x. [DOI] [Google Scholar]
- 13.Alebie G, Erko B, Aemero M, Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region. Ethiopia Parasites Vectors. 2014;7(1):15. doi: 10.1186/1756-3305-7-15(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hailu B, Berhanu E, Gebre M, Fekadu B. Decline of urinary schistosomiasis in Kurmuk town, western Ethio-Sudanese border. Ethiopia Ethiop. Med. J. 1996;34(1):47–49. [PubMed] [Google Scholar]
- 15.Itagaki H, Suzuki N, Ito Y, Hara T, Wonde T. Study on the Ethiopian freshwater molluscs especially on identification distribution and ecology of vector snails of human Schistosomiasi. Japanese J. Tropical Med. Hyg. 1975;3(2):107–134. doi: 10.2149/tmh1973.3.107. [DOI] [Google Scholar]
- 16.Gordy MA, Kish L, Tarrabain M. Hanington PC A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta. Canada Parasitol Res. 2016;115(10):3867–3880. doi: 10.1007/s00436-016-5152-9(2016). [DOI] [PubMed] [Google Scholar]
- 17.Abe, E.M., Guan, W., Guo, Y.-H., Kassegne, K., Qin, Z.-Q., Xu, J. et al. Differentiating snail intermediate hosts of Schistosoma spp. Using molecular approaches: Fundamental to successful integrated control mechanism in Africa, Infect. Dis. Poverty. 7(1), 29 10.1186/s40249-018-0401-z (2018). [DOI] [PMC free article] [PubMed]
- 18.Erko B, Tedla S, Petros B. Transmission of intestinal schistosomiasis in Bahir Dar, northwest. Ethiopia. Ethiop. Med. J. 1991;29(4):199–211. [PubMed] [Google Scholar]
- 19.Mekonnen Z, Haileselassie H, Medhin G, Erko B, Berhe N. Schistosomia mansoni focus in Mekele city, northern Ethiopia. Ethiop. Med. J. 2012;50(4):331–336. [PubMed] [Google Scholar]
- 20.Mulugeta M, Techalew S, Workneh T, Ashenafi T, Tesfaye K, Asrat H. Human intestinal Schistosomiasis in communities living near three rivers of Jimma town, south western Ethiopia. Ethiop. J. Health Sci. 2011;21(2):111–118. doi: 10.4314/ejhs.v21i2.69051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hailegebriel T, Nibret E, Munshea A. Prevalence of Schistosoma mansoni and associated risk factors in human and Biomphalaria snails in Ethiopia: A systematic review and Meta-analysis. Acta Parasitol. 2021 doi: 10.1007/s11686-021-00449-6. [DOI] [PubMed] [Google Scholar]
- 22.Melese, W. Ecosystem services and tourism potential in Lake Tana peninsula: Ethiopia review, J. Tourism Hosp. 6(6) 10.4172/2167-0269.1000324 (2017)
- 23.Abebe, A., Chalachew, A., Minwuyelet, M., Goraw, G. The fish and the fisheries of Lake Tana. In: Stave K. GG, Aynalem S, editor. Social and Ecological System Dynamics, (Springer, 2017).
- 24.Melese, W. Lake Tana as biosphere reserve: Review, J. Tourism Hosp. 6, 5 10.4172/2167-0269.1000310 (2017).
- 25.WHO A practical guide to identification ofAfrican freshwater snails. Danish Biharziasis Laboratory in Collaboration with World Health Organization. 1–13 (1980).
- 26.Falade MO, Otarigho B. Shell morphology of three medical important tropical freshwater pulmonate snails from five sites in South-Western Nigeria. Int. J. Zool. Res. 2015;11:140–150. doi: 10.3923/ijzr.2015.140.150. [DOI] [Google Scholar]
- 27.Brown DS. Freshwater snails of Africa and their medical importance. Taylor and Francis; 1994. [Google Scholar]
- 28.Jordaens K, Bruyndoncx L, Van Goethem J, Backeljau T. Morphological and anatomical differentiation of three land snails of the genus Rhynchotrochus (Gastropoda: Pulmonata: Camaenidae) J. Molluscan. Stud. 2008;75(1):1–8. doi: 10.1093/mollus/eyn035. [DOI] [Google Scholar]
- 29.Manyangadze T, Chimbari MJ, Rubaba O, Soko W. Mukaratirwa S Spatial and seasonal distribution of Bulinus globosus and Biomphalaria pfeifferi in Ingwavuma, uMkhanyakude district, KwaZulu-Natal South Africa: Implications for schistosomiasis transmission at micro-geographical scale. Parasites Vectors. 2021;14(1):222. doi: 10.1186/s13071-021-04720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian-Bi Y-NT, Webster B, Konan CK, Allan F, Diakité NR, Ouattara M, et al. Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d’Ivoire. Parasites Vectors. 2019;12(1):117. doi: 10.1186/s13071-019-3381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frandsen F, Christensen N. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trématode species of medical and veterinary importance. Acta Trop. 1984;4:181–202. [PubMed] [Google Scholar]
- 32.Fatima MA, Maikaje DB, Umar YA. Cercarial diversity in freshwater snails from selected freshwater bodies and its implication for veterinary and public health in Kaduna State Nigeria. World Acad. Sci., Eng. Technol. Int. J. Anim. Vet. Sci. 2018;12(2):52–58. [Google Scholar]
- 33.Anucherngchai S, Tejangkura T, Chontananarth T. Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin, Central Thailand. Asian Pacific J. Tropical Biomed. 2016;6(6):539–545. doi: 10.1016/j.apjtb.2016.01.015. [DOI] [Google Scholar]
- 34.Seid T, Jemal B, Delenasaw Y, Belayhun M, YihunDechassa AT, et al. Environmental determinants of distribution of freshwater snails and trematode infection in the Omo gibe River basin, southwest Ethiopia. Infect. Dis. Poverty. 2019;8(1):93. doi: 10.1186/s40249-019-0604-y(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dida GO, Gelder FB, Anyona DN, Matano A-S, Abuom PO, Adoka SO, et al. Distribution and abundance of schistosomiasis and fascioliasis host snails along the mara river in Kenya and Tanzania. Infect. Ecol. Epidemiol. 2014 doi: 10.3402/iee.v4.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ketema D, Etana J, Belayhun M, Zeleke M, Delenasaw Y, Yihun A, et al. Effects of land use on intermediate snail host fauna, abundance, distribution and cercariae infection rate in Omo-Gibe river basin Ethiopia. Res. Sq.: Preprent. 2020 doi: 10.21203/rs.2.22079/v1(2020). [DOI] [Google Scholar]
- 37.Magero, V. O., Kisara, S., Wade, C. M. Geographical distribution of Biomphalaria pfeifferi snails in East Africa bioRxiv Preprint. 2021:2021.11.04.467236 10.1101/2021.11.04.467236.
- 38.De Kock KN, Wolmarans CT, Bornman M. Distribution and habitats of the snail Lymnaea truncatula, intermediate host of the liver fluke Fasciola hepatica South Africa. J. S. Afr. Vet. Assoc. 2004;74(4):117–122. doi: 10.4102/jsava.v74i4.523. [DOI] [PubMed] [Google Scholar]
- 39.Salawu O, Odaibo A. Preliminary study on ecology of Bulinus jousseaumei in Schistosoma haematobium endemic rural community of Nigeria. Afr. J. Ecol. 2012;51(3):441–446. doi: 10.1111/aje.12054. [DOI] [Google Scholar]
- 40.Opisa S, Odiere MR, Jura WGZO, Karanja DMS. Mwinzi PNM Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu city, western Kenya. Parasites Vectors. 2011;4(1):226. doi: 10.1186/1756-3305-4-226(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousif F, Kamel G, el Emam M, Mohamed S. Ecology of Biomphalaria alexandrina the snail vector of Schistosoma mansoni in Egypt. J. Egypt Soc. Parasitol. 1993;23(1):29–42. [PubMed] [Google Scholar]
- 42.Gouvras, A. N., Allan, F., Kinung'hi, S., Rabone, M., Emery, A., Angelo, T., et al. Longitudinal survey on the distribution of Biomphalaria sudanica and B. choanomophala in Mwanza region, on the shores of Lake Victoria, Tanzania: implications for schistosomiasis transmission and control, Parasites Vectors. 10(1), 316 10.1186/s13071-017-2252-z (2017). [DOI] [PMC free article] [PubMed]
- 43.Ismail HA, Abed EEA, Young-Ha L, Mousab SE, Youngjin K, Seungman C, et al. Population dynamics of intermediate-host snails in the white nile river, Sudan: A year-round observational descriptive study. Korean J. Parasitol. 2021;59(2):121–129. doi: 10.3347/kjp.2021.59.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulkadir FM, Maikaje DB, Umar YA. Ecology and distribution of freshwater snails in Gimbawa dam, Kaduna state Nigeria. NJCR. 2017;22(2):98–106. [Google Scholar]
- 45.Odero, S.O., Ogonda, L., Sang, D., Munde, E.O., Shiluli, C., Chweya, P. Distribution of biomphalaria snails in associated vegetations and schistosome infection prevalence along the shores of lake victoria in mbita, Kenya: A cross-sectional study East Afr. Health Res. J. 3(2), 172–7 10.24248/eahrj-d-19-00013 (2019) [DOI] [PMC free article] [PubMed]
- 46.Rowel, C., Fred, B., Betson, M., Sousa-Figueiredo, J.C., Kabatereine, N.B., Stothard, J.R. Environmental epidemiology of intestinal schistosomiasis in Uganda: Population dynamics of Biomphalaria (Gastropoda: Planorbidae) in Lake albert and lake victoria with observations on natural infections with digenetic trematodes, Biomed. Res. Int. 2015, 717261 10.1155/2015/717261 (2015). [DOI] [PMC free article] [PubMed]
- 47.Sidy, B., Christopher, J. E. H, Cheikh Tidiane, B., Nicolas, J., Gilles, R,. Jason Robert, R. Seasonal Variations of Densities of Biomphalaria pfeifferi, the Intermediate Host of Schistosoma mansoni Parasite at the North of Senegal. In: Sajal R, Soumalya M, editors. Update on Malacology (2021).
- 48.Marie M.-A.S., El-Deeb. F. A. A., Hasheesh, W. S., Mohamed, R. A., Sayed, S. S. M. Impact of seasonal water quality and trophic levels on the distribution of various freshwater snails in four egyptian governorates, Appl. Ecol. Environ. Res. 3(4), 117–26 10.12691/aees-3-4-4 (2015).
- 49.Bakuza JS, Gillespie R, Nkwengulila G, Adam A, Kilbride E, Mable BK. Assessing S. mansoni prevalence in Biomphalaria snails in the Gombe ecosystem of western Tanzania: The importance of DNA sequence data for clarifying species identification. Parasites Vectors. 2017;10(1):584. doi: 10.1186/s13071-017-2525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alemayehu B, Tomass Z, Wadilo F, Leja D, Liang S, Erko B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone Southern Ethiopia. BMC Public Health. 2017;17:587. doi: 10.1186/s12889-017-4499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gashaw A, Zeleke M, Berhanu E. A new focus of Schistosoma mansoni in Hayk town, northeastern Ethiopia. BMC Res. Notes. 2015;8:22. doi: 10.1186/s13104-014-0965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hailegebriel T, Nibret E, Munshea A. Prevalence of Schistosoma mansoni and S haematobium in snail intermediate hosts in Africa: A systematic review and Meta-analysis. J. Trop. Med. 2020;2020:8850840. doi: 10.1155/2020/8850840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gashaw F, Aemero M, Legesse M, Petros B, Teklehaimanot T, Medhin G, et al. Prevalence of intestinal helminth infection among school children in Maksegnit and Enfranz Towns, northwestern Ethiopia, with emphasis on Schistosoma mansoni infection. Parasites Vectors. 2015;8(1):567. doi: 10.1186/s13071-015-1178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuss A, Mazigo HD, Mueller A. Malacological survey to identify transmission sites for intestinal schistosomiasis on Ijinga Island Mwanza, North-western. Tanzania Acta Trop. 2020;203:105289. doi: 10.1016/j.actatropica.2019.105289. [DOI] [PubMed] [Google Scholar]
- 55.Ayanda OI. Prevalence of snail vectors of schistosomiasis and their infection rates in two localities within Ahmadu bello university (ABU) Campus Zaria, Kaduna State, Nigeria. J. Cell Anim. Biol. 2009;3(4):58–61. doi: 10.5897/JCAB.9000122. [DOI] [Google Scholar]
- 56.Nzalawahe J. Trematode infections in freshwater snails and seasonal variations in Iringa and Arumeru districts. Tanzania TVJ. 2021 doi: 10.4314/tvj.v36i1.3. [DOI] [Google Scholar]
- 57.Ismail HAHA, Ahmed AEERM, Cha S, Jin Y. The life histories of intermediate hosts and parasites of Schistosoma haematobium and Schistosoma mansoni in the white Nile river Sudan. Int. J. Environ. Res. Public Health. 2022;19(3):1508. doi: 10.3390/ijerph19031508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okeke OC, Ubachukwu PO. Trematode infections of the fresh water snail Biomphalaria pfeifferi from a south-east Nigerian community with emphasis on cercariae of Schistosoma. J. Helminthol. 2017;91:295–301. doi: 10.1017/S0022149X16000353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.